Abstract
Background:
Patients with obstructive sleep apnea (OSA) are at increased risk of cardiovascular disease (CVD). The omega-3 fatty acid docosahexaenoic acid (DHA) is a major component of neural tissues, and supplementation with fish oils improves autonomic tone and reduces risk for CVD. A link between low DHA status and less mature sleep patterns was observed in newborns.
Methods:
We investigated the relations between red blood cell (RBC) levels of DHA and OSA severity in 350 sequential patients undergoing sleep studies. Severity categories were defined as none/mild, moderate, and severe, based on apnea hypopnea index (AHI) scores of 0 to 14, 15 to 34, and > 34, respectively.
Results:
After controlling for age, sex, race, smoking, BMI, alcohol intake, fish intake, and omega-3 supplementation, RBC DHA was inversely related with OSA severity. For each 1-SD increase in DHA levels, a patient was about 50% less likely to be classified with severe OSA. The odds ratios (95% CI) were 0.47 (0.28 to 0.80) and 0.55 (0.31 to 0.99) for being in the severe group versus the none/mild or moderate groups, respectively.
Conclusion:
These findings suggest that disordered membrane fatty acid patterns may play a causal role in OSA and that the assessment of RBC DHA levels might help in the diagnosis of OSA. The effects of DHA supplementation on OSA should be explored.
Citation:
Ladesich JB; Pottala JV; Romaker A; Harris WS. Membrane level of omega-3 docosahexaenoic acid is associated with severity of obstructive sleep apnea. J Clin Sleep Med 2011;7(4):391-396.
Keywords: Omega-3 fatty acids, docosahexaenoic acid, epidemiology, sleep disordered breathing, biomarkers
Obstructive sleep apnea (OSA) affects 15 to 20 million Americans, and, as shown in the Sleep Heart Health study, is associated with increased risk for cardiovascular mortality and morbidity.1 OSA is associated with a heightened inflammatory state,2 with autonomic dysfunction,3 and with increased risk for sudden cardiac death.4 Interestingly, low blood and tissue levels of omega-3 FAs (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) are also associated with a heightened inflammatory state,5,6 with autonomic dysfunction,7–9 and with increased risk for sudden cardiac death.10,11 In addition, DHA in particular is a major structural component of the brain, and low DHA intake is associated with a variety of cognitive/behavioral endpoints such as the development of attention in children,12 slowed learning,13 visual acuity,14 and possibly IQ.15 Exposure to lower omega-3 levels in utero is associated with less mature sleep patterns in newborn babies.16 This nexus suggests a possible biochemical link between omega-3 FA deficiency and OSA. The purpose of this study was to determine the extent to which OSA was associated with reduced omega-3 FA levels measured in a surrogate for tissue omega-3 levels, the RBC membrane.
METHODS
Subjects
All patients referred for routine sleep studies at Saint Luke's Hospital in Kansas City during 2007 were invited to participate in this study. Other than being willing to participate and to allow leftover blood from blood gas assays to be used for omega-3 FA testing, there were no inclusion/exclusion criteria. Informed consent allowing our team to utilize leftover blood from the arterial blood gas sample was obtained from all patients. This study was approved by the Institutional Review Board of Saint Luke's Hospital, and all subjects provided written, informed consent prior to participating.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Low tissue levels of omega-3 fatty acids may be part of the nexus of sleep disordered breathing, inflammation, autonomic dysfunction and cardiovascular disease. This study was done to examine the extent to which RBC omega-3 fatty acids were associated with the severity of OSA.
Study Impact: The demonstration of an inverse relationship between RBC omega-3 DHA levels and the severity of OSA suggests that membrane fatty acid composition could play a role in the etiology of the disease, and lays the groundwork for a clinical trial to determine if fish oil supplementation could beneficially impact OSA.
Obstructive apnea is the 10-sec cessation of airflow through the naso-oropharynx during sleep with continued respiratory muscle activity, and hypopnea (shallow breathing) is a 50% reduction in airflow associated with a 3% to 4% drop in desaturation compared to normal. The apnea-hypopnea index (AHI) is a measure of severity that combines apneas and hypopneas and divides the total by the number of hours of sleep. We placed subjects into 3 categories based upon the AHI: (1) none/mild apnea (AHI 0-14), (2) moderate apnea (AHI 15-34), and (3) severe apnea ≥ 35.17
Questionnaire
All patients filled out a questionnaire to assist with polysomnographic interpretation. To the original questionnaire, which focused broadly on sleep patterns, the 2 following questions were added to estimate omega-3 FA intake: “How many time in a month do you have a serving (meal) of tuna or other non-fried fish?” and “Do you take fish oil (omega-3) supplements?”
