Abstract
Study Objectives:
Approximately 30% of obstructive sleep apnea (OSA) patients have supine-predominant OSA, and simply avoiding supine sleep should normalise respiratory disturbance event rates. However, traditional supine-avoidance therapies are inherently uncomfortable, and treatment adherence is poor and difficult to monitor objectively. This study evaluated the efficacy of a novel, potentially more acceptable position monitor and supine-avoidance device for managing supine-predominant OSA and snoring.
Design and Setting:
In-laboratory evaluation of position recording accuracy versus video recordings (validation study), and randomized controlled crossover trial of active versus inactive supine-avoidance therapy in the home setting (efficacy study).
Patients:
17 patients undergoing in-laboratory sleep studies (validation) and 15 patients with supine-predominant OSA (efficacy).
Interventions:
Efficacy study: 1 week of inactive and 1 week of active treatment in randomized order, separated by 1 week.
Measurements and Results:
Agreement between 30-sec epoch-based posture classifications from device versus video records was high (median κ 0.95, interquartile range: 0.88-1.00), and there was good supine time agreement (bias 0.3%, 95%CI: −4.0% to 4.6%). In the efficacy study, apnea-hypopnea index (AHI) and snoring frequency were measured in-home using a nasal pressure and microphone based system during inactive and active treatment weeks. The position monitoring and supine alarm device markedly inhibited supine time (mean ± SEM 19.3% ± 4.3% to 0.4% ± 0.3%, p < 0.001) and reduced AHI (25.0 ± 1.7 to 13.7 ± 1.1 events/h, p = 0.030) but not snoring frequency.
Conclusions:
This new position monitoring and supine alarm device records sleep position accurately and improves OSA but not snoring in patients with supine-predominant OSA.
Citation:
Bignold JJ; Mercer JD; Antic NA; McEvoy RD; Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med 2011;7(4):376-383.
Keywords: Obstructive sleep apnea, positional therapy, snoring, supine posture, sleep position
Obstructive sleep apnea (OSA) is a prevalent and chronic disorder characterized by recurrent collapse of the upper airway during sleep producing frequent apnea and hypopnea events. Eleven percent of women and 25% of men aged 40 years or older have at least 15 apneas plus hypopneas per hour of sleep (apnea-hypopnea index, AHI).1 OSA is associated with multiple adverse outcomes, including pathological daytime sleepiness,2 a ∼2-fold increase in motor vehicle accidents,3 depression,4 hypertension,5 and all-cause mortality.6 The main treatments include continuous positive airway pressure (CPAP), mandibular advancement splint devices, weight loss, and upper airway or bariatric surgery. Upper airway function is typically more compromised in the supine posture, such that therapies designed to discourage supine sleep have a significant potential treatment role, as an adjunct or primary treatment in appropriately selected patients.
In OSA patients, unfavorable gravitational effects in supine sleep lead to an increased propensity for and frequency of upper airway collapse,7,8 higher airway opening pressures,9 and more prolonged and severe respiratory events with greater oxygen desaturation.10 Positional OSA, traditionally defined as supine AHI ≥ twice that of non-supine postures, has a prevalence of 50% to 60%.7,11,12 According to a more recent and stringent definition requiring a supine AHI ≥ twice that of non-supine AHI and normalization of AHI in non-supine postures,13 positional OSA patients who meet these criteria should remain within normal limits by using effective positional therapy alone to simply avoid supine sleep. Approximately 30% of clinic-referred OSA patients exhibit positional OSA on this basis.13,14
BRIEF SUMMARY
Current Knowledge/Study Rationale:Traditional supine avoidance strategies to reduce breathing disturbances in sleep are inherently uncomfortable, and treatment adherence may be poor and is difficult to objectively evaluate. This study assessed position monitoring accuracy and supine avoidance effectiveness of a new more comfortable supine avoidance monitor and alarm device.
Study Impact: Accurate and reliable position and device usage monitoring shown in this study are important for evaluating supine-avoidance treatment effectiveness and adherence in the longer term. Longer term data are still needed, but comfortable and effective supine-avoidance approaches such as this hold significant promise for treating patients with supine-predominant OSA.
