Abstract
Unintended anthropogenic deposition of sulfur (S) to forest ecosystems has a range of negative consequences, identified through decades of research. There has been far less study of purposeful S use in agricultural systems around the world, including the application of elemental sulfur (S0) as a quick-reacting fungicide to prevent damage to crops. Here we report results from a three-year study of the transformations and flows of applied S0 in soils, vegetation, and hydrologic export pathways of Napa Valley, CA vineyards, documenting that all applied S is lost from the vineyard ecosystem on an annual basis. We found that S0 oxidizes rapidly to sulfate (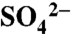 ) on the soil surface where it then accumulates over the course of the growing season. Leaf and grape tissues accounted for only 7–13% of applied S whereas dormant season cover crops accounted for 4–10% of applications. Soil S inventories were largely
) on the soil surface where it then accumulates over the course of the growing season. Leaf and grape tissues accounted for only 7–13% of applied S whereas dormant season cover crops accounted for 4–10% of applications. Soil S inventories were largely 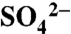 and ester-bonded sulfates; they decreased from 1,623 ± 354 kg ha-1 during the dry growing season to 981 ± 526 kg ha-1 (0–0.5 m) during the dormant wet season. Nearly all S applied to the vineyard soils is transported offsite in dissolved oxidized forms during dormant season rainstorms. Thus, the residence time of reactive S is brief in these systems, and largely driven by hydrology. Our results provide new insight into how S use in vineyards constitutes a substantial perturbation of the S cycle in Northern California winegrowing regions and points to the unintended consequences that agricultural S use may have at larger scales.
and ester-bonded sulfates; they decreased from 1,623 ± 354 kg ha-1 during the dry growing season to 981 ± 526 kg ha-1 (0–0.5 m) during the dormant wet season. Nearly all S applied to the vineyard soils is transported offsite in dissolved oxidized forms during dormant season rainstorms. Thus, the residence time of reactive S is brief in these systems, and largely driven by hydrology. Our results provide new insight into how S use in vineyards constitutes a substantial perturbation of the S cycle in Northern California winegrowing regions and points to the unintended consequences that agricultural S use may have at larger scales.
Keywords: sulfur cycling, sulfur isotopes, hydrologic response, preferential flow, irrigation
Three decades of research have characterized biogeochemical and ecological effects of anthropogenic sulfur (S) deposition on temperate forests in the northeastern United States and Europe. These studies demonstrated that high loadings of reactive S cause soil and surface water acidification (1, 2), base cation depletion in soils (3), changes to forest structure and function (4, 5), and production and bioavailability of other elements, including methyl mercury (6, 7). Whereas S deposition to and consequences for forested ecosystems are unintended, S is purposely applied in many agricultural ecosystems—sometimes as a fungicide, but more commonly as a pH regulator and vital plant nutrient (8). Thus far, research examining S cycling in agricultural systems has focused on crop S demands (9–12), the relative efficacy of sulfate (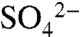 )-supplying fertilizers (13), and the effect of elemental S (S0) oxidation on soil pH and
)-supplying fertilizers (13), and the effect of elemental S (S0) oxidation on soil pH and 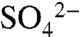 availability (14, 15).
availability (14, 15).
In recent years, regulations and low-S fuels have reduced “free” S supply via atmospheric deposition to croplands, leading to increased demand for S fertilizers (16, 17). The environmental consequences of agricultural S have been largely ignored, except for a few isolated studies, such as examining controls on hydrogen sulfide (H2S) emissions from rice paddies (18), and linking 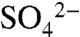 -rich agricultural effluent to production of methyl mercury and changes to the plant community composition in wetlands (19, 20). Targeted S additions may have consequences for soil and water quality that are similar to inadvertent S deposition, but the impacts have not been explored.
-rich agricultural effluent to production of methyl mercury and changes to the plant community composition in wetlands (19, 20). Targeted S additions may have consequences for soil and water quality that are similar to inadvertent S deposition, but the impacts have not been explored.
Vineyards present a particularly intriguing setting to study the fate and transport of S due to the magnitude of the applications and the dramatic contrasts in seasonal hydrologic conditions. In the Napa Valley of Northern California, 810.5 Mg of S0 are applied to the region’s 19,425 ha of vineyards (21) (Fig. 1). During the growing season, which coincides with the dry season in Northern California (April through October), growers broadcast spray S by tractor frequently (e.g., weekly), at low doses (6–14 kg S ha-1), coating vines and soil between trellises with a reactive skin. On average, these applications add up to 100–300 kg S ha-1 y-1, well in excess of the 1973 peak in annual 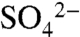 -S deposition to northeastern United States forests of 23 kg
-S deposition to northeastern United States forests of 23 kg 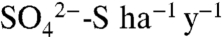 (22). Many growers irrigate vines weekly with drip irrigation systems, using water captured in reservoirs during the dormant (wet) season or pumped from groundwater reserves, creating variably saturated conditions in the soil (23). During the dormant season, S is not sprayed. Total dormant season rainfall ranges from 500 to 1,000 mm in Napa Valley (24), with storms often resulting in saturated conditions in near-surface soils. No study to date has systematically examined the fates and potential consequences of high S applications in these economically important agricultural regions.
(22). Many growers irrigate vines weekly with drip irrigation systems, using water captured in reservoirs during the dormant (wet) season or pumped from groundwater reserves, creating variably saturated conditions in the soil (23). During the dormant season, S is not sprayed. Total dormant season rainfall ranges from 500 to 1,000 mm in Napa Valley (24), with storms often resulting in saturated conditions in near-surface soils. No study to date has systematically examined the fates and potential consequences of high S applications in these economically important agricultural regions.
