Abstract
Ataxia telangiectasia (AT) is a rare genetic disorder characterized by immunodeficiency, early onset neurological degeneration, hypersensitivity to ionizing radiation and a high incidence of lymphoid cancers. The disease results from bi-allelic mutations in the AT mutated (ATM) gene involved in cell cycle checkpoint control and repair of DNA double-strand breaks. Evidence has been accumulating that oxidative stress is associated with AT and may be involved in the pathogenesis of the disease. This led to a hypothesis that antioxidant therapy may mitigate the symptoms of AT, especially neurological degeneration and tumorigenesis. Consequently, several studies examined the effect of antioxidants in Atm deficient mice used as an animal model of AT. N-acetyl-L-cysteine (NAC), EUK-189, tempol and 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl (CTMIO) have been tested for their chemopreventive properties and had some beneficial effects. In addition to antioxidants, cancer therapeutic agent dexamethasone was examined for cancer prevention in Atm deficient mice. Of the tested antioxidants, only NAC has wide clinical applications due to safety and efficacy and is available as an over-the-counter dietary supplement. In this article, we review chemoprevention studies in Atm deficient mice and, in more detail, our findings on the effect of NAC. The short-tem study showed that NAC suppressed genome rearrangements linked to cancer. The long-term study demonstrated that NAC reduced both the incidence and multiplicity of lymphoma.
Keywords: ataxia telangiectasia, Atm, mouse, antioxidants, chemoprevention, lymphoma
Introduction
Ataxia Telangiectasia (AT) is an autosomal recessive human disorder caused by mutational inactivation of the AT mutated (ATM) gene. It is a severe pleiotropic disease characterized by early onset progressive neurological degeneration, high incidence of cancer, immunodeficiency, oculocutaneous telangiectasias, growth retardation, endocrine abnormalities, infertility, and hypersensitivity to ionizing radiation (Boder, 1985; Boder et al. 1970; Gatti, 2001; Lavin et al. 1997; Meyn, 1999). The most prominent neuropathological manifestation of AT is atrophy of the cerebellar cortex associated with the loss of Purkinje and granule cells. An early sign of neurological degeneration is ataxia characterized by unstable gait and lack of coordination of head and eyes. About 40% of AT patients develop cancer, mostly in the lymphoid organs early in life and solid tumors at later age (Taylor et al. 1996; Xu, 1999). AT patients display a variety of lymphoid tumors including non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and several types of leukemia, most tumors being of T cell origin. AT patients suffer from increased mortality due to malignancy, infections of the respiratory system and various rare complications (Boder, 1975; Crawford et al. 2006). It is possible to alleviate some of the clinical symptoms of AT. For example, sinopulmonary infections respond well to antibiotics or modest improvements in neurological symptoms can sometimes be achieved by L-DOPA derivatives, dopamine agonists or steroids (Lavin et al. 2007). However, currently there is no therapy available to prevent cancer or progressive neurodegeneration. The median survival of AT patients is calculated to be 19–25 years (Crawford et al. 2006).
The gene defective in AT, ATM, encodes a phosphatidylinositol-3′ related kinase that is involved in cell cycle checkpoint and repair responses to DNA double-strand breaks (DSBs) via a series of phosphorylated intermediary proteins including p53, Chk2, Brca1 and Nbs1 (Lavin et al. 2005; Savitsky et al. 1995; Shiloh, 2003). A lack of ATM function results in genomic instability characterized by chromosome breaks, chromosome gaps, translocations and aneuploidy (Cohen et al. 1975; Gropp et al. 1967; Stumm et al. 2001). ATM deficiency is also associated with elevated oxidative stress. ATM deficient cells in culture are more sensitive to oxidative stress than normal cells, cells isolated from AT patients display elevated oxidative damage to lipids and DNA and AT patients have reduced plasma antioxidant concentrations (Reichenbach et al. 2002; Reichenbach et al. 1999; Yi et al. 1990). Further evidence that AT is linked to oxidative stress stems from studies with Atm deficient mice. Atm deficient mice exhibit elevated levels of reactive oxygen species (ROS), oxidative damage to proteins and DNA, lipid peroxidation and alterations in the levels and function of antioxidative enzymes (Barlow et al. 1999; Ito et al. 2007; Kamsler et al. 2001; Quick et al. 2001; Reliene et al. 2004b). Atm deficient mice largely recapitulate the human disease (Barlow et al. 1996; Borghesani et al. 2000; Elson et al. 1996; Xu et al. 1996). Similar to human AT phenotype, Atm deficient mice display growth retardation, infertility, immunodeficiency, radiosensitivity and malignant lymphomas (Barlow et al. 1996; Elson et al. 1996; Xu et al. 1996). Although Atm deficient mice do not show the gross cerebellar degeneration that characterizes the human disease, more subtle alterations in the cerebellum have been observed and are consistent with a mild decrease in their motor performance (Barlow et al. 1996; Borghesani et al. 2000; Kuljis et al. 1997).