Snoring is a cardinal feature of OSA and results from vibrations of a partially obstructed upper airway during sleep.15 Snoring may have deleterious health consequences in its own right, including vibration damage-induced carotid atherosclerosis16,17 and potentially carotid plaque rupture and stroke. Snoring very frequently disrupts the sleep of others, particularly affecting bed partner sleep and quality of life.18 While there are some anecdotal reports of marked reductions in snoring with supine sleep avoidance, only one study appears to have examined the impact of positional therapy on snoring in positional OSA patients via objective snoring measurements.19 This used indirect flow measurements without sound recordings, and found that snoring time remained unchanged with a severely restrictive positioning apparatus that was not tolerated by 22% (5/23) of patients studied.19
Several positional therapies have been devised, including the classic “tennis ball technique,” in which a tennis ball strapped to the back is used to discourage supine sleep. Although this technique is effective,20 recent data from our laboratory strongly suggest that patients rarely comply with this treatment longer term.21 After an average follow-up time of ∼30 months, only ∼6% of patients reported continuing use, the main reason for discontinuation being excessive discomfort associated with the ball-on-back design.21 While a minority of patients report having learned to avoid supine sleep without continued treatment,21,22 objective data to support the long-term success of supine avoidance and treatment adherence are currently lacking for supine-avoidance therapies in general.23
The aims of this study were to evaluate the position recording accuracy and acute treatment effectiveness of a novel body position orienting device. The device monitors sleep position and activates a vibration alarm on the sternum, designed to facilitate effective vibration transmission with minimal disturbance to others, to alert and discourage the patient from sleeping supine. An abstract of the preliminary results has previously been published.24
METHODS
This project was approved by the Repatriation General Hospital and University of Adelaide Human Research and Ethics Committees. All participants gave written informed consent.
Body Position Orienting Device
The position monitoring and supine alarm device (BuzzPOD, Gorman ProMed Pty Ltd, Victoria, Australia) is a small, light-weight, battery-powered device (80 × 40 × 20 mm, 50 g, 2 × AAA batteries) that is fastened to the chest using a Velcro strap (Figure 1). The device logs body posture (left, right, prone, supine) at 1 Hz, using internal position-sensitive tilt switches. A vibrating component can be programmed to remain inactive or to activate after the detection of 5 consecutive sec of supine sleep (to facilitate movement/turning without unnecessary vibrations). A patient event-mark button allows for recording of bed and rise times to identify the sleep period and facilitate monitoring of treatment adherence. The device can store 3 weeks of continuous position, alarm, and event data and is configured and data uploaded through custom software via universal serial bus (USB) connection and personal computer.
Figure 1.
Subject wearing the position monitoring and supine alarm device
Validation of Position Recording Accuracy
Seventeen patients undergoing routine diagnostic sleep studies at the Adelaide Institute for Sleep Health were recruited and wore the position monitoring device in addition to standard polysomnographic measures, with the device programmed to record sleep position without vibration. For comparison, an infrared video camera (ICX-IR480/25M, Finest Security System Co. Ltd, Songshan District, Taipei, Taiwan) inside the study bedroom and in-laboratory position sensor (Compumedics P/N 7000-0057-00 sampled at 1 Hz) was used to record sleep position throughout the night.
Data and Statistical Analysis
Device and in-lab posture data recorded at 1 Hz were classified as left, right, supine, or prone in each 30-sec epoch according to the predominant posture registered in each epoch. The same posture classifications were applied to video recordings on an equivalent 30-sec epoch basis using playback at 8 times scored normal speed by an investigator (JB) blinded to position monitoring device, and using in-lab position sensor data.
Agreement was evaluated using the group-averaged κ statistic25 (i.e., the chance-corrected proportion of agreement) determined from each epoch throughout the night within each patient. Kappa values can range from −1 (complete disagreement) through 0 (no agreement above that expected by chance) to +1 (perfect agreement). Agreement was also assessed via Bland-Altman analysis26 of supine time according to the 2 measurement methods.
Efficacy of Supine-Avoidance Treatment in Positional OSA Patients
Subjects
Positional OSA patients (overall AHI ≥ 15/h; supine AHI ≥ twice the non-supine AHI; ≥ 20 min of sleep in supine and non-supine postures; and non-supine AHI < 15/h) identified from diagnostic sleep studies conducted at the Adelaide Institute for Sleep Health between January and July 2009 were invited to participate. Apneas and hypopneas were scored from polysomnography records using the Chicago criteria.27 A cutoff of 15/h using these criteria corresponds to approximately 5/h using more recent AASM “recommended” criteria.28,29 Exclusion criteria were existing treatment(s) for OSA; mobility-limiting problems inhibiting lateral sleep; and a cardiac pacemaker (given unknown potential for device interference). Where applicable, patients' bed partners were also invited to participate, via informed consent, to complete questionnaires regarding patient snoring and potential sleep disturbance from the device.
Protocol
Patients were instructed to wear the position monitoring device each night in a 3-week in-home randomized controlled crossover trial. The trial comprised 1 week of active vibration and 1 week of inactive vibration in a random order, separated by an intervening washout week without vibration to control for potential carryover or training effects,30 with a previous study20 guiding the duration of the washout period. While difficult to blind patients to active versus inactive treatment, all information regarding treatment allocation and timing was withheld until study completion. Patients were aware of the positional nature of their OSA but were given no specific instructions regarding sleep position.
The position monitoring device recorded data continuously day and night. To mark the approximate overnight sleep period for data analysis, patients were instructed to press an event-marking button on the device each evening once after fitting the device for sleep and once on the final awakening the next morning. Patients were also asked to maintain a sleep diary and to rate their nightly sleep quality from 0 (“worst ever”) to 10 (“best ever”) throughout the study.
