Abstract
Heat shock proteins (HSPs) are a defined set of chaperones for maintaining proper functions of proteins. The HSP70 family, one of the most inducible families in response to stress, protects cells from stress-induced cell death. It has been documented that HSP70s are highly expressed in various types of cancer cells and make the cells resistant to adverse microenvironments, such as hypoxia and glucose starvation, which are common features in malignant progression. Over-expression of HSP70s is thus associated with tumor transformation and eventually results in a decrease of chemotherapy efficacy. Notably, the distribution of HSP70s is deregulated in cancer cells. It has been reported that HSP70s localize distinct organelles or are exported to humoral circulation during cancer development. Either surface or exported HSP70s play danger signals and trigger immune response to destroy the tumor cells. In this review, we lay out recent advances in the HSP70s-mediated cancer diagnosis and therapy. This review would be enlightening for clinical cancer medicine.
Introduction
In 1962, the chromosome puffs were observed in heat stressed Drosophila larvae and the encoded-genes were identified as heat shock proteins (HSPs).1,2 HSPs are also known to be mainly transactivated by heat shock factor to chaperone misfolded proteins and rescue cells from adverse environmental stresses, such as PH alteration, ischemia, hypoxia, osmotic and treatment with a number of metals.3 According to molecular weight, HSPs are classified into five families including high molecular mass HSP100s, HSP90s, HSP70s, HSP60s and small HSPs.3 Moreover, HSP100s function to disaggregate denatured proteins, while small HSPs inhibit aggregation of proteins.4–6 HSP90s support the folding of premature proteins and play important signal transducers in cell differentiation and proliferation.7 HSP70s coordinate with HSP40s to fold nascent polypeptide chains,8 while HSP60s, which is so-called chaperonin, and HSP10 are mostly responsible for folding mitochondrial proteins.9 Among them, HSP70s is the most important HSPs for protein folding and is highly associated with tumor progression, which may also provide therapeutic strategy of cancer.
Human Heat Shock Protein 70 Family
In humans, the HSP70 family encompasses multiple distinct genes, which encode a group of highly related proteins: mitochondria resided glucose-regulated protein 75 (GRP75, HSPA9B locus) contains N-terminal mitochondrial localization signal, endoplasmic reticulum (ER) resided glucose-regulated protein 78 (GRP78, HSPA5 locus) contains N-terminal ER localization signal and C-terminal ER retention signal, cytosol/nuclear resided HSC70s (the cognate/constitutive HSP70, HSPA1L, HSPA2 and HSPA8 locus), and also cytosol/nuclear resided but highly inducible and intronless iHSP70s (HSP70/72; HSPA1A, HSPA1B, and HSPA6 loci) (Table 1).10 Recent data indicates that some of HSP70s genes are significantly induced in response to distinct stresses in humans. Two of these genes, HSPA1A and HSPA1B, are found as a nearly identical tandem pair in the major histocompatibility complex at 6p21.3 region. HSPA1A and HSPA1B are coded for two almost identical proteins, the major inducible HSP70s in the stressed-cells.11,12 Another inducible gene of HSP70s, HSPA6, is located on chromosome 1 and is mainly induced by extreme temperature.13,14 However, the genes of HSC70s are ubiquitously expressed at low levels in most tissues, but show high expression level in specific tissues.10,15–17 On the other hand, specific compartment-localized HSP70s, the genes HSPA5 and HSPA9 are not only constitutively expressed, but also induced under specific stress. HSPA5 is specifically induced in response to ER stress, while HSPA9 is induced by glucose starvation and ionizing radiation.18–20 It indicates that HSP70s play crucial roles for cell survival under stresses. Additionally, HSP70s are upregulated in a number of cancer cells, suggesting that HSP70s allow the cells to adapt to adverse microenvironments, such as hypoxia and glucose starvation, which are common features in tumor transformation.
Table 1.
Classification of human heat shock protein 70 family.
| Protein | Gene locus symbol | Intracellular localization | Alternative name | PI/MW (kDa) | Inducibility | Reference |
|---|---|---|---|---|---|---|
| iHSP70s | ||||||
| HSP70-1 | HSPA1A | Nu/Cyto/Lyso | HSP72, Hsp70, Hsp70i, Hsp70-1a | 5.48/70.0 | Yes | 11, 12 |
| HSP70-2 | HSPA1B | Nu/Cyto/Lyso | HSP72, Hsp70, Hsp70-1b | 5.48/70.0 | Yes | 11, 12 |
| HSP70B′ | HSPA6 | Nu/Cyto | Hsp7070-6 | 5.76/70.4 | Yes | 13, 14 |
| HSC70s | ||||||
| HSC70 | HSPA8 | Nu/Cyto | Hsp70-8, HSP73 | 5.81/71.0 | No | 15 |
| HSP70-Hom | HSPA1L | Nu/Cyto | Hsp70-1l, Hsp70t | 5.48/70.0 | No | 16 |
| HSP70-3 | HSPA2 | Nu/Cyto | Hsp70-2, Hsp70-2b | 5.07/72.3 | No | 17 |
| GRP70s | ||||||
| GRP75 | HSPA9 | Mito | Mortalin, mtHsp75, Hsp70-9, PBP74 | 5.37/70.9 | Yes | 19, 20 |
| GRP78 | HSPA5 | ER | Bip, Hsp70-5 | 6.03/73.7 | Yes | 18 |
Abbreviations: Nu: nucleus; Cyto: cytoplasm; Mito: mitochondria; ER: endoplasmic reticulum; PBP: peptide binding protein; Bip: Immunoglobulin heavy chain-binding protein homolog.