Since oxidative stress has been evidenced in AT and oxidative stress is linked to neurodegenerative diseases and cancer, it has been suggested that it may contribute to neuropathological and malignant phenotype of AT, while antioxidants might alleviate these symptoms (Barzilai et al. 2002). This hypothesis has been tested in Atm deficient mice in several studies that examined the effect of anti-oxidants EUK-189, tempol, CTMIO and NAC (Browne et al. 2004; Gueven et al. 2006; Ito et al. 2007; Reliene et al. 2006; Schubert et al. 2004). In addition to antioxidants, dexamethasone (Dx), which is used in chemotherapy of hematological malignancies, was studied for cancer chemoprevention in Atm deficient mice (Yan et al. 2002).
Chemopreventive Agents
NAC
NAC is a low molecular weight thiol-containing molecule that is readily taken up by the cells (Kelly, 1998). It directly inhibits reactive electrophiles and ROS and can enhance the synthesis of glutathione (GSH) as a precursor of cysteine (De Flora et al. 2001). NAC has been used in the clinical practice more than 40 years and has found wide applications (Decramer et al. 2005; Kelly, 1998; Van Schooten et al. 2002). NAC has been used for the treatment of respiratory diseases as a mucolytic agent (Webb, 1962), for acetaminophen overdose, where it rescues from GSH depletion in the liver (Prescott et al. 1977), and is available as an over-the-counter dietary supplement. NAC is most frequently taken orally and thus, we examined the effect of NAC on Atm deficient mice by the oral route (Reliene et al. 2004b; Reliene et al. 2006). We gave NAC supplemented drinking water to Atm deficient mice from fertilization throughout their life. In this treatment scenario, Atm +/− mice were crossed with each other and dams were given NAC-containing drinking water throughout pregnancy and lactation. After weaning (at about 3 weeks of age) animals continued to receive NAC in their drinking water. The major reason to start antioxidant administration as early as from fertilization was to protect against genome rearrangements that can occur during mouse development and lead to carcinogenesis later in life.
Effect of NAC on cancer prevention
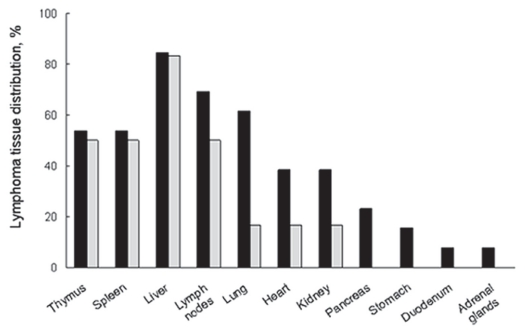
We found that NAC intake significantly increased the lifespan and reduced both the incidence and multiplicity of lymphoma in Atm deficient mice (Reliene et al. 2006). The mean survival of NAC treated mice was 68 weeks, while that of untreated mice was only 50 weeks (p = 0.03). We completed gross necropsy and histopathological examination to determine a possible cause of death. Consistent with previous studies, the most frequent tumor in Atm deficient mice was lymphoma (Barlow et al. 1996; Elson et al. 1996; Xu et al. 1996). Remarkably, the incidence of lymphoma in NAC treated mice decreased by two-fold (37.5 versus 76.5%, p = 0.02). We examined the lymphoma tissue distribution and found tumors in various organs in both NAC treated and control mice (Fig. 1). However, in NAC treated Atm deficient mice, the multiplicity of tumors decreased from 4.6 to 2.8 tumors per mouse (p = 0.038). Lymphoma burden was similar in the thymus, spleen and liver, while in other organs, such as lymph nodes, lung, heart, kidney, pancreas, stomach, duodenum and adrenal glands there were fewer or no tumors in the NAC treatment group (Fig. 1).
Figure 1. Lymphoma tissue distribution in untreated and NAC treated Atm deficient mice.
