Abstract
There are multiple genetic links between schizophrenia and a deficit of proline dehydrogenase (PRODH) enzyme activity. However, reports testing for an association of schizophrenia with the resulting proline elevation have been conflicting. The objectives of this study were to investigate whether hyperprolinemia is associated with schizophrenia, and to measure the relationship between plasma proline, and clinical features and symptoms of schizophrenia.
We performed a cross-sectional case-control study, comparing fasting plasma proline in 90 control subjects and 64 schizophrenic patients and testing for association of mild to moderate hyperprolinemia with schizophrenia. As secondary analyses, the relationship between hyperprolinemia and five measures of clinical onset, symptoms and outcome were investigated.
Patients had significantly higher plasma proline than matched controls (p<0.0001), and categorical analysis of gender adjusted hyperprolinemia showed a significant association with schizophrenia (OR 6.15, p=0.0003). Hyperprolinemic patients were significantly older at their first hospitalization (p=0.015 following correction for multiple testing). While plasma proline level was not related to total, positive or negative symptoms, hyperprolinemic status had a significant effect on length of hospital stay (p=0.005), following adjustment for race, BPRS score, and cross-sectional time from admission to proline measurement.
Mild to moderate hyperprolinemia is a significant risk factor for schizophrenia, and may represent an intermediate phenotype in the disease. Hyperprolinemic patients have a significantly later age of first psychiatric hospitalization, suggestive of later onset, and hospital stays 46% longer than non-hyperprolinemic subjects. These findings have implications in the etiology of schizophrenia, and for the clinical management of these patients.
Keywords: Proline, PRODH, outcome, etiology, intermediate phenotype, neuromodulator
1. Introduction
Schizophrenia is a severe psychiatric disorder of unknown cause, with a worldwide incidence of approximately 1%. There is a large increased risk of schizophrenia and other psychotic disorders in people with 22q11 deletion syndrome (22q11DS), with up to one third developing schizophrenia or schizoaffective disorder (Jacquet et al. 2002; Karayiorgou and Gogos 2004; Karayiorgou et al. 2010; Murphy et al. 1999; Scambler 2000). A common feature of 22q11DS is a hemizygous deletion of the proline dehydrogenase (PRODH) gene, which encodes the proline dehydrogenase enzyme, that catalyses the first step in proline catabolism (Mitsubuchi et al. 2008). Significantly, approximately 37-50% of patients with the 22q11 deletion (Goodman et al. 2000; Raux et al. 2007) have elevation of plasma proline that is between 2 -10 fold higher than the upper end of the normal range (Mitsubuchi et al. 2008), and plasma proline levels have been found to inversely correlate with intelligence quotient in patients with the 22q11DS velo-cardiofacial syndrome (Raux et al. 2007).
In addition to its proteogenic role, proline is a precursor of the neurotransmitter glutamate, and has several characteristics that suggest it functions as a CNS neuromodulator (Phang et al. 2001). Studies of elevated proline in humans and model systems illustrate some of the pathogenic properties of hyperprolinemia: In the hyperprolinemic PRO/RE mouse strain, elevated peripheral and CNS proline is associated with neurocognitive dysfunction, in the form of learning and memory deficits (Baxter et al. 1985; Davis et al. 1987). Deficiency of PRODH activity in the PRO/RE mouse, which results from a heterozygous nonsense Prodh mutation (the premature termination E453X variant (Gogos et al. 1999)), closely mimics the loss of PRODH activity and the 2-10 fold elevation of plasma proline observed in human hyperprolinemia type-I (HPI), which also arises from mutations in the PRODH gene (Mitsubuchi et al. 2008). Although variable, the neurological phenotype associated with HPI includes mental retardation and epilepsy (Afenjar et al. 2007; Mitsubuchi et al. 2008). Plasma proline elevations greater than 10-fold above the normal range are found in patients with hyperprolinemia type-II (HPII), caused by mutations in the ALDH4A1 gene that encodes Δ-1-pyrroline-5-carboxylate (P5C) dehydrogenase, which is immediately downstream of PRODH in proline catabolism. P5C dehydrogenase deficits and the resultant hyperprolinemia can lead to low IQ, seizures, and in some subjects, mild mental retardation (Flynn et al. 1989). Following chronic proline administration, rats with plasma proline levels consistent with human HPII developed behavioral and brain histological changes coupled with impairments of glutamate synthesis, all suggestive of neurological dysfunction (Shanti et al. 2004).
Evidence supporting the functional significance of hyperprolinemia in schizophrenia comes from two sources: Mice homozygous for the Prodh E453X mutation, have elevated plasma and brain proline, locally decreased CNS glutamate and γ-aminobutyric acid (GABA) (Gogos et al. 1999; Paterlini et al. 2005), and a deficit in sensorimotor gating shown as decreased prepulse inhibition of the acoustic startle response, that is a characteristic of schizophrenia (Braff et al. 1978). Moreover, a familial PRODH deletion and PRODH missense mutations that have been described in patients with schizophrenia (Bender et al. 2005; Jacquet et al. 2002), and that have been functionally related to both moderately and severely decreased PRODH enzyme activity in vitro (Bender et al. 2005), have also been associated with both HPI and moderate hyperprolinemia in schizophrenic patients (Jacquet et al. 2002). However, the conclusions of case-control studies evaluating peripheral proline levels as a risk factor for schizophrenia have been conflicting: Following measurement of plasma proline, Jacquet et al. did not detect an association between mild to moderate hyperprolinemia and schizophrenia in a mixed-gender study of Caucasian subjects, although they did report hyperprolinemia as a significant risk for schizoaffective disorder (Jacquet et al. 2005). This study concurred with a previous report, finding no significant difference in serum proline level across groups of control subjects, treated schizophrenics, naive schizophrenics and drug-free schizophrenic subjects (Rao et al. 1990). Conversely, a more recent study also measuring serum levels found a significant elevation of proline in schizophrenic patients when compared to controls, but only in female subjects (Tomiya et al. 2007). Despite these mixed findings, data continues to support a functional role for PRODH variants and hyperprolinemia in the etiology of schizophrenia (Kempf et al. 2008), although studies relating plasma proline level to the clinical symptoms of schizophrenia are lacking. The objective of this study was to test the hypothesis that elevated peripheral proline is associated with schizophrenia after adjusting for gender differences, and to explore the clinical effects of elevated proline levels in schizophrenic patients.
