Abstract
Sperm cells acquire hyperactivated motility as they ascend the female reproductive tract, which enables them to overcome barriers and penetrate the cumulus and zona pellucida surrounding the egg. This enhanced motility requires Ca2+ entry via cation channel of sperm (CatSper) Ca2+-selective ion channels in the sperm tail. Ca2+ entry via CatSper is enhanced by the membrane hyperpolarization mediated by Slo3, a K+ channel also present in the sperm tail. To date, no transmitter-mediated currents have been reported in sperm and no currents have been detected in the head or midpiece of mature spermatozoa. We screened a number of neurotransmitters and biomolecules to examine their ability to induce ion channel currents in the whole spermatozoa. Surprisingly, we find that none of the previously reported neurotransmitter receptors detected by antibodies alone are functional in mouse spermatozoa. Instead, we find that mouse spermatozoa have a cation-nonselective current in the midpiece of spermatozoa that is activated by external ATP, consistent with an ATP-mediated increase in intracellular Ca2+ as previously reported. The ATP-dependent current is not detected in mice lacking the P2X2 receptor gene (P2rx2−/−). Furthermore, the slowly desensitizing and strongly outwardly rectifying ATP-gated current has the biophysical and pharmacological properties that mimic heterologously expressed mouse P2X2. We conclude that the ATP-induced current on mouse spermatozoa is mediated by the P2X2 purinergic receptor/channel. Despite the loss of ATP-gated current, P2rx2−/− spermatozoa have normal progressive motility, hyperactivated motility, and acrosome reactions. However, fertility of P2rx2−/− males declines with frequent mating over days, suggesting that P2X2 receptor adds a selection advantage under these conditions.
Keywords: infertility, P2X channel, purinergic ion channel, reproduction
Spermatozoa become motile after they are deposited into the female reproductive tract. This initial motility requires intracellular ATP-dependent dynein motors and does not require calcium. As they ascend the female reproductive tract, spermatozoa undergo an incompletely understood set of changes, aggregately called capacitation, that enable them to fertilize the egg. Capacitation, at a minimum, involves enhancement in motility and the ability to release the acrosome. Hyperactivation of motility, in contrast to initial motility, requires calcium permeation through the cation channel of sperm (CatSper) channel complex (1–4). In mice, CatSper channel activation appears to be dependent on increasing alkalinity, beginning at the cervix and continuing into the uterus and oviduct. In human ejaculated sperm but not mouse epididymal sperm, progesterone also activates CatSper ion channels (5, 6). Many biomolecules are present in the female reproductive tract, such as hormones (e.g., progesterone, estradiol); growth factors [insulin-like growth factor and EGF (7)]; and neurotransmitters, such as glycine (8), glutamate (8), and GABA (9). Immunohistochemistry and cytochemistry studies have suggested that male germ cells and spermatozoa express several types of hormonal and neurotransmitter receptors (10, 11), but their function has never been directly measured.
In previous work, direct recordings of mouse epididymal sperm under a whole-cell voltage clamp reveal two primary currents: ICatSper (2) and IKSper (12). ICatSper is a complex ion channel encoded by four separate gene products that comprise the pore lining subunits (CatSper1–4) and at least three accessory membrane-spanning subunits: β, γ (13), and δ (14). The CatSper channel is activated by increasing alkalinity and is absolutely required for fertility in mammals, such as mice and humans (4, 14–19). IKSper (12) is encoded by mSlo3, a sperm-specific K+ channel with reduced K+ selectivity and sensitivity to pH (20, 21). In human spermatozoa, CatSper, a voltage-gated proton current (Hv1) (22), and an unknown K channel are present, but only the loss of CatSper is known to produce human male infertility to date. In human ejaculated spermatozoa, progesterone enhances gating of CatSper (5, 6). Here, we describe a third ion conductance directly measured in mouse spermatozoa, ATP-gated current (IATP), and show that it is a purinergic P2X2 receptor channel.
Results
ATP Activates an Inwardly Rectifying Current in the Midpiece of the Spermatozoa.