Only mice that had lymphoma are included in the calculation. Black bars depict untreated mice, gray bars show NAC treated mice. Lymph nodes affected were mesenteric and/or peripheral, thoratic and perirenal. Lymphoma in the heart was seen in epicardium and/or pericardium. Taken from (Reliene et al. 2007).
Subsequently the finding that NAC extends the reduced lifespan of Atm deficient mice was reproduced by another group of researchers (Ito et al. 2007). NAC was administered in drinking water and treatment was started from birth. In this study, the mean survival was approximately 20 weeks and 43 weeks in untreated Atm deficient mice and NAC treatment group, respectively.
Possible Mechanism of Lymphoma Prevention by NAC
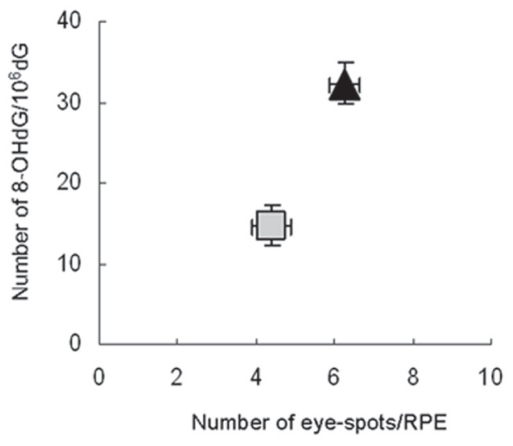
Several studies examined the molecular action mechanism of NAC in cancer prevention in Atm deficient mice. NAC reduced abnormally high DNA synthesis and ROS levels in lymphocytes from Atm deficient mice (Ito et al. 2007; Yan et al. 2001). ROS causes oxidative DNA damage, while upregulated DNA synthesis results in a lack of time required for repair of damaged DNA template before it is used for replication. Oxidative DNA damage is often translated into irreversible genome rearrangements during replication (Kuzminov, 2001). We found that NAC suppressed both oxidative DNA damage and DNA deletions in Atm deficient mice supporting the interpretation that DNA deletions may be a consequence of abnormal DNA synthesis and oxidative damage (Fig. 2) (Reliene et al. 2004b). These studies showed that NAC may reduce cancer incidence by reducing oxidative stress and genomic instability.
Figure 2. The correlation between oxidative DNA damage and the frequency of DNA deletions.
Oxidative DNA damage was determined as the number of oxidized guanine residues per 106 guanine residues (8-OHdG/106dG) using HPLC. The frequency of DNA deletions was determined as the number of eye-spots in the retinal pigment epithelium (RPE) of the eye. The eye-spots are derived from 70 kb DNA deletions at the pink-eyed unstable (pun) locus of the pink-eyed dilution (p) gene, which result in black pigment accumulation in the affected cells (Reliene et al. 2004a). Data for untreated mice are shown by a black triangle; results for NAC treated mice are shown by a gray rectangle. Taken from (Reliene et al. 2007).
Other studies demonstrated that NAC reduces the number of aberrant V(D)J rearrangements between T cell receptor (TCR) β and γ genes, which can cause lymphoma (Ito et al. 2007; Lista et al. 1997). In fact, tumors in Atm deficient mice exhibit abnormal TCR rearrangements suggesting that development of lymphoma may be driven by aberrant V(D)J recombination (Liyanage et al. 2000). NAC reduced the number of defective rearrangements and restored the decreased T cell numbers, which probably accounted for reduced lymphomagenesis (Ito et al. 2007).
ROS have also been proposed to be involved in tumor metastasis, a process that includes epithelial-mesenchymal transition, migration, invasion of the tumor cells and angiogenesis (Nishikawa et al. 2006; Wu, 2006). ROS can oxidize the critical target molecules and thereby play a role in the transcription and expression of genes implicated in tumor progression. NAC can counteract some effects of ROS in tumor progression. NAC has been reported to limit invasion of human bladder cancer cells by inhibiting both the production and activity of matrix metalloproteinase-9 involved in cancer invasion and metastasis (Kawakami et al. 2001). NAC inhibits vascular endothelial growth factor (VEGF) production and growth of angiogenesis-driven Kaposi’s sarcoma in nude mice (Albini et al. 2001), promotes anti-angiogenic factor angiostatin production and results in endothelial apoptosis and vascular collapse in an experimental breast cancer assay (Agarwal et al. 2004). We found that NAC reduced the multiplicity of lymphoma in Atm deficient mice, which may be explained by NAC’s anti-invasive and anti-angiogenic properties (Reliene et al. 2006). The effect was most pronounced in nonlymphoid organs supporting the observation of other studies that NAC exhibits an anti-metastatic effect.