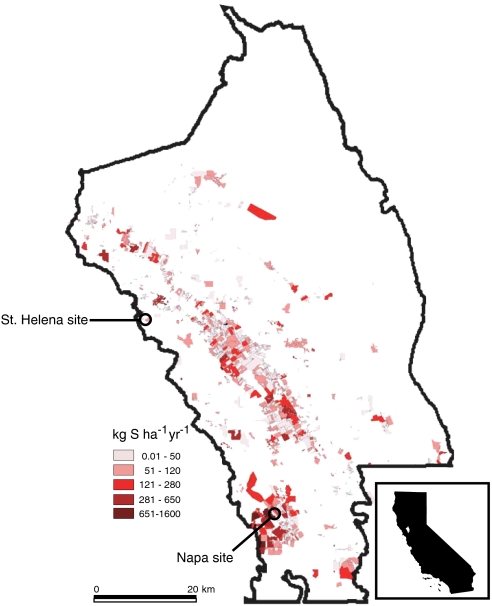
Fig. 1.
Elemental sulfur inputs to Napa County vineyards. Vineyards used in this study are marked with open circles. Inset shows Napa County within the state of California. Data are from the 2002–2003 growing season, the most current countywide dataset available at the time of our study (21). Vineyards occupy approximately 18% of the Napa River watershed.
In this paper, we present data characterizing (i) the major biogeochemical transformations of S occurring over the seasonal cycle, and (ii) the patterns of S input, storage, and export that determine the annual S budget in vineyards. Using two vineyard locations in Napa Valley, CA, we evaluate the immediate fates of applied S0 in soil and examine S retention and loss through soil, vegetation, and hydrologic pathways. Because this paper is a synthesis, it includes previously published data from Hinckley et al. (23, 25) along with analyses that have not yet been reported.
Physical Setting
We conducted this study at two primary research sites in Napa Valley, CA. Immediate oxidation of applied S0 during the growing season was examined at a 2-ha vineyard block in St. Helena, CA (N 38°49′, W -122.53′) chosen for its representative management (with respect to valley-wide average S applications and typical irrigation practices) and distance from marine sources of 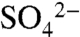 . This site is characterized by Boomer–Forward–Felta rocky loam soils on 30–50% slopes underlain by Franciscan mélange (includes greywacke, chert, serpentinite, and greenstone) (26). Vines are Cabernet Sauvignon grafted on AxR rootstock (a hybrid of Vitis vinifera and Vitis rupestris), planted in rows perpendicular to the dominant slope with predominately western exposure.
. This site is characterized by Boomer–Forward–Felta rocky loam soils on 30–50% slopes underlain by Franciscan mélange (includes greywacke, chert, serpentinite, and greenstone) (26). Vines are Cabernet Sauvignon grafted on AxR rootstock (a hybrid of Vitis vinifera and Vitis rupestris), planted in rows perpendicular to the dominant slope with predominately western exposure.
We measured flows of S into vegetation, soils, and solution waters for 3 y at a 1.5-ha vineyard block in Napa, CA (N 38°27′, W -122°33′). This site is characterized by Bressa–Dibble clay loam soils (0–0.4 m) underlain by a sandy clay hardpan that is consistent across lower Napa Valley and predates vineyard cultivation (26). The textural contrast forms the lower boundary of the rooting zone. Vines are Dijon 114 Pinot noir grafted on 101-14 rootstock, planted in rows perpendicular to the slope with 1.8 m spacing, and were 14 y old at the time of this study. During the dormant season, growers seed vineyard avenues with cover crops (i.e., Rosa, Trifolium, and Triticale spp.) to reduce erosion. During our study, growers applied 105 kg S ha-1 y-1 over the course of the growing season and used a standard weekly drip irrigation, 4 L h-1 vine-1 for 4 h. Following each growing season, they irrigated at the same rate for 8 h to flush nutrients below the rooting zone. A suite of tension and zero-tension lysimeters were distributed throughout the vineyard to measure soil water under saturated conditions and leachate under variably saturated conditions, respectively. We used these instruments to capture the hydrologic response and chemical evolution of solution waters during the period of study.
Results
Transformations of Applied S0.
At the St. Helena site, we evaluated the immediate transformation of applied S0 during the growing season using a combination of elemental measurements of soil (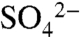 and total combustible S), pH, and X-ray absorption near-edge structure (XANES) spectroscopy. Following each of two applications of 6.7 kg S0 ha-1 to the vineyard, the majority of S0 oxidized during the first 30 min, as reported by Hinckley et al. (25). Soil pH decreased by 0.3 units and
and total combustible S), pH, and X-ray absorption near-edge structure (XANES) spectroscopy. Following each of two applications of 6.7 kg S0 ha-1 to the vineyard, the majority of S0 oxidized during the first 30 min, as reported by Hinckley et al. (25). Soil pH decreased by 0.3 units and 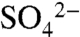 inventories increased by 5.5 kg
inventories increased by 5.5 kg 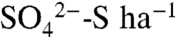 . The increase in the soil
. The increase in the soil 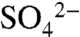 pool (Fig. 2A) scales to approximately 82% of the S applied, which is within the uncertainty of our soil pool estimates. Following each application event, pH rebounded to preapplication levels. The small fraction of applied S not recovered in the soil
pool (Fig. 2A) scales to approximately 82% of the S applied, which is within the uncertainty of our soil pool estimates. Following each application event, pH rebounded to preapplication levels. The small fraction of applied S not recovered in the soil 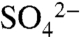 pool likely volatized or drifted beyond the field boundaries.
pool likely volatized or drifted beyond the field boundaries.
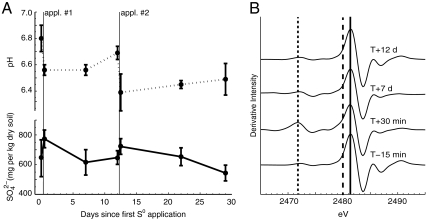
Fig. 2.
