Abstract
We describe a modified system for the precise delivery of small volumes of drugs to brain sites of behaving monkeys during simultaneous single-neuron electrophysiology. The system combines a conventional microelectrode for recording single neurons and a small gauge microsyringe in a durable design. It incorporates newly available microfluidic components to achieve high-precision fluidic control. The system is inexpensive, reusable and easy to fabricate; it minimizes neural tissue damage and achieves reliable single-neuron recordings at the injection site.
Keywords: Drug delivery, extracellular recording, awake animal, microinjection, monkey
1. Introduction
Studies of the neural mechanisms of behavior in monkeys primarily involve three major approaches: electrophysiological recordings, electrical microstimulation, and pharmacological manipulations of local neural activity. Combining these three approaches in the same study necessarily provides a more potent means of elucidating neural mechanisms (e.g. Sommer and Wurtz, 2006). To achieve this, several labs have previously developed “microinjectrode” systems for simultaneous drug delivery, neural recording and electrical microstimulation. Malpeli and Schiller’s method was the first to be used successfully in anesthetized monkeys (Malpelli and Schiller, 1979). Subsequently, Crist et al (1988) introduced a microinjectrode system that combined a recording electrode with an injection cannula for simultaneous drug injection and extracellular recording of neuronal activity for use in behaving monkeys (Crist, Yamasaki et al., 1988). Later, Dias and Segraves improved the fluidic control of that system (Dias and Segraves, 1997). Subsequently, Chen et al. modified the original Crist injectrodes to achieve better recording quality (Chen, Goffart et al., 2001). A number of other studies have employed different microinjectrode systems capable of altering both neuronal activity and behavior in animals (Table 1).
Table 1.
Comparison of six major parameters across several microinjectrode systems. The parameters listed in the first column are among the most relevant for successful long-term, reliable microinjection, and single-neuron recordings in behaving monkeys. A single plus in the row 1 indicates systems in which volume monitoring is accomplished only at the infusion pump stage, whereas two pluses represent systems in which the monitoring is also accomplished after the pump stage and closer to the infusion cannula. A single plus in row 2 indicates systems that use microwires for recording, whereas two pluses indicate systems using conventional electrodes with etched, tapered tips. Single pluses in row 4 indicate systems in which guide tubes (21G–25G) or pre-puncturing of the dura is needed for insertion of the microinjectrode; two pluses indicate that the microelectrode is protected by the small diameter (32G) infusion cannula. Pluses in row 5 indicate systems incorporating a proactive means of preventing the clogging of the cannula. The parameter in row 6 refers to the absolute distance between the microelectrode tip and the presumed center of the drug delivery volume. Columns marked “high” are systems in which the microelectrode and the injection cannula are attached side-by-side; all other systems employ coaxial arrangements.
| microinjection system | Crist, Yamasaki, Komatsu, and Wurtz, 1988 | Dias and Segraves, 1997 | Tokuno, Ikeuchi, et al., 1998 | Martin and Ghez, 1999 | Chen, Goffart and Sparks, 2001 | Kliem, Wichmann, 2004 | Noudoost and Moore, 2010 |
|---|---|---|---|---|---|---|---|
| important parameters | |||||||
| 1) Fluid volume monitoring | + | ++ | + | ++ | + | + | ++ |
| 2) Recording quality | + | + | + | + | ++ | ++ | ++ |
| 3) Maximum diameter inside the brain (μm) | 305 | 305 | 311+75 | 200 | 229 | 250+103 | 236 |
| 4) Microelectrode protection during dura-brain penetration | + | + | + | + | + | + | ++ |
| 5) Clog prevention | – | – | – | – | – | + | + |
| 6) Offset between infused drug and microelectrode | low | low | high | low | low | high | low |
Neurophysiological studies involving behaving monkeys are unique in that the preparation of each animal subject typically involves many months of training prior to physiological study. Once the physiological study begins the repeated delivery of electrodes, drugs or electrical current to the brain presumably results in cumulative deleterious effects on the neural tissue under study. Thus, it behooves the experimenter to minimize the amount of damage and maximize the number of appropriate measurements per experiment. Therefore, an ideal microinjectrode system should have the following characteristics: 1) A reliably high recording quality (i.e. single-neuron isolation), 2) Precise control of the drug volume, 3) Reliable drug delivery, and 4) A small diameter cannula-electrode assembly. The previously described microinjectrode systems differ in the degree to which they achieve the above characteristics. The original Crist et al. (1988) system, though effective, was not optimal, and there have since been a number of significant refinements. The systems described by Dias and Segraves (1997) and Martin and Ghez (1999) implement perhaps the best means of measuring the delivered drug volume by monitoring it immediately above the infusion cannula, though neither systems employ a single-neuron quality microelectrode. The systems described by Chen et al. (2001) and Kliem and Wichmann (2004) do employ single-neuron quality microelectrodes, yet they employ a less optimal means of monitoring the drug volume than the above mentioned systems. In the systems described by Tokuno et al. (1998) and Kliem and Wichmann (2004), a micro-wire or microelectrode is attached to the side of the drug cannula. This not only increases the width of the microinjectrode, but it also increases the distance between the neural recording and the center of the delivered drug volume. In the system described here, we have attempted to combine the optimal design features of the above systems into one that is both inexpensive and easy to assemble. Below, we describe the components, construction and use of this system. In addition, we provide more detailed, step-by-step instructions in the supplemental materials.