AHI and snoring were measured on either night 6 or 7 (to facilitate scheduling of concurrent patients) of active and inactive treatment weeks using a portable unattended type III monitor (Visi-lab Grey Flash, Stowood Scientific Instruments Ltd, UK). This monitor recorded airflow, blood oxygen saturation via a finger sensor (LNOP DCSC, Masimo, Irvine, CA, USA), and sound throughout the night using nasal cannulae (1616-TG, Salter Labs, Arvin, CA, USA) and an internal microphone connected to the cannulae port. This microphone primarily records tracheal sounds and snoring generated by the passage of air through the upper airway. To minimize background noise, patients were instructed to avoid using fans, televisions, and radios on AHI recording nights.
Where applicable, following both AHI recording nights, patients' bed partners completed the snoring scale score questionnaire31 adapted to apply to their partners' snoring the previous night. This questionnaire comprises a question each on the frequency, length, and loudness of snoring, with overall scores ranging from 0 (no snoring) to 9 (consistent loud snoring).31 Bed partners also rated how much the supine alarm had disturbed their own sleep using a visual analog scale ranging from 0 (“not at all”) to 10 (“very much”), with intermediate descriptors of “a little” (2-3), “somewhat” (5), and “much” (7-8).
Data Analysis
Nightly supine time as a percentage of the sleep period was determined based on the interval between successive event markings in conjunction with sleep diary-recorded bed and rise times. Patient noncompliance was assessed and deemed present if only one posture registered for an entire nightly period, strongly suggesting failure to wear the position monitoring device.
To investigate the temporal characteristics of supine periods during inactive and active treatment, the frequencies of transitions to the supine posture lasting ≥ 5 sec were averaged across nights within each condition. For inactive treatment, the average duration of these periods was calculated, while for active treatment, average alarm time was determined for alarm events of any length.
Type III monitor recordings were analyzed using the accompanying software (Download 3 v1.2, Stowood Scientific Instruments Ltd) following randomization of all fully de-identified records by another investigator to ensure analysis blinding. Raw signals were manually reviewed before analysis and periods in which the cannulae or finger sensor were detached from the patient or in which the patient was clearly not asleep (e.g., talking with partner in sound recording) were excluded. In the remaining periods, Download 3 automatically calculated the apnea index, hypopnea index, and AHI as the number of apneas, hypopneas, and apneas plus hypopneas, respectively, per hour of recording. Apnea and hypopnea were defined as a reduction in airflow amplitude of > 90% and of 50% to 90%, respectively, lasting 10-100 sec.27 Minimum overnight oxygen saturation and 4% oxygen desaturation index were also calculated. AHI values from this type III monitor have previously been validated against laboratory polysomnography-determined AHI, with an area under the receiver operator curve for AHI ≥ 10/h of 0.96.32
A “snore” was automatically scored when there was a spike in sound intensity ≥ 50 decibels (dB), whether during inspiration or expiration. This threshold was chosen because it has been used previously to identify snoring,33 and was above background noise (mean ± SD, 31.4 ± 1.8 dB) and normal breathing sounds (which registered up to 45 dB). Following automated analysis, sound recordings were manually reviewed, and identifiable artifacts such as teeth grinding were removed. Snoring was subsequently quantified by frequency (snores/h) and by mean duration (in milliseconds). While the analysis software did not allow snoring intensity to be examined directly, snoring frequency was calculated for “soft” (≥ 50, < 60 dB), “moderate” (≥ 60, < 70 dB) and “loud” (≥ 70 dB) snores as well as overall (≥ 50 dB). The 50, 60, and 70 dB cutoffs correspond approximately to the loudness of a quiet conversation, a ringing telephone, and a vacuum cleaner, respectively.34
Statistical Analysis
The primary endpoint was AHI, and all analyses were conducted on an intention-to-treat basis. The sample size chosen was based on the results of 2 previous crossover trials of positional therapy.20,35 Using AHI as the primary endpoint, we estimated that a within-subject difference of 9/h between active and inactive treatment (SD 11/h) could be detected with a sample size of 15, 2-tailed significance level of 5%, and power of 80%.
To test for potential learning and carryover effects on supine time among patients randomized to active treatment first, intervening supine time data were analyzed in isolation for treatment order and order by night effects using linear mixed model analysis with an autoregressive covariance structure (SPSS v17.0, SPSS Inc., Chicago, IL, USA). Mixed-model analysis was also used to compare AHI and other endpoints between conditions (inactive vs active) and, where applicable, across nights (1-7). Treatment order and AHI recording night (6 vs 7) were initially included as factors in relevant models to assess for order and night effects, but were subsequently removed when nonsignificant. Where applicable, Bonferroni-adjusted custom post hoc contrasts were used to further examine significant interaction and/or main effects. Unless otherwise indicated, data are expressed as mean ± SEM or, if non-normally distributed, as median (interquartile range, IQR). p < 0.05 was considered statistically significant.