Short-term fates of applied S0 in the vineyard setting. (A) Soil pH and 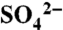 following two applications of S0 (n = 5), and (B) X-ray absorption near-edge structure spectroscopy results showing transformation of applied S0 (2,472.2 eV) to C-bonded S (2,480 eV, on the shoulder of the dominant peak at 2,482 eV), and
following two applications of S0 (n = 5), and (B) X-ray absorption near-edge structure spectroscopy results showing transformation of applied S0 (2,472.2 eV) to C-bonded S (2,480 eV, on the shoulder of the dominant peak at 2,482 eV), and 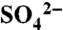 and ester sulfates (2,482 eV). Vertical lines mark the respective locations of these three peaks and time points are relative to the application of S0 (T = 0). [Data are republished here with kind permission from Springer Science+Business Media (25).]
and ester sulfates (2,482 eV). Vertical lines mark the respective locations of these three peaks and time points are relative to the application of S0 (T = 0). [Data are republished here with kind permission from Springer Science+Business Media (25).]
XANES spectroscopy was used to determine the species of S comprising soil reservoirs. Disappearance of the S0 peak in XANES spectra within 7 d of application suggested complete oxidation of S0 to 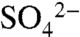 , consistent with measurements of soil pH and
, consistent with measurements of soil pH and 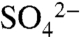 (25). Measurement of the total combustible S and
(25). Measurement of the total combustible S and 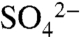 content of the bulk soils allowed for separation of
content of the bulk soils allowed for separation of 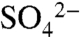 and ester sulfates (organic S) in the spectra, which both peak at 2,482 eV. After the complete oxidation of S0 (marked by the peak at 2,472.7 eV), the major forms of S in these soils are carbon-bonded S (7%, indicated by the peak at 2,480 eV),
and ester sulfates (organic S) in the spectra, which both peak at 2,482 eV. After the complete oxidation of S0 (marked by the peak at 2,472.7 eV), the major forms of S in these soils are carbon-bonded S (7%, indicated by the peak at 2,480 eV), 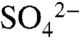 -S (8%), and ester sulfates (85%) (Fig. 2B). These data suggest that
-S (8%), and ester sulfates (85%) (Fig. 2B). These data suggest that 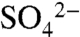 is transformed to ester-bonded sulfates during the growing season.
is transformed to ester-bonded sulfates during the growing season.
The Sulfur Budget.
To develop the field-scale S budget of the vineyard, we measured the S content of major pools and fluxes during the growing and dormant seasons at the Napa, CA study area. These pools and fluxes include aboveground vegetation tissues (leaves and fruit), soil, and soil solution waters (soil matrix water during saturated soil conditions and leachate during variably saturated conditions).
The total S content of vegetation tissues was a relatively small component of the vineyard S budget. During the growing season, S concentrations in vine tissues were 2 ± 0.2 g S kg-1 dry tissue and grapes were 0.4 ± 0.1 g kg-1 dry tissue (n = 5); together these account for 7–14 kg S ha-1. During the dormant season, S concentrations in cover crop tissues were 2.3 ± 0.1 g S kg-1 dry tissue (n = 5), constituting 4–10 kg S ha-1. The combined S contents of vine and cover crop tissues are equivalent to 10–23 % of the S0 applied annually.
Dissolved 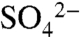 , total dissolved S (TDSu), and the 34S/32S ratio of
, total dissolved S (TDSu), and the 34S/32S ratio of 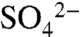 were measured on an event-basis from 2005 through 2007 in precipitation, irrigation, soil water, and leachate within the soil profile. During short (four-hour) irrigation events,
were measured on an event-basis from 2005 through 2007 in precipitation, irrigation, soil water, and leachate within the soil profile. During short (four-hour) irrigation events, 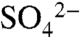 concentrations in leachate were low (4.5 ± 0.66 mg
concentrations in leachate were low (4.5 ± 0.66 mg 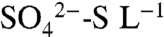 ), but increased to 13.7 ± 2.6 mg
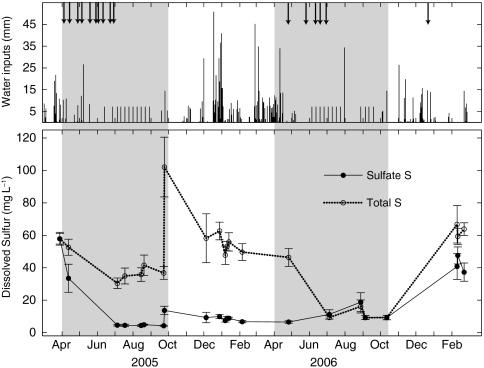
), but increased to 13.7 ± 2.6 mg 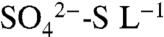 as the soil saturated during longer (> 8-h) irrigation events in 2005 and 2006 (Fig. 3; 23). In all years, dormant season storms flushed accumulated
as the soil saturated during longer (> 8-h) irrigation events in 2005 and 2006 (Fig. 3; 23). In all years, dormant season storms flushed accumulated 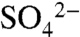 and organic S (the residual of TDSu-
and organic S (the residual of TDSu-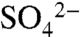 ) from the soil. Elevated concentrations of these constituents were measured during storm events in all three years, with dissolved organic S comprising 72.3 ± 22.7% of S species in solution (Fig. 3). During the wettest season of our study, October 2005 through May 2006, S species in soil and water did not return to growing season levels until applications commenced the following growing season. Based on average TDSu concentrations and water fluxes from the 3 y of study (see Methods), we estimate that 4 ± 1 kg S ha-1 in the growing season and 123 ± 40 kg S ha-1 in the dormant season are exported below the rooting zone via hydrologic pathways.
) from the soil. Elevated concentrations of these constituents were measured during storm events in all three years, with dissolved organic S comprising 72.3 ± 22.7% of S species in solution (Fig. 3). During the wettest season of our study, October 2005 through May 2006, S species in soil and water did not return to growing season levels until applications commenced the following growing season. Based on average TDSu concentrations and water fluxes from the 3 y of study (see Methods), we estimate that 4 ± 1 kg S ha-1 in the growing season and 123 ± 40 kg S ha-1 in the dormant season are exported below the rooting zone via hydrologic pathways.
Fig. 3.
