Abstract
Current classifications of pulmonary hypertension have contributed a great deal to our understanding of pulmonary vascular disease, facilitated drug trials, and improved our understanding of congenital heart disease in adult survivors. However, these classifications are not applicable readily to pediatric disease. The classification system that we propose is based firmly in clinical practice. The specific aims of this new system are to improve diagnostic strategies, to promote appropriate clinical investigation, to improve our understanding of disease pathogenesis, physiology and epidemiology, and to guide the development of human disease models in laboratory and animal studies. It should be also an educational resource. We emphasize the concepts of perinatal maladaptation, maldevelopment and pulmonary hypoplasia as causative factors in pediatric pulmonary hypertension. We highlight the importance of genetic, chromosomal and multiple congenital malformation syndromes in the presentation of pediatric pulmonary hypertension. We divide pediatric pulmonary hypertensive vascular disease into 10 broad categories.
Keywords: pulmonary hypertension, pulmonary hypertension in the newborn, pulmonary vascular disease, pediatric patient
INTRODUCTION
The classification of pulmonary hypertension conceived at the 1998 WHO Symposium in Evian[1] and the subsequent revisions and refinements that resulted from symposia in Venice[2] and Dana Point[3] have contributed greatly to the understanding of pulmonary vascular disease, facilitated drug trials and improved our understanding of congenital heart disease in adult survivors. However, these classifications are not applicable readily to pediatric disease.[4–7] The response to the debate on the classification of pediatric pulmonary hypertension at the Pulmonary Vascular Research Institute (PVRI) meeting in Lisbon in 2010 suggested that there was a widespread, well-recognized need for the development of a classification system of pediatric pulmonary hypertensive vascular disease specifically for use in children. Also, it was recognized that physicians, who care for adult survivors of pediatric disease, might be able also to use such a classification in their assessments. As a result, the PVRI Pediatric Taskforce was initiated. This paper summarizes the work of the PVRI Pediatric Taskforce as presented at the 2011 annual meeting of the PVRI in Panama.
DISCUSSION
Difficulties in applying the Dana Point Classification to pediatrics
The areas of particular difficulty in applying the Dana Point Classification[3] in pediatrics are mentioned briefly here and expanded upon under specific headings later in the article. The fetal origins of pulmonary vascular disease are important not only in pediatric diseases, but also in adults as perinatal events are likely to play a key role in establishing the risk for pulmonary hypertension. The Dana Point Classification does not acknowledge the potential importance of developmental mechanisms. Pulmonary hypertensive vascular disease, even when presenting in adulthood, maybe related to fetal, perinatal and early childhood development. The perinatal origins of systemic hypertension and coronary artery disease in adults are now well recognized.[8] Neonatal pulmonary vascular disease received inconsistent attention at Evian, Venice and Dana Point. In particular the concepts of perinatal maladaptation, maldevelopment and pulmonary hypoplasia as causative factors in neonatal pulmonary hypertension were not listed. Furthermore as a tool in the real life clinical assessment of the young child, the Dana Point Classification often does not reflect the complex heterogeneity of factors that contribute to pediatric pulmonary vascular disease[6] (Fig. 1). For instance in pediatric practice, patients are commonly evaluated for pulmonary hypertension who may have been born prematurely, with chromosomal or genetic anomalies, congenital cardiac defects, as well as, sleep disordered breathing, chronic aspiration and secondary parenchymal pulmonary disease.
Figure 1.
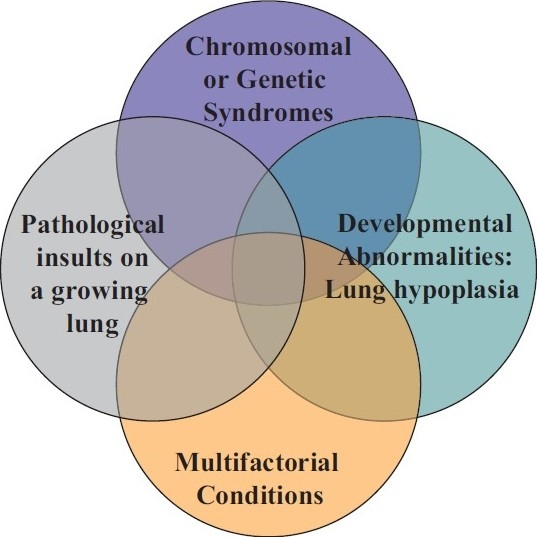
Venn diagram illustrating the heterogeneity and multifactorial elements in pediatric pulmonary hypertensive vascular disease.
Aims of the PVRI Panama Classification
The classification system that we propose is based firmly in clinical practice. The specific aims of this new system are to improve diagnostic strategies, to promote appropriate clinical investigation and to improve our understanding of disease pathogenesis, physiology and epidemiology and to guide the development of human disease models in laboratory and animal studies. It should be also an educational resource. This classification system unequivocally is not based on therapy of pulmonary hypertension or designed to be a therapeutic guide. The utility of an effective classification system lies in its ability to help us to make sense of our observations on each child, but be structured enough to permit unambiguous classification when possible but flexible enough to allow for the inclusion of as yet undiscovered ideas. Classifications are useful in medicine if they provide a framework for the diagnosis and management of a disease, and encourage epidemiological insight. A perfect classification, like the periodic table, would also have categories for as yet undiscovered disease or mechanisms of known disease complexes.
We acknowledge the great value of the Dana Point Classification.[3] Indeed, there are elements that we have left untouched. We are cognizant that if our suggested classification system has any merit it is because–to paraphrase Sir Isaac Newton in 1676–only by “standing on the shoulders of giants” have we been able to see further. With this in mind, we propose a new classification of pediatric pulmonary hypertensive vascular disease.
Overall schema
We have used the term pediatric pulmonary hypertensive vascular disease in preference to pulmonary hypertension to exclude patients with pulmonary hypertension but without an elevated pulmonary vascular resistance (Table 1). This occurs in children with large systemic to pulmonary connections. These children do not require drug therapy for pulmonary hypertension but rather benefit from timely and accurate closure of the defect. We do, however, wish to include children who have undergone various stages of single ventricle treatment who may have a symptomatically elevated pulmonary vascular resistance but with a mean pulmonary artery pressure <25 mmHg. Thus we suggest that pediatric pulmonary hypertensive vascular disease be defined as a mean pulmonary artery pressure >25 mmHg and a pulmonary vascular resistance index >3.0 Wood units m2 for biventricular circulations. We suggest that pulmonary hypertensive vascular disease following a cavopulmonary anastomosis be defined as a pulmonary vascular resistance index >3.0 Wood units m2 or a transpulmonary gradient >6 mmHg (mean pulmonary artery pressure minus mean left atrial pressure) even if the mean pulmonary artery pressure is <25 mmHg. We add the caveat that calculated pulmonary vascular resistance maybe increased, not only, by an increased transpulmonary gradient, but also, by decreased pulmonary blood flow. We acknowledge that pulmonary blood flow maybe difficult to estimate after a cavopulmonary anastomosis because of multiple sources of pulmonary blood flow.
Table 1.
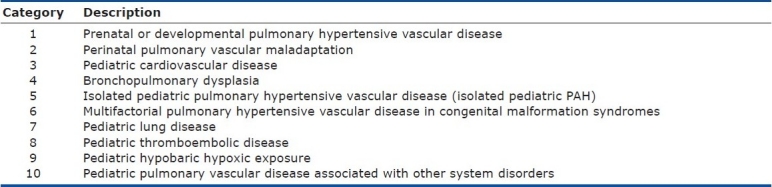
The broad schema of 10 basic categories of Pediatric Pulmonary Hypertensive Vascular Disease
The pulmonary artery occlusion, left atrial or systemic ventricular end diastolic pressures maybe increased or normal but these values are clearly important in considering the differential diagnosis.
We have divided pediatric pulmonary hypertensive vascular disease into 10 broad categories listed in order of frequency of presentation to the pediatric clinic (Table 1). There is no published all-inclusive epidemiological study or registry data on pediatric pulmonary hypertension. As far as we can tell the reports to date have excluded one or other of the categories in the classification system we present here. Therefore, when such data is available the order of the categories may need revision. We emphasize that we have attempted to provide a clinically useful classification (Table 2), which permits the categorization of patients with multifactorial causes of pulmonary hypertension especially when associated with a syndrome or chromosomal abnormality. To reflect the heterogeneity of pulmonary vascular disease in childhood we have included the possibility that a disease or condition may appear in different categories. This is particularly the case when a disease such as sickle cell, scimitar or antiphospholipid syndrome may cause different types of pulmonary hypertensive vascular disease.