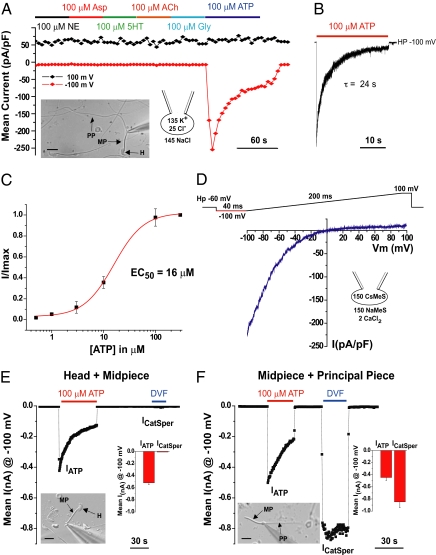
Mouse whole-spermatozoon currents were measured in response to repetitive voltage ramps over the physiological range as hormones and neurotransmitters were applied. Progesterone, adenosine, glycine, GABA, serotonin, norepinephrine, aspartate, and acetylcholine applications (Fig. 1A and Fig. S1A) induced no detectable current. In contrast, 100 μM ATP induced a large and fast-activating inward current (Fig. 1 A and B) that desensitized with a time constant (τ) of 24 s (Fig. 1B). Desensitization was less pronounced at lower ATP concentrations (Fig. S1B). ATP activation of the current was dose-dependent with an EC50 for peak current of 16 μM (Fig. 1C). To abrogate IKSper, Cs+ replaced K+ in the pipette solution, revealing that IATP strongly inwardly rectified (Fig. 1D).
Fig. 1.
ATP gates a mouse spermatozoan channel. (A) Responses to saturating concentrations of neurotransmitters: glycine, serotonin, norepinephrine, aspartate, acetylcholine, and ATP. Currents were evoked by 1-s voltage ramps from −100 to 100 mV applied every 4 s from a holding potential of −60 mV. Black diamonds are average currents at +100 mV; red diamonds are average currents at −100 mV (n = 6). ACh, acetylcholine; Asp, aspartate; Gly, glycine; HP, holding potential; 5-HT, serotonin; NE, norepinephrine. (Inset) Differential interference contrast (DIC) image of the whole spermatozoa under patch clamp showing the head, midpiece, and principal piece. H, head; MP, midpiece; PP, principal piece. (Scale bar: 10 μM.) (B) Fast-activating and slowly desensitizing (τ = 24 ± 4 s; n = 6) current evoked by ATP in a sperm cell held at −100 mV. (C) ATP concentration–response curve. Data points were fitted with the Hill equation. EC50 = 16 μM ATP (n = 8). I/Imax = ratio of measured current to maximum current. (D) Representative IATP in response to voltage ramp. Vm, membrane voltage. (E) IATP recordings from the head plus midpiece fragment. nA, nanoAmperes. (Inset) DIC image, summary histogram (n = 5). ICatSper, specific to the tail fragment, is not present in the head plus the midpiece. (F) ICatSper and IATP were recorded from the midpiece plus the principal piece. (Inset) DIC image, summary histogram (n = 4).
Sperm fragments containing only the head plus the midpiece or only the midpiece plus the principal piece were then used to determine the localization of IATP. ATP (100 μM) activated a large inwardly rectifying current in the head plus midpiece fragment, whereas, as expected, no ICatSper was detected (Fig. 1E). ICatSper and IATP were readily detected in the midpiece plus principal piece fragment (Fig. 1F). There was no statistical difference between IATP measured from the head plus the midpiece (−0.52 ± 0.03 nA) vs. from the midpiece plus principal piece (−0.45 ± 0.05 nA; mean ± SD); thus, we conclude that the strongly inwardly rectifying IATP originates from the midpiece of the mouse spermatozoon.
IATP Is a Cation Nonselective Ca2+ Permeable Current.