RESULTS
Validation of Position Recording Accuracy
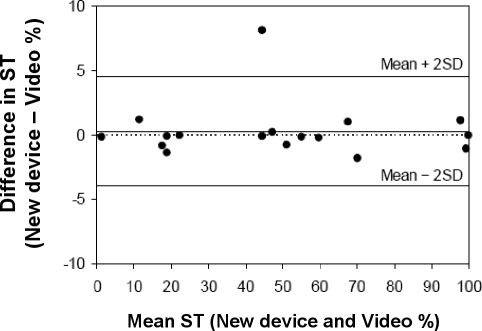
Patients were predominantly male (11/17 patients) with a mean ± SD age and body mass index of 49.7 ± 15.7 years and 34.6 ± 8.4 kg/m2, respectively. Based on a median of 830 epochs per patient (IQR: 715-905), the median κ statistic of posture recording agreement between the position monitoring device and video recordings was 0.95 (IQR 0.88-1.00), indicating close agreement. A Bland-Altman plot of supine time agreement is shown in Figure 2. The mean difference (bias) in supine time between the position monitoring device and video recordings was 0.3%, with 95% limits of agreement of −4.0% to 4.6%. Agreement between video and in-laboratory position sensor recordings were similar but appeared to be more variable (median κ 0.96, IQR 0.47-0.99, supine time bias 1.1%, 95% limits of agreement −13.6% to 15.5%).
Figure 2.
Bland-Altman plot of mean versus difference in supine time (ST) between the new position monitoring device and video recordings during in-laboratory diagnostic sleep studies (N = 17)
The top and bottom unbroken lines indicate the upper and lower 95% limits of agreement, respectively; the middle unbroken line indicates the mean difference; and the dotted line zero bias.
Efficacy of Supine Avoidance in Positional OSA Patients
Thirty-five positional OSA patients were approached. Ten declined (3 reported they rarely slept supine, 2 had upcoming travel commitments, and 5 gave no reason), and 9 were already on OSA treatment. Thus, 16 patients with supine-predominant OSA consented and were randomized into the trial, with all but one completing the study protocol. This patient withdrew on the first night due to intolerance of the position monitoring device chest strap and vibration alarm, leaving no data available for analysis. All 15 patients who completed the study contributed AHI and snoring data, but supine time data were not available for one patient because of a position monitoring device upload failure. Seven patient bed partners completed the questionnaires.
Table 1 shows baseline and in-laboratory diagnostic sleep study characteristics of the 15 patients who completed the trial. Patients were predominantly male, middle-aged, and overweight and had mild OSA strongly associated with the supine position. AHI was not statistically significantly different between REM and NREM sleep.
Table 1.
Baseline demographic and clinical characteristics of patients completing the efficacy trial (n = 15)
| Variable | Value |
|---|---|
| Patient Characteristics | |
| Age (y) | 58.2 ± 13.9 |
| Sex (M/F) | 13/2 |
| Weight (kg) | 83.5 ± 13.1 |
| Height (cm) | 169.8 ± 10.5 |
| BMI (kg/m2) | 28.8 ± 2.5 |
| Laboratory sleep study | |
| Total sleep time (h) | 6.7 ± 1.2 |
| Sleep efficiency (%) | 81.4 ± 9.2 |
| Supine sleep time (h) | 2.3 ± 1.3 |
| Supine sleep (%) | 36.4 ± 20.6 |
| Arousal Index (events/h) | |
| Total | 17.3 ± 6.9 |
| Respiratory | 5.3 ± 3.3 |
| Spontaneous | 6.0 ± 2.4 |
| Apnea-hypopnea Index (events/h) | |
| Total | 24.1 ± 10.5 |
| REM | 30.5 ± 18.4 |
| NREM | 22.8 ± 10.8 |
| Supine | 51.3 ± 23.3 |
| Non-supine | 9.7 ± 3.9* |
| REM: NREM ratio | 1.5 (IQR 0.8 - 2.2) |
| Supine: non-supine ratio | 5.3 (IQR 3.3 - 9.2) |
Values are mean ± SD or median (IQR).
M, male; F, female; BMI, body mass index; AHI, apnea-hypopnea index.
p < 0.001 vs supine.
Based on the presence of event marks and intervening position movements, the position monitoring device was worn on 81% ± 10% of all active treatment nights, 91% ± 5% of intervening nights, and 83% ± 8% of inactive treatment nights. Total use over the full 3 weeks of the trial was 85% ± 6% of nights, with average hours of use per night of 6.8 ± 0.6 h (including zero hours when not worn) and 8.0 ± 0.3 h when worn. Patients' self-reported sleep quality was not statistically significantly different between nights or conditions: active 6.4 ± 0.2 vs inactive 6.2 ± 0.2. There were no order (carryover) effects for any endpoint among patients commencing active treatment first.