Table 2.
Detailed Classification of pediatric pulmonary hypertensive vascular disease

CATEGORY 1
Prenatal or developmental pulmonary vascular disease
Perhaps the most striking difference between the adult and childhood onset of pulmonary hypertensive vascular disease is that during fetal, neonatal and early postnatal life the pulmonary vasculature is exposed to pathological and/or environmental insults while it is still growing and maturing. This may result in maladaptation, maldevelopment or growth arrest. Natural attempts at recovery from insults may be influenced by the ongoing developmental and maturational signals. This may result in unique and different sequelae than those seen in adults exposed to a similar insult (Table 2). The lung-vascular unit is composed of alveolus, bronchiole, capillary, arteriole, venule and lymphatic channel and the development of each is dependent upon another.[9] Disease of one element in the lung-vascular unit may affect other components as for example in persistent pulmonary hypertension of the newborn, bronchopulmonary dysplasia[10] (Fig. 2) and alveolar capillary dysplasia with misalignment of the pulmonary veins.[11]
Figure 2.
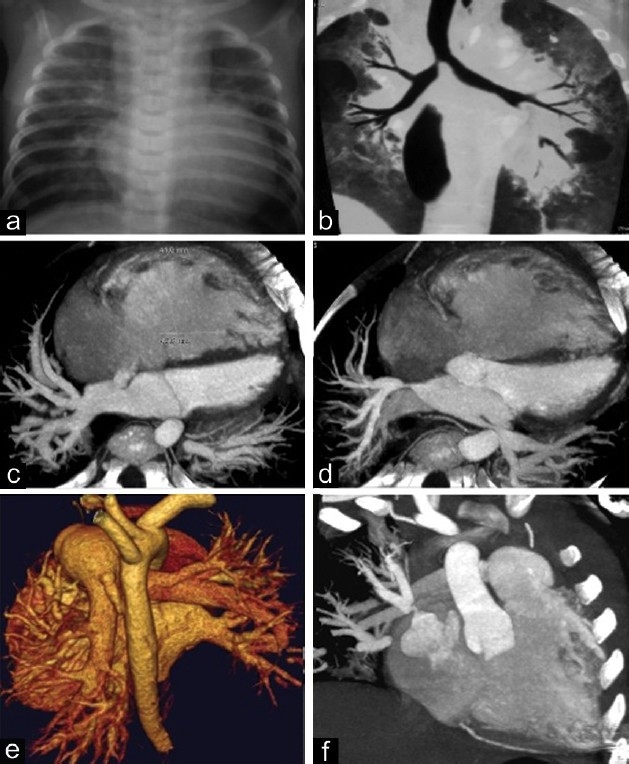
This figure illustrates the complexity of pulmonary hypertensive vascular disease in a two-year-old infant with bronchopulmonary dysplasia. (a) chest X ray, cardiomegaly and parenchymal lung infiltrates; (b) lung CT scan, showing lung extensive parenchymal damage with areas of atelectasis and emphysema; (c) CT angiogram, showing right ventricular and right atrial dilatation, and atrial septal defect; (d) CT angiogram showing left and right upper pulmonary vein stenosis; (e) reconstructed CT image showing persistent ductus arteriosus; and (f) CT angiogram showing the severe stenosis of right upper pulmonary vein.
In utero, the fetal pulmonary circulation is characterized by high pulmonary artery pressure and markedly elevated pulmonary vascular resistance. In the first hours after birth, dramatic respiratory and circulatory events cause pulmonary vasodilation and favorable remodeling of the pulmonary vascular bed, which reduce pulmonary vascular resistance and lead to an increase in pulmonary blood flow. If successful transition of the pulmonary circulation occurs the pulmonary artery mean pressure decreases in the first three weeks of life to 10-20 mmHg, similar to adult levels.[12] In young children total pulmonary vascular resistance indexed is similar to adults.[13] Yet despite this physiological adaptation with reduction in pulmonary vascular resistance the ultra structural appearance of smooth muscle cells does not closely resemble that of the adult until about 2 years of age.[14] Fetal growth factors may influence postnatal pulmonary vascular form and function.[10]
It is clear that pediatric pulmonary hypertension specialists manage increasing numbers of neonates and children whose pulmonary hypertension may have fetal origins. In particular the association of pre-eclampsia and bronchopulmonary dysplasia,[15] and disorders associated with lung hypoplasia and diseases associated with pulmonary vascular disease in utero.[11,16–25] Pulmonary hypoplasia, the result of growth arrest, is an important concept in any classification system of neonatal pulmonary hypertensive disease. Pulmonary hypertensive vascular disease in children may occur against a background of varying degrees of pulmonary hypoplasia. This has been especially well documented particularly in congenital heart disease[9] congenital diaphragmatic hernia[26] and Down syndrome.[27,28] It is also relevant that alveolarisation and pulmonary vascular development may continue through the first 8 years of life.[9] The normal rate of vascular growth and changes in the cross sectional area of the pulmonary vascular bed at birth or during the first years of life is unknown. Notably lung hypoplasia may be found in around 10% of neonatal autopsies and in up to 50% of neonates with congenital anomalies.[28,29] It is possible that diverse post natal pulmonary vascular insults, even those resulting in adult onset disease, are more likely to result in pulmonary hypertension if the subject was born with a pulmonary vascular cross sectional area below the 3rd percentile. Thus the likelihood of developing pulmonary hypertension throughout life may be related to the initial surface area at birth, with the effects of each successive insult at least partly due to the balance between pulmonary vascular reserve and rate of pulmonary vascular attrition due to the pathological insult be it genetic, epigenetic or environmental.
CATEGORY 2
Perinatal pulmonary vascular maladaptation
This category contains only the syndrome of persistent pulmonary hypertension of the newborn (PPHN) (Table 2). We recognize that there is considerable debate about the origins of PPHN and that it may reflect in utero pulmonary vascular disease.[30] Clinical observations that neonates with severe PPHN who die during the first days after birth already have pathologic signs of chronic pulmonary vascular disease suggest that intrauterine events may play an important role in this syndrome.[30–32] Adverse intrauterine stimuli during late gestation, such as abnormal blood flow, changes in substrate or hormone delivery to the lung, chronic hypoxia, chronic systemic hypertension, inflammation or others, may potentially alter lung vascular function and structure, contributing to abnormalities of postnatal adaptation.[33,34] It seems likely that as the mechanisms of PPHN become understood better it will become necessary to reassess the classification. However, at present most would recognize PPHN as a disorder of transition from intra to extra uterine life.[35–43]
Neonates born at high altitude frequently need more time to adapt to ex-utero life; some of them require supplementary oxygen for a few weeks. The pulmonary pressures remain increased above the normal age specific values for altitude, at this time. There is a delay in the pulmonary arterial remodeling after birth in those born at high altitude.[44] However, we have acknowledged the considerable, even fatal effect that birth at very high altitudes (≥ 2,500m) may impose in the early postnatal period. These newer observations[4,45] contrast with previous reports.[46] We suggest that PPHN is a disease of the first 30 days of life that usually presents at, or within a few days, after birth. However, we recognize that it would be prudent to accelerate and broaden the diagnostic evaluation of any neonate presenting with symptomatic pulmonary hypertension outside the first week of life as the etiology may not be PPHN.
CATEGORY 3
Pediatric cardiovascular disease
Pediatric cardiovascular disease may be the most common disorder globally causing pulmonary vascular disease in children (Table 2).[47–49]
The list of cardiac abnormalities and diseases is more comprehensive in this section of the classification than in the Dana Point Classification but we have maintained the basic structure of the Dana point classification as it pertains to shunts.[5,7,50,51] We considered the essential outcome of the diagnostic work up of a child with a shunt and elevated pulmonary vascular resistance index is to conclude whether or not the child should undergo cardiac repair or further evaluation. There is considerable interest in evaluating if a course of medical therapy will enable surgical repair in certain patients with borderline pulmonary vascular resistances.