2. Material and methods
2.1 Construction of Microinjectrode
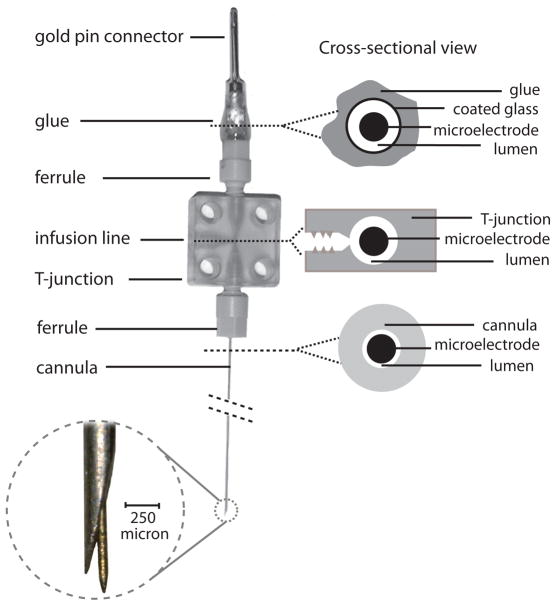
Our microinjectrode consists of a 32-gauge (236 μm outer diameter) stainless steel, beveled-tip cannula (Cadence Science Co., Lake Success, NY ) containing a 75 μm, commercially available epoxy-coated, tungsten microelectrode (FHC Inc., Bowdoinham, ME). Microfluidic components obtained from Labsmith Inc. (Livermore, CA) are used to complete the overall assembly (Fig. 1). LabSmith microfluidic components are designed with low “dead-volumes” and are optimized for leak-free connectivity even at high pressure. The cannula is first attached by a plastic ferrule (Fig. 2A) to the bottom side of a Cilux T-junction (Fig. 2B). Next, the microelectrode is back-loaded through the cannula to the top side of the T-junction where is positioned inside a ~1.5 cm piece of polyimide-coated glass tubing (Fig. 2C) (OD: 360±10 μm, ID: 150±4 μm; Polymicro Technologies, Phoenix, AZ) and the second ferrule. This small piece of glass tubing holds the microelectrode and the gold pin connector steady during soldering and gluing. It is later sealed by glue and is only used for stability. We use “long-axis” to refer to the sides of the three-way connector through which the electrode passes; the “short-axis” is used to interface with the microinjection circuit. The end of the microelectrode is cut, stripped of insulation and then soldered to a gold pin connector. The pin connector, polyimide tubing and second ferrule are all glued together at the top of the second ferrule. Although the second ferrule is a standard “off-the-shelf” ferrule made by Labsmith, in our construction, the first ferrule is custom-made from a Labsmith “plug”. Specifically, a 32-gauge (or smaller) hole is drilled through the plug to make a ferrule with a smaller opening than the pre-made ferrules, which are 30-gauge. Once the electrode is soldered and glued in place and the ferrule is tightened to the T-junction, the tip of the microelectrode should protrude from the tip of the cannula’s bevel at a desired distance (e.g. 100–300 μm). Since the microelectrode’s protrusion from the cannula’s tip is determined by the second ferrule’s attachment to the T-junction, the microelectrode can be withdrawn inside the cannula by unscrewing the second ferrule slightly (but not completely). In our construction the electrode tip can be withdrawn almost completely with about 2 turns of the ferrule. By withdrawing the microelectrode during positioning of the microinjectrode within neural tissue, its tip can be completely protected from damage. In our experience, reliable multi-unit activity recording is attainable even after 4–6 experiments with a single microinjectrode. After microinjectrode positioning, the ferrule can be re-tightened to return the microelectrode tip to its recording position outside the cannula. The microinjectrode can be mounted on a conventional micromanipulator to advance it through the dura and into neural tissue. We used a standard hydraulic “microdrive” in our arrangement (Narishige International USA, INC., East Meadow, NY. Catalog number: MO-96). The microdrive was custom modified to hold the microinjectrode via screws fastened through two corner holes in the three-way connector (Figure 3).