Inputs and solution sulfur dynamics during the period of study. Inputs are precipitation and irrigation water (bars), and elemental sulfur (arrows). Solution includes leachate measured during irrigation events in the growing season (shaded panels) and soil water measured below the rooting zone during the dormant season (white panels). Note the two long (≥8-h) irrigation events on September 25, 2005; August 1, 2006; and October 10, 2006. Values are the average of ≥16 measurements from unique instruments per sample date, ± 1 SD. 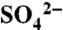 -S data are from Hinckley et al. (23) and are republished/modified by permission of the American Geophysical Union.
-S data are from Hinckley et al. (23) and are republished/modified by permission of the American Geophysical Union.
The δ34S of 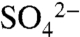 in soil and leachate waters, reported in Hinckley et al. (23), is consistent with the seasonal pattern of soil S transport. The average δ34S of leachate during four-hour irrigation events was low (7.2 ± 3.5‰; Table 1), close to the value of irrigation water (5.7 ± 0.9‰). A leachate isotopic composition close to that of the source water indicates that most water captured below the rooting zone during short irrigations moved there via preferential flow paths, with minimal soil S interaction. In contrast, the S isotopic ratio of soil waters under saturated conditions increased to 13.2 ± 1.4‰, reflecting mobilization of stored soil S.
in soil and leachate waters, reported in Hinckley et al. (23), is consistent with the seasonal pattern of soil S transport. The average δ34S of leachate during four-hour irrigation events was low (7.2 ± 3.5‰; Table 1), close to the value of irrigation water (5.7 ± 0.9‰). A leachate isotopic composition close to that of the source water indicates that most water captured below the rooting zone during short irrigations moved there via preferential flow paths, with minimal soil S interaction. In contrast, the S isotopic ratio of soil waters under saturated conditions increased to 13.2 ± 1.4‰, reflecting mobilization of stored soil S.
Table 1.
Stable isotope values of sulfate (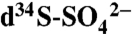 ) in solution measured in Napa, CA [republished/modified by permission of the American Geophysical Union (23)]
) in solution measured in Napa, CA [republished/modified by permission of the American Geophysical Union (23)]
| Component | δ34S* |
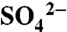 † †
|
n‡ |
| S0 application | −1.5 | NA | 4 |
| Precipitation | 5.5 | 6.7 | 1§ |
| Irrigation water | 5.7(0.9) | 7.1(2.4) | 8 |
| Leachate (4-h)¶ | 7.2(3.5) | 4.5(0.7) | 10 |
| Leachate (8-h)¶ | 9.5(3.6) | 13.7(2.6) | 19 |
| Soil water∥ | 13.2(1.4) | 56.0(18.0) | 74 |
*Units are mean( ± 1 SD).
†Units are mean( ± 1 SD), mg L-1.
‡Number of samples.
§In order to have enough material for analysis, samples of precipitation and irrigation water.
¶Sampled below the vine rooting zone during irrigation events of 4- and 8-h.
∥Sampled below the vine rooting zone during dormant season storm events.
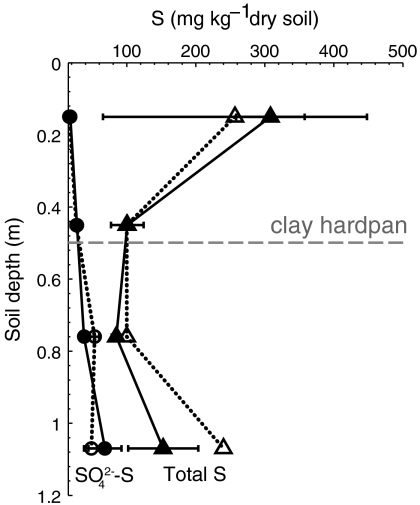
Soil S samples collected before and after the dormant season document the net effect of S transport patterns. Sulfate-S did not differ significantly before and after the dormant season. Within the soil profile, it increased slightly with depth (Fig. 4), potentially due to reprecipitation of CaSO4 in the hardpan (e.g., 27). Unlike 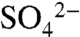 -S, total S content was higher in surface soils (0–0.4 m) than at depth (0.9–1.2 m), and total S content of surface soils decreased from 1623 ± 354 kg ha-1 at the end of the growing season to 981 ± 526 kg ha-1 at the end of the dormant season (Table 2), as determined by scaling soil S content by the measured bulk density of the soil horizons. Whereas the data were highly spatially variable (probably due to variation in S cycling processes within the soil profile), the difference between the mean values, though not statistically significant, supports that export of stored organic S in solution waters occurs during the dormant season.
-S, total S content was higher in surface soils (0–0.4 m) than at depth (0.9–1.2 m), and total S content of surface soils decreased from 1623 ± 354 kg ha-1 at the end of the growing season to 981 ± 526 kg ha-1 at the end of the dormant season (Table 2), as determined by scaling soil S content by the measured bulk density of the soil horizons. Whereas the data were highly spatially variable (probably due to variation in S cycling processes within the soil profile), the difference between the mean values, though not statistically significant, supports that export of stored organic S in solution waters occurs during the dormant season.
Fig. 4.
Sulfate-S and total S through the soil profile at the Napa, CA study site. Values are mean ± 1 SD, n = 6. Closed symbols represent postgrowing season values, and open symbols represent postdormant season values.
Table 2.
Annual vineyard budget measured in Napa, CA
| Component | kg S ha-1* | Soil gain/loss† | |
| Growing season | Sulfur applications | 105 | + |
| Irrigation water | 21(1) | + | |
| Vine leaves | 5–11 | 0 | |
| Soil (0–0.5 m) | 1623(354) | 0 | |
| Wine grapes | 2–3 | - | |
| Leachate | 4(1) | - | |
| Dormant season | Precipitation | 1(0.5) | + |
| Cover crop | 4-10 | 0 | |
| Soil (0–0.5 m) | 981(526) | 0 | |
| Soil water | 123(40) | - | |
| Annual inputs: | 127(1) | ||
| Annual outputs: | 129–130(40) |
*Total S is expressed as mean values (± 1 SD), except for biomass estimates, which are expressed as a range.
†+, −, and 0 denote gain, loss, and no change.