The interaction between congenital heart disease and genetic factors often makes it difficult to classify the cause of the pulmonary hypertensive vascular disease with certainty. For instance how should a child with an atrioventricular canal defect and BMPR 2 mutation be classified?[52] Or how should we classify a child with a minor cardiac shunt and a coexistent genetic or chromosomal anomaly? The classification allows for this eventuality and this area will become clarified in the future as we seek genetic links between congenital heart and pulmonary vascular disease.
Persistent or late presenting pulmonary vascular disease after atrial or arterial switch for transposition of the great arteries with an intact septum is recognized with such increasing frequency that we have specified the condition in the classification.[53–55]
The classical Eisenmenger syndrome is well recognized as a multisystem disorder. However, the differentiation between complex and simple is clinically extremely important for both survival and functional level.[56] Some studies have suggested that children with Eisenmenger may have a more rapid clinical decline than adults.[57] There is growing concern that children with repaired congenital shunts and either persistent or recurrent pulmonary hypertension fare worse than patients with either Eisenmenger or idiopathic pulmonary hypertension.[47] It is likely that this subgroup will need further refinement in the future.
The category entitled pulmonary venous hypertension includes in addition the cardiomyopathies, both acquired and congenital.[58,59]
Pulmonary vascular disease following staged surgery for single ventricle: The use of pulmonary hypertension specific agents in the treatment of children and adults following the Glenn or Fontan type surgery is widespread. Preliminary data from the Spanish registry suggests that 14% of children receiving sildenafil or bosentan have a single ventricle type lesion. The interaction of the pulmonary and systemic circulations when the kinetic energy for blood flow through both circulations is derived from a single ventricular mass (and without a dedicated subpulmonary ventricle) is complex and pulmonary vascular resistance plays an important physiologic role.[60–62] Recent studies have suggested that exercise intolerance,[63,64] and even plastic bronchitis[65,66] and protein losing enteropathy[67] may be due in part to an increased pulmonary vascular resistance.[61,68]
Hypobaric hypoxic exposure and congenital heart disease: We have included congenital heart disease at high altitude under Category 9 because high altitude may affect the incidence as well as the anatomy of the ductus arteriosus.[69] This pertains also to children with trisomy 21 born at high altitude. In addition, pulmonary vascular reactivity testing (including prolonged hyperoxia testing) and management criteria may differ from those used at sea level.[4,44,45,69–72]
CATEGORY 4
Bronchopulmonary dysplasia
Bronchopulmonary dysplasia (Table 2) remains the most common sequela after preterm birth, causing persistent cardiorespiratory problems throughout childhood and is growing as a significant problem in adulthood.[73,74] Twelve percent (12%) of births are premature and place the patient at risk of bronchopulmonary dysplasia or chronic lung disease of prematurity. Bronchopulmonary dysplasia is a complex disorder and much more than chronic parenchymal lung disease secondary to ventilation strategies. Bronchopulmonary dysplasia, although it has changed over the decades, is characterized by an arrest of vascular and alveolar lung growth,[75–78] which often has prenatal origins.[15] Thus a patient with bronchopulmonary dysplasia may have pulmonary hypertension due to decreased vascular growth compounded by intermittent or chronic hypoxia, hypercarbia due to lung and airway injury, a systemic to pulmonary shunt, diastolic cardiac dysfunction and pulmonary vein stenosis[79–83] (Fig. 2).
CATEGORY 5
Isolated pediatric pulmonary hypertensive vascular disease or isolated pediatric pulmonary arterial hypertension
The category for isolated pulmonary hypertensive vascular disease or isolated pulmonary arterial hypertension (Table 2) resembles closely the Dana Point Classifica-tion.[84–86] However, we suggest that the term “idiopathic” be reserved for those cases with truly “idiopathic” pulmonary hypertension i.e. unassociated with any other genetic, chromosomal etc. abnormality. In pediatrics the difficulties are encountered with a classification system if “idiopathic” pulmonary arterial hypertension is diagnosed together with a genetic defect or chromosomal syndrome.[6]
Some drugs reported to cause pulmonary hypertension in children are different or (because they are used infrequently in pediatrics) less well validated from those described in adults.[87–92]
CATEGORY 6
Multifactorial causes of pulmonary hypertension associated with congenital malformation syndromes
We are recognizing more frequently that children born with congenital malformations (Table 2) often suffer from pulmonary vascular disease due to a number of contributing factors. Examples include CHARGE, VACTERL, Down syndrome and Di George spectrum of disorders.[23,93–106] In addition, pulmonary vascular disease secondary to a shunt maybe more rapidly progressive in patients with genetic syndromes.[107]
CATEGORY 7
Pediatric lung disease
The co-existence of certain lung diseases with pulmonary hypoplasia is recognized increasingly in children (Table 2). The classification of interstitial lung disease also suggests that lung hypoplasia and growth arrest are a common feature of a number of childhood interstitial lung diseases.[18] Pulmonary hypertension has a profound impact on the outcome of interstitial lung disease.[18] Genetic causes of lung disease are recognized and may have an impact on the prenatal pulmonary vasculature.[33,34,108,109]
CATEGORY 8
Pediatric thromboembolic disease
There is a lower incidence of pulmonary hypertension due to thromboembolic disease in children compared to adults. The associated or predisposing diseases associated with pulmonary thromboembolism in children are also in general different.[110–115] Although surgical options for chronic thromboembolic pulmonary hypertension have been explored less well in children, the success of surgical treatment of this disease in adults should encourage considering such an option in certain cases in the pediatric population (Table 2).[116,117]
CATEGORY 9
Pediatric hypobaric hypoxic exposure
Hypobaric hypoxic exposure or pulmonary hypertension due to high altitude (Table 2) was considered by those on the task force with extensive clinical experience working at high altitude to be sufficiently different from other forms of pulmonary arterial hypertension to justify inclusion as a separate category. These differences include hypoxia in the absence of parenchymal lung disease, different genetic aspects, and different treatment strategies.[4,44–46,70,72,118–126]
CATEGORY 10
Pediatric pulmonary hypertensive vascular disease associated with other system disorders
Here we have listed disorders (Table 2), which may be complicated by or associated with pulmonary hypertension.[100,127,148–155] We draw attention to unique aspects of pediatric disease such as extrahepatic portal hypertension, which may occur secondary to portal vein thrombosis following umbilical line placement and be overlooked as liver function tests may be normal.