2. Methods
2.1 Subjects and Recruitment
Male and female, African American, Caucasian and Hispanic patients, aged 18-65, were recruited from inpatient wards at Bellevue Hospital Center (BHC). A significant effect of valproic acid (VPA) on plasma proline level was previously reported (Jacquet et al. 2005), and therefore schizophrenic subjects treated with VPA at the time of enrollment were excluded. Patient screening and recruitment was not dependent on their length of stay in the hospital at the time of recruitment, and thus cross-sectional data were generated. Patients received a standardized hospital diet based upon ADA Guidelines of 20% protein, 25% fat and 55% carbohydrates. Psychiatric symptoms were measured using the Brief Psychiatric Rating Scale (BPRS), the Schedule for Assessment of Positive Symptoms (SAPS), the Schedule for Assessment of Negative Symptoms (SANS), and schizophrenia diagnoses were confirmed using the Structured Clinical Interview for DSM IV Disorders (SCID).
Controls were recruited from the BHC community, with recruitment targeted to reflect the patients on age, race/ethnicity, and gender. A SCID-NP interview was conducted for all controls, who were excluded if they reported symptoms from modules A-D. All subjects completed general questionnaires, self-reporting race, and documenting diagnostic and medical history information for common diseases and prescription medication use. Capacity to give informed consent was determined in accordance with the New York University (NYU) IRB regulations. After description of the study to the subjects, written informed consent was obtained from all subjects in accordance with all institutional IRB guidelines and regulations.
2.2 Determination of Plasma Proline Levels
For all subjects, a fasting morning blood draw was performed and heparinized blood samples sent to ARUP Laboratories (500 Chipeta Way, SLC, UT84108) for quantitative plasma amino acid analysis (reference number 0080710). Proline was measured in μmoles/liter (μM).
2.3 Statistical Analysis
Group differences were tested using the Satterthwaite t-test or ANOVA with a correction for multiple testing (assuming normality of continuous variables), and using the χ2 or Fisher exact test where the expected cell size was <5 (categorical variables).
Tests of normality (n=154, p<0.001) and inspection of the proline distribution suggested non-symmetry with a positive skew and heavier than normal tails. Therefore, proline levels were compared across groups using the Mann-Whitney and Kruskal-Wallis non-parametric tests, and the Spearman’s rank correlation coefficient to assess relationships with continuous variables. To adjust for previously reported gender differences (Jacquet et al. 2005), Jacquet et al.’s criteria were employed to define hyperprolinemic status as a proline level two standard deviations (SDs) or more above the gender-specific mean of controls (Jacquet et al. 2005).
We sought to determine the effect of plasma proline on five clinical measures collected, using a generalized linear modeling (GLM) approach, employing a maximum-likelihood estimation to summarize the relationship between hyperprolinemia and the clinical outcomes of total BPRS, SAPS, and SANS scores, age at first hospitalization, and length of hospital stay (LOHS). To model LOHS, subjects were excluded from analysis if they were transferred to another treatment facility (n=19), as discharge due to improvement could not be considered. Distributional assumptions were tested for each dependent variable using the Anderson-Darling test (Supplementary Data S1). For models that passed criteria (a relationship with hyperprolinemia when alpha <0.1), medication (CPZ equivalent daily dose), severity of illness (total BPRS, SAPS, and SANS scores), history of alcohol abuse/dependence, smoking status, prior housing status before admission, plus the demographic variables age, race, gender, current occupational status (currently working or attending school compared to those currently unemployed), and highest education level reached (excluding subjects still in education) were assessed as possible covariates. Due to the cross-sectional nature of the data collection, the variable of time from admission to proline measurement was also evaluated as a covariate in the LOHS model. To assess utility in adjusting the dependent variable, each covariate was entered into a bivariate analysis, and terms found to have p values of <0.10 carried forward to a multivariate model, where we examined the effect of plasma proline on LOHS while controlling for significant potential confounding variables (p<0.05). Final model selection and fit were determined using Akaike’s information criterion and the Likelihood Ratio test ([−2ln(likelihood for null model/likelihood for alternative model)]), which tested for the significant influence of covariates plus the main explanatory variable in sequential models. Outliers in the data were characterized by Cook’s distance values (Di>4/n), and assessment of the absolute value of DFBETAs for the intercept and each independent variable. Coefficients were retransformed back to the original units. Assumptions of independence and homoscedasticity of errors were met for all models and there were no signs of multicollinearity between predictor variables. Bonferroni corrections of final models were employed to adjust for multiple clinical measures hypothesis testing of our secondary outcomes (n=5). Statistical analysis was performed in SAS v9.1, Stata IC v10.1, and R v2.10.1.
3 Results
3.1 Sample Characteristics
64 schizophrenic patients and 90 healthy controls met the study criteria and were included in the analysis. Subject’s demographic characteristics are shown in Table 1. Subjects were matched on gender, ethnicity, and age. There were no significant differences between study groups on the presence of common diseases, prescription of common medications, or on alcohol or substance abuse and/or dependence. However there was a significant difference in smoking status, as more patients reported that they were current or previous smokers (p<0.0001). Schizophrenic patients were relatively short-stay inpatients (mean length of hospital stay 42±27 days), recruited following psychiatric hospitalization to the BHC primary care facility. Clinical characteristics and medication profiles of the patients are shown in Tables 2 and 3.
Table 1.