Discussion
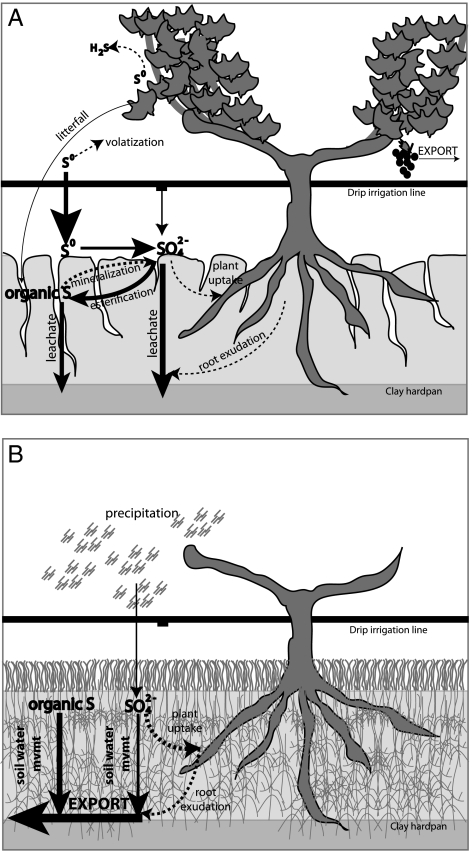
The goal of our study was to understand the transformations, fates, and residence times of the large, highly reactive S loads applied to vineyards each year. In general, the growing season is characterized by rapid biogeochemical transformations following the application of S0 and use of deficit drip irrigation to sustain vines (Fig. 5A), whereas the dormant season is characterized by export of oxidized S species during rainstorms of variable size and duration (Fig. 5B). We found that on an annual basis, S inputs to the vineyard system are roughly balanced by total S export (Table 2).
Fig. 5.
Sulfur transformations and flows in the vineyard setting. (A) The growing season, showing drip irrigation, S0 application, and prevalence of preferential flow paths (e.g., cracks and macropores), and (B) the dormant season, showing cover crop, and lateral transport of S and water caused by large storm events. Size of arrows and text indicates the relative magnitude of each pool and pathway. Dashed arrows depict inferred pathways.
The immediate oxidation of S0 to 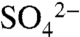 that we observed in vineyard soils is consistent with studies of S0 transformations in other agricultural systems (e.g., 13–15). However, winegrowers apply S more frequently and in a form with a smaller grain size to quickly kill any powdery mildew present, in contrast to most fertilizer S products designed to provide a slow release of
that we observed in vineyard soils is consistent with studies of S0 transformations in other agricultural systems (e.g., 13–15). However, winegrowers apply S more frequently and in a form with a smaller grain size to quickly kill any powdery mildew present, in contrast to most fertilizer S products designed to provide a slow release of 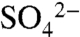 to plants. Consistent with changes in soil
to plants. Consistent with changes in soil 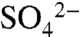 , we observed highly dynamic pH effects, with rapid decreases after S application, followed by rebound over the next 7 d (Fig. 2A). The soils in this study had high base saturation with robust buffering capacity—a feature characteristic of Northern California soils, and likely an important factor driving pH increases between S applications. Moreover, conversion of
, we observed highly dynamic pH effects, with rapid decreases after S application, followed by rebound over the next 7 d (Fig. 2A). The soils in this study had high base saturation with robust buffering capacity—a feature characteristic of Northern California soils, and likely an important factor driving pH increases between S applications. Moreover, conversion of 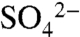 to ester sulfates provides an additional mechanism for neutralizing acidity generated by oxidation of S0.
to ester sulfates provides an additional mechanism for neutralizing acidity generated by oxidation of S0.
With rapid oxidation to mobile forms, high losses of S in leachate waters during irrigation events might be expected. However, Hinckley et al. (23) found that the use of drip or deficit irrigation during the growing season prevents mobilization of soil S forms; losses during the growing season are primarily low-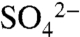 irrigation water that is rapidly transported via preferential flow paths in the near surface (23) (Fig. 3). Thus, irrigation management results in minimal S leaching and transport during the growing season, a result that is also likely under the dry farming management common in many Napa Valley vineyards. As a result, applied S0 accumulates in the surface soil and is not mobilized at the vineyard scale until large (> 8 h) irrigation events or the first dormant season rainstorms (Fig. 3).
irrigation water that is rapidly transported via preferential flow paths in the near surface (23) (Fig. 3). Thus, irrigation management results in minimal S leaching and transport during the growing season, a result that is also likely under the dry farming management common in many Napa Valley vineyards. As a result, applied S0 accumulates in the surface soil and is not mobilized at the vineyard scale until large (> 8 h) irrigation events or the first dormant season rainstorms (Fig. 3).
Soil S may be incorporated into living biomass, adsorbed onto iron and aluminum oxides, transformed by microbes into gaseous and organic forms, or transported in solution via hydrologic pathways. In vineyards, the S content of leafy tissues represents a small fraction of the total annual budget (10–23% of the annual application). In contrast, in their studies of forested systems of the northeastern United States, Likens et al. (3) reported that the majority of S deposition is taken up and cycles through living and dead organic material before returning to the soil, slowing S release over years to decades. On the other hand, vineyards are similar to forested systems in that S uptake is largely an internal process; with the exception of grapes (2–3 kg S ha-1, 1.9–2.8% of the annual application), leaf and woody tissues are returned to the soil each season.