Common Functions of HSP70s: Chaperone and Anti-apoptosis
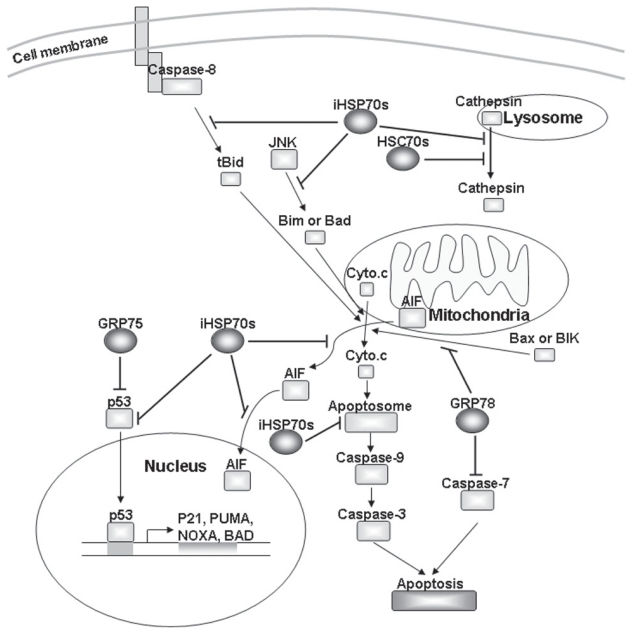
HSP70s function as chaperones to control protein quality or interact with a number of key regulators to modulate cell proliferation, survival, and death.21,22 The primary structure of HSP70s consists of around 45 kD ATPase domain and around 25 kD C-terminal peptide binding domain. The C-terminal domain can be further divided into two subdomains: peptide binding subdomain (15kD) and C-terminal subdomain (10 kD).23 While ATPase domain stimulates ATP hydrolysis to provide energy for protein folding, EEVD sequence of C-terminal subdomain recruits carboxyl terminus of HSC70-interacting protein with ubiquitin E3-ligase activity (CHIP) to trigger degradation of those malfolded proteins, which cannot be recovered.24 HSP70s also associate with co-chaperones including HSP40s and nucleotide exchange factors to efficiently stimulate protein folding. The chaperone activity of HSP70s is also required for blocking apoptosis caused by various stimuli, since deletion of ATPase domain or C-terminal EEVD sequence diminishes the anti-apoptotic effect in response to stress.25 However, while iHSP70s serve as safeguards to protect cells from apoptosis in a chaperone-dependent manner, iHSP70s interfere c-Jun N-terminal kinase (JNK) phosphorylation and abrogate JNK-mediated apoptosis in a chaperone independent manner (Fig. 1).26
Figure 1. Protective mechanisms of HSP70s in stress-induced cell death.
HSP70s block the activation of several death factors to attenuate stress-induced cell death. iHSP70s inhibit caspase-8, JNK, p53, AIF and apoptosome formation to protect cell from apoptosis. Moreover, both iHSP70s and HSC70s stabilize lysosomal membrane and decrease cathepsin-dependent cell death. Besides, GRP78 diminishes caspase-dependent apoptosis by inhibiting Bax, BIK and caspase-7 activity. In addition, GRP75 traps p53 in the cytoplasm so that p53 cannot turn on the pro-apoptotic genes.
It has been found that the HSP70s prevent stress-induced apoptosis through either mitochondria- dependent or independent pathways (Fig. 1). HSP70s can block activation of the death factors to allow the cells resistance to stress-induced apoptosis.21 For instance, iHSP70s attenuate the release of cytochrome c by blocking cleavage of BH3-interacting domain death agonist (Bid) from caspase-8.27 iHSP70s coupled with HSP40 prevent Bax mitochondrial translocation.28 Moreover, iHSP70s interact with Apaf-1 to forbid its association with procaspase-9.29 iHSP70s and HSP70-3 also diminish cell death in caspase- independent pathway by inhibiting apoptosis inducing factor (AIF) and maintaining lysosomal membrane, respectively.30,31 Furthermore, HSP70-3 siRNA induced p53 expression in HeLa cells, whereas siRNA of iHSP70s did not alter the p53 expression.32 Also, PUMA, NOXA and Bcl2 antagonist of cell death (BAD) are critical mediators in apoptosis and transactivated by p53,33,34 suggesting that HSP70-3 suppresses p53 expression to decrease stress-induced apoptosis.
On the other hand, overexpression of GRP78 suppresses caspase-7/12 activation or stabilizes Raf-1 to maintain mitochondrial permeability, thus diminishing apoptosis in cells treated with ER stress inducers or genotoxic agents.35–38 Recombinant GRP78 also reduces cytochrome c-induced caspase-3 activation in vitro.39 Moreover, introduction of antisense GRP78 into cells decreases cell viability during ER stress stimuli.40 Knockdown of GRP78 by siRNA arrests cell growth and increases activation of apoptosis regulator Bax or BCL2-interacting killer (BIK) in stress-induced apoptosis.41,42 Besides, GRP75 interacts with p53 in the cytoplasm to sequester its apoptotic function, thus reducing cell death caused by serum starvation.43 Recent studies show that deregulation of HSP70s expression or functions is associated with diseases such as autoimmunity, neurodegenerative diseases, and tumor transformation, particularly in malignant progression.3,44 It suggests that overexpression of HSP70s in tumor cells confers resistance to adverse microenvironments to promote tumor transformation and decrease chemotherapy efficacy.
Involvement of HSP70s in Cancer
Overexpression of HSP70s is significantly associated with tumor transformation since HSP70s may block apoptosis to adapt adverse microenvironments as mentioned above or chaperone the mutated proteins of cancer cells, such as mutated p53, which is observed in approximately 50% cancers.21,45 Moreover, the level of HSP70s is reportedly increased in a variety of tumor cells or tissues as followings (Table 2).
Table 2.
Association of HSP70s in cancers.