Laboratory Methods
Blood gases are routinely collected at the beginning of each sleep study to correlate pulse oximetry data with direct measurements. Blood was collected in heparin in a standard blood gas syringe. After gas analysis, the remaining blood was sent to the research lab for RBC FA analysis. The RBC pellet was isolated by centrifugation, and an aliquot was frozen at −70°C. RBC FAs were determined as previously described.18 Briefly, an RBC aliquot was heated with boron trifluoride methanol for 10 min at 100°C. After cooling, the fatty acid methyl esters thus generated were extracted with hexane, and analyzed by flame ionization gas chromatography. FAs were identified by comparison with known standards, and their composition reported as weight percent of total FA. The sum of EPA+DHA in erythrocyte membranes is referred to as the “omega-3 index.”19 Personnel analyzing the FAs were blind to apnea status.
Statistical Methods
During 2007, we were able to collect 377 usable samples for this cross-sectional study. Due to limited numbers in some ethnic groups, 27 samples were excluded (i.e., Asians, Polynesians, Hispanics, Native Americans, and no race given) which resulted in Caucasians (n = 305) and African Americans (n = 45) for statistical analyses. Alcohol consumption was categorized as rarely, monthly, weekly, or daily. Fish intake was grouped as none, < 1/week, ≥ 1/week, or ≥ 2/week. Age and the natural log transformation of BMI were normally distributed. Smoking, gender, and fish oil supplement use were dichotomous predictor variables. Differences in patient characteristics and fatty acids across apnea severity levels were univariately tested using Kruskal-Wallis test and χ2 test for continuous and categorical data, respectively.
A polychotomous (i.e., 3 groups) logistic regression model was used to predict the sleep apnea severity category from the fatty acid measurements while controlling for patient characteristics. The initial model included all 8 factors mentioned above without any FAs. Then DHA and EPA (separately and combined) were added to the model. A 2-sided critical level of 0.05 was used to ascribe significance. LOWESS nonparametric regression, and 95% confidence bands were used to smooth the individual predicted probabilities as a function of DHA levels.20
Additionally a lognormal regression model was used to determine the amount of variance in DHA attributable to variability in the subject characteristics. Analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC).
RESULTS
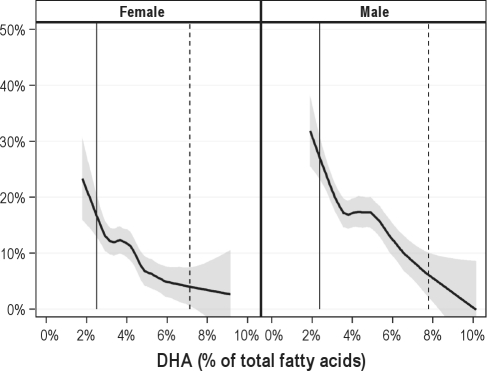
Of the patient characteristics assessed, only age, BMI, and male gender were associated with OSA severity (Table 1). Of the RBC FAs, only DHA was significantly associated with OSA severity, and because of its major contribution to the omega-3 index, the latter was also associated with severity (Table 2). The likelihood of having severe compared with none/mild OSA was reduced by 53% (odds ratios [95% CI] = 0.47 [0.28 to 0.80] for each 1-SD increase in DHA levels (Table 3). The likelihood of severe compared with moderate OSA was reduced by 45% (0.55 [0.31 to 0.99]). The adjusted model predicts that about 17% of women with a RBC DHA level of 2.5% would have severe OSA, but as the DHA level rises to about 7%, the prevalence decreases to around 4%. For men, the estimated prevalences were 27% for those with a low RBC DHA and only 6% for those with a high DHA (Figure 1).
Table 1.