In any soil system, redox state is a strong determinant of the fate of S within the soil matrix. When oxygen concentrations are low, microbial reduction of sulfate is favorable and comprises a significant component of the S cycle in many S-rich environments, both managed (e.g., 18) and unmanaged (28, 29). We observed an increase in the δ34S isotopic ratio of soil water 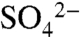 as it moved below the rooting zone (23), an effect that has been attributed to sulfate reduction in other environments (e.g., 30). However, vineyard soils are generally unsaturated during the growing season and contain very limited quantities of organic material. Based on these characteristics, we would expect the soils to remain oxygenated, limiting the potential for microbial S reduction. Indeed, in a companion study, we found little S reduction under laboratory-maintained reducing conditions (31), suggesting that in situ S reduction is minimal. Based on these results, we expect that gaseous losses of reduced S from vineyard soils are not a significant component of the overall S budget. The exception occurs if the powdery mildew organism (Unicula necator) is present at the time of S0 applications (32), which releases H2S upon contact with S0. The flux of gaseous S from the vines has not been quantified.
as it moved below the rooting zone (23), an effect that has been attributed to sulfate reduction in other environments (e.g., 30). However, vineyard soils are generally unsaturated during the growing season and contain very limited quantities of organic material. Based on these characteristics, we would expect the soils to remain oxygenated, limiting the potential for microbial S reduction. Indeed, in a companion study, we found little S reduction under laboratory-maintained reducing conditions (31), suggesting that in situ S reduction is minimal. Based on these results, we expect that gaseous losses of reduced S from vineyard soils are not a significant component of the overall S budget. The exception occurs if the powdery mildew organism (Unicula necator) is present at the time of S0 applications (32), which releases H2S upon contact with S0. The flux of gaseous S from the vines has not been quantified.
Alternatively, the increase in δ34S of soil water 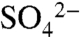 may be due to input of soil S minerals, unmeasured sulfate reduction, or the transformation of soil
may be due to input of soil S minerals, unmeasured sulfate reduction, or the transformation of soil 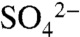 to ester sulfates, which are > 80% of soil S. Although we find that ester sulfate formation is an important process in vineyards and dissolved organic S is the dominant fraction of solution S mobilized during dormant season storms, it has not been cited previously as a major fractionating process. Therefore, the change in soil water isotopic composition that we observed begs further study.
to ester sulfates, which are > 80% of soil S. Although we find that ester sulfate formation is an important process in vineyards and dissolved organic S is the dominant fraction of solution S mobilized during dormant season storms, it has not been cited previously as a major fractionating process. Therefore, the change in soil water isotopic composition that we observed begs further study.
Both solution data (Fig. 3) and soil S inventories (Fig. 4) indicate that S accumulates during the growing season and is removed during the wet season. Spatial and temporal variability—in both S cycling and the location of preferential flow paths (e.g., macropore and bypass flow)—likely contribute to the uncertainty associated with field-scale S export. However, the overall patterns in our data indicate clearly that S transport beyond the vineyard extent is the major fate of S inputs on an annual timescale, and is largely controlled by water availability (Fig. 5B).
Whereas some of our findings are applicable to other agricultural systems where S is used extensively, vineyards are unique in that the cumulative applications of S across hundreds of fields, coupled with changes to the flows of water, constitute a seasonally coordinated hydro-biogeochemical manipulation at the regional scale. Whereas variability in management practices (e.g., S applications and irrigation strategies), soil properties, and rainfall may affect local vineyard S budgets, there are few mechanisms that could operate in any place to promote S retention at the field scale. Furthermore, S use and the general pattern of dry growing seasons followed by wet dormant seasons are consistent across the region. Thus, it is likely that in Napa Valley, the regional perturbation of the S cycle, modulated by the interplay between S management and rainfall, results in the export of the majority of applied S from vineyards, with potentially unintended consequences at the basin scale.
Our study points to the need for greater attention to the consequences of agricultural S use at local to regional scales globally. Sulfur use is not unique to vineyards or Northern California; it is commonly used both as a major nutrient input, and as a “carrier” for other necessary amendments, such as nitrogen and potassium, in a variety of agricultural systems. Although the impacts of its use may be minimal within local fields, transport to downgradient aquatic ecosystems with fluctuating redox conditions could be environmentally disruptive. Future research should explore the consequences of high 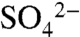 and organic S loads to aquatic ecosystems; redox conditions may be low enough that increased inputs cause large alterations in biogeochemistry, especially mobilization of mercury and other heavy metals.
and organic S loads to aquatic ecosystems; redox conditions may be low enough that increased inputs cause large alterations in biogeochemistry, especially mobilization of mercury and other heavy metals.
Methods
Short-Term Fates of Applied S at the St. Helena, CA Site.
Immediately prior to and following two applications of S0, surface soil samples (0–0.02 m) were analyzed for pH, extractable sulfate (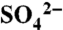 ), total combustible S, and S speciation using XANES spectroscopy at 30 min, 2 d, 7 d, 12 d, and 19 d after each application. A detailed description of these measurements and processing of XANES spectra is in Hinckley et al. (25).
), total combustible S, and S speciation using XANES spectroscopy at 30 min, 2 d, 7 d, 12 d, and 19 d after each application. A detailed description of these measurements and processing of XANES spectra is in Hinckley et al. (25).
Flows of S into Soils, Vegetation, and Hydrological Pathways at the Napa, CA Site.
Soil sampling was conducted on 19–21 October 2005 (postgrowing season) and 9–11 April 2007 (postdormant season) to characterize soil physical and chemical properties. Six cores (0.2 m diam. × 1.2 m depth) were collected in four 0.3 m sections. Sulfate was extracted according to (33, 34) and extracts were subsequently measured on an ion chromatograph (IC) at Stanford University. In preparation for analysis of total S, plant material was removed and soils were sieved (< 2 mm), oven-dried at 60 °C for 24 h, and ground to a fine powder using a rolling mill. Total S was measured by combustion on an elemental analyzer connected to a Micromass Optima isotope ratio mass spectrometer at the US Geological Survey (USGS) Menlo Park Stable Isotope Laboratory in California. Sulfur content of the top 0.5 m was calculated using the measured bulk density value of 1,100 kg m-3 to scale to the hectare.
Vine leaf and grape tissues were collected immediately prior to harvest on August 11, 2007, freeze-dried, ground with a mortar and pestle, and total S was determined by combustion, as described above. We used net primary productivity data (35, 36) to scale total S contents of vine and grape tissues to the hectare. These measurements take into account pruning of leaves and clusters, and have been used in calculation of other regional nutrient budgets (37).