CONCLUSION
We propose a comprehensive classification of pediatric pulmonary hypertension that includes pulmonary vascular hypertensive disorders occurring throughout early life from the neonate to adolescent. We emphasize the importance of prenatal and perinatal influences, including maldevelopment and lung hypoplasia, that may contribute to pulmonary vascular disease. We suggest that pediatric pulmonary hypertensive vascular disease be defined as a mean pulmonary artery pressure >25 mmHg and a pulmonary vascular resistance index >3.0 Wood units m2 for biventricular circulations. We suggest that following a cavopulmonary anastomosis pulmonary hypertensive vascular disease be defined as a pulmonary vascular resistance index >3.0 Wood units m2 or a transpulmonary gradient >6 mmHg even if the mean pulmonary artery pressure is <25 mmHg. We have classified pediatric pulmonary hypertensive vascular disease into 10 broad categories. The classification we propose is based firmly on clinical practice. The specific aims are to improve diagnostic strategies, promote clinical investigation and understanding of pathogenesis, physiology and epidemiology, and to guide the development of human disease models in laboratory and animal studies. We hope, at the least, that this classification system will serve as a catalyst for improvement and lead ultimately to better outcomes for our patients. If there are omissions or improvements to be made, we encourage interested readers to let us know through the PVRI website (http://pvri.info/home)
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rich S. Primary pulmonary hypertension: Executive summary from the world symposium-primary pulmonary hypertension 1998. Paper presented at: World Health Organisation, 1998; Evian [Google Scholar]
- 2.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43(Suppl 12):5S–12. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Díaz G. Aspectos Generales; Definición Clasificación y Epidemiología. In: Díaz G, Sandoval J, Sola A, editors. Hipertensión Pulmonar en Niños. Bogotá: Editorial Médica Distribuna; 2011. pp. 9–19. [Google Scholar]
- 5.van Albada ME, Berger RM. Pulmonary arterial hypertension in congenital cardiac disease--the need for refinement of the Evian-Venice classification. Cardiol Young. 2008;18:10–7. doi: 10.1017/S1047951107001849. [DOI] [PubMed] [Google Scholar]
- 6.van Loon RL, Roofthooft MT, van Osch-Gevers M, Delhaas T, Strengers JL, Blom NA, et al. Clinical characterization of pediatric pulmonary hypertension: Complex presentation and diagnosis. J Pediatr. 2009;155:176–182.e1. doi: 10.1016/j.jpeds.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Schulze-Neick I, Beghetti M. Classifying pulmonary hypertension in the setting of the congenitally malformed heart--cleaning up a dog's dinner. Cardiol Young. 2008;18:22–5. doi: 10.1017/S1047951107001850. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(Suppl 6):588S–95. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 9.Reid LM. Lung growth in health and disease. Br J Dis Chest. 1984;78:113–34. [PubMed] [Google Scholar]
- 10.Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol. 2010;661:323–35. doi: 10.1007/978-1-60761-500-2_21. [DOI] [PubMed] [Google Scholar]
- 11.Eulmesekian P, Cutz E, Parvez B, Bohn D, Adatia I. Alveolar capillary dysplasia: A six-year single center experience. J Perinat Med. 2005;33:347–52. doi: 10.1515/JPM.2005.067. [DOI] [PubMed] [Google Scholar]
- 12.Rowe R, James L. The normal pulmonary arterial pressure during the first year of life. J Pediatr. 1957;51:1–4. doi: 10.1016/s0022-3476(57)80273-x. [DOI] [PubMed] [Google Scholar]
- 13.Lock J, Einzig S, Moller J. Hemodynamic responses to exercise in normal children. Am J Cardiol. 1978;41:1278–84. doi: 10.1016/0002-9149(78)90886-x. [DOI] [PubMed] [Google Scholar]
- 14.Haworth SG. Pulmonary hypertension in childhood. Eur Respir J. 1993;6:1037–43. [PubMed] [Google Scholar]
- 15.Hansen AR, Barnes CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr. 2010;156:532–6. doi: 10.1016/j.jpeds.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Maeno YV, Kamenir SA, Sinclair B, van der Velde ME, Smallhorn JF, Hornberger LK. Prenatal features of ductus arteriosus constriction and restrictive foramen ovale in d-transposition of the great arteries. Circulation. 1999;99:1209–14. doi: 10.1161/01.cir.99.9.1209. [DOI] [PubMed] [Google Scholar]
- 17.Castillo M, Vade A, Lim-Dunham JE, Masuda E, Massarani-Wafai R. Pulmonary interstitial glycogenosis in the setting of lung growth abnormality: Radiographic and pathologic correlation. Pediatr Radiol. 2010;40:1562–5. doi: 10.1007/s00247-010-1670-2. [DOI] [PubMed] [Google Scholar]
- 18.Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, et al. Diffuse lung disease in young children: Application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–8. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Ursell PC, Adatia I, Hislop AA, Giannikopoulos P, Hornberger LK. Prenatal diagnosis of primary pulmonary hypoplasia in fraternal twins. Ultrasound Obstet Gynecol. 2010;35:113–6. doi: 10.1002/uog.7520. [DOI] [PubMed] [Google Scholar]
- 20.Inwald D, Brown K, Gensini F, Malone M, Goldman A. Open lung biopsy in neonatal and paediatric patients referred for extracorporeal membrane oxygenation (ECMO) Thorax. 2004;59:328–33. doi: 10.1136/thx.2003.010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu S, Shao L, Kilbride H, Zwick DL. Haploinsufficiencies of FOXF1 and FOXC2 genes associated with lethal alveolar capillary dysplasia and congenital heart disease. Am J Med Genet A. 2010;152:1257–62. doi: 10.1002/ajmg.a.33378. [DOI] [PubMed] [Google Scholar]
- 22.Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84:780–91. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw-Smith C. Genetic factors in esophageal atresia, tracheo-esophageal fistula and the VACTERL association: Roles for FOXF1 and the 16q24.1 FOX transcription factor gene cluster, and review of the literature. Eur J Med Genet. 2010;53:6–13. doi: 10.1016/j.ejmg.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vassal HB, Malone M, Petros AJ, Winter RM. Familial persistent pulmonary hypertension of the newborn resulting from misalignment of the pulmonary vessels (cogenital alveolar capillary dysplasia) J Med Genet. 1998;35:58–60. doi: 10.1136/jmg.35.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, et al. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: A model of alveolar capillary dysplasia? Circ Res. 2004;94:1115–23. doi: 10.1161/01.RES.0000125624.85852.1E. [DOI] [PubMed] [Google Scholar]
- 26.Kitagawa M, Hislop A, Boyden EA, Reid L. Lung hypoplasia in congenital diaphragmatic hernia: A quantitative study of airway, artery, and alveolar development. Br J Surg. 1971;58:342–6. doi: 10.1002/bjs.1800580507. [DOI] [PubMed] [Google Scholar]
- 27.Cooney TP, Thurlbeck WM. Pulmonary hypoplasia in Down's syndrome. N Engl J Med. 1982;307:1170–3. doi: 10.1056/NEJM198211043071902. [DOI] [PubMed] [Google Scholar]
- 28.Sherer DM, Davis JM, Woods JR., Jr Pulmonary hypoplasia: A review. Obstet Gynecol Surv. 1990;45:792–803. doi: 10.1097/00006254-199011000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Askenazi SS, Perlman M. Pulmonary hypoplasia: Lung weight and radial alveolar count as criteria of diagnosis. Arch Dis Child. 1979;54:614–8. doi: 10.1136/adc.54.8.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy JD, Vawter GF, Reid LM. Pulmonary vascular disease in fatal meconium aspiration. J Pediatr. 1984;104:758–62. doi: 10.1016/s0022-3476(84)80962-2. [DOI] [PubMed] [Google Scholar]