Figure 1.
Schematic of the microinjectrode. A thin microelectrode is passed through a 32G cannula (OD: 236 μm). The cannula is connected to a T-junction via a ferrule. The electrode is passed through a T-junction and a polyimide-coated glass tube and its end is soldered to a gold pin. The polyimide tubing, gold pin, and ferrule are all glued together. Thus unscrewing the ferrule retracts the microelectrode tip back into the cannula. On right, cross-sections through different parts of microinjectrode are shown. Top cross-section is a cut above the top ferrule. The lumen in this section is only open to the T-junction since its top part is sealed by glue. The middle cross-section is through the T-junction. Polyimide tubing connects to the opening of T-junction by a ferrule. Bottom cross-section is through the cannula. Enlarged view of the microelectrode and cannula tips shows their relative position and size.
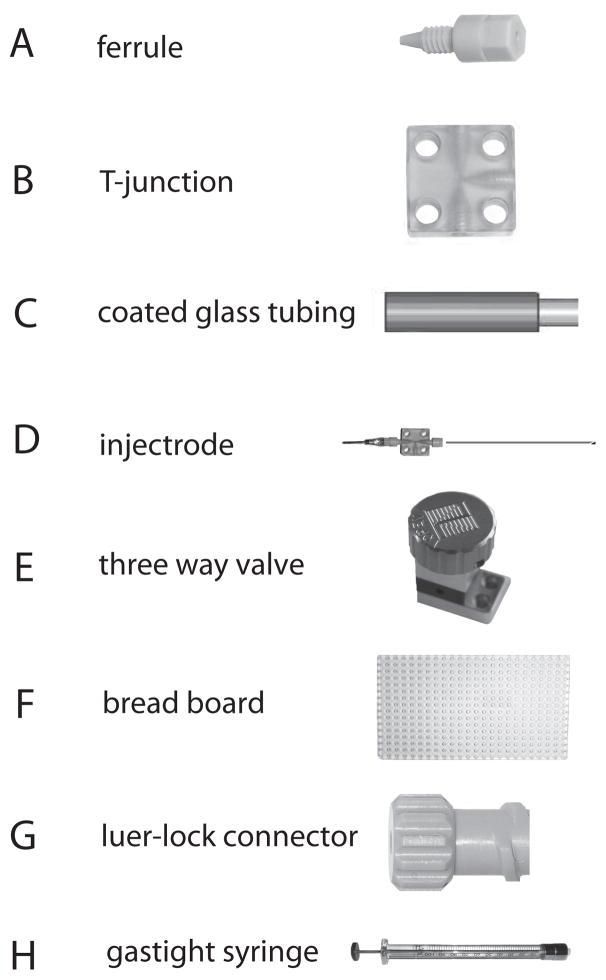
Figure 2.
Key components of microinjection circuit. Ferrules (A), T-junctions (B), breadboard (F), three-way valve (E), and luer-lock connectors (G) are obtained from Labsmith Co.. Polyimide-coated, glass tubing (C) is obtained from Polymicro Co., and the gastight syringe (H) from Hamilton Co.. The microinjectrode (D) is assembled from various parts as described in figure 1.