Baseline characteristics of subjects by sleep apnea severitya
| Variable | None/Mild (n = 228) | Moderate (n = 70) | Severe (n = 52) | p-valueb |
|---|---|---|---|---|
| Age (y), mean (SD) | 50 (14) | 58 (13) | 54 (14) | 0.0003 |
| Body mass index (kg/m2), mean (SD) | 34 (8.5) | 34 (7.1) | 37 (9.1) | 0.03c |
| Male | 122 (54)d | 50 (71) | 36 (69) | 0.008 |
| Currently smoking | 30 (13) | 11 (16) | 6 (12) | 0.77 |
| Fish oil supplement | 67 (29) | 24 (34) | 14 (27) | 0.62 |
| Ethnicity | - | - | - | 0.47 |
| African American | 32 (14) | 9 (13) | 4 (7.7) | - |
| Caucasian | 196 (86) | 61 (87) | 48 (92) | - |
| Alcohol consumption | - | - | - | 0.55 |
| Rarely | 98 (43) | 29 (41) | 24 (46) | - |
| Monthly | 48 (21) | 12 (17) | 9 (17) | - |
| Weekly | 51 (22) | 12 (17) | 10 (19) | - |
| Daily | 26 (11) | 14 (20) | 5 (9.6) | - |
| Fish meals per month | - | - | - | 0.15 |
| None | 75 (33) | 24 (34) | 23 (44) | - |
| Less than 1/week | 86 (38) | 20 (29) | 18 (35) | - |
| At least 1/week | 56 (25) | 20 (29) | 7 (13) | - |
| At least 2/week | 11 (4.8) | 6 (8.6) | 3 (5.8) | - |
Sample sizes: age, male, ethnicity, and BMI = 350; Smoking = 340: Supplements = 345; Alcohol = 338; Fish meals = 349.
One-way nonparametric ANOVA and χ2 tests for continuous and categorical variables, respectively
Loge(BMI) was tested
n (%) for all variables except age and BMI. Bolded values are statistically significant.
Table 2.
Fatty acids (as % total FA) by sleep apnea severity level (N = 350)
| Fatty Acids | Structure | None/Mild (n =228) | Moderate (n = 70) | Severe (n = 52) | p-valuea |
|---|---|---|---|---|---|
| Total saturated | - | 39 (38,40)b | 39 (38,40) | 40 (39,40) | 0.06 |
| Myristic acid | C14:0 | 0.35 (0.28,0.46) | 0.35 (0.29,0.45) | 0.32 (0.28,0.41) | 0.28 |
| Palmitic acid | C16:0 | 21 (20,22) | 21 (20,22) | 21 (20,23) | 0.16 |
| Stearic acid | C18:0 | 18 (16,18) | 17 (16,18) | 17 (16,18) | 0.91 |
| Lignoceric acid | C24:0 | 0.38 (0.25,0.58) | 0.35 (0.24,0.58) | 0.33 (0.26,0.54) | 0.53 |
| Total monounsaturated | - | 15 (14,16) | 15 (14,16) | 15 (14,16) | 0.13 |
| Palmitoleic acid | C16:1 | 0.32 (0.22,0.44) | 0.31 (0.24,0.41) | 0.35 (0.23,0.54) | 0.30 |
| Oleic acid | C18:1 | 14 (13,15) | 14 (13,15) | 14 (13,15) | 0.10 |
| Eicosenoic acid | C20:1 | 0.16 (0.13,0.18) | 0.15 (0.13,0.18) | 0.16 (0.13,0.19) | 0.90 |
| Nervonic acid | C24:1 | 0.38 (0.24,0.56) | 0.33 (0.23,0.54) | 0.32 (0.26,0.52) | 0.64 |
| Total trans unsaturated | - | 1.9 (1.6,2.3) | 1.9 (1.6,2.5) | 1.9 (1.7,2.2) | 0.62 |
| trans Palmitoleic acid | C16:1t | 0.11 (0.09,0.13) | 0.10 (0.08,0.13) | 0.11 (0.09,0.13) | 0.59 |
| transOleic acid | C18:1t | 1.6 (1.3,1.9) | 1.6 (1.3,2.1) | 1.5 (1.3,1.7) | 0.53 |
| transLinoleic | C18:2t | 0.26 (0.21,0.32) | 0.26 (0.21,0.32) | 0.26 (0.21,0.31) | 0.95 |