| Protein | Findings | Cancer type | Reference |
|---|---|---|---|
| iHSP70s | HSP70-1 and/or -2 expression are increased in the cancer | Breast cancer | 52 |
| Colon cancer | 48 | ||
| Gastric cancer | 46 | ||
| Melanoma | 50 | ||
| Tumorigenic cells (HeLa, MCF-7, PC-3, HuH-7, SGC-7901) | 32, 59 | ||
| HSP70-2 polymorphism associates with the cancer | Nasopharynx cancer | 55 | |
| HSP70-1 and/or -2 decrease chemotherapy efficiency | Bladder cancer (BIU-87) | 58 | |
| HSC70s | HSP70-3 expression is increased in the cancer and reduce cell death | Bladder cancer | 31 |
| HSP70-3 regulates cell proliferation | Cervix cancer (HeLa) | 32 | |
| GRP75 | GRP75 expression is elevated in the cancer | Brain cancer | 62 |
| Breast cancer | 63 | ||
| Colon cancer | 63 | ||
| Elevated GRP75 in cancer cells decreases survival of the patient | Colon cancer | 64 | |
| GRP78 | GRP78 expression is increased in the cancer | Liver cancer | 75 |
| Lung cancer | 72 | ||
| Colon cancer | 70 | ||
| Gastric cancer | 68, 71 | ||
| GRP78 shortens the time of recurrence | Breast cancer | 66 |
iHSP70s
Several reports have shown that increased iHsp70s expression is elevated in a variety of malignant tumors, such as colorectal, esophageal and gastric cancer.46–48 The expression level of HSP70-1 and/or -2 is elicited in either pancreatic cancer cells49 or melanoma cell lines.50 The importance of these findings is illustrated by the fact that high levels of expression of HSP70-1 and/or HSP70-2 are correlated with hepatoma progression51 and increased drug resistance of human breast cancer.52,53 Overexpression of HSP70-1 and/or HSP70-2 expands the tumor size, metastasis and resistance to chemotherapy.54 The polymorphism of HSP70-2 is associated with nasopharyngeal carcinoma55 and increases the viability of patients with breast cancer.56 Specifically, the cytoplasmic HSP70-1 and/or -2 are more abundant in the colorectal carcinoma than in the normal mucosa.57 Also, depletion of the genes HSPA1A and/or HSPA1B by antisense nucleotides in human bladder cancer cell lines BIU-87 sensitizes mitomycin C-based chemotherapy.58 Knockdown of the genes HSPA1A and/or HSPA1B by siRNA arrests cell cycle at G2/M phase to diminish cell proliferation of tumorigenic cells including HeLa, MCF-7, PC-3, HuH-7 and gastric cancer SGC-7901 cells, whereas the effect is not observed in nontumorigenic HBL-100 cells.32,59 However, siRNA of the gene HSPA6 reveals no effect in both HeLa and HBL-100 cells.32
HSC70s
Compared with peripheral blood mononuclear cells (PBMC), HSC70 shows high levels of expression in the epithelial cancer cells including oral cancer (OSC20 and Ca9-22), colon cancer (SW620) and stomach cancer (MKN45).60 The gene HSPA2 is elevated in invasive bladder cancer and tissue from patients with breast cancer.31,32 Nevertheless, HSPA1L shows no correlation with prostate cancer risk.61
GRP75
GRP75 shows induced-expression in many types of brain tumors including meningiomas, neurinomas, pituitary adenomas and metastases.62 The mRNA level of GRP75 is upraised in tumor tissues of breast, brain, and colon tumors in the mouse model.63 The gene HSPA9 expression in most of tumor or immortalized cell is higher than that in normal cells.63 Malignancy of breast cancer is enhanced in GRP75-overexpressed cells.63 Besides, elevated expression of GRP75 decreases survival of the patient with colon cancer.64
GRP78
The microenvironment in tumor transformation induces ER stress response to stimulate GRP78 expression. The mRNA and protein level of GRP78 in the tumor tissue are much higher than that in the normal tissue of patients with lung cancer, indicating that the level of GRP78 greatly correlates with malignant progression.65 Besides, the expression level of GRP78 is significantly associated with a shorter time of recurrence in patients with breast cancer.66 The elevated protein level of GRP78 is also observed in resected tissue from patients with a number of cancer types including liver, lung, prostate, colon, and gastric cancer,67–71 whereas the mRNA level had no difference in colon cancer, suggesting that posttranscriptional regulation may be involved in the GRP78 expression.72 A recent report shows that GRP78 inhibits BIK, a BH3-only proapoptotic protein in ER, or molecularly chaperone Raf-1 to diminish stress-induced apoptosis in human breast cancer MCF-7/BUS cells or non-small cell lung cancer H460 cells.38,73 Therefore, it indicates that GRP78 could be a treatment marker in chemotherapy.
Heat shock factor 1 (HSF1) and activating transcription factor 6 (ATF6), the transcription factors of iHSP70s and GRP78, respectively, are overexpressed in some certain cancer cells.74,75 This is probably why the expression level of HSP70s is elevated in tumor cells. Additionally, since the anti-apoptotic and chaperone functions of HSP70s promote tumor transformation, the induction of HSP70s play pivotal roles in carcinogenesis.66,76 It may also provide diagnostic markers of cancer.
Distribution of HSP70s in Cancer
Besides the high level expression of HSP70s in tumor cells, HSP70s have also been observed to distribute distinct subcellular localization.77–79 HSP70s associate with either tumor-specific antigen to localize on the surface or proapoptotic protein to inhibit its function, which may provide a pharmacological strategy in cancer therapy.
iHSP70s
In addition to nucleus/cytoplasm localization, the minority of HSP70-1 and/or HSP70-2 associated with tumor specific antigens localizes on the cell surface and serves as a danger signal to trigger an immune response in patients with cancer.80 Cell surface-localized HSP70-1, HSP70-2 or its derived peptide TKDNNLLGRFELSG (TKD, aa 450–463) interact with CD94 of natural killer (NK) cells to trigger the granzyme B released from NK cells.81 The released granzyme B is internalized into tumor cells by interacting with surface-localized HSP70-1 and/or HSP70-2 and initiates apoptosis in a perforin-independent manner.81 However, distinct length of TKD-related peptides or TKD-equivalent peptides derived from HSP70-Hom or HSC70 cannot activate NK cells.82 Moreover, HSP70-1 and/or HSP70-2 stimulate pro-inflammatory cytokines secretion of antigen presenting cells (APC)s through binding with Toll-like receptors.83 Interestingly, HSP70-1 and/or HSP70-2 also exist at high levels in the sera of prostate cancer patients.84
HSC70s
HSC70 has been found on the cell surface as HSP70-1 and/or HSP70-2 and is released to extracellular space in the K562 erythroleukemic cells treated with pro-inflammatory cytokine interferon-gamma (IFN-γ).78 Likewise, HSC70 co-localizes with tumor antigens and MHC class I molecules in the exosome released from 2 different types of MHC-mouse cell lines including CT26 (H-2d MHC) and TA3HA (H-2a MHC).85 The HSC70-contained exosomes activate dendritic cells and suppress tumor size in the colon cancer-bearing mice.85
GRP75
GRP75 localizes in duplicated centrosome at late G1, S, and G2 phases of the cell cycle, whereas it dissociates with unduplicated centrosome at M phase.86 GRP75 is tyrosine phosphorylated at G1 phase in the cells exposed to fibroblast growth factor (FGF) and correlated with FGF-1 induced cell growth.87 Moreover, GRP75 interacts with mot-2-binding site (aa 323–337) of p53 to trap p53 in the cytoplasm and inhibit its apoptotic function.88
GRP78
GRP78 is observed in the other sub-cellular compartments except ER. Surface-localized GRP78 is increased in LNCaP prostate cancer cells treated with synthetic androgen R1881.79 The peptide sequence LIGRTWNDPSVQQDIKFL(aa 98-115) of surface-localized GRP78 binds to receptor binding domain (RBD) of α2-macroglobulin (α2M*) and serves as a receptor of α2M* to promote cell growth via PI3K/AKT dependent pathway.89,90 Moreover, GRP78 accumulates in the cytoplasm in hepatocellular carcinoma (HCC).75 Besides, recent study shows that autoantibody against GRP78 is increased in sera of the patients with prostate cancer.90 Further, the antibody isolated from the patients enhances the proliferation of 1-LN cells expressing GRP78 on the cell surface.90 The anti-GRP78 antibody also prevents apoptosis in cells exposed to tumor necrosis factor α90.