Demographic Characteristics of Schizophrenic Patients (SZ) and Healthy Control Subjects, n=154
| Characteristic n |
SZ n=64 |
Control n=90 |
Proba |
|---|---|---|---|
| Females, % (n) | 51.6 (33) | 51.1 (46) | .9560 |
| Ethnicity, % (n) | .9775 | ||
| African American | 32.8 (21) | 34.4 (31) | |
| Caucasian | 34.4 (22) | 33.3 (30) | |
| Hispanic | 32.8 (21) | 32.2 (29) | |
| Age (years), mean ± SD | 38.5 ± 11.3 | 37.9 ± 12.0 | .7187 |
| Body Mass Index, mean ± SD | 27.2 ± 5.4 | 26.4 ± 5.0 | .3663 |
| Smoking Status, % (n) | <.0001* | ||
| Current or Previous | 60.93 (39) | 24.4 (22) | |
| Never Smoked | 34.38 (22) | 74.5 (67) | |
| Not reported | 4.69 (3) | 1.1 (1) | |
| History of Alcoholism, % (n) | .7087 | ||
| Abuse | 9.4 (6) | 6.7 (6) | |
| Dependence | 6.3 (4) | 4.4 (4) | |
| Neither | 84.4 (54) | 88.9 (80) | |
| History of Substance Abuse, % (n) | .1279 | ||
| Abuse | 7.8 (5) | 3.3 (3) | |
| Dependence | 14.1 (9) | 6.7 (6) | |
| Neither | 78.1 (50) | 90.0 (81) | |
| History of Seizures, % (n) | 1.6 (1) | 0 (0) | .4106 |
| Asthma, % (n) | 7.9 (5) | 9.0 (8) | .7368 |
| IDD, % (n)b | 4.8 (3) | 0 (0) | .0692 |
| NIDD, % (n)c | 7.9 (5) | 4.4 (4) | .4855 |
| Common Medication, % (n) | |||
| Antibiotics | 3.1 (2) | 0 (0) | .1711 |
| Antilipidemics | 6.3 (4) | 4.4 (4) | .7192 |
| Antihypertensives | 9.4 (6) | 3.3 (3) | .1644 |
| Antivirals | 1.6 (1) | 1.1 (1) | .9999 |
| Steroids | 6.3 (4) | 1.1 (1) | .1609 |
p-values calculated by Satterthwaite t-test, Fisher exact test, or Chi-Square
=significant values when comparing SZ patients to controls.
IDD = Insulin Dependent Diabetes
NIDD = Non-Insulin Dependent Diabetes
Table 2.
Clinical Characteristics of Schizophrenic Subjects (n=64)
| Characteristic | Mean | SD | Min | Max |
|---|---|---|---|---|
| Age at First Hospitalizationa | 24.6 | 7.5 | 14 | 44 |
| Length of Hospital Stay (days) | 42.2 | 27.4 | 8 | 135 |
| BPRSb Total Symptoms | 32.6 | 8.2 | 18 | 56 |
| SAPSc Total Symptoms | 15.1 | 10.2 | 1 | 51 |
| SANSd Total Symptoms | 15.3 | 10.9 | 0 | 56 |
| SZ Subtype, % (n) | % (n) | |||
| Disorganized | 14.1 (9) | |||
| Catatonic | 0 (0) | |||
| Paranoid | 34.4 (22) | |||
| Residual | 4.7 (3) | |||
| Undifferentiated | 46.9 (30) |
n = 47 for whom this characteristic could be obtained
Brief Psychiatric Rating Scale
Schedule for Assessment of Positive Symptoms
Schedule for Assessment of Negative Symptoms
Table 3.
Medication of Schizophrenic Subjects (n=64)
| Neuroleptic Medications Received |
% (n) | ||||
|---|---|---|---|---|---|
| Neuroleptic Type | |||||
| Typical only | 15.6 (10) | ||||
| Atypical only | 68.7 (44) | ||||
| Both | 14.1 (9) | ||||
| None | 1.6 (1) | ||||
|
Neuroleptic Poly-pharmacy and
Dose |
Subjects (n) | Mean | SD | Min | Max |
| Total number of NL administered | 63 | 1.2 | 0.5 | 0 | 3 |
| Daily CPZE dosea,c | 63 | 550.2 | 358.3 | 0 | 2100 |
| Normalized daily NL doseb,c | 63 | 62.7 | 37.6 | 0 | 269.2 |
| Medication Dose (mg) | Subjects (n) | Mean | SD | Min | Max |
| Aripiprazole | 7 | 35.0 | 21.2 | 5 | 75 |
| Clozapine | 4 | 300.0 | 196.9 | 125 | 575 |
| Fluphenazine | 1 | 10.0 | 10 | 10 | |
| Haloperidol | 16 | 9.3 | 4.5 | 1 | 20 |
| Paliperidone | 1 | 9.0 | 9 | 9 | |
| Olanzapine | 13 | 21.5 | 11.1 | 5 | 40 |
| Perphenazine | 2 | 18.0 | 8.5 | 12 | 24 |
| Quetiapine | 8 | 520.0 | 312.9 | 60 | 1050 |
| Risperidone | 24 | 4.5 | 1.4 | 1 | 8 |
| Mood Stabilizers: Dose (mg) | Subjects (n) | Mean | SD | Min | Max |
| Total number of mood stabilizers administeredd |
0.1 | 0.3 | 0 | 1 | |
| Lithium | 5 | 1140.0 | 134.2 | 900 | 1200 |
| Lamotrigine | 2 | 100.0 | 70.7 | 50 | 150 |
| None | 57 |
NLs - neuroleptic drugs
Chlorpromazine (CPZ) equivalent dose, n=63 as one subject’s NL had no CPZ equivalent.
Percent of the n=64 group maximum daily dose for each NL medication. The summed percentages across all NLs taken were calculated for each individual.
CPZE and normalized daily neuroleptic dose were highly correlated, r2 =0.92, p< 0.0001 (Supplementary Data S2).