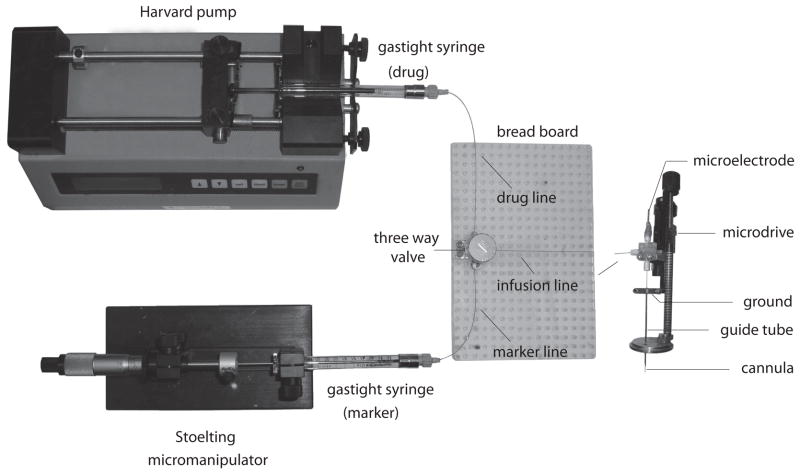
Figure 3.
Arrangement of the microinjection circuit. The three-way valve is mounted on a breadboard and is connected to the infusion, drug and marker lines. Loading of volumes into the circuit is accomplished with the Harvard pump. When the circuit is loaded with a drug, the movement of that drug column inside the infusion line is controlled by a Stoelting micromanipulator and measured in the marker line. The infusion line connects to the microinjectrode, which is mounted on a hydraulic microdrive (e.g. Narishige International USA, INC., East Meadow, NY. Catalog number: MO-96). The electrode holder of the microdrive is custom modified in order to accommodate the three-way Cilux connector of the microinjectrode. The microinjectrode cannula sits inside a larger gauge guide tube. During penetration of the dura and brain, the microelectrode is retracted into the cannula and then extended once the cannula is in place at the brain site under study.
2.2 Construction of the Microinjection circuit
Once the microinjectrode assembly is completed (Fig. 2D), its remaining, short-axis opening can be connected via a ferrule to the “infusion line” of the microinjection circuit. The infusion line is made up of an appropriate length (~ 1.5 m) of polyimide-coated (flexible) glass tubing that is connected (via ferrule) to a three-way valve (Fig. 2E) mounted on a microfluidic breadboard (Fig. 2F). (We used glass tubing because, unlike Teflon or PE tubing, glass tubing is more internally rigid and is therefore more resistant to hysteresis fluid flow hysteresis.) The two remaining inputs of the three-way valve are attached (via tubing and ferrules) to two luer-lock connectors (Fig. 2G), which are in turn connected to two gas-tight Hamilton syringe (100–1000 μL ) (Fig. 2H). The two syringes contain the infused drug and a volume marker to respectively make up the loading and marker line inputs to the three-way valve (Fig. 3). The loading line is driven by a Harvard pump (Harvard Apparatus Inc., Holliston, MA) and the marker line, which is used during drug infusion, is controlled manually by Stoelting micromanipulator (Stoelting Co., Wood Dale, IL).
2.3 Measurement of infused volume
The success of our microinjection system relies primarily on optimizing fluidic control both by using high precision, low “dead-volume” components (e.g. three-way valve, T-junction) which are now commercially available (LabSmith, inc. Livermore, CA) and by keeping the diameter of the entire circuit relatively constant from the luer-lock to the tip of the microinjectrode. As a result, measurement of the volume of drug delivered is both easy and accurate. The infusion line is used to fill the circuit with drug solution, from three-way valve to cannula tip, prior to insertion into brain. Once filled, the marker line serves to both inject the solution into the brain and to accurately measure the injected volume in tubing of similar diameter as the cannula. To observe the volume injected, the marker line can be filled with an oil/dye combination to form a distinct border that can be monitored (through the glass tubing) during drug delivery (similar to Dias and Segraves, 1997 and Martin and Ghez 1999). The oil, which is of low-viscosity (~1 centiStoke), is first loaded into the marker line to a point at least passing through the three-way valve and emerging on the cannula side. Next, the dye, which is a dark-colored food coloring, is then introduced to the marker line with the Hamilton syringe, which is mounted on the Stoelting manual infusion/withdrawal pump. The resulting oil/dye border, which can be visualized through the polyimide glass tubing, can now be used to monitor the delivered drug volume. A plastic ruler can be fixed underneath the tubing on the breadboard for measurement. Fixed distances along the marker line correspond linearly to fixed volumes of fluids; e.g., for the tubing described here a 1 mm movement of the marker corresponds to 17 nL of infused drug. In practice, there may be multiple oil/dye borders within the marker line, each of which can be used to track the flow of drug. But, the oil must remain in front of the dye prior to the three-way valve to prevent the dye from mixing with the drug. The reliability of fluidic control in our system can be confirmed by observing and measuring, the formation of droplets at the cannula tip under a dissecting microscope. We found that the diameter of the droplet could be rapidly and consistently increased or decreased by moving the marker, with no observable hysteresis, and no significant drift when held stable for up to 1 hour.