In addition to distinct intracellular distribution of HSP70s, HSP70s are exported to serum in cancer patients.91,92 Also, anti-HSP70s antibodies have been determined in some certain cancers.84,90 Although the function of HSP70s release or autoantibody production needs more studies to examine, it implies that they can be treated as diagnostic or prognostic markers in cancer therapy.
HSP70s in Cancer Therapy
Since overexpression and dysregulation of HSP70s are involved in tumor transformation, HSP70s play the role of danger signals in patients with cancer. According to the features of HSP70s in cancer, HSP70s—based cancer therapy has been verified in some certain cancers (Table 3).
Table 3.
Potential HSP70s-based cancer therapy.
| Protein | Mechanism of action | Therapy | Reference |
|---|---|---|---|
| iHSP70s | Activate NK cells against cancer cells with surface iHSP70s | IL-2/TKD peptide (immunotherapy) |
93 94 |
| Inhibit iHSP70s expression | Antisense iHSP70s cDNA | 53 | |
| siRNA | 49 | ||
| Quercetin | 49 | ||
| Cardenolide (UNBS1450) | 97 | ||
| Triptolide | 96 | ||
| HSC70s | Activate CTL | HSC70-derived peptide (immunotherapy) | 78 |
| Inhibit HSC70s expression | siRNA | 32 | |
| GRP75 | Inhibit GRP75 expression | ribozyme | 98 |
| siRNA | 99 | ||
| Disrupt interaction between GRP75 and p53 | MKT-077 | 100 | |
| GRP75 binding peptide | 88 | ||
| GRP78 | Inhibit gene expression | siRNA | 71, 101 |
| The binding peptide conjugated with cytotoxic peptide kill the cancer cells with surface localized-GRP78 | GRP78 binding peptides | 105, 106 | |
| Cleave GRP78 at Leu 416 | SubAB | 104 | |
| Block GRP78 in the ER | MDA-7/IL-24 recombinant adenoviruses | 102 |
iHSP70s
Ex vivo activation of NK cells from patients with metastasized colon and lung cancer by IL-2/TKD peptide treatment has been tested for its tolerability, feasibility, and safety in phase I clinical trial.93 The activated NK cells show cytolytic activity to surface HSP70 (HSP70-1 and/or HSP70-2) positive tumor cells in vivo and most patients experienced no negative side effects. Furthermore, the IL-2/TKD peptide- activated NK cells, but not T lymphocyte, which decreased the tumor weight and liver metastasis, thereby increasing the survival rate of pancreatic tumor-bearing mice.94 Introduction of antisense cDNA against HSPA1A and/or HSPA1B to nude mice efficiently diminishes the tumor growth of glioblastoma, breast and colon cancer.53 Depletion of HSPA1A and/or HSPA1B by siRNA or HSP70s by quercetin, which is an inhibitor of HSF1,95 raises the apoptosis of human pancreatic cancer cells including MiaPaCa-2 and Panc-1 in a caspase-dependent manner.49 Also, injection of quercetin into the mouse xenografted with MiaPaCa-2 tumor cells significantly attenuates the tumor size.49 Likewise, triptolide derived from Triptergium wilfordii blocks HSF1 transcriptional activity without affecting its activation and DNA binding activity, thus diminishing the genes HSPA1A and/or HSPA1B expression and enhances stress-induced apoptosis.96 Moreover, cardenolide (UNBS1450), a potent anti-cancer drug for the paclitaxel-and oxaliplatin-resistant tumor, attenuates both mRNA and protein levels of HSP70-1 and/or HSP70-2 in human non-small cell lung cancers partly through suppression of NFAT5/TonEBP, which regulates transcriptional levels of iHSP70s.97 It supports the notion that induction of iHSP70s associates with not only malignant progression, but also drug resistance. However, since HSF1 transactivates most of inducible HSPs genes, inhibiting HSF1 not only block iHSP70s expression, but other inducible HSPs, which may appear side effect in the treatment.