Patients receiving valproic acid (VPA) at the time of recruitment were excluded from analyses. Of the VPA-untreated subjects (n=64), four subjects had received VPA during their hospitalization, but prior to their enrollment into the study (one subject: last received VPA 5 days prior to enrollment, two subjects: 10 days prior, and one subject: 14 days prior).
3.2 Association between Plasma Proline Level and Schizophrenia
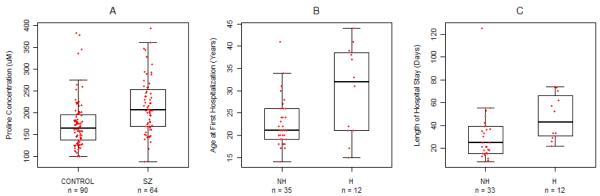
Schizophrenic patients had significantly higher fasting plasma proline levels than controls (Figure 1A, p<0.0001). Previously, studies have reported the effects of gender (Bremer et al. 1981; Jacquet et al. 2005) and alcohol use (Walter et al. 2008) on proline level, and so we examined the effect of these two confounds on our finding of elevated proline in schizophrenia. Proline was higher in males than in females; significantly higher in controls (204.41±61.59 versus 145.46±28.9, Mann-Whitney z=5.58, p<0.0001) with a trend toward significance in the SZ group (229.26±59.17 versus 203.24±64.76, z=1.87, p=0.06). Importantly, the finding of significantly higher proline in schizophrenic patients compared to controls remained following a gender-stratified analysis (males: z=−2.35, p=0.019, females: z=−4.48, p<0.0001). A relationship with alcohol use was only observed in controls; ten controls reporting alcohol abuse or dependence had significantly higher proline than eighty controls with no abuse or dependence (203.5±38.8 versus 170.63±56.9, Mann-Whitney z=2.55, p=0.0106). In the patient group, no significant differences were observed between alcohol use groups (z=1.507, p=0.132). These data are consistent with a recent study suggesting effects only of current alcohol on proline level (Walter et al. 2008) and are perhaps indicative of inpatient’s lack of access to alcohol.
Figure 1. Hyperprolinemia in Schizophrenia, and the Effect on Age at First Hospitalization (AFH) and Length of Hospital Stay (LOHS).
1a Fasting Plasma Proline in SZ and Control Groups. The boxplot illustrates the significant difference between control (174.28±55.97) and SZ patient (215.84± 63.00) groups, Mann-Whitney z=−4.58, p<0.0001. 1b. Bivariate Relationship Between AFH and Hyperprolinemia. Hyperprolinemic SZ patients have a significantly later age of first hospitalization (29.9±10.2 years) compared to non-hyperprolinemic patients (22.2±5.4 years), log-normal model: z=3.37, 1df, p=0.001. Age at first hospitalization could not be determined for 17 subjects. 1c) Bivariate Relationship Between LOHS and Hyperprolinemia. The duration of their hospital stay is longer for hyperprolinemic SZ patients (47.0±19.7 days), compared to non-hyperprolinemic patients (30.1±21.9 days), gamma-log model: z=2.38, 1df, p=0.017. 19 subjects were excluded from analysis because they were transferred or discharged to another treatment facility.
A gamma-log model showed no effect of AFH on LOHS for all 35 subjects for whom AFH could be determined and who were not transferred to a second care facility (z=1.35, 1df, p=0.178), or for the subset of 9 hyperprolinemic subjects (z=1.04, 1df, p=0.298).
Key: SZ: Schizophrenia, NH: Non-hyperprolinemic, H: Hyperprolinemic, AFH: Age at first hospitalization, LOHS: length of hospital stay, IQR: interquartile range. Red jittered points represent individual subject data. The horizontal line within each box represents the group mean (mean ± SD reported). The box indicates the IQR. The whiskers extend to the most extreme data point which is <1.5 times the IQR.
We also performed an investigation of other potential confounds: Proline levels did not differ across ethnic groups (n=154, Kruskal-Wallis χ2=1.955, 2df, p=0.45), or between subjects who had previously or currently smoked and those that had never smoked (n=154, Mann-Whitney z=−1.071, p=0.28). Moreover, a significant difference in proline level between schizophrenics and controls remained after stratifying analysis by smoking status (non-smokers p=0.005, current/previous smokers p=0.016). In the patient group, there was also no relationship between proline level and education (rho=−0.1056, p=0.51, n=41). As previously reported (Jacquet et al. 2005), there was no relationship between proline and age in the study sample (n=154, Spearman’s rho=0.04, p=0.66).
3.3 Association of Hyperprolinemic Status with Schizophrenia
Subjects with hyperprolinemia were identified following a gender-specific adjustment for proline differences (Jacquet et al. 2005). The distribution of hyperprolinemic subjects was significantly different between controls and schizophrenic subjects (n=5/90 and 17/64 respectively, 1df, OR=6.15, p=0.0003, 95% CI 1.99-22.4). Thus, subjects with hyperprolinemia have six times greater odds of schizophrenia. This result is unlikely confounded by racial/ethnic, smoking status, or age group differences. In addition, this result remained significant following analysis where the 20 subjects who reported alcohol abuse or dependence were excluded (1df, OR= 6.31, p=0.0005, 95% CI 1.99-23.41). Of interest, there was a trend towards significance for subjects who were hyperprolinemic to be sampled in the early part of their hospitalization, compared to those who were not hyperprolinemic, who were sampled later in their hospitalization (proportion of stay=time from admission to proline measurement/total hospital stay: 0.38±0.26, n=17 versus 0.49±0.25, n=47 Mann-Whitney z=−1.7, p=0.089).