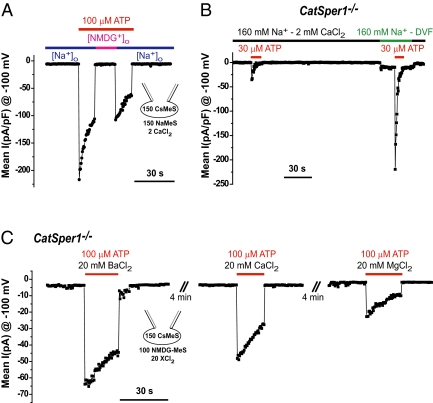
Replacement of bath cations by N-methyl-d-glucamine (NMDG+) abolished IATP (Fig. 2A), suggesting that IATP is cationic. To characterize IATP cation selectivity, we used spermatozoa from CatSper1−/− mice to prevent potential contamination of currents by ICatSper. Removal of Ca2+ from the bath solution [2 mM EGTA, nominally divalent-free solution (DVF)] further increased the current amplitude of the monovalent IATP (Fig. 2B), consistent with Ca2+ binding to the pore. Inward rectification persisted in monovalent solution (Fig. S2A). Currents activated by ATP were also measured with only divalent cations in the bath solution. Inwardly rectifying Ba2+, Ca2+, and Mg2+ currents triggered by ATP were recorded as shown in Fig. 2C and Fig. S2 B and C. We conclude that sperm IATP is an intrinsically rectifying, nonselective, Ca2+-permeant cationic current. These properties are consistent with purinergic ionotropic channels.
Fig. 2.
Cationic nonselective divalent-permeant IATP. (A) No significant IATP was seen when extracellular cations (Na+ and Ca2+) were replaced by NMDG-Cl. (B) Reduction of extracellular divalent cations (Ca2+) in the DVF solution increased the monovalent (Na+) current activated by ATP. (C) Divalent cationic currents elicited by ATP. IATP conducted Ba2+ > Ca2+ > Mg2+, consistent with P2X ion channels.
Pharmacological Properties of Spermatozoa IATP.
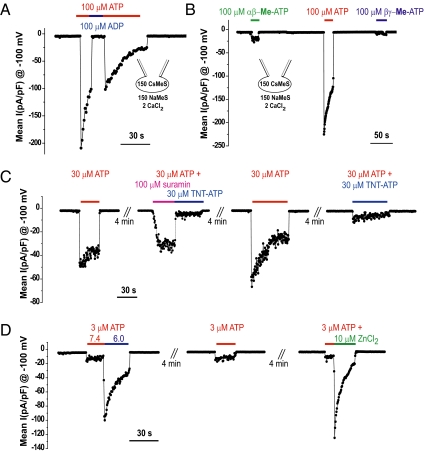
Extracellular ATP activates the seven members of the purinergic ionotropic family (P2X1–7) of ligand-gated ion channels (23). Each subtype has a unique pharmacology that enables partial dissection of the molecular identity of IATP in the mouse spermatozoa. ATP, but not ADP, UTP, α,β-methylene-ATP, β,γ-methylene-ATP, benzoylbenzoyl-ATP, or 2-methylthio-ATP (Fig. 3B and Fig. S3A), activated mouse sperm IATP. The purinergic antagonist trinitrophenyl-ATP reversibly blocked sperm IATP (Fig. S3B), in contrast to suramin, pyridoxalphosphate-6-azophenyl-28,48-disulfonate, and NF023, a suramin analog, (Fig. 3B). This pharmacological profile suggests that either P2X2 or P2X4 receptor may mediate sperm IATP. Although P2X2 and P2X4 are both potentiated by Zn2+ (24, 25), only P2X2 homomeric channels are potentiated by acidic pH (25, 26). We found that extracellular acidification (pH 6.0) and Zn2+ (10 μM) increased IATP by threefold (Fig. 3C). Taken together, these results point to P2X2 as the purinergic receptor most likely responsible for IATP.
Fig. 3.
Pharmacological characterization of IATP. (A) Fast exchange perfusion system application of ATP analogs. ATP activated IATP, which was not activated when ADP replaced ATP. Short ramp protocol (200 ms from −100 to 100 mV, holding potential = −60 mV) was applied every 0.5 s. pA; pF. (B) α,β-Methylene–ATP and β,γ-methylene–ATP evoked currents were 7% and 4% of those evoked by ATP. αβ-Me, α,β-methylene; βγ-Me, β,γ-methylene. (C) Block of IATP by 100 μM suramin was 44 ± SEM% compared with ∼90% block by 30 μM trinitrophenyl-ATP (TNT-ATP). (D) Acid pH (6.0) and 10 μM Zn2+ potentiated IATP approximately fivefold in mouse sperm cells (n = 5).
Expression of ATP Receptor in Mouse Sperm Cells.