2.4 Loading of infused drug
Prior to each experiment the drug to be infused is loaded throughout the microinjection circuit with a Harvard pump when the three-way valve is open to connect loading line with the infusion line. To minimize damage to microinjectrode-microinjection assembly and the introduction of air bubbles in the circuit, the infusion line is loaded at a very slow rate (0.5–1 μL/min). To ensure that the circuit is completely loaded, a volume of drug that exceeds the capacity of the circuit is delivered. For example, since a 1.5 m infusion line (ID= 150 μm) contains 25.5 μL, 100 μL of drug run through the circuit will ensure that it is completely filled and that the drug is flowing from the tip of the cannula. Drug flow during loading can be monitored with the aid of a dissecting microscope. Prior to loading the drug into the infusion line, the line is thoroughly flushed with a liquid disinfectant/sterilant (e.g. Nolvasan) followed by sterile saline and finally air using the same procedure described for loading the drug. In addition, a sterilization filter (Millipore Co., Billerica, MA) can be placed in the luer-lock junction with the drug syringe for delivery of sterile drug.
2.5 Experimental setup
Once the microinjection circuit is cleaned and loaded, and the marker line is prepared, the control of drug flow with the manual infusion/withdrawal pump from the tip of the microinjectrode should be confirmed. The three-way valve should now be opened to connect the marker and infusion lines. The formation and disappearance of a droplet of drug under manual control can be visualized with the dissecting microscope. At this stage, the drug “zero point” can be measured at the oil/dye border in the marker line. The drug can then be retracted to a desired, known point prior to infusion. At this point, the top ferrule on the microinjectrode can be loosened to retract the electrode into the cannula prior to penetration of the microinjectrode into neural tissue. Once the cannula is safely within the brain, the ferrule can be re-tightened and the microinjectrode is ready for recording and microinfusion. The cannula serves as the infusion route when the ferrule is tightened and as a protection for the electrode tip when ferrule is loosened. Thus, unlike some of the previous methods (e.g. Crist, Yamasaki et al., 1988) there is no need to pre-puncture the dura before advancing the electrode.
3. Results
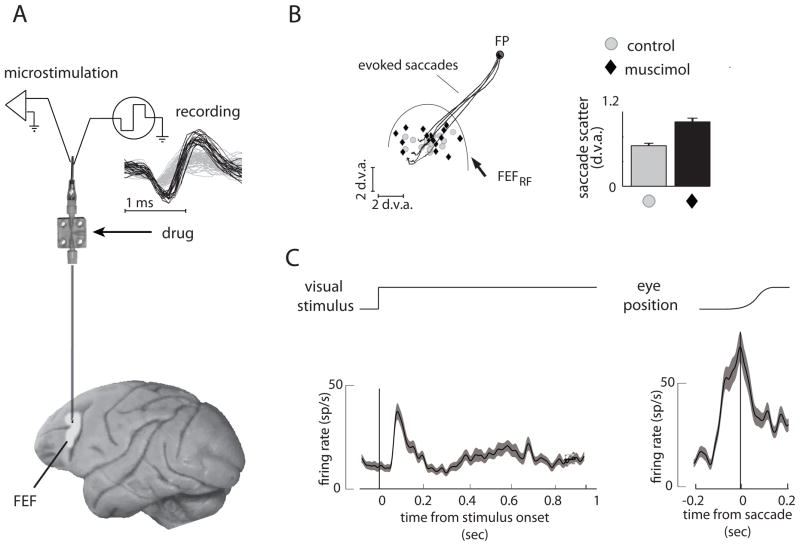
Our microinjectrode system was used to pharmacologically manipulate neuronal activity within the FEF of behaving monkeys (Noudoost and Moore, 2008; Noudoost and Moore, submitted). Figure 4 shows examples of a single-neuron isolation, responses of an FEF neuron during visually guided saccades, saccades evoked with electrical microstimulation of the recording site, and the behavioral effects of local inactivation of the FEF recording site with the GABAa agonist muscimol. The FEF has a known involvement in the planning and generation of saccades (Bruce, Goldberg et al., 1985) and it has been shown that local inactivation of the FEF increases the scatter of visually guided saccades (Dias and Segraves, 1999). In our experiments, good single-neuron isolations could be achieved consistently from the injection site (>90% of recordings in 4 monkeys) (Fig. 4A). Thus, the response properties of neurons and the vectors of saccades evoked with electrical microstimulation at the site could be characterized prior to microinjection (Fig. 4B, C). Following the infusion of 500 nL of muscimol (5 mg/ml) at the FEF site, there was a significant increase in the scatter of endpoints of saccades made to targets located in the part of space represented by neurons at the infusion site. In a separate study, we observed highly consistent effects of various drugs (GABAergic or dopaminergic) on oculomotor behavior (Noudoost and Moore, 2008; Noudoost and Moore, submitted).