3.4 Testing the effects of potential medication confounds
As described, schizophrenic subjects were excluded from analysis if they were currently receiving the mood stabilizer VPA, due to the known influence of VPA on plasma proline (Jacquet et al. 2005). Four subjects had received VPA during their current hospitalization; although the VPA treatment had ended 5-14 days prior to enrollment into the study (see Table 3). The significant association of hyperprolinemia with schizophrenia remained after removing all four patients (1 hyperprolinemic, 3 non- hyperprolinemic patients) from analysis (n=150, 1df, OR=6.18, p=0.005). In the patient group (n=64) there was no significant difference in the proportion of hyperprolinemic patients receiving other mood stabilizer medications compared to those receiving no mood-stabilizers (p=1.0). With regards to neuroleptic use, only one patient did not receive neuroleptic medication prior to blood draw. However, as for mood-stabilizers, there was no significant difference in the proportion of hyperprolinemic subjects receiving only atypical neuroleptics compared to those receiving typicals only (n=13/44 versus n=2/10, p=0.71), and there were no proline differences in the atypical-only versus typical-only groups (z=−0.56, p=0.58, n=54). There was also no relationship between proline and two independent summary measures of neuroleptic dose; daily CPZ equivalents (rho=−0.06, p=0.62, n=63), or normalized daily neuroleptic dose (see Table 3 and Supplementary Data S2, rho=−0.06, p=0.62, n=63), although the measures themselves were highly correlated. In the patient group, there was no significant difference in the proportion of hyperprolinemic subjects versus non-hyperprolinemic subjects receiving the anticholinergic benztropine (n=2/17 versus n=12/47, p=0.32), and there was no relationship with proline level and benztropine dose (rho=−0.18, p=0.54, n=14). None of the control subjects reported benztropine use. Similarly, use of antidepressants (n=2/17 versus n=6/47, p=0.99), or benzodiazepines (n=3/17 versus n=8/47, p=0.99) did not differ significantly in the hyperprolinemic versus non-hyperprolinemic patient groups. In patients there was also no relationship between proline level and education (rho=−0.1056, p=0.51, n=41). These data suggest that the association between hyperprolinemia and schizophrenia does not arise from mood stabilizer and/or neuroleptic use, and is consistent with published studies (Jacquet et al. 2005).
3.5 Patient characteristics associated with Hyperprolinemia
Initial analysis of the data illustrated a significant relationship between hyperprolinemia and age at first hospitalization (AFH) (Figure 1B, p=0.001). Only the variable of age passed covariate evaluation for an effect on AFH (p=0.055), but was subsequently removed from the final log-normal AFH model based upon the LRT (p>0.05). Following adjustment for gender, due to the known effects of gender on age at first onset and hospitalization (Rabinowitz, Levine, and Hafner 2006), and a correction for multiple testing, the significant relationship remained (p=0.015). Retransformation of the hyperprolinemia coefficient predicted that hyperprolinemic patients (mean age at first hospitalization = 29.9+10.2 years) were, on average, 7 years older than non-hyperprolinemic subjects (mean age =22.7±5.4) when they were first hospitalized.
We also observed a significant bivariate relationship between hyperprolinemia and LOHS (Figure 1C, p=0.017). For analysis of the LOHS outcome variable, we excluded subjects who were transferred or discharged to another care facility such as a state psychiatric hospital (n=19), as we reasoned that these subjects may not have achieved a degree of improvement to allow for interpretation of LOHS as clinically relevant. To further model LOHS, a gamma distribution was determined a good-fit to characterize the outcome, with a log link function to specify the relationship with the explanatory variables: Variables that passed criteria from the bivariate screen are detailed in Supplementary Data S3. Hyperprolinemic status was found to have a significant effect on the outcome of LOHS, when adjusted for the time to blood draw, BPRS score, and race (Table 4), and further adjustment for multiple testing (p=0.005). Retransformation of the coefficients predicted that patients with hyperprolinemia stayed in the hospital on average an additional 13 days longer than non-hyperprolinemic patients, keeping the variables of time, BPRS, and race constant. Because of the small sample size, model interactions with the main effects were not statistically evaluated (see Supplementary Data S4 for further discussion of model interactions).
Table 4.
Multivariate Modeling of Length of Hospital Stay (LOHS).
| Final Model**a Log likelihood (LL): −168.19 | ||||||
|---|---|---|---|---|---|---|
| Variable | b | se | z | pb | LR χ2 | pc |
| Constant | 1.67 | 0.251 | 6.66 | <0.001 | ||
| Timed (mean = 14.91 days) | 0.029 | 0.005 | 5.92 | <0.001 | 40.38 | <0.001 |
| Race | ||||||
| Hispanic (n=15) v Caucasian (n=16) |
0.322 | 0.132 | 2.45 | 0.014 | ||
| Hispanic (n=15) v African American (n=14) |
0.586 | 0.132 | 4.43 | <0.001 | ||
| BPRS Total Score (mean = 32.3) | 0.028 | 0.007 | 3.87 | <0.001 | ||
| Hyperprolinemia (Yes, n=12 v No, n=33) |
0.408 | 0.120 | 3.38 | 0.001 | 10.67 | 0.0011 |
n=45, df=5, χ2=51.05, p<0.0001
Gamma distribution with log link
probability >|z|
probability of likelihood ratio (LR) statistic (p> χ2)
Time from admission to proline measurement
There was no significant difference in the proportion of subjects with hyperprolinemia across schizophrenia subtypes (disorganized n=3/9, catatonic 0/0, paranoid 5/22, residual 2/3, undifferentiated 7/30, p=0.38), and proline levels did not differ across subtype (Kruskal-Wallis χ2=0.75, 3df, p=0.86). In the patient group, there was no significant bivariate relationship between hyperprolinemia and measures of symptom severity: BPRS total (1df, p=0.48, n=64), SAPS total (1df, p=0.40, n=64), or SANS total score (1df, p=0.40, n=64).
4. Conclusions
Schizophrenic patients had significantly elevated fasting plasma proline levels, compared to matched control subjects. The confounding effects of alcohol and gender on plasma proline ( 1981; Jacquet et al. 2005; Walter et al. 2008), were evaluated: alcohol abuse and dependence analysis confirmed that alcohol use did not drive our finding of elevated proline in patients, and gender-stratified analysis demonstrated a significant plasma proline elevation in schizophrenia, in both males and females. This is consistent with a report by Tomiya et al., who measured serum proline elevation in both male and female schizophrenic patients when compared to controls (Tomiya et al. 2007), although the small sample size they employed likely contributed to the insignificant finding in males.