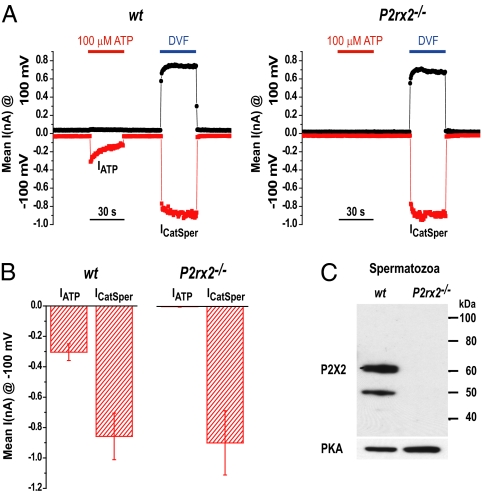
We detected two transcripts encoding P2X2 receptors in mouse testis (Fig. S4A), in agreement with a previous report (27). P2X2 protein was detected by Western blotting in wt mouse testis and spermatozoa (Fig. 4C and Fig. S4B). P2X2 antibody recognized a prominent band migrating at ∼70 kDa in testis lysates (Fig. S4B), as well as bands of 64 and 57 kDa, consistent with the N-linked glycosylation pattern previously shown for P2X2 receptors (28). However, only the 60- and 52-kDa bands were detected in lysates from isolated spermatozoa, suggesting that P2X2 was not glycosylated under these conditions or was partially proteolyzed (Fig. 4C, the lower band is consistent with the molecular mass predicted for the primary amino acid sequence, 485 amino acids of P2X2a). Confirming the absence of P2X2 protein in the KO mice, no P2X2-reactive bands were observed from lysed spermatozoa from P2rx2−/− males. Unfortunately, the P2X2 antibody was inadequate for immunocytochemistry.
Fig. 4.
Absence of IATP in P2rx2−/− spermatozoa. (A) Spermatozoa (wt and P2rx2−/− ) were recorded blinded to genotype. ICatSper and IATP are from a 400-ms voltage ramp from −100 to 100 mV every 0.5 s (holding potential = 0 mV). Each symbol represents the current amplitude at −100 mV (red squares) and +100 mV (black circles). (B) Average IATP and ICatSper measured from wt (−319 ± 54 pA and −858 ± 153 pA, respectively; n = 7) and P2rx2−/− (−1.2 ± 4 pA and −898 ± 200 pA, respectively; n = 5) sperm cells. pA. (C) Sperm cell lysates from wt and P2rx2−/− mice were analyzed by Western blotting using anti-P2X2 antibody. Protein kinase A regulatory subunit type II was the loading control. PKA, protein kinase A.
P2X2 Receptor Channels Mediate IATP in Mouse Spermatozoa.
We cloned a longer transcript corresponding to the cDNA of the P2X2a receptor from a mouse testis library. Expression in HEK-293T cells reconstituted the essential properties of sperm IATP (Fig. S5). Most importantly, we found that mice lacking the P2rx2 gene lack IATP (Fig. 4 A and B), whereas ICatSper remained unchanged. These results identify the P2X2 receptor channel as underlying the large ionotropic purinergic current in mouse spermatozoa.
P2X2: Spermatozoan Function.
P2rx2−/− mice exhibited no defects in sperm morphology, sperm count, motility, or percent of sperm undergoing the acrosome reaction (Figs. S6 and S7). Capacitated sperm reportedly swim faster in the presence of millimolar levels of ATP (29). In agreement with this report, we observed slightly faster swimming with 1 mM ATP (Fig. S6C), but hyperactivated motility was not significantly different in wt and P2rx2−/− sperm cells (Fig. S6 C–E). In addition, in vitro fertilization (IVF) assays were not affected by the presence or absence of apyrase (an ATP diphosphohydrolase; Table S1). Finally, male fertility was similar in P2rx2−/− (7.2 pups per litter) and wt mice (7.8 pups per litter).