Immediately prior to mowing on March 15, 2005, cover crop tissues were harvested from 0.5 × 0.5 m quadrats (n = 5) randomly located within the study area. Samples were dried at 60 °C for 24 h, weighed to determine dry mass per area, and subsamples were ground for analysis using a mortar and pestle. Total S was determined by combustion, as for soil and vine tissues, and dry biomass weights were used to scale total S to the hectare.
Across the study area, we installed both tension and zero-tension lysimeters below the majority of the rooting zone (at the soil textural contrast) to capture solution losses during irrigation and storm events from 2005 through 2007. Tension lysimeters captured soil water from the soil matrix during saturated conditions (i.e., long irrigation events and dormant season storms) and zero-tension lysimeters sampled leachate—primarily preferential flow—during irrigation events. Complete description of the hydrologic study has been reported previously (23). Precipitation, irrigation water, soil water, and leachate samples were frozen until analysis for 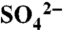 on an IC and total S on an inductively coupled plasma spectrophotometer at Stanford University. All solution samples were prepared for δ34S of
on an IC and total S on an inductively coupled plasma spectrophotometer at Stanford University. All solution samples were prepared for δ34S of 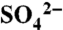 at the USGS (Menlo Park, CA) using BaCl crystals to precipitate BaSO4, as described in Hinckley et al. (23). The data were corrected to Cañ˜on Diablo Troilite (CDT) using standard material NBS-127 (at 21.3‰), along with two in-house standards, and are reported here in delta notation (δ34S) in parts per thousand (‰).
at the USGS (Menlo Park, CA) using BaCl crystals to precipitate BaSO4, as described in Hinckley et al. (23). The data were corrected to Cañ˜on Diablo Troilite (CDT) using standard material NBS-127 (at 21.3‰), along with two in-house standards, and are reported here in delta notation (δ34S) in parts per thousand (‰).
To calculate total dissolved S (TDSu, 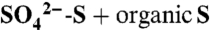 ) in leachate collected during irrigation events, we assumed that zero-tension lysimeters capture a representative TDSu concentration and solution volume and scaled these values, as described in ref. 23. Losses during the 8-h irrigation were calculated separately and added to this value. Soil water S mobilized during the dormant season was calculated using the average TDSu value across all saturated events multiplied by water export (Q). We determined water export using the water balance equation:
) in leachate collected during irrigation events, we assumed that zero-tension lysimeters capture a representative TDSu concentration and solution volume and scaled these values, as described in ref. 23. Losses during the 8-h irrigation were calculated separately and added to this value. Soil water S mobilized during the dormant season was calculated using the average TDSu value across all saturated events multiplied by water export (Q). We determined water export using the water balance equation:
| [1] |
and assumed that soil moisture storage, ΔSM, was the same at the beginning and end of the dormant season period. Precipitation (P) and evapotranspiration (ETA) data for this site are available online (22).
Inputs of S in irrigation and precipitation water were calculated as the average TDSu concentration in each constituent multiplied by the total water inputs (irrigation data supplied by the growers at the Napa, CA study site, precipitation data by ref. 24). Growers at the Napa, CA study site supplied S application data for the study period; total (i.e., cumulative over the growing season) S applications were consistent during the period of study.
Data are reported as mean ± 1 SD, except where noted. Uncertainty was propagated through the S budget calculations using the formula:
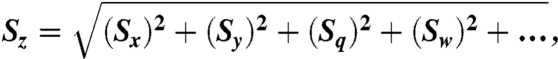 |
[2] |
where Sz is the total uncertainty, and Sx,y,q,w are the uncertainties associated with each mean value in the expression.
Acknowledgments.
We thank Ashley Anderson, Benjamin Falk, Kirk Grace, Sandra L. Perry, and Debby Zygielbaum for site access. We also thank Scott Fendorf and Keith Loague, who contributed to supporting studies; and Matthew Long, Kimberly Nicholas, and Peter Vitousek, who provided helpful comments and discussion on early drafts. Charles Driscoll and Carol Kendall critically reviewed the final manuscript. E.S.H was supported by an Environmental Protection Agency Science to Achieve Results (STAR) Fellowship and a grant from the Geological Society of America, as well as Stanford University funds to P.A.M.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cronan CS, Schofield CL. Relationships between aqueous aluminum and acidic deposition in forested watersheds of North America and northern Europe. Environ Sci Technol. 1990;24:1100–1105. [Google Scholar]
- 2.Charles DF, Christie S. Acidic Deposition and Aquatic Ecosystems: Regional Case Studies. New York: Springer; 1991. p. 747. [Google Scholar]
- 3.Likens GE, et al. The biogeochemistry of sulfur at Hubbard Brook. Biogeochemistry. 2002;60:235–316. [Google Scholar]
- 4.Shortle WC, Smith KT, Minocha R, Lawrence GB, David MB. Acidic deposition, cation mobilization, and biochemical indicators of stress in healthy red spruce. J Environ Qual. 1997;26:871–876. [Google Scholar]
- 5.Horsley SB, Long RP, Bailey SW, Hallett RA, Wargo PM. Health of eastern North American sugar maple forests and factors affecting decline. North J Appl For. 2002;11:34–44. [Google Scholar]