- 31.Geggel RL, Reid LM. The structural basis of PPHN. Clin Perinatol. 1984;11:525–49. [PubMed] [Google Scholar]
- 32.Haworth SG, Reid L. Persistent fetal circulation: Newly recognized structural features. J Pediatr. 1976;88:614–20. doi: 10.1016/s0022-3476(76)80021-2. [DOI] [PubMed] [Google Scholar]
- 33.Guillot L, Carre A, Szinnai G, Castanet M, Tron E, Jaubert F, et al. NKX2 1 mutations leading to surfactant protein promoter dysregulation cause interstitial lung disease in “Brain-Lung-Thyroid Syndrome”. Hum Mutat. 2010;31:E1146–62. doi: 10.1002/humu.21183. [DOI] [PubMed] [Google Scholar]
- 34.Boggaram V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond) 2009;116:27–35. doi: 10.1042/CS20080068. [DOI] [PubMed] [Google Scholar]
- 35.Kinsella JP, Neish SR, Shaffer E, Abman SH. Low-dose inhalational nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340:819–20. doi: 10.1016/0140-6736(92)92687-b. [DOI] [PubMed] [Google Scholar]
- 36.Kinsella JP, Abman SH. Recent developments in the pathophysiology and treatment of persistent pulmonary hypertension of the newborn. J Pediatr. 1995;126:853–64. doi: 10.1016/s0022-3476(95)70197-4. [DOI] [PubMed] [Google Scholar]
- 37.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990;259:H1921–7. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 38.Steinhorn RH, Kinsella JP, Pierce C, Butrous G, Dilleen M, Oakes M, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009;155:841–847.e1. doi: 10.1016/j.jpeds.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med. 2010;11(Suppl 2):S79–84. doi: 10.1097/PCC.0b013e3181c76cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thebaud B, Tibboel D. Pulmonary hypertension associated with congenital diaphragmatic hernia. Cardiol Young. 2009;19(Suppl 1):49–53. doi: 10.1017/S1047951109003965. [DOI] [PubMed] [Google Scholar]
- 41.Kinsella JP, Ivy DD, Abman SH. Pulmonary vasodilator therapy in congenital diaphragmatic hernia: Acute, late, and chronic pulmonary hypertension. Semin Perinatol. 2005;29:123–8. doi: 10.1053/j.semperi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Chang AC, Macrae D. Neonates with congenital cardiac defects and pulmonary hypertension. Cardiol Young. 2009;19(Suppl 1):4–7. doi: 10.1017/S1047951109003874. [DOI] [PubMed] [Google Scholar]
- 43.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: A pilot randomized blinded study. Pediatrics. 2006;117:1077–83. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 44.Peñaloza D, Sime F, Ruiz L. Pulmonary hemodynamics in children living at high altitudes. High Alt Med Biol. 2008;9:199–207. doi: 10.1089/ham.2008.1004. [DOI] [PubMed] [Google Scholar]
- 45.Díaz G, Márquez A. Hipertensión Pulmonar en Niños a Moderada Altura en Hipertensión Pulmonar en Niños. In: Díaz G, Sandoval J, Sola A, editors. Hipertensión Pulmonar en Niños. Bogotá: Editorial Médica Distribuna; 2011. pp. 266–84. [Google Scholar]
- 46.Gamboa R, Marticorena E. Pulmonary arterial pressure in newborn infants in high altitude. Arch Inst Biol Andina. 1971;4:55–66. [PubMed] [Google Scholar]
- 47.Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: The UK Pulmonary Hypertension Service for Children 2001-2006. Heart. 2009;95:312–7. doi: 10.1136/hrt.2008.150086. [DOI] [PubMed] [Google Scholar]
- 48.Adatia I, Kothari SS, Feinstein JA. Pulmonary hypertension associated with congenital heart disease: Pulmonary vascular disease: The global perspective. Chest. 2010;137(Suppl 6):52S–61. doi: 10.1378/chest.09-2861. [DOI] [PubMed] [Google Scholar]
- 49.Fasnacht MS, Tolsa JF, Beghetti M. The Swiss registry for pulmonary arterial hypertension: The paediatric experience. Swiss Med Wkly. 2007;137:510–3. doi: 10.4414/smw.2007.11895. [DOI] [PubMed] [Google Scholar]
- 50.Adatia I, Mullen M, Kulik TJ. Pulmonary venous hypertension or pulmonary hypertension due to left heart disease. Prog Pediatr Cardiol. 2009;27:35–42. [Google Scholar]
- 51.Kulik T, Mullen M, Adatia I. Pulmonary arterial hypertension associated with congenital heart disease. Prog Pediatr Cardiol. 2009;27:25–33. doi: 10.1016/j.ppedcard.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts KE, McElroy JJ, Wong WP, Yen E, Widlitz A, Barst RJ, et al. BMPR2 mutations in pulmonary arterial hypertension with congenital heart disease. Eur Respir J. 2004;24:371–4. doi: 10.1183/09031936.04.00018604. [DOI] [PubMed] [Google Scholar]
- 53.Cordina R, Celermajer D. Late-onset pulmonary arterial hypertension after a successful atrial or arterial switch procedure for transposition of the great arteries. Pediatr Cardiol. 2010;31:238–41. doi: 10.1007/s00246-009-9597-9. [DOI] [PubMed] [Google Scholar]
- 54.Kumar A, Taylor G, Sandor G, Patterson M. Pulmonary vascular disease in neonates with transposition of the great arteries and intact ventricular septum. Br Heart J. 1993;69:442–5. doi: 10.1136/hrt.69.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sreeram N, Petros A, Peart I, Arnold R. Progressive pulmonary hypertension after the arterial switch procedure. Am J Cardiol. 1994;73:620–1. doi: 10.1016/0002-9149(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 56.Daliento L, Somerville J, Presbitero P, Menti L, Brach-Prevert S, Rizzoli G, et al. Eisenmenger syndrome factors relating to deterioration and death. Eur Heart J. 1998;19:1845–55. doi: 10.1053/euhj.1998.1046. [DOI] [PubMed] [Google Scholar]
- 57.van Loon RL, Hoendermis ES, Duffels MG, Vonk-Noordegraaf A, Mulder BJ, Hillege HL, et al. Long-term effect of bosentan in adults versus children with pulmonary arterial hypertension associated with systemic-to-pulmonary shunt: Does the beneficial effect persist? Am Heart J. 2007;154:776–82. doi: 10.1016/j.ahj.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Bograd AJ, Mital S, Schwarzenberger JC, Mosca RS, Quaegebeur JM, Addonizio LJ, et al. Twenty-year experience with heart transplantation for infants and children with restrictive cardiomyopathy: 1986-2006. Am J Transplant. 2008;8:201–7. doi: 10.1111/j.1600-6143.2007.02027.x. [DOI] [PubMed] [Google Scholar]
- 59.Daftari B, Alejos JC, Perens G. Initial experience with sildenafil, bosentan, and nitric oxide for pediatric cardiomyopathy patients with elevated pulmonary vascular resistance before and after orthotopic heart transplantation. J Transplant. 2010;2010:656984. doi: 10.1155/2010/656984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Leval MR. The Fontan circulation: A challenge to William Harvey? Nat Clin Pract Cardiovasc Med. 2005;2:202–8. doi: 10.1038/ncpcardio0157. [DOI] [PubMed] [Google Scholar]
- 61.de Leval MR, Deanfield JE. Four decades of Fontan palliation. Nat Rev Cardiol. 2010;7:520–7. doi: 10.1038/nrcardio.2010.99. [DOI] [PubMed] [Google Scholar]
- 62.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–8. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 63.La Gerche A, Gewillig M. What limits cardiac performance during exercise in normal subjects and in healthy fontan patients? Int J Pediatr 2010. 2010;pii:791291. doi: 10.1155/2010/791291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–7. doi: 10.1093/eurheartj/ehn215. [DOI] [PubMed] [Google Scholar]
- 65.Humpl T, Reyes JT, Holtby H, Stephens D, Adatia I. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension: Twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation. 2005;111:3274–80. doi: 10.1161/CIRCULATIONAHA.104.473371. [DOI] [PubMed] [Google Scholar]