Mitochondria and P2X2 channels are localized to the sperm midpiece, where ATP activation of these channels increases Ca2+ influx. Increases in intracellular Ca2+ energizes sperm mitochondria (30), presumably as a consequence of Ca2+-dependent potentiation of enzymes in the Kreb's cycle (e.g., pyruvate dehydrogenase) (31). Thus, we investigated whether purinergic agonists increase mitochondrial energy production. Initial basal ATP levels (0.6 ± 0.2 vs. 0.7 ± 0.1 nmol per million sperm cells) were similar in spermatozoa from wt and P2rx2−/− mice, which was unchanged after 30 min or 1 h of incubation under capacitating conditions. Removal of carbon sources from the incubating medium human tubal fluid (HTF) resulted in loss of wt and P2rx2−/− spermatozoa motility. Readdition of metabolic substrates or ATP rapidly recovered sperm motility; 300 μM ATP restored sperm motility to levels comparable to sperm incubated in complete HTF medium (Movies S1–S6). Finally, we found no difference in restoration of motility by addition of ATP to sperm cells from wt and P2rx2−/− mice. Interestingly, a rapid increase in intracellular ATP [(ATP)i] was observed in wt and P2rx2−/− mouse spermatozoa 1 min after ATP addition, presumably attributable to the rapid transmembrane ATP transporter adenine nucleotide translocator 4 (32). The speed and efficiency of ATP uptake prevented us from determining the relative enhancement of energy production by Ca2+ entry through P2X2 channels.
We searched for an ATP source in the mouse female reproductive tract. ATP levels in uterine fluid recovered after mating were <1 μM. Oviductal fluid from superovulated virgin female mice did not evoke sperm IATP in patch-clamp recordings. Cumulus cells or ovulated eggs were unlikely to be an ATP source because apyrase did not inhibit IVF. Because of the presence of natural extracellular ATPases, the most likely source of ATP is from transient cellular release after collision of spermatozoa with cells along the route of fertilization. However, using “patch-cramming” (33), a method in which the patch-clamped spermatozoon or a Hek-293T cell transfected with mouseP2X2 cDNA is forced into a cell, cumulus-oocyte complex (COC), or oocyte (absent its zona pellucida) in this case, failed to activate IATP. Osmotic swelling or shrinking of the egg or COC also yielded negative results. These results suggest that the female reproductive tract might not be the primary site of sperm P2X2 receptor function.
The P2X2 receptor is potentiated by acidic pH or 10 μM Zn2+ (Fig. 3D), conditions also found in the epididymal lumen (34–37). The epididymis is responsible for sperm transport, concentration, storage, and maturation. We surmised that the midpiece-localized P2X2 channel might potentiate the maturation process to facilitate sperm supply and might be evident in repetitive mating, providing a selective advantage to sperm that can boost mitochondrial ATP production under conditions of high sexual demand. The ideal experimental design is isolation of ejaculated sperm repeatedly and testing their function in vitro. Because the collection method for mouse semen is not well established, we conducted repetitive mating with females to evaluate sperm fertility in vivo. Indeed, after repetitive mating, there is a significant decrease in P2rx2−/− male fertility, as shown in Table 1. The wt and P2rx2−/− males mated with a superovulated female every other day until completion of five successful matings, as judged by the presence of the vaginal plug. The fertility of P2rx2−/− males was comparable to that of wt males in the initial mating, as assessed with conventional breeding tests. Interestingly, P2rx2−/− male fertility gradually declined over repeated mating, whereas wt male fertility remained at ∼90% (Table 1). This result suggests that P2X2 receptor enhances the continuous production of functional sperm under conditions of high demand.
Table 1.
Male fertility after multiple sequential matings
|
wt |
P2rx2−/− |
|||||||||
| Mating* | Live eggs per female (mean ± SD)† | Total eggs | Fertilized eggs | Unfertilized eggs | Fertilized eggs, % | Live eggs per female (mean ± SD)† | Total eggs | Fertilized eggs | Unfertilized eggs | Fertilized eggs, % |
| First | 18.6 ± 12.6 | 93 | 84 | 9 | 90 | 22.0 ± 15.8 | 110 | 101 | 9 | 92 |
| Second | 25.2 ± 11.5 | 126 | 75 | 51 | 60 | 28.6 ± 15.2 | 143 | 121 | 22 | 85 |
| Third | 28.8 ± 10.5 | 144 | 132 | 12 | 92 | 34.8 ± 6.1 | 174 | 130 | 44 | 75 |
| Fourth | 28.4 ± 13.6 | 142 | 134 | 8 | 94 | 23.2 ± 5.8 | 116 | 70 | 46 | 60 |
| Fifth | 24.2 ± 5.2 | 121 | 110 | 11 | 91 | 21.8 ± 14.6 | 109 | 49 | 60 | 45 |
wt and P2rx2−/− males were cohabited with a superovulated female. Oviducts of females with a coupling plug were flushed with PBS to recover unfertilized eggs and fertilized eggs (2-cell embryos) at 1.5 days postcoitus. The first five successful mating were analyzed for fertility.