We also performed a categorical analysis of proline. Using criteria to define gender-adjusted mild to moderate hyperprolinemia (Jacquet et al. 2005), we demonstrated a highly significant association with schizophrenia, with 26.6% of the patients defined as hyperprolinemic compared to 5.6% of controls. Potential medication-based confounds on this association were investigated. VPA-treated patients were excluded from the study and non-VPA mood stabilizer use did not have a significant effect on proline level. While the effect of neuroleptics on proline was difficult to truly assess because all but one schizophrenic patient was receiving neuroleptics, there was no evidence to suggest the proportion of hyperprolinemic subjects differed in the atypical versus typical neuroleptic use groups. There was also no relationship between proline level and two independent measures of neuroleptic dose. In summary, elevated proline and mild hyperprolinemia were significantly associated with schizophrenia in this inpatient sample, and this finding is unlikely confounded by gender, alcohol use, or patient medication.
Interestingly, an association of hyperprolinemia with schizoaffective disorder but not with schizophrenia was previously reported (Jacquet et al. 2005). Jacquet et al’s., predominately paranoid schizophrenic sample had subtypes different to those reported here (p<0.001), although we did not detect differences in proline level across subtypes. Additionally, Jacquet et al. sampled a Caucasian population, whereas African American, Caucasian and Hispanic subjects were recruited for this study. Although we found no significant differences between ethnic/racial groups on proline level, we cannot rule out the possibility that the different subject groups, coupled with recruitment from different treatment settings (Jacquet et al. 2005), may account for the discrepant findings. One potential limitation of our study design was that data on the socioeconomic status of all study subjects was not collected and analyzed. However, in the patient group there was no relationship between proline level and the highest level of education reached. Moreover, proline levels were measured following an overnight fast, and therefore potential influences of socioeconomic status on, for example, diet, may be reduced, lessening the impact on our primary finding of an association between schizophrenia and hyperprolinemia.
Considering sources of the proline elevation, PRODH gene variants are a potential candidate, as variants have been identified in schizophrenia. For example PRODH variants were found in 36% of a schizophrenic patient sample (Jacquet et al. 2002), of which over 40% would be predicted to have low enzyme activity and elevated proline (Bender et al. 2005). There is also a strong association between schizophrenia and 22q11DS and/or microdeletions of 22q11 encompassing the PRODH locus (Karayiorgou et al. 2010), and it has been suggested that 22q11DS may be under-diagnosed (McDonald-McGinn et al. 2005). However, whilst our study subjects were not genotyped for PRODH variants, based upon the frequency of subjects with hyperprolinemia (26.6%), abnormal proline homeostasis may also be implicated, rather than higher than expected prevalence of 22q11DS or functional PRODH nucleotide variants (Guilmatre et al. 2010).
This is one of the first studies to explore the association between hyperprolinemia and clinical characteristics in a schizophrenic patient sample. While hyperprolinemia was not associated with total, positive, or negative symptoms, we demonstrated that schizophrenic patients with hyperprolinemia are significantly older at their first psychiatric hospitalization (29.9 years) when compared to non-hyperprolinemic patients (22.7 years), after adjusting for gender. Although not an exact measure of onset, previous studies have shown a strong relationship between age at first hospitalization and age of onset in both genders ((Rabinowitz et al. 2006) and references therein), and thus our finding suggests a later age of onset in subjects with elevated proline. Interestingly, the largest study of VCFS patients reported a significantly later onset of schizophrenia in the 22q11DS patients (mean age 26 years) compared to a control group of unrelated schizophrenic patients (mean age 19 years)(Murphy et al. 1999). That study along, with our finding, may thus point to etiological differences between patients with and without hyperprolinemia: Clinically elevated peripheral proline is reflected by elevation in the CNS (Baxter et al. 1985; Dingman and Sporn 1959; Efron 1965; Gogos et al. 1999; Jacquet et al. 2003; Shanti et al. 2004), and we hypothesize that the elevated plasma proline in schizophrenia also reflects elevated CNS levels in these subjects. Speculatively, it may be that chronically elevated CNS proline increases risk for development of schizophrenia, but that long-term exposure is necessary for this effect to manifest.
We also found that the presence of mild to moderate hyperprolinemia in schizophrenic patients predicts a significantly longer hospital stay. LOHS is a useful measure of time to clinical benefit and discharge (Centorrino et al. 2004; Wassef et al. 2005), and our finding of hospitalizations that were on average two weeks longer for hyperprolinemic subjects, which represents nearly 50% longer hospitalization periods, highlights a subset of patients with substantial increases in life disruption and inconvenience, and has important clinical and economic ramifications. Although a caveat to this data interpretation arises due to the cross-sectional nature of the study measures, our significant finding remained after adjustment for the time from admission to proline measurement. Intriguingly our data also showed a trend towards significance for hyperprolinemic subjects to be sampled earlier in their hospital stay, when compared to non-hyperprolinemic subjects. A longitudinal study investigating proline level over the course of an individual patient’s hospitalization, that also explores the relationship with clinical improvement (as measured by the change in a clinical severity scale, such as the BPRS) between admission and discharge, would be an optimal and warranted approach to further explore our findings.