HSC70s
Like HSP70-1 and/or HSP70-2, extracellular HSC70 has been determined to function as a cytokine to activate an immune response leading to tumor suppression.78 HSC70-derived peptide EYKGETKSF (aa 106–114) or FDNRMVNHF (aa 233–241) activate cytotoxic T lymphocyte (CTL) in the peripheral blood mononuclear cells of epithelial cancer patients.60 Besides, inhibiting the gene HSPA8 expression by siRNA shows anti-proliferative effects in both tumorigenic and non-tumorigenic cells.32 Nevertheless, HSP70-3 depletion arrests the cell cycle at G1 phase of HeLa cells, whereas the effect is not observed in normal cells. Depletion of the gene HSPA2 induces expression of macrophage inhibitory cytokine-1, a target of p53, in both p53 dependent and independent pathways to arrest cell cycle at G1 phase.32 Suppression of the gene HSPA2 expression also results in cathepsin-dependent cell death via permeabilizing the lysosomal membrane.31
GRP75
Several studies have shown the importance of the interaction between GRP75 and p53 in carcinogenesis.43,88 Therefore, suppression of the gene HSPA9 expression or interference with interaction would be a therapeutic strategy against cancer. Indeed, knockdown of the gene HSPA9 by ribozyme or siRNA reduces cell growth and viability of human cancer cells.98,99 Disrupting the interaction between GRP75 and p53 by the potent anti-cancer drug, MKT-077,100 or GRP75 binding peptide, activates endogenous p53, thus preventing cell growth of osteosarcoma and breast carcinoma cells.88
GRP78
Since GRP78 associates with tumor transformation and cancer therapy, it provides a good therapeutic target for several potent anti-cancer drugs. For instance, suppression of HSPA5 by siRNA increases sensitivity of tumor cells to etoposide in mice xenografted with human breast cancer.101 Moreover, Melanoma differentiation-associated gene-7/interleukin-24 (MDA-7/IL-24) and its derived peptide M4 interact with GRP78 in ER lumen to trigger ER stress response, which in turn promotes apoptosis of cancer cells.102 Injection of MDA-7/IL-24 recombinant adenoviruses (Ad. Mda-7) into breast tumor-bearing mice results in a decrease of tumor volume.102 In phase I clinical trial of Ad. Mda-7 in patients with melanoma, it has been determined to be safe and efficient in inducing apoptosis of melanoma.103 Additionally, SubAB, a toxin from the highly virulent strain of Escherichia coli, cleaves GRP78 at Leu 416 site to destroy its function, which may provide a therapeutic application.104 On the other hand, surface-localized GRP78 could be utilized as a carrier of anti-cancer drugs. The cyclic 13-mer oligopeptide, Pep42, interacts with surface-localized GRP78 and internalizes to lysosome of tumor cells in a clathrin-dependent manner.105 Pep42 also targets tumor tissue in the xenograft mice with melanoma cells. Furthermore, Pep42 conjugated with the apoptosis-inducing oligopeptide (AIO) or photosensitizer decreases viability of tumor cells, such as A549 and HepG2 cells; whereas the effect is not observed in normal cells.105 Likewise, the GRP78 binding peptides, WIFPWIQL or WDLAWMFRLPVG, fused with AIO decreases the tumor size in the xenograft mice with prostate and breast cancers.106
While some of the surface-localized HSP70s (HSP70-1, HSP70-2, and HSC70) serve as danger signals in cancer patients, surface-localized GRP78 serves as a receptor of α2M* to promote cancer cell growth. Inhibiting HSP70s genes expression by siRNA or inhibitors might also diminish the danger signal from the surface-localized HSP70. Therefore, HSP70s-based cancer therapy may depend on the specificity of induced or surface-localized HSP70s in the patient.
Conclusion and Perspective
While HSP70s play pivotal roles for general peptide folding to maintain normal physiological function of cells, dysregulation of expressional and spatial control of HSP70s causes tumor transformation and decreases chemotherapy efficacy. Therefore, comprehension of molecular mechanisms regarding gene regulation and protein function of HSP70s might provide a paradigm for cancer therapy. Although plenty of studies about HSP70s-based cancer therapy have been determined, most of the studies are still in cell or preclinical models. The efficacy of HSP70s-based cancer therapy needs to be further verified in the clinical trial. On the other hand, since overexpressed HSP70s demonstrate variable localization as mentioned above and some of HSP70s reveal posttranslational modification in tumor cells,107 the relevance between post-translational modification and distribution of HSP70s remains unclear. To determine the detailed mechanism of HSP70s in tumor progression, HSP70s-based chaperosome using a proteomics approach would be informative in the regulation of HSP70s in cancers, which in turn would shed light on the cancer therapy.
Acknowledgments
This work was supported by National Institutes of Health Grants (R01-AI067395-01, R21-R022754-01, and R21-I58002-01). We thank Mandy Kao and Paul Kotol for critical reading of the manuscript.
References
- 1.Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experimentia. 1962;18:571–3. [Google Scholar]
- 2.Moran L, Mirault ME, Arrigo AP, Goldschmidt-Clermont M, et al. Heat shock of Drosophila melanogaster induces the synthesis of new messenger RNAs and proteins. Philos Trans R Soc Lond B Biol Sci. 1978;283:391–406. doi: 10.1098/rstb.1978.0044. [DOI] [PubMed] [Google Scholar]
- 3.Macario AJ, Conway de Macario E. Sick chaperones, cellular stress, and disease. N Engl J Med. 2005;353:1489–501. doi: 10.1056/NEJMra050111. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Sha B. Cloning, expression, purification and preliminary X-ray crystallographic studies of Escherichia coli Hsp100 ClpB N-terminal domain. Acta Crystallogr D Biol Crystallogr. 2001;57:1933–5. doi: 10.1107/s0907444901017322. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Sha B. Cloning, expression, purification and preliminary X-ray crystallographic studies of Escherichia coli Hsp100 ClpB nucleotide-binding domain 1 (NBD1) Acta Crystallogr D Biol Crystallogr. 2001;57:909–11. doi: 10.1107/s0907444901007296. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, et al. Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res. 2006;1089:67–78. doi: 10.1016/j.brainres.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 7.van der Straten A, Rommel C, Dickson B, Hafen E. The heat shock protein 83 (Hsp83) is required for Raf-mediated signalling in Drosophila. Embo J. 1997;16:1961–9. doi: 10.1093/emboj/16.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaner L, Morano KA. All in the family: atypical Hsp70 chaperones are conserved modulators of Hsp70 activity. Cell Stress Chaperones. 2007;12:1–8. doi: 10.1379/CSC-245R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohfeld J, Hartl FU. Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. J Cell Biol. 1994;126:305–15. doi: 10.1083/jcb.126.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–10. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Milner CM, Campbell RD. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–51. doi: 10.1007/BF00187095. [DOI] [PubMed] [Google Scholar]