The studies reviewed in this article show that NAC significantly reduces lymphomagenesis in Atm deficient mice but these positive effects by no means suggest that NAC administration can replace the missing Atm protein. It has been recently reported that ATM prevents cancer progression through detection and response to oncogene-induced DNA replication stress and DNA damage (Bartkova et al. 2005). In this report, NAC had only a marginal effect suggesting that DNA damage caused by hyperproliferative oncogenic stimuli cannot be suppressed by antioxidants. Similarly in our studies the cancer frequency and multiplicity were significantly reduced but still much elevated above wildtype levels.
EUK-189
EUK-189, a salen-manganese compound with catalase and superoxide dismutase activities, has been previously shown to be neuroprotective in animal models characterized by oxidative damage (Doctrow et al. 2002; Melov et al. 2001). Atm deficient mice were treated with EUK-189 from 40 days of age via an osmotic pump implanted subcutaneously. The EUK-189 treatment improved performance on a rotarod and showed a trend towards prolonged life span (p = 0.08) (Browne et al. 2004). When the study was terminated at 5 months, 31% vehicle-treated and 56% EUK-189-treated animals were still alive (Browne et al. 2004).
Tempol
Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) is a stable nitroxide free radical and superoxide dismutase mimetic (Damiani et al. 2000; Hahn et al. 1994; Mitchell et al. 1990). Tempol detoxifies oxygen metabolites, oxidizes redox-active trace metal ions, reduces quinone radicals and, in biological systems, is itself reduced by GSH and ascorbic acid (Branca et al. 1988; Krishna et al. 1992; Krishna et al. 1996; May et al. 2005). Tempol mixed in a mouse chow was chronically administered to Atm deficient mice either from fertilization or from weaning (Schubert et al. 2004). Tempol had no effect, when the treatment was started from fertilization. The intake of tempol food significantly increased the life span (mean survival 62 versus 30 weeks) in the second treatment scenario. Tempol reduced ROS levels, protein oxidation and restored mitochondrial membrane potential in thymocytes implying that chemoprevention by tempol is associated with its antioxidant activity. However, tempol treatment also reduced cell number in the thymus and decreased weight gain in Atm deficient mice. These effects were explained by tempol anti-proliferative activity and unknown effects, respectively. A possibility of caloric restriction was ruled out, mainly because no decrease in the intake of food containing tempol was observed.
CTMIO
Like tempol, CTMIO belongs to a class of stable nitroxide free radicals (Damiani et al. 2000; Hahn et al. 1994; Mitchell et al. 1990). The effect of CTMIO intake through drinking water was recently examined in Atm deficient mice (Gueven et al. 2006). The treatment was started immediately after weaning. CTMIO prolonged the survival of Atm deficient mice resulting in the median survival of 54 weeks versus 16 weeks. CTMIO chemoprevention mechanism does not appear to involve apoptosis, as tumors from CTMIO treated mice did not show higher levels of apoptosis compared to tumors from untreated Atm deficient mice. However, it was not shown whether CTMIO induced apoptosis in mice without tumors.
Dx
Dx is a synthetic glucocorticoid hormone used in the chemotherapy of hematological malignancies (Goss, 1992; Keating et al. 1997) for its anti-inflammatory and cytotoxic effects (Greenstein et al. 2002). In a chemoprevention study with Dx, the untreated Atm−/− mice died at 2.2–4.8 month of age, while 50% of Dx treated Atm−/− mice survived up to 6 months when treatment was started at 4 weeks of age, and all Atm−/− mice survived for 10 months when the treatment began at 2 weeks of age, implying that Dx is more effective when given during earlier postnatal stages than later (Yan et al. 2002).