Proline has several properties that are similar to classical excitatory amino acid neurotransmitters, such as its release at the synapse after K+-induced depolarization, its synthesis within synaptosomes and its uptake into synaptosomes by a high-affinity Na-dependent transport system (Nadler 1987; Nadler et al. 1992; Nickolson 1982; Yoneda and Roberts 1982). In addition, the PROT high affinity proline transporter is differentially expressed in a subpopulation of excitatory nerve terminals and proline can modulate glutamatergic neurotransmission, further supporting a CNS neurotransmission-related role for proline (Cohen and Nadler 1997a; Cohen and Nadler 1997b; Fremeau et al. 1992; Phang et al. 2001; Renick et al. 1999; Shafqat et al. 1995; Velaz-Faircloth et al. 1995). Based upon our significant findings of elevated proline in patients with schizophrenia, of later age at first hospitalization in hyperprolinemic subjects, and if confirmed, the finding that hyperprolinemia is associated with delayed patient hospital discharge following improvement, we propose that elevated proline is a risk factor for schizophrenia and may represent an intermediate phenotype of a distinct etiological subtype of the disorder, providing insight into the etiology of schizophrenia and potentially a target for new therapeutic strategies. Further study of hyperprolinemia in schizophrenia, and also schizoaffective disorder (Jacquet et al. 2005), is warranted to elucidate whether proline elevation and a theorized dysregulation of CNS neurotransmission propagates the disease or symptom onset, or is simply a marker of psychiatric illness.
Supplementary Material
Acknowledgments
The authors would like to thank Ellie DeCandia RN and the nursing staff of the New York University CTSI, for their invaluable assistance during this study, and Dr Karen Duff for critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afenjar A, Moutard ML, Doummar D, Guet A, Rabier D, Vermersch AI, et al. Early neurological phenotype in 4 children with biallelic PRODH mutations. Brain Dev. 2007;29(9):547–52. doi: 10.1016/j.braindev.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Baxter CF, Baldwin RA, Davis JL, Flood JF. High proline levels in the brains of mice as related to specific learning deficits. Pharmacol Biochem Behav. 1985;22(6):1053–9. doi: 10.1016/0091-3057(85)90316-8. [DOI] [PubMed] [Google Scholar]
- Bender HU, Almashanu S, Steel G, Hu CA, Lin WW, Willis A, et al. Functional consequences of PRODH missense mutations. Am J Hum Genet. 2005;76(3):409–20. doi: 10.1086/428142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L, et al. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15(4):339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Bremer HJ, Duran M, Kamerling JP, Przyrembel H, Wadman SK, et al. Disturbances of amino acid metabolism: Clinical chemistry and diagnosis. Urban & Schwarzenberg, Inc.; Baltimore-Munich: 1981. [Google Scholar]
- Centorrino F, Goren JL, Hennen J, Salvatore P, Kelleher JP, Baldessarini RJ. Multiple versus single antipsychotic agents for hospitalized psychiatric patients: case-control study of risks versus benefits. Am J Psychiatry. 2004;161(4):700–6. doi: 10.1176/appi.ajp.161.4.700. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Nadler JV. Proline-induced potentiation of glutamate transmission. Brain Res. 1997a;761(2):271–82. doi: 10.1016/s0006-8993(97)00352-1. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Nadler JV. Proline-induced inhibition of glutamate release in hippocampal area CA1. Brain Res. 1997b;769(2):333–9. doi: 10.1016/s0006-8993(97)00721-x. [DOI] [PubMed] [Google Scholar]
- Davis JL, Pico RM, Flood JF. Differences in learning between hyperprolinemic mice and their congenic controls. Behav Neural Biol. 1987;48(1):128–37. doi: 10.1016/s0163-1047(87)90649-2. [DOI] [PubMed] [Google Scholar]
- Dingman W, Sporn MB. The penetration of proline and proline derivatives into brain. J Neurochem. 1959;4(2):148–53. doi: 10.1111/j.1471-4159.1959.tb13184.x. [DOI] [PubMed] [Google Scholar]
- Efron ML. Familial hyperprolinemia. Report of a second case, associated with congenital renal malformations, hereditary hematuria and mild mental retardation, with demonstration of an enzyme defect. N Engl J Med. 1965;272:1243–54. doi: 10.1056/NEJM196506172722401. [DOI] [PubMed] [Google Scholar]
- Flynn MP, Martin MC, Moore PT, Stafford JA, Fleming GA, Phang JM. Type II hyperprolinaemia in a pedigree of Irish travellers (nomads) Arch Dis Child. 1989;64(12):1699–707. doi: 10.1136/adc.64.12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Caron MG, Blakely RD. Molecular cloning and expression of a high affinity L-proline transporter expressed in putative glutamatergic pathways of rat brain. Neuron. 1992;8(5):915–26. doi: 10.1016/0896-6273(92)90206-s. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Santha M, Takacs Z, Beck KD, Luine V, Lucas LR, et al. The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat Genet. 1999;21(4):434–9. doi: 10.1038/7777. [DOI] [PubMed] [Google Scholar]
- Goodman BK, Rutberg J, Lin WW, Pulver AE, Thomas GH. Hyperprolinaemia in patients with deletion (22)(q11.2) syndrome. J Inherit Metab Dis. 2000;23(8):847–8. doi: 10.1023/a:1026773005303. [DOI] [PubMed] [Google Scholar]
- Guilmatre A, Legallic S, Steel G, Willis A, Di Rosa G, Goldenberg A, et al. Type I hyperprolinemia: genotype/phenotype correlations. Hum Mutat. 2010;31(8):961–965. doi: 10.1002/humu.21296. [DOI] [PubMed] [Google Scholar]
- Jacquet H, Raux G, Thibaut F, Hecketsweiler B, Houy E, Demilly C, et al. PRODH mutations and hyperprolinemia in a subset of schizophrenic patients. Hum Mol Genet. 2002;11(19):2243–9. doi: 10.1093/hmg/11.19.2243. [DOI] [PubMed] [Google Scholar]