- 12.Shu CW, Cheng NL, Chang WM, Tseng TL, et al. Transactivation of hsp70-1/2 in geldanamycin-treated human non-small cell lung cancer H460 cells: involvement of intracellular calcium and protein kinase C. J Cell Biochem. 2005;94:1199–209. doi: 10.1002/jcb.20348. [DOI] [PubMed] [Google Scholar]
- 13.Leung TK, Hall C, Rajendran M, Spurr NK, et al. The human heat-shock genes HSPA6 and HSPA7 are both expressed and localize to chromosome 1. Genomics. 1992;12:74–9. doi: 10.1016/0888-7543(92)90409-l. [DOI] [PubMed] [Google Scholar]
- 14.Narita N, Noda I, Ohtsubo T, Fujieda S, et al. Analysis of heat-shock related gene expression in head-and-neck cancer using cDNA arrays. Int J Radiat Oncol Biol Phys. 2002;53:190–6. doi: 10.1016/s0360-3016(02)02727-x. [DOI] [PubMed] [Google Scholar]
- 15.Dworniczak B, Mirault ME. Structure and expression of a human gene coding for a 71 kd heat shock ‘cognate’ protein. Nucleic Acids Res. 1987;15:5181–97. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goate AM, Cooper DN, Hall C, Leung TK, et al. Localization of a human heat-shock HSP 70 gene sequence to chromosome 6 and detection of two other loci by somatic-cell hybrid and restriction fragment length polymorphism analysis. Hum Genet. 1987;75:123–8. doi: 10.1007/BF00591072. [DOI] [PubMed] [Google Scholar]
- 17.Bonnycastle LL, Yu CE, Hunt CR, Trask BJ, et al. Cloning, sequencing, and mapping of the human chromosome 14 heat shock protein gene (HSPA2) Genomics. 1994;23:85–93. doi: 10.1006/geno.1994.1462. [DOI] [PubMed] [Google Scholar]
- 18.Lee AS. The ER. chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–81. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Gao CX, Zhang SQ, Yin Z, Liu W. Molecular chaperone GRP75 reprove cells from injury caused by glucose deprivation. Shi Yan Sheng Wu Xue Bao. 2003;36:381–7. [PubMed] [Google Scholar]
- 20.Sadekova S, Lehnert S, Chow TY. Induction of PBP74/mortalin/Grp75, a member of the hsp70 family, by low doses of ionizing radiation: a possible role in induced radioresistance. Int J Radiat Biol. 1997;72:653–60. doi: 10.1080/095530097142807. [DOI] [PubMed] [Google Scholar]
- 21.Beere HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115:2633–9. doi: 10.1172/JCI26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song J, Takeda M, Morimoto RI. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat Cell Biol. 2001;3:276–82. doi: 10.1038/35060068. [DOI] [PubMed] [Google Scholar]
- 23.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li RF, Zhang F, Lu YJ, Sui SF. Specific interaction between Smad1 and CHIP: a surface plasmon resonance study. Colloids Surf B Biointerfaces. 2005;40:133–6. doi: 10.1016/j.colsurfb.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Mosser DD, Caron AW, Bourget L, Meriin AB, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–59. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volloch V, Gabai VL, Rits S, Sherman MY. ATPase activity of the heat shock protein hsp72 is dispensable for its effects on dephosphorylation of stress kinase JNK and on heat-induced apoptosis. FEBS Lett. 1999;461:73–6. doi: 10.1016/s0014-5793(99)01428-3. [DOI] [PubMed] [Google Scholar]
- 27.Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2002;22:3415–24. doi: 10.1128/MCB.22.10.3415-3424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotoh T, Terada K, Oyadomari S, Mori M. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- 29.Beere HM, Wolf BB, Cain K, Mosser DD, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–75. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 30.Gurbuxani S, Schmitt E, Cande C, Parcellier A, et al. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene. 2003;22:6669–78. doi: 10.1038/sj.onc.1206794. [DOI] [PubMed] [Google Scholar]
- 31.Daugaard M, Kirkegaard-Sorensen T, Ostenfeld MS, Aaboe M, et al. Lens epithelium-derived growth factor is an Hsp70-2 regulated guardian of lysosomal stability in human cancer. Cancer Res. 2007;67:2559–67. doi: 10.1158/0008-5472.CAN-06-4121. [DOI] [PubMed] [Google Scholar]
- 32.Rohde M, Daugaard M, Jensen MH, Helin K, et al. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19:570–82. doi: 10.1101/gad.305405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu F, Watts RN, Zhang XD, Borrow JM, et al. Involvement of BH3-only proapoptotic proteins in mitochondrial-dependent Phenoxodiol-induced apoptosis of human melanoma cells. Anticancer Drugs. 2006;17:1151–61. doi: 10.1097/01.cad.0000231484.17063.9a. [DOI] [PubMed] [Google Scholar]
- 34.Jiang P, Du W, Heese K, Wu M. The Bad guy cooperates with good cop p53: Bad is transcriptionally up-regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol Cell Biol. 2006;26:9071–82. doi: 10.1128/MCB.01025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–14. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- 36.Reddy RK, Mao C, Baumeister P, Austin RC, et al. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–24. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Zhang H, Dong Y, Park YM, et al. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005;65:9073–9. doi: 10.1158/0008-5472.CAN-05-2016. [DOI] [PubMed] [Google Scholar]
- 38.Shu CW, Sun FC, Cho JH, Lin CC, et al. GRP78 and Raf-1 cooperatively confer resistance to endoplasmic reticulum stress-induced apoptosis. J. Cell. Physiol. 2007 doi: 10.1002/jcp.21340. In press. [DOI] [PubMed] [Google Scholar]
- 39.Rao RV, Peel A, Logvinova A, del Rio G, et al. Coupling endoplasmic reticulum stress to the cell death program: role of the ER. chaperone GRP78. FEBS Lett. 2002;514:122–8. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyake H, Hara I, Arakawa S, Kamidono S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000;77:396–408. doi: 10.1002/(sici)1097-4644(20000601)77:3<396::aid-jcb5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Zu K, Bihani T, Lin A, Park YM, et al. Enhanced selenium effect on growth arrest by BiP/GRP78 knockdown in p53-null human prostate cancer cells. Oncogene. 2006;25:546–54. doi: 10.1038/sj.onc.1209071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–11. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pshezhetsky AV. Proteomic analysis of vascular smooth muscle cells treated with ouabain. Methods Mol Biol. 2007;357:253–69. doi: 10.1385/1-59745-214-9:253. [DOI] [PubMed] [Google Scholar]