*To complete five successful matings, wt and P2rx2−/− males required 6.0 ± 0.71 and 7.4 ± 2.1 (mean ± SD) cohabitations, respectively.
†Average number of live eggs from five mated females.
Discussion
Four lines of evidence demonstrate that homomeric P2X2 receptors mediate mouse sperm IATP. First, the P2X2 receptor protein is highly expressed in spermatozoa. Second, ATP evokes IATP in sperm cells with an EC50 of 16 μM; the current is rapidly activating and slowly desensitizes (τ = 24 s). These characteristics mimic heterologously expressed P2X2 and P2X4 homomeric channels (23, 38). Third, IATP's potentiation by both Zn2+ and acidic pH is unique to P2X2 among members of the P2X family (23, 38). Finally, and most importantly, IATP is absent in P2rx2−/− mouse spermatozoon.
What is the functional importance of P2X2 receptor/channels in the spermatozoan midpiece? We postulate that sperm count and/or motility of P2rx2−/− males declines with frequent matings within a short period, resulting in reduced fertility. In laboratories, pups are counted to assess fertility after paired matings for several months. Once a male impregnates the female, the male mouse is relieved for 3 wk before the next mating. This is not the case in the wild, where there is a selective advantage for male mice that can mate repetitively with several females. Given the high evolutionary pressure on gametes, we suggest that P2X2 presence in spermatozoa provides a selective advantage by providing Ca2+ to drive mitochondrial ATP production and enhance sperm fitness. The unanswered question is the source of this extracellular ATP.
Materials and Methods
Reagents.
Unless otherwise indicated, reagents were obtained from Sigma–Aldrich. Apyrase (grade II) was used at a final concentration of 0.4 U/mL. Purinergic receptor agonists were prepared as 1,000-fold concentrated stocks in a 20-mM Hepes solution (pH 7.4) and diluted in the appropriate solution just before use.
Animals.
Male mice of the following strains were used: wt C57BL/6J (The Jackson Laboratory), B6D2F1 (The Jackson Laboratory), B6.129-P2rx2tm1Ckn/J (congenic strain after 7 backcrosses with C57BL/6J, named P2rx2−/−; The Jackson Laboratory), and CatSper1−/−. CD-1 females (Charles River) were used for fertility tests. All animal studies were carried out with the approval of the Children's Hospital Boston Animal Care and Use Committee. Experiments involving wt/mutant paired preparations were done in a blinded manner.
Electrophysiology.
Sperm cells were isolated from the corpus epididymis from C57BL/6, CatSper1−/−, or P2rx2−/− mice 3–8 mo of age. Whole-cell recordings were made on noncapacitated spermatozoa as reported (2). The standard bath solution (HS) contained 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 20 mM Hepes, 5 mM glucose, 10 mM lactic acid, and 1 mM Na pyruvate (pH 7.4) with NaOH. To allow calcium and pH to fluctuate during receptor activation, a weakly pH and Ca2+ buffered pipette solution was used in the initial screening experiments containing 150 mM K-methanesulfonate (MeSO3), 5 mM KCl, 1 mM K4-BAPTA [1,2-bis(o-aminophenoxy)ethane-tetraacetic acid], 5 mM Hepes, and 5 mM MES [2-(N-morpholino)ethanesulfonic acid] (pH 7.0). To characterize IATP, we used a low Cl− bath solution (to reduce any Cl− conductances) that contained 150 mM Na-methanesulfonate (Na-MeSO3), 2 mM CaCl2, 10 mM Na-Hepes, and 10 mM MES (pH 7.4 or 6.0). In experiments designed to measure ICatSper or the effect of divalent ions, we used a DVF containing 150 mM Na-MeSO3, 2 mM Na3HEDTA [(hydroxyethyl)ethylenediaminetriacetic acid], 2 mM EGTA, and 20 mM Hepes (pH 7.4) with NaOH. Divalent solutions were as follows: 110 mM NMDG+, 10 mM Hepes, 10 mM MES, and 20 mM BaCl2 (or 20 mM CaCl2 or mM 20 MgCl2) (pH 7.4) with methanesulfonic acid. The standard pipette solution contained 120 mM Cs-MeSO3, 5 mM CsCl, 10 mM Cs4-BAPTA, 10 mM Hepes, and 10 mM MES (pH 7.2). Solutions were applied to sperm cells (lifted from the coverslips) initially by bath perfusion. For fast solution exchange (∼1 s), an array of hollow quartz fibers was positioned near the sperm cell. After break-in, the access resistance was 25–80 MΩ.