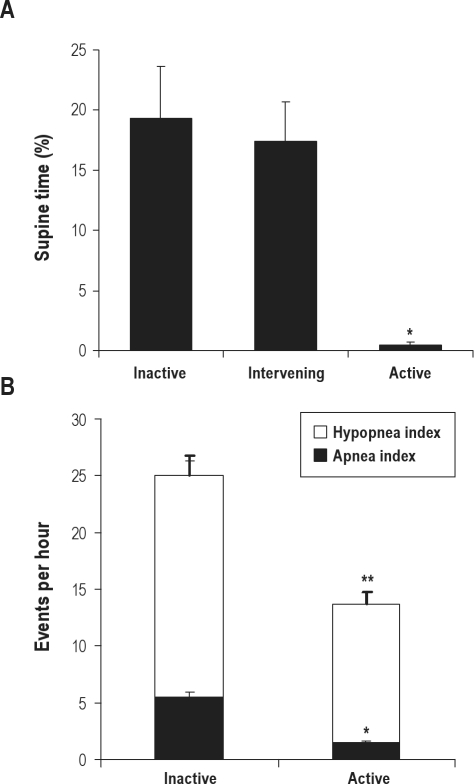
Figure 3 shows supine time averaged across all nights during each condition. There was a statistically significant effect of treatment (p < 0.001), with lower supine times on active vs inactive (p < 0.001) and active vs intervening (p < 0.001) nights. There were no statistically significant differences between inactive and intervening weeks, or between-night or treatment by night effects. Supine sleep time during in-laboratory diagnostic sleep study was statistically significantly greater compared to in-home supine time with inactive treatment (36.6% ± 5.7% vs 19.3% ± 4.3%, p = 0.002).
Figure 3.
(A) Percent supine time during inactive, intervening and active supine avoidance treatment weeks in positional obstructive sleep apnea patients (N = 14), *p < 0.001 vs inactive and intervening. (B) Apnea index, hypopnea index, and total AHI (thick error bar) during inactive and active treatment (N = 15). *apnea, hypopnea, and total AHI different vs inactive treatment (p < 0.05). Values are mean ± SEM.
The frequency of transitions to the supine position was not different between inactive and active treatment (1.7 ± 0.5 vs 1.3 ± 0.3 transitions/h). During inactive treatment supine periods lasted 8.2 ± 1.9 min. During active treatment, average alarm and supine time (excluding programmed 5-sec delay) was 9.2 ± 4.1 sec before transition to another posture.
Total AHI was reduced in the order of 45% with active treatment (p = 0.03), via reduced apnea (p = 0.02) and hypopnea indices (p = 0.04, Figure 3). There was a significant corresponding decrease in 4% oxygen desaturation index (5.5 ± 0.3 to 3.4 ± 0.2 dips/h, p = 0.014) and increase in minimum overnight oxygen saturation (84.3% ± 1.3% to 88.3% ± 0.9%, p = 0.02). Although the group mean AHI was substantially reduced with active treatment, the number of patients achieving an AHI < 15/h was not different between active (10/15) and inactive treatment (9/15).
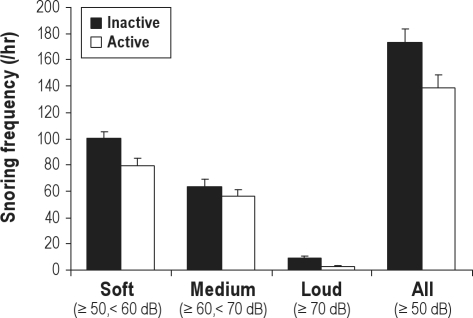
There was a trend for an overall reduction in snoring frequency in the order of 20% with active treatment (Figure 4), but this was not statistically significant (p = 0.084), and there was no treatment by snoring intensity interaction effect. There were also no differences in mean snore duration (inactive 1126 ± 169 vs active 1082 ± 96 msec), or bed partner snoring scale scores (3.0 ± 0.9 vs 3.0 ± 0.9, n = 7) to support reduced snoring with supine-avoidance. Bed partners' sleep disturbance from the supine vibration alarm increased slightly but significantly from 0.7 ± 0.6 to 2.9 ± 0.8 (p = 0.01), corresponding to “a little” disturbance.
Figure 4.
Frequency of soft (≥ 50, < 60 dB), moderate (' 60, < 70 dB), loud (≥ 70 dB), and all (≥ 50 dB) snores during inactive and active supine-avoidance treatment in positional obstructive sleep apnea patients (N = 15)
Values are mean ± SEM.
DISCUSSION
This study demonstrates the clinical utility of this position monitoring and supine alarm device as an accurate monitor of overnight posture and a highly effective supine-avoidance therapy, almost completely abolishing the supine posture and significantly reducing overnight AHI, albeit with persistent snoring in positional OSA patients.