- 6.Driscoll C, Driscoll KM, Mitchell MJ, Raynal DJ. Effects of acidic deposition on forest and aquatic ecosystems in New York State. Environ Pollut. 2003;123:327–336. doi: 10.1016/s0269-7491(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 7.Driscoll C, et al. Mercury contamination in forest and freshwater ecosystems in the northeastern United States. Bioscience. 2007;57:17–28. [Google Scholar]
- 8.Ober JA. Materials Flow of Sulfur. Reston, VA: USGS; 2002. Open-File Report 02-298. [Google Scholar]
- 9.Haneklaus S, Bloem E, Schnug E. History of sulfur deficiency in crops. In: Jez J, editor. Sulfur: A Missing Link Between Soils, Crops, and Nutrition. Madison, WI: Soil Science Society of America; 2008. pp. 45–58. [Google Scholar]
- 10.Naaem HA. Sulfur nutrition and wheat quality. In: Jez J, editor. Sulfur: A Missing Link Between Soils, Crops, and Nutrition. Madison, WI: Soil Science Society of America; 2008. pp. 153–170. [Google Scholar]
- 11.Aulakh MS, Chhibba IM. Sulfur in soils and responses of crops to its application in Punjab. Fert News. 1992;37:33–45. [Google Scholar]
- 12.Aulakh MS, Pasricha NS. Role of sulphur in the production of the grain legumes. Fert News. 1986;31:31–35. [Google Scholar]
- 13.Slaton NA, Ntamatungiro S, Wilson CE, Jr, Norman RJ. Research Series 460. Fayetteville, AR: 1998. Rice Research Series 1997. Arkansas Agricultural Experiment Station. [Google Scholar]
- 14.Germida JJ, Janzen HH. Factors affecting the oxidation of elemental sulfur in soils. Nutr Cycl Agroecosys. 1993;35:101–114. [Google Scholar]
- 15.Slaton NA, Norman RJ, Gilmour JT. Oxidation rates of commercial elemental sulfur products applied to an alkaline silt loam from Arkansas. Soil Sci Soc Am J. 2001;65:239–243. [Google Scholar]
- 16.Irwin JG, Campbell G, Vincent K. Trends in sulphate and nitrate wet deposition over the United Kingdom: 1986–1999. Atmos Environ. 2002;36:2867–2879. [Google Scholar]
- 17.Kelly TD, Matos GR. Historical statistics for mineral and material commodities in the United States: US Geological Survey Data Series. USGS Report Number 140. 2009. http://pubs.usgs.gov/ds/2005/140/
- 18.Wind T, Conrad R. Localization of sulfate reduction in planted and unplanted rice field soil. Biogeochemistry. 1997;37:253–278. [Google Scholar]
- 19.Bates AL, Orem WH, Harvey JW, Spiker EC. Tracing sources of sulfur in the Florida Everglades. J Environ Qual. 2002;31:287–299. doi: 10.2134/jeq2002.2870. [DOI] [PubMed] [Google Scholar]
- 20.National Research Council. Progress Toward Restoring the Everglades. Washington, DC: The National Academies Press; 2010. pp. 190–197. [Google Scholar]
- 21.California Department of Pesticide. California Department of Pesticide Regulation (CDPR) http://calpip.cdpr.ca.gov/cfdocs/calpip/prod/main.cfm.
- 22.Driscoll C, et al. Acidic deposition in the northeastern United States: Sources and inputs, ecosystem effects, and management strategies. Bioscience. 2001;51:180–198. [Google Scholar]
- 23.Hinckley ES, Kendall C, Loague K. Not all water becomes wine: Sulfur as an opportune tracer of hydrochemical losses from vineyards. Water Resour Res. 2008;44 10.1029/2007WR006672. [Google Scholar]
- 24.California Irrigation Management Information System (CIMIS) CIMIS Data. http://www.cimis.water.ca.gov/cimis/data.jsp.
- 25.Hinckley ES, Fendorf S, Matson PA. Short-term fates of high sulfur inputs in Northern California vineyard soils. Nutr Cycl Agroecosyst. 2010 10.1007/s10705-010-9383-3. [Google Scholar]
- 26.USDA. Soil Survey of Napa County. Napa, CA: Natural Resources Conservation Service; 1978. [Google Scholar]
- 27.Gomez-Miguel V, Peres Arias J, Guerrero F, Roquero C. Universidad Politécnica de Madrid; 1984. The soils and water table properties of the Polder area “Castillo de Dona Blanca,” Puerto de Santa Maria, Cadiz Escuela Técnica Superior de Ingenieros Agrónomos. [Google Scholar]
- 28.Howarth R. Pyrite: Its rapid formation in a salt marsh and its importance in ecosystem metabolism. Science. 1979;203:49–51. doi: 10.1126/science.203.4375.49. [DOI] [PubMed] [Google Scholar]
- 29.Canfield DE, DesMarais DJ. Aerobic sulfate reduction in microbial mats. Science. 1991;251:1471–1473. doi: 10.1126/science.11538266. [DOI] [PubMed] [Google Scholar]
- 30.Alewell C, Gehre M. Patterns of stable S isotopes in a forested catchment as indicators for biological S turnover. Biogeochemistry. 1999;47:317–331. [Google Scholar]
- 31.Hinckley ES. Stanford, CA: Stanford University; 2009. Biogeochemical and hydrologic sulfur dynamics in an agricultural system. PhD dissertation. [Google Scholar]
- 32.Williams JS, Cooper RM. The oldest fungicide and newest phytoalexin—a reappraisal of the fungitoxicity of elemental sulphur. Plant Pathol. 2004;53(3):263–279. [Google Scholar]
- 33.Schumacher BA, et al. Laboratory Methods for Soil and Foliar Analysis in Long-Term Environmental Monitoring Programs. Washington, DC: Environmental Protection Agency; 1995. [Google Scholar]
- 34.Tan Z, McLaren RG, Cameron KC. Forms of sulfur extracted from soils after different methods of sample preparation. Aust J Soil Res. 1994;32:823–834. [Google Scholar]
- 35.Williams LE. Grape. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plants and Crops: Source-Sink Relationship. New York: Marcel Dekker; 1996. pp. 851–881. [Google Scholar]
- 36.Williams LE. Growth and development of grapevines. In: Christensen LP, editor. Raisin Production Manual. Oakland, CA: University of California Agriculture and Natural Resources; 2000. pp. 17–23. [Google Scholar]
- 37.Kroodsma DA, Field CB. Carbon sequestration in California agriculture, 1980–2000. Ecol Appl. 2006;16:1975–1985. doi: 10.1890/1051-0761(2006)016[1975:csica]2.0.co;2. [DOI] [PubMed] [Google Scholar]