Whole-cell currents were recorded from intact epididymal spermatozoa. To record from the head plus the midpiece or the midpiece plus the principle piece, beheaded and tail-less sperm cells were prepared by trituration. This allowed us to determine currents from each section of spermatozoa (2). All experiments were performed at 22–24 °C. The whole-cell currents were recorded using an Axopatch 200B amplifier (Molecular Devices), acquired with Clampex 9 (pClamp9 Software; Molecular Devices), and analyzed with Origin software (OriginLab). Signals were low-pass filtered at 2 kHz and sampled at 10 kHz. Data are given as mean ± SD.
Western Blotting.
Cauda epididymal spermatozoa isolated from wt and P2rx2−/− mice were incubated at room temperature for 5 min in lysis buffer containing 1% SDS and 20 mM Tris-Cl (pH 8.0). After centrifugation at 15,000 × g for 3 min, the supernatant (containing 106 sperm) was incubated in lithium dodecyl sulfate (LDS) sample buffer (Invitrogen) at 70 °C for 5 min, followed by Western blotting with anti-P2X2 antibody (Chemicon/Millipore) and anti-protein kinase A regulatory subunit type II (Santa Cruz Biotechnology) as a loading control. BenchMark (Invitrogen) was used as a molecular weight marker.
Fertility Tests.
Female CD-1 mice (7–9 wk old) were superovulated by i.p. injections of 5 IU pregnant mare serum gonadotropin (PMSG) (Calbiochem), followed 48 h later by 5 IU human chorionic gonadotropic (hCG) (Calbiochem). The wt and P2rx2−/− males (3–4 mo old) cohabited with single females overnight, and the coupling plug formation was checked the following morning. Frequent mating in shorter periods results in failure of vaginal plug formation, suggesting suppression of mating behavior (probably related to behavioral cues). Our experimental design used males with successful mating histories in short periods. We learned that wt males are able to mate every other day when cohabited with females, suggesting that a 1-d relief period is sufficient for normal mating behavior. Mating was repeated every other day until completion of five successful matings. Mated females were killed, and two-cell embryos and unfertilized eggs were collected from the oviduct by flushing with PBS at ∼1.5 d postcoitus; by that time, all fertilized eggs should have developed to two-cell embryos. Fertility rate was calculated after subtracting the number of degenerating or dead eggs, which appeared dark and flattened by phase contrast microscopy. Some of degenerating eggs may result from low-quality sperm fused to healthy eggs. If this is the case, fertility rates could be overestimated.
Supplementary Material
Acknowledgments
We thank Thomas E. Finger for generously providing us with P2X2/P2X3 double-KO mice (used in the initial screening). We thank the Mental Retardation/Developmental Disabilities Research Center Molecular Genetics Core Facility at Children's Hospital. This work was supported by National Institutes of Health Grant P30-HD18655 and the Bill and Melinda Gates Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111695108/-/DCSupplemental.