- 66.Apostolopoulou SC, Papagiannis J, Rammos S. Bosentan induces clinical, exercise and hemodynamic improvement in a pre-transplant patient with plastic bronchitis after Fontan operation. J Heart Lung Transplant. 2005;24:1174–6. doi: 10.1016/j.healun.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 67.Uzun O, Wong JK, Bhole V, Stumper O. Resolution of protein-losing enteropathy and normalization of mesenteric Doppler flow with sildenafil after Fontan. Ann Thorac Surg. 2006;82:e39–40. doi: 10.1016/j.athoracsur.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 68.Ovaert C, Thijs D, Dewolf D, Ottenkamp J, Dessy H, Moons P, et al. The effect of bosentan in patients with a failing Fontan circulation. Cardiol Young. 2009;19:331–9. doi: 10.1017/S1047951109990023. [DOI] [PubMed] [Google Scholar]
- 69.Heath A, Stewart K, Mendes J, Ramirez M, Freudenthal F. Does High altitude protect against irreversible pulmonary hypertension? PVRI Rev. 2010;2:131–3. [Google Scholar]
- 70.Sime F, Banchero N, Peñaloza D, Gamboa R, Cruz J, Marticorena E. Pulmonary hypertension in children born and living at high altitudes. Am J Cardiol. 1963;11:143–9. doi: 10.1016/0002-9149(63)90054-7. [DOI] [PubMed] [Google Scholar]
- 71.Penaloza D, Arias-Stella J, Sime F, Recavarren S, Marticorena E. The Heart and pulmonary circulation in children at high altitudes: physiological, anatomical, and clinical observations. Pediatrics. 1964;34:568–82. [PubMed] [Google Scholar]
- 72.Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: Healthy highlanders and chronic mountain sickness. Circulation. 2007;115:1132–46. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- 73.Cutz E, Chiasson D. Chronic lung disease after premature birth. N Engl J Med. 2008;358:743–5. doi: 10.1056/NEJMc073362. author reply 745-6. [DOI] [PubMed] [Google Scholar]
- 74.Mourani PM, Mullen M, Abman SH. Pulmonary hypertension in bronchopulmonary dysplasia. Prog Pediatr Cardiol. 2009;27:43–8. [Google Scholar]
- 75.Stenmark KR, Abman SH. Lung vascular development: Implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–61. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 76.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 77.Bancalari E, Claure N. Definitions and diagnostic criteria for bronchopulmonary dysplasia. Semin Perinatol. 2006;30:164–70. doi: 10.1053/j.semperi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–55. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 79.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: Clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–9. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 80.Krishnan U, Krishnan S, Gewitz M. Treatment of pulmonary hypertension in children with chronic lung disease with newer oral therapies. Pediatr Cardiol. 2008;29:1082–6. doi: 10.1007/s00246-008-9260-x. [DOI] [PubMed] [Google Scholar]
- 81.Drossner DM, Kim DW, Maher KO, Mahle WT. Pulmonary vein stenosis: Prematurity and associated conditions. Pediatrics. 2008;122:e656–61. doi: 10.1542/peds.2008-0075. [DOI] [PubMed] [Google Scholar]
- 82.Seale AN, Webber SA, Uemura H, Partridge J, Roughton M, Ho SY, et al. Pulmonary vein stenosis: The UK, Ireland and Sweden collaborative study. Heart. 2009;95:1944–9. doi: 10.1136/hrt.2008.161356. [DOI] [PubMed] [Google Scholar]
- 83.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154:379–84. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moledina S, Hislop AA, Foster H, Schulze-Neick I, Haworth SG. Childhood idiopathic pulmonary arterial hypertension: A national cohort study. Heart. 2010;96:1401–6. doi: 10.1136/hrt.2009.182378. [DOI] [PubMed] [Google Scholar]
- 85.Girerd B, Montani D, Coulet F, Sztrymf B, Yaici A, Jais X, et al. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am J Respir Crit Care Med. 2010;181:851–61. doi: 10.1164/rccm.200908-1284OC. [DOI] [PubMed] [Google Scholar]
- 86.Fraisse A, Jais X, Schleich JM, di Filippo S, Maragnes P, Beghetti M, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis. 2010;103:66–74. doi: 10.1016/j.acvd.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Mathew R, Huang J, Katta US, Krishnan U, Sandoval C, Gewitz MH. Immunosuppressant-induced endothelial damage and pulmonary arterial hypertension. J Pediatr Hematol Oncol. 2011;33:55–8. doi: 10.1097/MPH.0b013e3181ec0ede. [DOI] [PubMed] [Google Scholar]
- 88.Yildizdas D, Erdem S, Kucukosmanoglu O, Yilmaz M, Yuksel B. Pulmonary hypertension, heart failure and neutropenia due to diazoxide therapy. Adv Ther. 2008;25:515–9. doi: 10.1007/s12325-008-0049-3. [DOI] [PubMed] [Google Scholar]
- 89.Silvani P, Camporesi A, Mandelli A, Wolfler A, Salvo I. A case of severe diazoxide toxicity. Paediatr Anaesth. 2004;14:607–9. doi: 10.1111/j.1460-9592.2004.01276.x. [DOI] [PubMed] [Google Scholar]
- 90.Karaman MG, Atalay F, Tufan AE, Erdogan A. Pulmonary arterial hypertension in an adolescent treated with methylphenidate. J Child Adolesc Psychopharmacol. 2010;20:229–31. doi: 10.1089/cap.2009.0095. [DOI] [PubMed] [Google Scholar]
- 91.Lewman LV. Fatal pulmonary hypertension from intravenous injection of methylphenidate (Ritalin) tablets. Hum Pathol. 1972;3:67–70. doi: 10.1016/s0046-8177(72)80054-6. [DOI] [PubMed] [Google Scholar]
- 92.Barst RJ, Abenhaim L. Fatal pulmonary arterial hypertension associated with phenylpropanolamine exposure. Heart. 2004;90:e42. doi: 10.1136/hrt.2004.036491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel MS, Taylor GP, Bharya S, Al-Sanna’a N, Adatia I, Chitayat D, et al. Abnormal pericyte recruitment as a cause for pulmonary hypertension in Adams-Oliver syndrome. Am J Med Genet. 2004;129A:294–9. doi: 10.1002/ajmg.a.30221. [DOI] [PubMed] [Google Scholar]
- 94.Bergman JE, Blake KD, Bakker MK, du Marchie Sarvaas GJ, Free RH, van Ravenswaaij-Arts CM. Death in CHARGE syndrome after the neonatal period. Clin Genet. 2010;77:232–40. doi: 10.1111/j.1399-0004.2009.01334.x. [DOI] [PubMed] [Google Scholar]
- 95.Baskar S, Kulkarni ML, Kulkarni AM, Vittalrao S, Kulkarni PM. Adams-Oliver syndrome: Additions to the clinical features and possible role of BMP pathway. Am J Med Genet A. 2009;149:1678–84. doi: 10.1002/ajmg.a.32938. [DOI] [PubMed] [Google Scholar]
- 96.Hess OM, Steurer J, Goebel NH, Kuhlmann U, Krayenbuhl HP. Goldenhar syndrome. Schweiz Med Wochenschr. 1979;109:19–23. [PubMed] [Google Scholar]
- 97.Argueta-Morales IR, Meador LC, Nykanen DG, DeCampli WM. Infantile form of scimitar syndrome with contralateral pulmonary vein stenosis. Pediatr Cardiol. 2010;31:550–2. doi: 10.1007/s00246-009-9630-z. [DOI] [PubMed] [Google Scholar]
- 98.Salerno T, Guccione P, Malena S, Cutrera R. Horseshoe lung associated with unique left pulmonary vein: An unreported association. Pediatr Cardiol. 2010;31:905–7. doi: 10.1007/s00246-010-9735-4. [DOI] [PubMed] [Google Scholar]
- 99.Cua CL, Blankenship A, North AL, Hayes J, Nelin LD. Increased incidence of idiopathic persistent pulmonary hypertension in Down syndrome neonates. Pediatr Cardiol. 2007;28:250–4. doi: 10.1007/s00246-006-0011-6. [DOI] [PubMed] [Google Scholar]
- 100.Levine OR, Harris RC, Blanc WA, Mellins RB. Progressive pulmonary hypertension in children with portal hypertension. J Pediatr. 1973;83:964–72. doi: 10.1016/s0022-3476(73)80530-x. [DOI] [PubMed] [Google Scholar]
- 101.Simeoni S, Puccetti A, Chilosi M, Tinazzi E, Prati D, Corrocher R, et al. Type 1 neurofibromatosis complicated by pulmonary artery hypertension: A case report. J Med Invest. 2007;54:354–8. doi: 10.2152/jmi.54.354. [DOI] [PubMed] [Google Scholar]