| Total omega-3 polyunsaturated | - | 7.4 (6.6, 9.1) | 7.7 (6.7, 9.8) | 6.8 (6.0, 8.4) | 0.04 |
| α-Linolenic acid | C18:3 | 0.20 (0.17,0.26) | 0.21 (0.17,0.26) | 0.20 (0.16,0.27) | 0.90 |
| Eicosapentaenoic acid (EPA) | C20:5 | 0.53 (0.38,0.81) | 0.56 (0.43,0.87) | 0.51 (0.42,0.77) | 0.29 |
| Docosapentaenoic acid | C22:5 | 2.6 (2.3,3.0) | 2.8 (2.4,3.0) | 2.5 (2.2,2.9) | 0.34 |
| Docosahexaenoic acid (DHA) | C22:6 | 4.1 (3.4, 5.3) | 4.5 (3.3, 5.8) | 3.6 (2.9,4.7) | 0.03 |
| Omega-3 Index (EPA+DHA) | - | 4.7 (3.8,6.1) | 4.9 (3.8,6.7) | 4.0 (3.5, 5.3) | 0.04 |
| Total n-6 polyunsaturated | - | 37 (35,38) | 36 (34,38) | 36 (34,38) | 0.22 |
| Linoleic acid | C18:2 | 12 (11,13) | 12 (10,13) | 12 (11,13) | 0.11 |
| y-Linolenic acid | C18:3 | 0.11 (0.09,0.14) | 0.10 (0.08,0.14) | 0.11 (0.09,0.14) | 0.49 |
| Eicosadienoic acid | C20:2 | 0.24 (0.21,0.26) | 0.23 (0.21,0.25) | 0.23 (0.21,0.25) | 0.40 |
| Eicosatrienoic acid | C20:3 | 1.6 (1.3,1.9) | 1.5 (1.3,1.7) | 1.4 (1.3,1.8) | 0.37 |
| Arachidonic acid | C20:4 | 17 (16,19) | 17 (16,19) | 17 (17,18) | 0.93 |
| Docosatetraenoic acid | C22:4 | 4.0 (3.3,4.5) | 3.7 (3.1,4.4) | 4.3 (3.7,4.5) | 0.06 |
| Docosapentaenoic acid | C22:5 | 0.74 (0.57,0.87) | 0.70 (0.52,0.84) | 0.74 (0.65,0.85) | 0.23 |
One-way nonparametric ANOVA test
Median (Interquartile range). Bolded values are statistically significant.
Table 3.
Odds ratios from the polychotomous logistic regression model (N = 334a)
| Variable | Overall p-value | Moderate vs. None/mild (95% CI) | Severe vs. None/mild (95% CI) | Severe vs. Moderate (95% CI) |
|---|---|---|---|---|
| Age per 5 yrs | < 0.0001 | 1.34 (1.18 to 1.52) | 1.30 (1.12 to 1.51) | 0.97 (0.82 to 1.16) |
| Log(BMI) [kg/m2] per 1 SD | 0.0001 | 1.42 (0.99 to 2.02) | 2.40 (1.58 to 3.63) | 1.69 (1.04 to 2.74) |
| Male | 0.0004 | 1.62 (1.16 to 2.27) | 1.96 (1.30 to 2.94) | 1.21 (0.75 to 1.93) |
| Currently smoking | 0.36 | 1.35 (0.89 to 2.06) | 1.15 (0.69 to 1.91) | 0.85 (0.48 to 1.49) |
| Fish oil supplement | 0.46 | 0.84 (0.59 to 1.18) | 0.82 (0.55 to 1.23) | 0.98 (0.62 to 1.57) |
| African American | 0.45 | 0.98 (0.62 to 1.56) | 0.68 (0.38 to 1.24) | 0.70 (0.36 to 1.37) |
| Alcohol consumption | 0.94 | b | b | b |
| Fish meals per month | 0.41 | b | b | b |
| Each fatty acid below was individually added to the above model (effects are for a 1 SD increase) | ||||
| Docosahexaenoic acid (DHA) | 0.02 | 0.85 (0.59 to 1.22) | 0.47 (0.28 to 0.80) | 0.55 (0.31 to 0.99) |
| Eicosapentaenoic acid (EPA) | 0.42 | 1.15 (0.83 to 1.59) | 0.77 (0.42 to 1.40) | 0.67 (0.36 to 1.25) |
| Omega-3 Index (EPA+DHA) | 0.04 | 0.91 (0.63 to 1.32) | 0.48 (0.27 to 0.85) | 0.53 (0.29 to 0.97) |
Although fatty acid data were available for all 350 subjects, complete data for regression modeling were available for 334.