- Jacquet H, Berthelot J, Bonnemains C, Simard G, Saugier-Veber P, Raux G, et al. The severe form of type I hyperprolinaemia results from homozygous inactivation of the PRODH gene. J Med Genet. 2003;40(1):e7. doi: 10.1136/jmg.40.1.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet H, Demily C, Houy E, Hecketsweiler B, Bou J, Raux G, et al. Hyperprolinemia is a risk factor for schizoaffective disorder. Mol Psychiatry. 2005;10(5):479–85. doi: 10.1038/sj.mp.4001597. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Gogos JA. The molecular genetics of the 22q11-associated schizophrenia. Brain Res Mol Brain Res. 2004;132(2):95–104. doi: 10.1016/j.molbrainres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11(6):402–16. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf L, Nicodemus KK, Kolachana B, Vakkalanka R, Verchinski BA, Egan MF, et al. Functional polymorphisms in PRODH are associated with risk and protection for schizophrenia and fronto-striatal structure and function. PLoS Genet. 2008;4(11):e1000252. doi: 10.1371/journal.pgen.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, et al. The 22q11.2 deletion in African-American patients: an underdiagnosed population? Am J Med Genet A. 2005;134(3):242–6. doi: 10.1002/ajmg.a.30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsubuchi H, et al. Inborn errors of proline metabolism. J Nutr. 2008;138(10):2016S–2020S. doi: 10.1093/jn/138.10.2016S. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardiofacial syndrome. Arch Gen Psychiatry. 1999;56(10):940–5. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Nadler JV. Sodium-dependent proline uptake in the rat hippocampal formation: association with ipsilateral-commissural projections of CA3 pyramidal cells. J Neurochem. 1987;49(4):1155–60. doi: 10.1111/j.1471-4159.1987.tb10006.x. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Bray SD, Evenson DA. Autoradiographic localization of proline uptake in excitatory hippocampal pathways. Hippocampus. 1992;2(3):269–78. doi: 10.1002/hipo.450020306. [DOI] [PubMed] [Google Scholar]
- Nickolson VJ. “On” and “off” responses of K+-induced synaptosomal proline release: involvement of the sodium pump. J Neurochem. 1982;38(1):289–92. doi: 10.1111/j.1471-4159.1982.tb10885.x. [DOI] [PubMed] [Google Scholar]
- Paterlini M, Zakharenko SS, Lai WS, Qin J, Zhang H, Mukai JW, Zakharenko SS, Lai WS, Qin J, Zhang H, Mukai JW, et al. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat Neurosci. 2005;8(11):1586–94. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- Phang JM, Hu CA, Valle D. Disorders of proline and hydroxyproline metabolism. Eigth Edition McGraw-Hill; New York: 2001. [Google Scholar]
- Rabinowitz J, Levine SZ, Hafner H. A population based elaboration of the role of age of onset on the course of schizophrenia. Schizophr Res. 2006;88(1-3):96–101. doi: 10.1016/j.schres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Rao ML, Gross G, Strebel B, Bräunig P, Huber G, Klosterkötter J. Serum amino acids, central monoamines, and hormones in drug-naive, drug-free, and neuroleptic-treated schizophrenic patients and healthy subjects. Psychiatry Res. 1990;34:243–257. doi: 10.1016/0165-1781(90)90003-n. [DOI] [PubMed] [Google Scholar]
- Raux G, Bumsel E, Hecketsweiler B, van Amelsvoort T, Zinkstok J, Manouvrier-Hanu S, et al. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Hum Mol Genet. 2007;16(1):83–91. doi: 10.1093/hmg/ddl443. [DOI] [PubMed] [Google Scholar]
- Renick SE, Kleven DT, Chan J, Stenius K, Milner TA, Pickel VM, et al. The mammalian brain high-affinity L-proline transporter is enriched preferentially in synaptic vesicles in a subpopulation of excitatory nerve terminals in rat forebrain. J Neurosci. 1999;19(1):21–33. doi: 10.1523/JNEUROSCI.19-01-00021.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambler PJ. The 22q11 deletion syndromes. Hum Mol Genet. 2000;9(16):2421–6. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- Shafqat S, Faircloth M, Henzi VA, Whitney KD, Yang-Feng TL, Seldin MF, et al. Human brain-specific L-proline transporter: molecular cloning, functional expression, and chromosomal localization of the gene in human and mouse genomes. Mol Pharmacol. 1995;48(2):219–29. [PubMed] [Google Scholar]
- Shanti ND, Shashikumar KC, Desai PV. Influence of proline on rat brain activities of alanine aminotransferase, aspartate aminotransferase and acid phosphatase. Neurochem Res. 2004;29(12):2197–206. doi: 10.1007/s11064-004-7026-2. [DOI] [PubMed] [Google Scholar]
- Tomiya M, Fukushima T, Watanabe H, Fukami G, Fujisaki M, Iyo M, et al. Alterations in serum amino acid concentrations in male and female schizophrenic patients. Clin Chim Acta. 2007;380(1-2):186–90. doi: 10.1016/j.cca.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Velaz-Faircloth M, Guadano-Ferraz A, Henzi VA, Fremeau RT. Mammalian brain-specific L-proline transporter. Neuronal localization of mRNA and enrichment of transporter protein in synaptic plasma membranes. J Biol Chem. 1995;270(26):15755–61. doi: 10.1074/jbc.270.26.15755. [DOI] [PubMed] [Google Scholar]
- Walter H, Schlaff WB, Lesch OM, Vitek L, Zima T, Hartl D, et al. Breath alcohol level and plasma amino acids: a comparison between older and younger chronic alcohol-dependent patients. Alcohol Alcohol. 2008;43(6):653–7. doi: 10.1093/alcalc/agn076. [DOI] [PubMed] [Google Scholar]
- Wassef AA, Winkler DE, Roache AL, Abobo VB, Lopez LM, Averill JP, et al. Lower effectiveness of divalproex versus valproic acid in a prospective, quasi-experimental clinical trial involving 9,260 psychiatric admissions. Am J Psychiatry. 2005;162(2):330–9. doi: 10.1176/appi.ajp.162.2.330. [DOI] [PubMed] [Google Scholar]
- Yoneda Y, Roberts E. A new synaptosomal biosynthetic pathway of proline from ornithine and its negative feedback inhibition by proline. Brain Res. 1982;239(2):479–88. doi: 10.1016/0006-8993(82)90523-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.