Conclusion
The effect of NAC, EUK-189, tempol, and CTMIO was studied in Atm deficient mice to understand whether antioxidant therapy has a potential in the management of AT. All the described compounds had some beneficial effects, particularly, in extending the life span and reducing lymphomagenesis. Of the tested antioxidants, only NAC has a long history of safety and efficacy in the clinical settings. Therefore, NAC has a strong potential to emerge as a dietary supplement against high risk of cancer in AT and possibly other oxidative stress linked disorders. At present there is an ongoing clinical trial in pediatric AT patients, where a cocktail of antioxidants including NAC, is employed (personal communication with Dr. G. Berry, Thomas Jefferson University Medical College, also see http://www.treat-at.org). The trial is being conduced in Philadelphia, PA, and is a result of the combined efforts of the national organization to Treat-AT, Dr. Gerald Berry and his colleagues at DuPont Children’s and Children’s Hospital of Philadelphia, and SHS International Ltd (Liverpool, UK). The aim of this study is to determine whether lymphocytes from AT patients show abnormal levels of ROS and increased apoptosis and whether chronic broad antioxidant therapy retards development of lymphocyte and cerebellar dysfunction or arrest destruction of these tissues.
Acknowledgments
This work is supported by grants from the National Institute of Environmental Health Sciences (NIH RO1 grant No. ES09519) and the American Institute for Cancer Research both to RHS, a post-doctoral research fellowship of the Lymphoma Research Foundation Elizabeth Banks Jacobs and Byron Wade Strunk Memorial Fellowship to RR.
References
- Agarwal A, Munoz-Najar U, Klueh U, et al. N-acetyl-cysteine promotes angiostatin production and vascular collapse in an orthotopic model of breast cancer. Am J Pathol. 2004;164:1683–96. doi: 10.1016/S0002-9440(10)63727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Morini M, D’Agostini F, et al. Inhibition of angiogenesis-driven Kaposi’s sarcoma tumor growth in nude mice by oral N-acetylcysteine. Cancer Res. 2001;61:8171–8. [PubMed] [Google Scholar]
- Barlow C, Dennery PA, Shigenaga MK, et al. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc Natl Acad Sci USA. 1999;96:9915–9. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–71. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- Boder E. Ataxia-telangiectasia: some historic, clinical and pathologic observations. Birth Defects Orig Artic Ser. 1975;11:255–70. [PubMed] [Google Scholar]
- Boder E. Ataxia-telangiectasia: an overview. Kroc Found Ser. 1985;19:1–63. [PubMed] [Google Scholar]
- Boder E, Sedgwick RP. Ataxia-telangiectasia. (Clinical and immunological aspects) Psychiatr. Neurol. Med. Psychol. Beih. 1970;13(14):8–16. [PubMed] [Google Scholar]
- Borghesani PR, Alt FW, Bottaro A, et al. Abnormal development of Purkinje cells and lymphocytes in Atm mutant mice. Proc Natl Acad Sci USA. 2000;97:3336–41. doi: 10.1073/pnas.050584897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca M, Denurra T, Turrini F. Reduction of nitroxide free radical by normal and G6PD deficient red blood cells. Free Radic Biol Med. 1988;5:7–11. doi: 10.1016/0891-5849(88)90057-3. [DOI] [PubMed] [Google Scholar]
- Browne SE, Roberts LJ, Dennery PA, et al. Treatment with a catalytic antioxidant corrects the neurobehavioral defect in ataxia-telangiectasia mice. Free Radic Biol Med. 2004;36:938–42. doi: 10.1016/j.freeradbiomed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Shaham M, Dagan J, et al. Cytogenetic investigations in families with ataxia-telangiectasia. Cytogenet Cell Genet. 1975;15:338–56. doi: 10.1159/000130530. [DOI] [PubMed] [Google Scholar]
- Crawford TO, Skolasky RL, Fernandez R, et al. Survival probability in ataxia telangiectasia. Arch Dis Child. 2006;91:610–1. doi: 10.1136/adc.2006.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Kalinska B, Canapa A, et al. The effects of nitroxide radicals on oxidative DNA damage. Free Radic Biol Med. 2000;28:1257–65. doi: 10.1016/s0891-5849(00)00242-2. [DOI] [PubMed] [Google Scholar]
- De Flora S, Izzotti A, D’Agostini F, et al. Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis. 2001;22:999–1013. doi: 10.1093/carcin/22.7.999. [DOI] [PubMed] [Google Scholar]