The position monitoring device showed high posture classification agreement (average κ 0.95) and supine time measurements with negligible systematic bias, with limits of agreement within 5% compared to simultaneous in-laboratory video recordings. Accurate position monitoring is an important consideration with this form of supine-avoidance treatment, given that false omissions would allow patients to sleep untreated when supine, and false detections would unnecessarily disturb patient and (potentially) bed partner sleep.
The ability to reliably and accurately record position in the home setting is a major advantage of this device over previous supine-avoidance approaches, since the ongoing evaluation of supine avoidance effectiveness and treatment adherence otherwise remain inevitably speculative. While longer-term treatment adherence and effectiveness of this and other supine-avoidance treatments remain largely unknown, the ability to continuously log overnight body position should allow for objective measures of long-term outcomes in patients with supine-predominant OSA and/or snoring, an area of sleep medicine for which data are currently lacking.23
In this study, supine time during inactive treatment was in the order of 20%, consistent with 10% to 34% reported in the few previous in-home studies,20,36 and presumably reflective of typical overnight supine time in the home setting. In contrast, supine sleep time in diagnostic in-laboratory sleep studies in the same patients was significantly higher (∼35%), consistent with previous reports of high in-laboratory supine sleep times (50-60%).30,36 This likely indicates a combination of verbal encouragement to sleep supine in the clinical laboratory setting, designed to examine worst-case respiration in sleep, plus restricted movement imposed by leads attached to patients.37 Such in-laboratory bias towards supine sleep may overestimate the therapeutic potential of supine-sleep avoidance in the more typical home environment. Consistent with previous studies of supine sleep avoidance therapies,19,20,35 AHI was reduced in the order of 50%, despite only 19% supine time during inactive treatment, underscoring the supine predominance of AHI in positional OSA patients. On the basis of in-laboratory recordings and by experimental design, all patients in this study were expected to achieve an AHI < 15 events/h with abolition of ∼35% supine time. Although group data suggest normalization of AHI in most patients, AHI responses were highly variable, and the number of patients achieving AHI < 15/h with active treatment (10/15) was not different from that with inactive treatment (9/15), indicating substantial AHI variability, and potential confounding of patient selection by elevated supine time in the laboratory setting. Evaluation of more typical supine time in the home versus in-laboratory setting may be needed to more appropriately target supine-avoidance treatment to those patients most likely to benefit.
Snoring
Overall snoring frequency appeared to be reduced in the order of 20% or ∼40 snores/h, but this and all other indices of snoring improvement, including bed partners' satisfaction regarding snoring, showed no improvement with supine-avoidance treatment. This finding is consistent with another study that examined the impact of positional therapy on objective measurements of snoring in positional OSA patients, which also found no significant change with treatment.19 In a less direct evaluation, Nakano and coworkers38 also found no significant difference in snoring intensity or snoring time between the supine and lateral sleep postures in 51 OSA patients, although no distinction was made between patients with and without positional OSA.
Snoring was reported to be relatively mild in terms of bed partner complaints. Nevertheless, persistent snoring with supine-avoidance therapy may limit the utility of this approach in patients with supine-predominant OSA. Snoring is one of the primary complaints in the clinical presentation of OSA that frequently disrupts bed partner sleep, affecting their own quality of life18 and that of the snorer. Snoring may also contribute to cardiovascular risk.16,17 Effective positional therapy to reduce AHI but with only a small reduction in snoring may be of greatest benefit to patients who regularly sleep alone (5/15 patients in this study and 17% to 25% in others39,40). However, for the majority of positional OSA patients, adjunct or alternative therapies are likely necessary to relieve snoring if snoring is a primary complaint or significant clinical concern.
The reasons for persistent snoring in positional OSA patients despite highly effective supine-avoidance and significantly reduced AHI are not entirely clear. While not statistically significant, and potentially a type II error, a 20% reduction in snoring frequency and/or small downward shift in the frequency of louder snores might be all that should be expected from a ∼20% reduction in supine time in positional OSA patients selected on the basis of the supine predominance of AHI and not snoring. Unlike apneas, which arise from complete obstruction of the upper airway and relative silence until apnea resolution, hypopneas, which dominate AHI, are associated with partial airway occlusion more relevant to snoring.15 Although hypopneas were significantly reduced and might be expected to improve snoring indices, partial upper airway collapse and flow limitation are likely prevalent during periods of relative breathing stability between conventionally scored respiratory events. At least in this positional OSA patient group, partial upper airway collapse, flow limitation, and snoring may therefore persist via conversion of apneas and more severe hypopneas into snoring events, continued snoring between respiratory events, and potentially reduced arousals and non-flow limited breaths during transient wakefulness. This does not discount that snoring may nevertheless be substantially resolved in patients with milder upper airway dysfunction and supine-predominant snoring. Further studies are needed to separately evaluate the supine-predominance of snoring and AHI to most appropriately target supine-avoidance treatment.