Figure 4.
A sample experiment in which single-neuron recording, electrical microstimulation and microinjection was carried out in the frontal eye field (FEF). (A) Examples of single-neuron waveforms (black traces) isolated from background (gray traces) are shown at top. (B) Response field of neurons at the FEF site (FEFRF) and the endpoints of saccades evoked by microstimulation are shown. Black traces show trajectories of microstimulation-evoked saccades. Gray circles indicate landing points of visually-guided saccades to targets within the FEFRF. Visually guided saccades were more scattered after infusion of 500 nl of 5 mg/ml muscimol (black diamonds). The bars at right show mean±SEM of saccade scatter (before= 0.68±0.25 d.v.a.; after= 1.08±0.36 d.v.a.; p=0.005, Wilcoxon sign-rank test). (C) Responses of a single-neuron to a visual stimulus (left) and at the time of a saccade (right) recorded before infusion. Abbreviation: d.v.a., degrees of visual angle.
4. Discussion
4.1 Potential pitfalls
Among the possible pitfalls in the construction of the microinjectrode, perhaps the biggest is a poor glue seal at the top ferrule, where the electrode is soldered to the gold pin connector (Fig. 1). Since this is often the weakest joint in the circuit, care should be taken to form a good seal in order to prevent leakage. In addition, minimizing the pressure inside of the circuit during loading will prevent leaks from forming. A second possible pitfall is that the three-way valves can get clogged with glass particles from polyimide-coated tubing if the tubing breaks inside of the valve-ferrule junction. To prevent this, the ends of the tubing should be free of cracks, burrs and fragments. Lastly, the distance between the electrode tip and the cannula tip must be optimized to both enable the microstimulation functionality while minimizing the offset between the center of the drug volume and the recording site. If the microelectrode tip is too close to the cannula tip (e.g. <100 μm) then the distance between electrical current flow from the microelectrode tip (source) to the cannula (sink) will be too short to stimulate a sufficiently large volume of tissue (Crist, Yamasaki et al., 1988). On the other hand, if the microelectrode is too far from the cannula tip then it will not be possible to assess the neurophysiological effects of the infused drug at the center of the drug volume.
4.2 Possible improvements
In spite of the apparent advantages of our microinjectrode system over that of others (Table 1), there are nevertheless a few ways in which our system might be improved. For example, although we have employed 32 gauge cannulae (~230 μm OD), we could have easily used smaller gauge cannulae, such as 33 or 34G, which correspond to ~200 or ~180 μm OD, respectively. Presumably this would minimize further the damage to neural tissue with repeated penetrations. Second, the precise measurement of fluid volume can easily be improved by a factor of four by simply employing polyimide-coated tubing with a 75 μm ID rather than 150 μm. Third, as a means of avoiding leaks in the system as result of excessive pressure within the circuit, one could insert a pressure gauge in series with the infusion line. Although we almost never observed evidence of clogging in our system (<3/82 experiments), the pressure gauge could nonetheless be used to minimize spikes in fluidic pressure, particularly when the infused substance is highly viscous (e.g. tracers, viruses). Lastly, the system can be extended to accommodate more complicated fluidic systems, for example involving multiple experimental drugs in one experiment.