References
- 1.Carlson AE, et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci USA. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 3.Qi H, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA. 2007;104:1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren D, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 6.Strünker T, et al. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 7.Revelli A, et al. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40–53. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris SE, Gopichandran N, Picton HM, Leese HJ, Orsi NM. Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology. 2005;64:992–1006. doi: 10.1016/j.theriogenology.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Burrello N, et al. Human follicular fluid stimulates the sperm acrosome reaction by interacting with the gamma-aminobutyric acid receptors. Fertil Steril. 2004;82(Suppl 3):1086–1090. doi: 10.1016/j.fertnstert.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Meizel S. The sperm, a neuron with a tail: ‘Neuronal’ receptors in mammalian sperm. Biol Rev Camb Philos Soc. 2004;79:713–732. doi: 10.1017/s1464793103006407. [DOI] [PubMed] [Google Scholar]
- 11.Naz RK, Sellamuthu R. Receptors in spermatozoa: Are they real? J Androl. 2006;27:627–636. doi: 10.2164/jandrol.106.000620. [DOI] [PubMed] [Google Scholar]
- 12.Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA. 2007;104:7688–7692. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Xia J, Cho KH, Clapham DE, Ren D. CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J Biol Chem. 2007;282:18945–18952. doi: 10.1074/jbc.M701083200. [DOI] [PubMed] [Google Scholar]
- 14.Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun. 2011;2:153–165. doi: 10.1038/ncomms1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avenarius MR, et al. Human male infertility caused by mutations in the CATSPER1 channel protein. Am J Hum Genet. 2009;84:505–510. doi: 10.1016/j.ajhg.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hildebrand MS, et al. Genetic male infertility and mutation of CATSPER ion channels. Eur J Hum Genet. 2010;18:1178–1184. doi: 10.1038/ejhg.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro B, Kirichok Y, Chung JJ, Clapham DE. Ion channels that control fertility in mammalian spermatozoa. Int J Dev Biol. 2008;52:607–613. doi: 10.1387/ijdb.072554bn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci USA. 2001;98:12527–12531. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quill TA, et al. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc Natl Acad Sci USA. 2003;100:14869–14874. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santi CM, et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584:1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci USA. 2011;108:5879–5884. doi: 10.1073/pnas.1100240108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lishko PV, Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J Physiol. 2010;588:4667–4672. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 24.Wildman SS, King BF, Burnstock G. Modulation of ATP-responses at recombinant rP2X4 receptors by extracellular pH and zinc. Br J Pharmacol. 1999;126:762–768. doi: 10.1038/sj.bjp.0702325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wildman SS, King BF, Burnstock G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br J Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King BF, Wildman SS, Ziganshina LE, Pintor J, Burnstock G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br J Pharmacol. 1997;121:1445–1453. doi: 10.1038/sj.bjp.0701286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koshimizu TA, et al. Carboxyl-terminal splicing enhances physical interactions between the cytoplasmic tails of purinergic P2X receptors. Mol Pharmacol. 2006;69:1588–1598. doi: 10.1124/mol.105.019802. [DOI] [PubMed] [Google Scholar]
- 28.Torres GE, Egan TM, Voigt MM. N-Linked glycosylation is essential for the functional expression of the recombinant P2X2 receptor. Biochemistry. 1998;37:14845–14851. doi: 10.1021/bi981209g. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Miranda E, et al. Extracellular adenosine 5′-triphosphate alters motility and improves the fertilizing capability of mouse sperm. Biol Reprod. 2008;79:164–171. doi: 10.1095/biolreprod.107.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia J, Reigada D, Mitchell CH, Ren D. CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation. Biol Reprod. 2007;77:551–559. doi: 10.1095/biolreprod.107.061358. [DOI] [PubMed] [Google Scholar]
- 31.Wan B, LaNoue KF, Cheung JY, Scaduto RC., Jr Regulation of citric acid cycle by calcium. J Biol Chem. 1989;264:13430–13439. [PubMed] [Google Scholar]
- 32.Kim YH, et al. Compartmentalization of a unique ADP/ATP carrier protein SFEC (Sperm Flagellar Energy Carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev Biol. 2007;302:463–476. doi: 10.1016/j.ydbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer RH. Patch cramming: Monitoring intracellular messengers in intact cells with membrane patches containing detector ion channels. Neuron. 1990;4:335–341. doi: 10.1016/0896-6273(90)90046-i. [DOI] [PubMed] [Google Scholar]
- 34.Levine N, Kelly H. Measurement of pH in the rat epididymis in vivo. J Reprod Fertil. 1978;52:333–335. doi: 10.1530/jrf.0.0520333. [DOI] [PubMed] [Google Scholar]
- 35.Mawson CA, Fischer MI. Zinc content of the genital organs of the rat. Nature. 1951;167:859. doi: 10.1038/167859a0. [DOI] [PubMed] [Google Scholar]
- 36.Pastor-Soler N, Piétrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology (Bethesda) 2005;20:417–428. doi: 10.1152/physiol.00036.2005. [DOI] [PubMed] [Google Scholar]
- 37.Stoltenberg M, Ernst E, Andreasen A, Danscher G. Histochemical localization of zinc ions in the epididymis of the rat. Histochem J. 1996;28:173–185. doi: 10.1007/BF02331441. [DOI] [PubMed] [Google Scholar]
- 38.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.