Tolerability of the Position Monitor and Supine Alarm
This is the first trial to evaluate patient compliance with positional therapy objectively, rather than by patient self-report, which may be unreliable. Although one patient withdrew on the first night of active treatment, overall treatment compliance was high and was not different between active and inactive treatment, indicating that most patients tolerate active therapy in the short-term. Compliance with traditional tennis ball based supine-avoidance appears to be very poor, primarily due to its inherent discomfort.21,22 Being fastened to the chest rather than the back, this new position monitoring device may be more comfortable, and the vibratory alarm less intrusive to the bed partner potentially facilitating improved longer-term compliance. A vibratory stimulus may also be more effective than an auditory alarm, given the presence of competing loud snoring and the potential for comorbid hearing loss.41 Although bed partners reported higher sleep disturbance during active treatment, this corresponded to only “a little” disturbance, and alarms were relatively infrequent. Nevertheless, further trials are clearly needed to investigate longer-term treatment compliance and acceptability.
Methodological Considerations
The main weakness of this study was the use of a type III device to estimate AHI using the total sleep period including any wake time as the denominator. We specifically chose the home setting most relevant to OSA and snoring and elected to estimate AHI with a simplified device that included objective snoring measurements, but without EEG, EOG, and EMG leads associated with full polysomnography, as these further restrict body movements and likely confound the study of posture and intervention effects of primary interest in this study. Polysomnography leads likely contributed to bias towards increased supine time in the laboratory setting in this and in other studies,37 and may overestimate the potential efficacy of positional therapies in the home environment. However, in the absence of sleep measurements it is possible that disrupted sleep with more wake time could have artifactually lowered AHI with active versus inactive treatment. Several observations strongly argue against this and the main findings are consistent with similar studies.19,20,35 The frequency of posture transitions was similar, and patient-reported sleep quality was not different between inactive and active treatment. In addition, alarms were infrequent and of short duration during active treatment. Of particular note is that snoring frequency did not change with active treatment, a finding consistent with another report,38 and difficult to reconcile on the basis of increased wake time. Finally, if AHI on active treatment exclusively represents non-supine events without changes in wake time, the addition of 19% supine time (ST) to a non-supine AHI (AHINS) of ∼14 events/h with a 5.3-fold higher supine versus non-supine AHI ratio (AHIRatio) would be expected to increase total AHI to ∼25 events/h (Expected AHITotal = AHINS × AHIRatio × ST + AHINS × (1-ST) = 14 × 5.3 × 0.19 + 14 × 0.81 = 25.4). This is entirely consistent with supine sleep avoidance with minimal additional wake time with active treatment. Any increase in wake time should have further reduced AHI calculated using the total sleep period as the denominator.
In summary, these data indicate this new position monitoring and supine alarm device accurately monitors sleep position and is an effective therapy for reducing AHI, but without relieving snoring, at least in patients with supine-predominant OSA. Effects on sleep quality and longer-term outcomes of effectiveness, adherence, and bed partner tolerance compared to alternative therapies remain to be established for positional OSA patients and for positional snorers without OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This project was supported by a Flinders Medical Centre Foundation Grant. The authors are very grateful to Michael Gorman for providing the position monitoring devices and technical support, and to Dr. Lyn Davies for advice on snoring analysis. This work was performed at Adelaide Institute for Sleep Health, Repatriation General Hospital.
REFERENCES
- 1.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–7. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 3.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–15. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 7.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–4. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 8.Isono S, Tanaka A, Nishino T. Lateral position decreases collapsibility of the passive pharynx in patients with obstructive sleep apnea. Anesthesiology. 2002;97:780–5. doi: 10.1097/00000542-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Neill AM, Angus SM, Sajkov D, McEvoy RD. Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1997;155:199–204. doi: 10.1164/ajrccm.155.1.9001312. [DOI] [PubMed] [Google Scholar]
- 10.Oksenberg A, Khamaysi I, Silverberg DS, Tarasiuk A. Association of body position with severity of apneic events in patients with severe nonpositional obstructive sleep apnea. Chest. 2000;118:1018–24. doi: 10.1378/chest.118.4.1018. [DOI] [PubMed] [Google Scholar]