4.3 Experimental use of microinjectrode system
As illustrated by the results depicted in figure 4, our microinjectrode system can be used to functionally characterize the physiological properties of neurons at an injection site prior to drug delivery. This can be done either with electrical microstimulation, which is advantageous within motor-related structures (e.g. FEF) where movements can readily be evoked, or via high-quality single or multi-unit recording, which is valuable within sensory structures (e.g. visual cortex). For example, a reliably high recording quality would allow one to confirm the similarity of visual selective properties of nearby neurons within presumed “face patches” within the inferotemporal cortex (Tsao, Freiwald et al., 2006; Afraz, Kiani et al., 2006) prior to pharmacological inactivation.
In general, the use of volume drug delivery in the study of the action of drugs on neuronal activity is suboptimal, for example in comparison with microiontophoresis (Hicks, 1984). Volume drug delivery is problematic because it presumably displaces neurons relative to the cannula and the electrode tip during the infusion. Thus, the maintenance of extracellular single-neuron isolations is virtually impossible to achieve with any confidence. As a result, comparison of activity after drug infusion with baseline (“control”) activity for individual neurons is equally unfeasible. Nevertheless, one can perform population-level comparisons, in which the drug and control measurements are treated independently. Such an approach would allow one to simultaneously obtain both neuronal and behavioral effects and to correlate them. For example, one could simultaneously test the effects of blocking GABA-mediated inhibition on direction of motion tuning of area MT neurons (Herrero, Roberts et al., 2008), and on visual motion perception in monkeys. Such an experiment would not be possible with microiontophoresis because the volume of delivered drug is typically far too small to alter behavior.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant EY014924, NSF Grant IOB-0546891, The McKnight Endowment for Neuroscience (T.M.) and IBRO research fellowship (B.N.). We thank Doug S. Aldrich for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afraz SR, Kiani R, Esteky H. Microstimulation of inferotemporal cortex influences face categorization. Nature. 2006;442:692–695. doi: 10.1038/nature04982. [DOI] [PubMed] [Google Scholar]
- 2.Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- 3.Chen LL, Goffart L, Sparks DL. A simple method for constructing microinjectrodes for reversible inactivation in behaving monkeys. J Neurosci Methods. 2001;107:81–85. doi: 10.1016/s0165-0270(01)00354-5. [DOI] [PubMed] [Google Scholar]
- 4.Crist CF, Yamasaki DS, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods. 1988;26:117–122. doi: 10.1016/0165-0270(88)90160-4. [DOI] [PubMed] [Google Scholar]
- 5.Dias EC, Segraves MA. A pressure system for the microinjection of substances into the brain of awake monkeys. J Neurosci Methods. 1997;72:43–47. doi: 10.1016/s0165-0270(96)00154-9. [DOI] [PubMed] [Google Scholar]
- 6.Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophysiol. 1999;81:2191–2214. doi: 10.1152/jn.1999.81.5.2191. [DOI] [PubMed] [Google Scholar]
- 7.Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hicks TP. The history and development of microiontophoresis in experimental neurobiology. Prog Neurobiol. 1984;22:185–240. doi: 10.1016/0301-0082(84)90019-4. [DOI] [PubMed] [Google Scholar]
- 9.Kliem MA, Wichmann T. A method to record changes in local neuronal discharge in response to infusion of small drug quantities in awake monkeys. J Neurosci Methods. 2004;138:45–49. doi: 10.1016/j.jneumeth.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Malpeli JG, Schiller PH. A method of reversible inactivation of small regions of brain tissue. J Neurosci Methods. 1979;1(2):145–159. doi: 10.1016/0165-0270(79)90011-6. [DOI] [PubMed] [Google Scholar]
- 11.Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- 12.Noudoost B, Moore T. Effects of frontal eye field activation and inactivation on visual responses of area v4 neurons. Program No. 464.5/JJ6; 2008 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2008. Online. [Google Scholar]
- 13.Noudoost B, Moore T. Control of visual information by prefrontal dopamine. doi: 10.1038/nature09995. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- 15.Tokuno H, Ikeuchi Y, Nambu A, Akazawa T, Imanishi M, Hamada I, Hasegawa N. A modified microsyringe for extracellular recording of neuronal activity. Neurosci Res. 1998;31:251–255. doi: 10.1016/s0168-0102(98)00041-8. [DOI] [PubMed] [Google Scholar]
- 16.Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.