- 102.Stewart DR, Cogan JD, Kramer MR, Miller WT, Jr, Christiansen LE, Pauciulo MW, et al. Is pulmonary arterial hypertension in neurofibromatosis type 1 secondary to a plexogenic arteriopathy? Chest. 2007;132:798–808. doi: 10.1378/chest.06-3017. [DOI] [PubMed] [Google Scholar]
- 103.Engel PJ, Baughman RP, Menon SG, Kereiakes DJ, Taylor L, Scott M. Pulmonary hypertension in neurofibromatosis. Am J Cardiol. 2007;99:1177–8. doi: 10.1016/j.amjcard.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 104.Aoki Y, Kodama M, Mezaki T, Ogawa R, Sato M, Okabe M, et al. von Recklinghausen disease complicated by pulmonary hypertension. Chest. 2001;119:1606–8. doi: 10.1378/chest.119.5.1606. [DOI] [PubMed] [Google Scholar]
- 105.Banka S, Newman WG, Ozgul RK, Dursun A. Mutations in the G6PC3 gene cause Dursun syndrome. Am J Med Genet A. 2010;152:2609–11. doi: 10.1002/ajmg.a.33615. [DOI] [PubMed] [Google Scholar]
- 106.Scurr I, Wilson L, Lees M, Robertson S, Kirk E, Turner A, et al. Cantu syndrome: Report of nine new cases and expansion of the clinical phenotype. Am J Med Genet A. 2011;155:508–18. doi: 10.1002/ajmg.a.33885. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki K, Yamaki S, Mimori S, Murakami Y, Mori K, Takahashi Y, et al. Pulmonary vascular disease in Down's syndrome with complete atrioventricular septal defect. Am J Cardiol. 2000;86:434–7. doi: 10.1016/s0002-9149(00)00960-7. [DOI] [PubMed] [Google Scholar]
- 108.Matsumura Y, Ban N, Inagaki N. Aberrant catalytic cycle and impaired lipid transport into intracellular vesicles in ABCA3 mutants associated with nonfatal pediatric interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L698–707. doi: 10.1152/ajplung.90352.2008. [DOI] [PubMed] [Google Scholar]
- 109.Park SK, Amos L, Rao A, Quasney MW, Matsumura Y, Inagaki N, et al. Identification and characterization of a novel ABCA3 mutation. Physiol Genomics. 2010;40:94–9. doi: 10.1152/physiolgenomics.00123.2009. [DOI] [PubMed] [Google Scholar]
- 110.Babyn PS, Gahunia HK, Massicotte P. Pulmonary thromboembolism in children. Pediatr Radiol. 2005;35:258–74. doi: 10.1007/s00247-004-1353-y. [DOI] [PubMed] [Google Scholar]
- 111.Tavil B, Kuskonmaz B, Kiper N, Cetin M, Gumruk F, Gurgey A. Pulmonary thromboembolism in childhood: A single-center experience from Turkey. Heart Lung. 2009;38:56–65. doi: 10.1016/j.hrtlng.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 112.Brandstetter Y, Weinhouse E, Splaingard ML, Tang TT. Cor pulmonale as a complication of methylmalonic acidemia and homocystinuria (Cbl-C type) Am J Med Genet. 1990;36:167–71. doi: 10.1002/ajmg.1320360208. [DOI] [PubMed] [Google Scholar]
- 113.Staser JA, Alam T, Applegate K. Calcified pulmonary thromboembolism in a child with sickle cell disease: Value of multidetector CT in patients with acute chest syndrome. Pediatr Radiol. 2006;36:561–3. doi: 10.1007/s00247-006-0161-y. [DOI] [PubMed] [Google Scholar]
- 114.Bonderman D, Wilkens H, Wakounig S, Schafers HJ, Jansa P, Lindner J, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33:325–31. doi: 10.1183/09031936.00087608. [DOI] [PubMed] [Google Scholar]
- 115.Branch GL, Castle RF. Thromboembolic complications in primary endocardial fibroelastosis. J Pediatr. 1966;69:250–8. doi: 10.1016/s0022-3476(66)80328-1. [DOI] [PubMed] [Google Scholar]
- 116.Johnson JN, Driscoll DJ, McGregor CG. Pulmonary thromboendarterectomy in adolescents and young adults. Pediatr Pulmonol. 2010;45:614–8. doi: 10.1002/ppul.21231. [DOI] [PubMed] [Google Scholar]
- 117.Lambert V, Durand P, Devictor D, Planche C, Serraf A. Unilateral right pulmonary thromboendarterectomy for chronic embolism: A successful procedure in an infant. J Thorac Cardiovasc Surg. 1999;118:953–4. doi: 10.1016/s0022-5223(99)70067-x. discussion 57. [DOI] [PubMed] [Google Scholar]
- 118.Groves BM, Droma T, Sutton JR, McCullough RG, McCullough RE, Zhuang J, et al. Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J Appl Physiol. 1993;74:312–8. doi: 10.1152/jappl.1993.74.1.312. [DOI] [PubMed] [Google Scholar]
- 119.Fagan KA, Weil JV. Potential genetic contributions to control of the pulmonary circulation and ventilation at high altitude. High Alt Med Biol. 2001;2:165–71. doi: 10.1089/152702901750265279. [DOI] [PubMed] [Google Scholar]
- 120.Yaron M, Niermeyer S, Lindgren KN, Honigman B, Strain JD, Cairns CB. Physiologic response to moderate altitude exposure among infants and young children. High Alt Med Biol. 2003;4:53–9. doi: 10.1089/152702903321488988. [DOI] [PubMed] [Google Scholar]
- 121.Maggiorini M, Leon-Velarde F. High-altitude pulmonary hypertension: A pathophysiological entity to different diseases. Eur Respir J. 2003;22:1019–25. doi: 10.1183/09031936.03.00052403. [DOI] [PubMed] [Google Scholar]
- 122.Fasules JW, Wiggins JW, Wolfe RR. Increased lung vasoreactivity in children from Leadville, Colorado, after recovery from high-altitude pulmonary edema. Circulation. 1985;72:957–62. doi: 10.1161/01.cir.72.5.957. [DOI] [PubMed] [Google Scholar]
- 123.Gabry AL, Ledoux X, Mozziconacci M, Martin C. High-altitude pulmonary edema at moderate altitude (< 2,400 m; 7,870 feet): A series of 52 patients. Chest. 2003;123:49–53. doi: 10.1378/chest.123.1.49. [DOI] [PubMed] [Google Scholar]
- 124.Das BB, Wolfe RR, Chan KC, Larsen GL, Reeves JT, Ivy D. High-altitude pulmonary edema in children with underlying cardiopulmonary disorders and pulmonary hypertension living at altitude. Arch Pediatr Adolesc Med. 2004;158:1170–6. doi: 10.1001/archpedi.158.12.1170. [DOI] [PubMed] [Google Scholar]
- 125.Durmowicz AG. Pulmonary edema in 6 children with Down syndrome during travel to moderate altitudes. Pediatrics. 2001;108:443–7. doi: 10.1542/peds.108.2.443. [DOI] [PubMed] [Google Scholar]
- 126.Sui GJ, Liu YH, Cheng XS, Anand IS, Harris E, Harris P, et al. Subacute infantile mountain sickness. J Pathol. 1988;155:161–70. doi: 10.1002/path.1711550213. [DOI] [PubMed] [Google Scholar]
- 127.Ivy DD, Feinstein JA, Humpl T, Rosenzweig EB. Non-congenital heart disease associated pediatric pulmonary arterial hypertension. Prog Pediatr Cardiol. 2009;27:13–23. doi: 10.1016/j.ppedcard.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cataltepe S, van Marter LJ, Kozakewich H, Wessel DL, Lee PJ, Levy HL. Pulmonary hypertension associated with nonketotic hyperglycinaemia. J Inherit Metab Dis. 2000;23:137–44. doi: 10.1023/a:1005613715351. [DOI] [PubMed] [Google Scholar]
- 129.del Toro M, Arranz JA, Macaya A, Riudor E, Raspall M, Moreno A, et al. Progressive vacuolating glycine leukoencephalopathy with pulmonary hypertension. Ann Neurol. 2006;60:148–52. doi: 10.1002/ana.20887. [DOI] [PubMed] [Google Scholar]
- 130.Leal GN, de Paula AC, Leone C, Kim CA. Echocardiographic study of paediatric patients with mucopolysaccharidosis. Cardiol Young. 2010;20:254–61. doi: 10.1017/S104795110999062X. [DOI] [PubMed] [Google Scholar]
- 131.Ueno M, Murakami T, Takeda A, Kubota M. Efficacy of oral sildenafil in a beraprost-treated patient with severe pulmonary hypertension secondary to type I glycogen storage disease. Circ J. 2009;73:1965–8. doi: 10.1253/circj.cj-08-0181. [DOI] [PubMed] [Google Scholar]
- 132.Hino T, Hayashida A, Okahashi N, Wada N, Watanabe N, Obase K, et al. Portopulmonary hypertension associated with congenital absence of the portal vein treated with bosentan. Intern Med. 2009;48:597–600. doi: 10.2169/internalmedicine.48.1715. [DOI] [PubMed] [Google Scholar]
- 133.Ohno T, Muneuchi J, Ihara K, Yuge T, Kanaya Y, Yamaki S, et al. Pulmonary hypertension in patients with congenital portosystemic venous shunt: A previously unrecognized association. Pediatrics. 2008;121:e892–9. doi: 10.1542/peds.2006-3411. [DOI] [PubMed] [Google Scholar]