- 11.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112:629–39. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 12.Richard W, Kox D, den Herder C, Laman M, van Tinteren H, de Vries N. The role of sleep position in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2006;263:946–50. doi: 10.1007/s00405-006-0090-2. [DOI] [PubMed] [Google Scholar]
- 13.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130–7. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 14.Dyonzak J, Cartwright RD. Prevalence of positional differences in obstructive sleep apnea. Sleep Res. 1993;22:191. [Google Scholar]
- 15.Lugaresi E. Snoring. Electroencephalogr Clin Neurophysiol. 1975;39:59–64. doi: 10.1016/0013-4694(75)90127-3. [DOI] [PubMed] [Google Scholar]
- 16.Amatoury J, Howitt L, Wheatley JR, Avolio AP, Amis TC. Snoring-related energy transmission to the carotid artery in rabbits. J Appl Physiol. 2006;100:1547–53. doi: 10.1152/japplphysiol.01439.2005. [DOI] [PubMed] [Google Scholar]
- 17.Lee SA, Amis TC, Byth K, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31:1207–13. [PMC free article] [PubMed] [Google Scholar]
- 18.Parish JM, Lyng PJ. Quality of life in bed partners of patients with obstructive sleep apnea or hypopnea after treatment with continuous positive airway pressure. Chest. 2003;124:942–7. doi: 10.1378/chest.124.3.942. [DOI] [PubMed] [Google Scholar]
- 19.Loord H, Hultcrantz E. Positioner--a method for preventing sleep apnea. Acta Otolaryngol. 2007;127:861–8. doi: 10.1080/00016480601089390. [DOI] [PubMed] [Google Scholar]
- 20.Skinner MA, Kingshott RN, Filsell S, Taylor DR. Efficacy of the ′tennis ball technique' versus nCPAP in the management of position-dependent obstructive sleep apnoea syndrome. Respirology. 2008;13:708–15. doi: 10.1111/j.1440-1843.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 21.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5:428–30. [PMC free article] [PubMed] [Google Scholar]
- 22.Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: A 6-month follow-up study. Laryngoscope. 2006;116:1995–2000. doi: 10.1097/01.mlg.0000237674.66716.a7. [DOI] [PubMed] [Google Scholar]
- 23.Chan AS, Lee RW, Cistulli PA. Non-positive airway pressure modalities: mandibular advancement devices/positional therapy. Proc Am Thorac Soc. 2008;5:179–84. doi: 10.1513/pats.200707-104MG. [DOI] [PubMed] [Google Scholar]
- 24.Bignold JJ, Mercer JD, Gorman M, McEvoy RD, Antic NA, Catcheside PG. Validation and performance of a novel positional therapy device for supine-dependent obstructive sleep apnoea. Sleep Biol Rhythms. 2009;7:A39–A40. [Google Scholar]
- 25.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 26.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 27.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 28.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iber C, Ancoli-Israel S, Chesson A, Quan S. 1st ed. Westchester: IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 30.Cartwright RD, Lloyd S, Lilie J, Kravitz H. Sleep position training as treatment for sleep apnea syndrome: a preliminary study. Sleep. 1985;8:87–94. doi: 10.1093/sleep/8.2.87. [DOI] [PubMed] [Google Scholar]
- 31.Lim PV, Curry AR. A new method for evaluating and reporting the severity of snoring. J Laryngol Otol. 1999;113:336–40. doi: 10.1017/s0022215100143919. [DOI] [PubMed] [Google Scholar]
- 32.Roebuck T, Ho S, Langan-Fox J, et al. Portable sleep monitoring: Is the “Greyflash” (Level III) device useful? Sleep Biol Rhythms. 2009;7:A70. [Google Scholar]
- 33.Hoffstein V, Mateika S, Nash S. Comparing perceptions and measurements of snoring. Sleep. 1996;19:783–9. [PubMed] [Google Scholar]
- 34.South T. Oxford: Elselvier Butterworth-Heinemann; 2004. Managing noise and vibration at work: a practical guide to assessment, measurement and control. [Google Scholar]
- 35.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115:771–81. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 36.Hartse KM, Logan MB, Branham GH, Eisenbeis JF. Unattended home monitoring in the evaluation of sleep apnea: is it equal to formal polysomnography? Sleep Res. 1996;25:251. [Google Scholar]
- 37.Metersky ML, Castriotta RJ. The effect of polysomnography on sleep position: possible implications on the diagnosis of positional obstructive sleep apnea. Respiration. 1996;63:283–7. doi: 10.1159/000196561. [DOI] [PubMed] [Google Scholar]
- 38.Nakano H, Ikeda T, Hayashi M, Ohshima E, Onizuka A. Effects of body position on snoring in apneic and nonapneic snorers. Sleep. 2003;26:169–72. doi: 10.1093/sleep/26.2.169. [DOI] [PubMed] [Google Scholar]
- 39.Grunstein RR, Stenlof K, Hedner JA, Sjostrom L. Impact of self-reported sleep-breathing disturbances on psychosocial performance in the Swedish Obese Subjects (SOS) Study. Sleep. 1995;18:635–43. doi: 10.1093/sleep/18.8.635. [DOI] [PubMed] [Google Scholar]
- 40.Hagert B, Wahren LK, Wikblad K, Odkvist L. Patients' and cohabitants' reports on snoring and daytime sleepiness, 1-8 years after surgical treatment of snoring. ORL J Otorhinolaryngol Relat Spec. 1999;61:19–24. doi: 10.1159/000027633. [DOI] [PubMed] [Google Scholar]
- 41.Hoffstein V, Haight J, Cole P, Zamel N. Does snoring contribute to presbycusis? Am J Respir Crit Care Med. 1999;159:1351–4. doi: 10.1164/ajrccm.159.4.9808147. [DOI] [PubMed] [Google Scholar]