- 44.Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18:444–52. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Soussi T. p53 alterations in human cancer: more questions than answers. Oncogene. 2007;26:2145–56. doi: 10.1038/sj.onc.1210280. [DOI] [PubMed] [Google Scholar]
- 46.Wang XP, Liao J, Liu GZ, Wang XC, et al. Co-expression of heat shock protein 70 and glucose-regulated protein 94 in human gastric carcinoma cell line BGC-823. World J Gastroenterol. 2005;11:3601–4. doi: 10.3748/wjg.v11.i23.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang XP, Liu GZ, Song AL, Chen RF, et al. Expression and significance of heat shock protein 70 and glucose-regulated protein 94 in human esophageal carcinoma. World J Gastroenterol. 2005;11:429–32. doi: 10.3748/wjg.v11.i3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang XP, Qiu FR, Liu GZ, Chen RF. Correlation between clinicopathology and expression of heat shock protein 70 and glucose-regulated protein 94 in human colonic adenocarcinoma. World J Gastroenterol. 2005;11:1056–9. doi: 10.3748/wjg.v11.i7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–25. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 50.Dressel R, Johnson JP, Gunther E. Heterogeneous patterns of constitutive and heat shock induced expression of HLA-linked HSP70-1 and HSP70-2 heat shock genes in human melanoma cell lines. Melanoma Res. 1998;8:482–92. doi: 10.1097/00008390-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Shen Y, Tang WX, Chen L, et al. Separation and identification of the exosomes derived from a mouse hepatoma carcinoma cell line (H22) and initial investigation of their protein composition. Zhonghua Gan Zang Bing Za Zhi. 2007;15:437–40. [PubMed] [Google Scholar]
- 52.Vargas-Roig LM, Gago FE, Tello O, Aznar JC, et al. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1998;79:468–75. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 53.Nylandsted J, Wick W, Hirt UA, Brand K, et al. Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res. 2002;62:7139–42. [PubMed] [Google Scholar]
- 54.Garrido C, Schmitt E, Cande C, Vahsen N, et al. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle. 2003;2:579–84. [PubMed] [Google Scholar]
- 55.Jalbout M, Bouaouina N, Gargouri J, Corbex M, et al. Polymorphism of the stress protein HSP70-2 gene is associated with the susceptibility to the nasopharyngeal carcinoma. Cancer Lett. 2003;193:75–81. doi: 10.1016/s0304-3835(02)00697-3. [DOI] [PubMed] [Google Scholar]
- 56.Mestiri S, Bouaouina N, Ahmed SB, Khedhaier A, et al. Genetic variation in the tumor necrosis factor-alpha promoter region and in the stress protein hsp70-2: susceptibility and prognostic implications in breast carcinoma. Cancer. 2001;91:672–8. doi: 10.1002/1097-0142(20010215)91:4<672::aid-cncr1050>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 57.Milicevic ZT, Petkovic MZ, Drndarevic NC, Pavlovic MD, et al. Expression of heat shock protein 70 (HSP70) in patients with colorectal adenocarcinoma--immunohistochemistry and Western blot analysis. Neoplasma. 2007;54:37–45. [PubMed] [Google Scholar]
- 58.He LF, Guan KP, Yan Z, Ye HY, et al. Enhanced sensitivity to mitomycin C by abating heat shock protein 70 expression in human bladder cancer cell line of BIU-87. Chin Med J (Engl) 2005;118:1965–72. [PubMed] [Google Scholar]
- 59.Zhao ZG, Shen WL. Heat shock protein 70 antisense oligonucleotide inhibits cell growth and induces apoptosis in human gastric cancer cell line SGC-7901. World J Gastroenterol. 2005;11:73–8. doi: 10.3748/wjg.v11.i1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azuma K, Shichijo S, Takedatsu H, Komatsu N, et al. Heat shock cognate protein 70 encodes antigenic epitopes recognised by HLA-B 4601-restricted cytotoxic T lymphocytes from cancer patients. Br J Cancer. 2003;89:1079–85. doi: 10.1038/sj.bjc.6601203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sfar S, Saad H, Mosbah F, Chouchane L. Association of HSP70-hom genetic variant with prostate cancer risk. Mol Biol Rep. 2007 doi: 10.1007/s11033-007-9107-1. [DOI] [PubMed] [Google Scholar]
- 62.Takano S, Wadhwa R, Yoshii Y, Nose T, et al. Elevated levels of mortalin expression in human brain tumors. Exp Cell Res. 1997;237:38–45. doi: 10.1006/excr.1997.3754. [DOI] [PubMed] [Google Scholar]
- 63.Wadhwa R, Takano S, Kaur K, Deocaris CC, et al. Upregulation of mortalin/mthsp70/Grp75 contributes to human carcinogenesis. Int J Cancer. 2006;118:2973–80. doi: 10.1002/ijc.21773. [DOI] [PubMed] [Google Scholar]
- 64.Dundas SR, Lawrie LC, Rooney PH, Murray GI. Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J Pathol. 2005;205:74–81. doi: 10.1002/path.1672. [DOI] [PubMed] [Google Scholar]
- 65.Wang Q, He Z, Zhang J, Wang Y, et al. Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev. 2005;29:544–51. doi: 10.1016/j.cdp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Lee E, Nichols P, Spicer D, Groshen S, et al. GRP78 as a Novel Predictor of Responsiveness to Chemotherapy in Breast Cancer. Cancer Res. 2006;66:7849–53. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Jiang Y, Jia Z, Li Q, et al. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. 2006;23:401–10. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 68.Pootrakul L, Datar RH, Shi SR, Cai J, et al. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12:5987–93. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 69.Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–38. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 70.Uramoto H, Sugio K, Oyama T, Nakata S, et al. Expression of endoplasmic reticulum molecular chaperone Grp78 in human lung cancer and its clinical significance. Lung Cancer. 2005;49:55–62. doi: 10.1016/j.lungcan.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Zhang F, Hamanaka RB, Bobrovnikova-Marjon E, Gordan JD, et al. Ribosomal stress couples the unfolded protein response to p53-dependent cell cycle arrest. J Biol Chem. 2006;281:30036–45. doi: 10.1074/jbc.M604674200. [DOI] [PubMed] [Google Scholar]