- 134.Iqbal CW, Krowka MJ, Pham TH, Freese DK, El Youssef M, Ishitani MB. Liver transplantation for pulmonary vascular complications of pediatric end-stage liver disease. J Pediatr Surg. 2008;43:1813–20. doi: 10.1016/j.jpedsurg.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 135.Newman B, Feinstein JA, Cohen RA, Feingold B, Kreutzer J, Patel H, et al. Congenital extrahepatic portosystemic shunt associated with heterotaxy and polysplenia. Pediatr Radiol. 2010;40:1222–30. doi: 10.1007/s00247-009-1508-y. [DOI] [PubMed] [Google Scholar]
- 136.Alghamdi MH, Steinraths M, Panagiotopoulos C, Potts JE, Sandor GG. Primary pulmonary arterial hypertension and autoimmune polyendocrine syndrome in a pediatric patient. Pediatr Cardiol. 2010;31:872–4. doi: 10.1007/s00246-010-9704-y. [DOI] [PubMed] [Google Scholar]
- 137.Xing Y, Song HM, Wu XY, He YY, Wei M. Clinical analysis of pulmonary arterial hypertension secondary to connective tissue disease in children. Zhonghua Er Ke Za Zhi. 2008;46:822–6. [PubMed] [Google Scholar]
- 138.Haworth SG, Hislop A, Reid L. Editorial: Progressive pulmonary hypertension in children with portal hypertension. J Pediatr. 1974;84:783–5. doi: 10.1016/s0022-3476(74)80044-2. [DOI] [PubMed] [Google Scholar]
- 139.Silver MM, Bohn D, Shawn DH, Shuckett B, Eich G, Rabinovitch M. Association of pulmonary hypertension with congenital portal hypertension in a child. J Pediatr. 1992;120:321–9. doi: 10.1016/s0022-3476(05)80455-x. [DOI] [PubMed] [Google Scholar]
- 140.Naeye RL. “Primary” pulmonary hypertension with coexisting portal hypertension.A retrospective study of six cases. Circulation. 1960;22:376–84. doi: 10.1161/01.cir.22.3.376. [DOI] [PubMed] [Google Scholar]
- 141.Saunders JB, Constable TJ, Heath D, Smith P, Paton A. Pulmonary hypertension complicating portal vein thrombosis. Thorax. 1979;34:281–3. doi: 10.1136/thx.34.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bower JS, Dantzker DR, Naylor B. Idiopathic pulmonary hypertension associated with nodular pulmonary infiltrates and portal venous thrombosis. Chest. 1980;78:111–3. doi: 10.1378/chest.78.1.111. [DOI] [PubMed] [Google Scholar]
- 143.Cohen MD, Rubin LJ, Taylor WE, Cuthbert JA. Primary pulmonary hypertension: An unusual case associated with extrahepatic portal hypertension. Hepatology. 1983;3:588–92. doi: 10.1002/hep.1840030419. [DOI] [PubMed] [Google Scholar]
- 144.Edwards BS, Weir EK, Edwards WD, Ludwig J, Dykoski RK, Edwards JE. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol. 1987;10:1233–8. doi: 10.1016/s0735-1097(87)80123-7. [DOI] [PubMed] [Google Scholar]
- 145.Yutani C, Imakita M, Ishibashi-Ueda H, Okubo S, Naito M, Kunieda T. Nodular regenerative hyperplasia of the liver associated with primary pulmonary hypertension. Hum Pathol. 1988;19:726–31. doi: 10.1016/s0046-8177(88)80180-1. [DOI] [PubMed] [Google Scholar]
- 146.Wanless IR, Albrecht S, Bilbao J, Frei JV, Heathcote EJ, Roberts EA, et al. Multiple focal nodular hyperplasia of the liver associated with vascular malformations of various organs and neoplasia of the brain: A new syndrome. Mod Pathol. 1989;2:56–62. [PubMed] [Google Scholar]
- 147.Padeh S, Laxer RM, Silver MM, Silverman ED. Primary pulmonaryy hypertension in a patient with systemic-onset juvenile arthritis. Arthritis Rheum. 1991;34:1575–9. doi: 10.1002/art.1780341216. [DOI] [PubMed] [Google Scholar]
- 148.Connor P, Veys P, Amrolia P, Haworth S, Ashworth M, Moledina S. Pulmonary hypertension in children with Evans syndrome. Pediatr Hematol Oncol. 2008;25:93–8. doi: 10.1080/08880010801888253. [DOI] [PubMed] [Google Scholar]
- 149.Nagai H, Yasuma K, Katsuki T, Shimakura A, Usuda K, Nakamura Y, et al. Primary antiphospholipid syndrome and pulmonary hypertension with prolonged survival.A case report. Angiology. 1997;48:183–7. doi: 10.1177/000331979704800213. [DOI] [PubMed] [Google Scholar]
- 150.Espinosa G, Cervera R, Font J, Asherson RA. The lung in the antiphospholipid syndrome. Ann Rheum Dis. 2002;61:195–8. doi: 10.1136/ard.61.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sharma S, Kirpalani AL, Kulkarni A. Severe pulmonary hypertension in a young patient with end-stage renal disease on chronic hemodialysis. Ann Pediatr Cardiol. 2010;3:184–6. doi: 10.4103/0974-2069.74055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123:1577–82. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]
- 153.Unal A, Sipahioglu M, Oguz F, Kaya M, Kucuk H, Tokgoz B, et al. Pulmonary hypertension in peritoneal dialysis patients: Prevalence and risk factors. Perit Dial Int. 2009;29:191–8. [PubMed] [Google Scholar]
- 154.Amin M, Fawzy A, Hamid MA, Elhendy A. Pulmonary hypertension in patients with chronic renal failure: Role of parathyroid hormone and pulmonary artery calcifications. Chest. 2003;124:2093–7. doi: 10.1378/chest.124.6.2093. [DOI] [PubMed] [Google Scholar]
- 155.Havlucu Y, Kursat S, Ekmekci C, Celik P, Serter S, Bayturan O, et al. Pulmonary hypertension in patients with chronic renal failure. Respiration. 2007;74:503–10. doi: 10.1159/000102953. [DOI] [PubMed] [Google Scholar]
- 156.Adatia I. Pulmonary veno-occlusive disease. In: Freedom RM, Yoo SJ, Mikailian H, Williams WG, editors. The natural and modified history of congenital heart disease. New York: Futura; 2004. p. 513. [Google Scholar]
- 157.Kobayashi D, Cook A, Williams D. Pulmonary hypertension secondary to partial pulmonary venous obstruction in a child with Cantú syndrome. Pediatr Radiol. 2010;45:727–9. doi: 10.1002/ppul.21215. [DOI] [PubMed] [Google Scholar]
- 158.Gewillig M, Brown SC, De Catte L, Debeer A, Eyskens B, Cossey V, et al. Premature foetal closure of the arterial duct: Clinical presentations and outcome. Eur Heart J. 2009;30:1530–6. doi: 10.1093/eurheartj/ehp128. [DOI] [PubMed] [Google Scholar]
- 159.Van Marter LJ, Leviton A, Allred EN, Pagano M, Sullivan KF, Cohen A, et al. Persistent pulmonary artery hypertension of the newborn and smoking and aspirin and nonsteroidal anti inflammatory drug consumption during pregnancy. Pediatrics. 1996;97:658–63. [PubMed] [Google Scholar]
- 160.Siu KL, Lee WH. Maternal diclofenac sodium ingestion and severe neonatal pulmonary hypertension. J Paediatr Child Health. 2004;40:152–3. doi: 10.1111/j.1440-1754.2004.00319.x. [DOI] [PubMed] [Google Scholar]
- 161.Schiessl B, Schneider KT, Zimmerman A, Kainer F, Friese K, Oberhoffer R. Prenatal constriction of the fetal ductus arteriosus-related to maternal pain medication? Z Geburtshilfe Neonatol. 2005;209:65–8. doi: 10.1055/s-2005-864116. [DOI] [PubMed] [Google Scholar]
- 162.Alano MA, Ngougmna E, Ostrea EM, Jr, Konduri GG. Analysis of nonsteroidal anti-inflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics. 2001;107:519–23. doi: 10.1542/peds.107.3.519. [DOI] [PubMed] [Google Scholar]
- 163.Zender M, Klinge J, Kruger C, Singer H, Scharf J. Severe pulmonary hypertension in a neonate caused by premature closure of the ductus arteriosus following maternal treatment with diclofenac: A case report. J Perinat Med. 1998;26:231–4. [PubMed] [Google Scholar]
- 164.Mas C, Menahem S. Premature in utero closure of the ductus arteriosus following maternal ingestion of sodium diclofenac. Aust N Z J Obstet Gynaecol. 1999;39:106–7. doi: 10.1111/j.1479-828x.1999.tb03456.x. [DOI] [PubMed] [Google Scholar]
- 165.Talati AJ, Salim MA, Korones SB. Persistent pulmonary hypertension after maternal naproxen ingestion in a term newborn: A case report. Am J Perinatol. 2000;17:69–71. doi: 10.1055/s-2000-9271. [DOI] [PubMed] [Google Scholar]