- Decramer M, Rutten-van Molken M, Dekhuijzen PN, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–60. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- Doctrow SR, Huffman K, Marcus CB, et al. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J Med Chem. 2002;45:4549–58. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- Elson A, Wang Y, Daugherty CJ, et al. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci USA. 1996;93:13084–9. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti RA. The inherited basis of human radiosensitivity. Acta Oncol. 2001;40:702–11. doi: 10.1080/02841860152619115. [DOI] [PubMed] [Google Scholar]
- Goss PE. New perspectives in the treatment of non-Hodgkin’s lymphoma. Semin Oncol. 1992;19:23–9. [PubMed] [Google Scholar]
- Greenstein S, Ghias K, Krett NL, et al. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res. 2002;8:1681–94. [PubMed] [Google Scholar]
- Gropp A, Flatz G. Chromosome breakage and blastic transformation of lymphocytes in ataxia-telangiectasia. Humangenetik. 1967;5:77–9. doi: 10.1007/BF00286217. [DOI] [PubMed] [Google Scholar]
- Gueven N, Luff J, Peng C, et al. Dramatic extension of tumor latency and correction of neurobehavioral phenotype in Atm-mutant mice with a nitroxide antioxidant. Free Radic Biol Med. 2006;41:992–1000. doi: 10.1016/j.freeradbiomed.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Hahn SM, Krishna CM, Samuni A, et al. Potential use of nitroxides in radiation oncology. Cancer Res. 1994;54:2006s–10s. [PubMed] [Google Scholar]
- Ito K, Takubo K, Arai F, et al. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J Immunol. 2007;178:103–10. doi: 10.4049/jimmunol.178.1.103. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Daily D, Hochman A, et al. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 2001;61:1849–54. [PubMed] [Google Scholar]
- Kawakami S, Kageyama Y, Fujii Y, et al. Inhibitory effect of N-acetylcysteine on invasion and MMP-9 production of T24 human bladder cancer cells. Anticancer Res. 2001;21:213–9. [PubMed] [Google Scholar]
- Keating MJ, McLaughlin P, Cabanillas F. Low-grade non-Hodgkin’s lymphoma—development of a new effective combination regimen (fludarabine, mitoxantrone and dexamethasone; FND) Eur J Cancer Care (Engl) 1997;6:21–6. doi: 10.1111/j.1365-2354.1997.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–27. [PubMed] [Google Scholar]
- Krishna MC, Grahame DA, Samuni A, et al. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of super-oxide. Proc Natl Acad Sci USA. 1992;89:5537–41. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna MC, Russo A, Mitchell JB, et al. Do nitroxide antioxidants act as scavengers of O2-. or as SOD mimics. J Biol Chem. 1996;271:26026–31. doi: 10.1074/jbc.271.42.26026. [DOI] [PubMed] [Google Scholar]
- Kuljis RO, Xu Y, Aguila MC, et al. Degeneration of neurons, synapses, and neuropil and glial activation in a murine Atm knockout model of ataxia-telangiectasia. Proc Natl Acad Sci USA. 1997;94:12688–93. doi: 10.1073/pnas.94.23.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzminov A. DNA replication meets genetic exchange: chromosomal damage and its repair by homologous recombination. Proc Natl Acad Sci USA. 2001;98:8461–8. doi: 10.1073/pnas.151260698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin MF, Birrell G, Chen P, et al. ATM signaling and genomic stability in response to DNA damage. Mutat Res. 2005;569:123–32. doi: 10.1016/j.mrfmmm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Gueven N, Bottle S, et al. Current and potential therapeutic strategies for the treatment of ataxia-telangiectasia. Br. Med. Bull. 2007;81(82):129–47. doi: 10.1093/bmb/ldm012. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- Lista F, Bertness V, Guidos CJ, et al. The absolute number of trans-rearrangements between the TCRG and TCRB loci is predictive of lymphoma risk: a severe combined immune deficiency (SCID) murine model. Cancer Res. 1997;57:4408–13. [PubMed] [Google Scholar]
- Liyanage M, Weaver Z, Barlow C, et al. Abnormal rearrangement within the alpha/delta T-cell receptor locus in lymphomas from Atm-deficient mice. Blood. 2000;96:1940–6. [PubMed] [Google Scholar]
- May JM, Qu ZC, Juliao S, et al. Ascorbic acid decreases oxidant stress in endothelial cells caused by the nitroxide tempol. Free Radic Res. 2005;39:195–202. doi: 10.1080/10715760400019661. [DOI] [PubMed] [Google Scholar]