- 72.Xing X, Lai M, Wang Y, Xu E, et al. Overexpression of glucose-regulated protein 78 in colon cancer. Clin Chim Acta. 2006;364:308–15. doi: 10.1016/j.cca.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 73.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67:3734–40. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 74.Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, et al. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol. 2000;156:857–64. doi: 10.1016/S0002-9440(10)64954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shuda M, Kondoh N, Imazeki N, Tanaka K, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER. stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–14. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 76.Ermakova SP, Kang BS, Choi BY, Choi HS, et al. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 77.Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–79. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- 78.Barreto A, Gonzalez JM, Kabingu E, Asea A, et al. Stress-induced release of HSC70 from human tumors. Cell Immunol. 2003;222:97–104. doi: 10.1016/s0008-8749(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 79.Whitaker HC, Stanbury DP, Brinham C, Girling J, et al. Labeling and identification of LNCaP cell surface proteins: a pilot study. Prostate. 2007;67:943–54. doi: 10.1002/pros.20580. [DOI] [PubMed] [Google Scholar]
- 80.Radons J, Multhoff G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc Immunol Rev. 2005;11:17–33. [PubMed] [Google Scholar]
- 81.Gross C, Koelch W, DeMaio A, Arispe N, et al. Cell. surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem. 2003;278:41173–81. doi: 10.1074/jbc.M302644200. [DOI] [PubMed] [Google Scholar]
- 82.Multhoff G, Pfister K, Gehrmann M, Hantschel M, et al. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones. 2001;6:337–44. doi: 10.1379/1466-1268(2001)006<0337:amhpsn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Asea A, Rehli M, Kabingu E, Boch JA, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 84.Abe M, Manola JB, Oh WK, Parslow DL, et al. Plasma levels of heat shock protein 70 in patients with prostate cancer: a potential biomarker for prostate cancer. Clin Prostate Cancer. 2004;3:49–53. doi: 10.3816/cgc.2004.n.013. [DOI] [PubMed] [Google Scholar]
- 85.Cho JA, Yeo DJ, Son HY, Kim HW, et al. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114:613–22. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 86.Ma Z, Izumi H, Kanai M, Kabuyama Y, et al. Mortalin controls centrosome duplication via modulating centrosomal localization of p53. Oncogene. 2006;25:5377–90. doi: 10.1038/sj.onc.1209543. [DOI] [PubMed] [Google Scholar]
- 87.Mizukoshi E, Suzuki M, Misono T, Loupatov A, et al. Cell-cycle dependent tyrosine phosphorylation on mortalin regulates its interaction with fibroblast growth factor-1. Biochem Biophys Res Commun. 2001;280:1203–9. doi: 10.1006/bbrc.2001.4225. [DOI] [PubMed] [Google Scholar]
- 88.Kaul SC, Aida S, Yaguchi T, Kaur K, et al. Activation of wild type p53 function by its mortalin-binding, cytoplasmically localizing carboxyl terminus peptides. J Biol Chem. 2005;280:39373–9. doi: 10.1074/jbc.M500022200. [DOI] [PubMed] [Google Scholar]
- 89.Misra UK, Gonzalez-Gronow M, Gawdi G, Wang F, et al. A novel receptor function for the heat shock protein Grp78: silencing of Grp78 gene expression attenuates alpha2M*-induced signalling. Cell Signal. 2004;16:929–38. doi: 10.1016/j.cellsig.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez-Gronow M, Cuchacovich M, Llanos C, Urzua C, et al. Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 2006;66:11424–31. doi: 10.1158/0008-5472.CAN-06-1721. [DOI] [PubMed] [Google Scholar]
- 91.Takashima M, Kuramitsu Y, Yokoyama Y, Iizuka N, et al. Proteomic analysis of autoantibodies in patients with hepatocellular carcinoma. Proteomics. 2006;6:3894–900. doi: 10.1002/pmic.200500346. [DOI] [PubMed] [Google Scholar]
- 92.Zhong L, Peng X, Hidalgo GE, Doherty DE, et al. Antibodies to HSP70 and HSP90 in serum in non-small cell lung cancer patients. Cancer Detect Prev. 2003;27:285–90. doi: 10.1016/s0361-090x(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 93.Krause SW, Gastpar R, Andreesen R, Gross C, et al. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase i trial. Clin Cancer Res. 2004;10:3699–707. doi: 10.1158/1078-0432.CCR-03-0683. [DOI] [PubMed] [Google Scholar]
- 94.Stangl S, Wortmann A, Guertler U, Multhoff G. Control of metastasized pancreatic carcinomas in SCID/beige mice with human IL-2/TKD-activated NK cells. J Immunol. 2006;176:6270–6. doi: 10.4049/jimmunol.176.10.6270. [DOI] [PubMed] [Google Scholar]
- 95.Hosokawa N, Hirayoshi K, Kudo H, Takechi H, et al. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol. 1992;12:3490–8. doi: 10.1128/mcb.12.8.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–22. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 97.Mijatovic T, Mathieu V, Gaussin JF, De Neve N, et al. Cardenolide-induced lysosomal membrane permeabilization demonstrates therapeutic benefits in experimental human non-small cell lung cancers. Neoplasia. 2006;8:402–12. doi: 10.1593/neo.05850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wadhwa R, Ando H, Kawasaki H, Taira K, et al. Targeting mortalin using conventional and RNA-helicase-coupled hammerhead ribozymes. EMBO Rep. 2003;4:595–601. doi: 10.1038/sj.embor.embor855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wadhwa R, Takano S, Taira K, Kaul SC. Reduction in mortalin level by its antisense expression causes senescence-like growth arrest in human immortalized cells. J Gene Med. 2004;6:439–44. doi: 10.1002/jgm.530. [DOI] [PubMed] [Google Scholar]
- 100.Deocaris CC, Widodo N, Shrestha BG, Kaur K, et al. Mortalin sensitizes human cancer cells to MKT-077-induced senescence. Cancer Lett. 2007;252:259–69. doi: 10.1016/j.canlet.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 101.Dong D, Ko B, Baumeister P, Swenson S, et al. Vascular targeting and antiangiogenesis agents induce drug resistance effector GRP78 within the tumor microenvironment. Cancer Res. 2005;65:5785–91. doi: 10.1158/0008-5472.CAN-05-0754. [DOI] [PubMed] [Google Scholar]
- 102.Gupta P, Walter MR, Su ZZ, Lebedeva IV, et al. BiP/GRP78 Is an Intracellular Target for MDA-7/IL-24 Induction of Cancer-Specific Apoptosis. Cancer Res. 2006;66:8182–91. doi: 10.1158/0008-5472.CAN-06-0577. [DOI] [PubMed] [Google Scholar]
- 103.Cunningham CC, Chada S, Merritt JA, Tong A, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–59. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 104.Montecucco C, Molinari M. Microbiology: death of a chaperone. Nature. 2006;443:511–2. doi: 10.1038/443511a. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y, Steiniger SC, Kim Y, Kaufmann GF, et al. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol Pharm. 2007;4:435–47. doi: 10.1021/mp060122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, et al. Cell. surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–84. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 107.Barati MT, Rane MJ, Klein JB, McLeish KR. A proteomic screen identified stress-induced chaperone proteins as targets of Akt phosphorylation in mesangial cells. J Proteome Res. 2006;5:1636–46. doi: 10.1021/pr0502469. [DOI] [PMC free article] [PubMed] [Google Scholar]