- Melov S, Doctrow SR, Schneider JA, et al. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci. 2001;21:8348–53. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyn MS. Ataxia-telangiectasia, cancer and the pathobiology of the ATM gene. Clin Genet. 1999;55:289–304. doi: 10.1034/j.1399-0004.1999.550501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Samuni A, Krishna MC, et al. Biologically active metal-independent superoxide dismutase mimics. Biochemistry. 1990;29:2802–7. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Hashida M. Inhibition of tumour metastasis by targeted delivery of antioxidant enzymes. Expert Opin Drug Deliv. 2006;3:355–69. doi: 10.1517/17425247.3.3.355. [DOI] [PubMed] [Google Scholar]
- Prescott LF, Park J, Ballantyne A, et al. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2:432–4. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- Quick KL, Dugan LL. Superoxide stress identifies neurons at risk in a model of ataxia-telangiectasia. Ann Neurol. 2001;49:627–35. [PubMed] [Google Scholar]
- Reichenbach J, Schubert R, Schindler D, et al. Elevated oxidative stress in patients with ataxia telangiectasia. Antioxid Redox Signal. 2002;4:465–9. doi: 10.1089/15230860260196254. [DOI] [PubMed] [Google Scholar]
- Reichenbach J, Schubert R, Schwan C, et al. Anti-oxidative capacity in patients with ataxia telangiectasia. Clin Exp Immunol. 1999;117:535–9. doi: 10.1046/j.1365-2249.1999.01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reliene R, Bishop AJ, Aubrecht J, et al. In vivo DNA deletion assay to detect environmental and genetic predisposition to cancer. Methods Mol Biol. 2004a;262:125–39. doi: 10.1385/1-59259-761-0:125. [DOI] [PubMed] [Google Scholar]
- Reliene R, Fischer E, Schiestl RH. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in atm-deficient mice. Cancer Res. 2004b;64:5148–53. doi: 10.1158/0008-5472.CAN-04-0442. [DOI] [PubMed] [Google Scholar]
- Reliene R, Schiestl RH. Antioxidant N-acetyl cysteine reduces incidence and multiplicity of lymphoma in Atm deficient mice. DNA Repair (Amst) 2006;5:852–9. doi: 10.1016/j.dnarep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Reliene R, Schiestl RH. Antioxidants suppress lymphoma and increase longevity in Atm-deficient mice. J Nutr. 2007;137:229S–32S. doi: 10.1093/jn/137.1.229S. [DOI] [PubMed] [Google Scholar]
- Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–53. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Schubert R, Erker L, Barlow C, et al. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum Mol Genet. 2004;13:1793–802. doi: 10.1093/hmg/ddh189. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–68. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Stumm M, Neubauer S, Keindorff S, et al. High frequency of spontaneous translocations revealed by FISH in cells from patients with the cancer-prone syndromes ataxia telangiectasia and Nijmegen breakage syndrome. Cytogenet Cell Genet. 2001;92:186–91. doi: 10.1159/000056900. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Metcalfe JA, Thick J, et al. Leukemia and lymphoma in ataxia telangiectasia. Blood. 1996;87:423–38. [PubMed] [Google Scholar]
- Van Schooten FJ, Besaratinia A, De Flora S, et al. Effects of oral administration of N-acetyl-L-cysteine: a multi-biomarker study in smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:167–75. [PubMed] [Google Scholar]
- Webb WR. Clinical evaluaton of a new mucolytic agent, acetylcysteine. J Thorac Cardiovasc Surg. 1962;44:330–43. [PubMed] [Google Scholar]
- Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Xu Y. ATM in lymphoid development and tumorigenesis. Adv Immunol. 1999;72:179–89. doi: 10.1016/s0065-2776(08)60020-6. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ashley T, Brainerd EE, et al. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–22. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- Yan M, Kuang X, Qiang W, et al. Prevention of thymic lymphoma development in Atm−/− mice by dexamethasone. Cancer Res. 2002;62:5153–7. [PubMed] [Google Scholar]
- Yan M, Qiang W, Liu N, et al. The ataxia-telangiectasia gene product may modulate DNA turnover and control cell fate by regulating cellular redox in lymphocytes. Faseb J. 2001;15:1132–8. doi: 10.1096/fj.00-0601com. [DOI] [PubMed] [Google Scholar]
- Yi M, Rosin MP, Anderson CK. Response of fibroblast cultures from ataxia-telangiectasia patients to oxidative stress. Cancer Lett. 1990;54:43–50. doi: 10.1016/0304-3835(90)90089-g. [DOI] [PubMed] [Google Scholar]