Odds ratios not listed since overall effect was not significant, and these 2 variables each had 4 levels. Bolded values are statistically significant.
Figure 1.
Predicted probabilities of having severe sleep apnea based on 8 patient characteristics and the level of DHA (as a % of total fatty acids in RBC)
Grouped by gender using LOWESS smoothing with 95% confidence band (shaded). The 5th and 95th percentiles are indicated with solid and dashed vertical lines, respectively.
The factors influencing DHA were explored (after natural log transformation of the latter to improve model assumptions). The variability in the 8 patient characteristics explained 38% of the variability in DHA, most of which (30%) was explained by fish intake and fish oil supplementation. The significant direct multiplicative factors were age (1.03 per 5 years, p < 0.0001), being African American (1.14, p = 0.009), fish intake (up to 1.10, p = 0.03), and taking fish oil supplements (1.39, p < 0.0001). The only significant inverse association with DHA was that with smoking (0.88, p = 0.006).
DISCUSSION
Based upon several similar links to cardiovascular disease risk, we hypothesized that an inverse relationship would exist between OSA severity and RBC DHA levels. Our findings support this hypothesis. For every 1-SD increase in RBC DHA, there was an approximately 50% reduction in the likelihood of having severe OSA.
A large body of epidemiological studies supports the view that omega-3 fatty acids reduce risk for cardiovascular disease, and specifically for sudden cardiac death.21,22 Siscovick et al. showed that patients with an omega-3 index of 3.3% were about 3 times as likely to have had a primary cardiac arrest as those with an omega-3 index of 5.0%.11 Risk for sudden cardiac death was about 10 times as high in Physicians' Health Study participants who had a baseline omega-3 index of 3.6% as those with an index of 6.9%.10 The reduced atherosclerotic burden seen in Japanese subjects appears to be due to their higher intake of omega-3 fatty acids.23 Three major (N > 6000 patients) randomized controlled trials with omega-3 fatty acids have shown significant reductions in either total mortality24,25 or major adverse cardiac events.26 Hence, higher tissue levels of omega-3 fatty acids are cardioprotective.
The link between low omega-3 FA levels and sudden cardiac death is particularly strong, and suggests effects on electrical signaling and membrane stability. One of the proposed mechanisms by which these FAs operate is by altering autonomic tone. Higher intakes of omega-3 FAs or higher blood levels are both associated with reduced heart rate and increased heart rate variability. In addition, low omega-3 FA blood levels or dietary intakes have been associated with neuropsychiatric conditions such as depression, bipolar disorder, mood, and even increased risk for dementia.27–29 Since OSA is also associated with increased risk for sudden cardiac death,10,11 depression,30 cognitive decline,31 and metabolic disorders,32 a link with omega-3 FA status may exist.
A relation between DHA status and sleep disturbances was also reported by Cheruku et al. who studied sleep patterns in newborn babies delivered from 17 women as a function of plasma phospholipid DHA levels.16 They found that babies born to mothers with DHA levels above the median (above 3% of total phospholipid FAs) had more mature sleep wake cycles than those born to mothers with lower DHA levels. The authors indicated that more mature sleep patterns suggested more mature central nervous system development. While sleep patterns early in life may have little direct relations with the pathology associated with OSA in adults, these findings, at the very least, suggest that DHA levels may influence sleep physiology.
A low DHA could be a cause, a consequence or coincidental to OSA severity. Because DHA is a major constituent in neural membranes and has been shown to alter neuronal signaling pathways,33 changes in DHA levels could have pathophysiological consequences. Recently, Laville et al. reported that hamsters raised on an omega-3 FA deficient diet had higher diurnal and nocturnal locomotor activities that were associated with decreased levels of pineal melatonin during the dark phase and with increased levels of dopamine and its metabolites in the striatum. They concluded that omega-3 FA deficiency diminished melatonin rhythms, weakened endogenous functioning of the circadian clock, and thereby produced nocturnal sleep disturbances. Indeed, fatty acid metabolites (prostaglandin D2, anandamide, and 2-arachidonyl glycerol) are known to play a role in regulating the wake-sleep cycle.34 Nevertheless, the extent to which RBC DHA levels directly reflect pineal and striatal DHA levels, and how variations in brain DHA could directly contribute to OSA are not clear. Alternatively, it is theoretically possible that lower levels of omega-3 fatty acids in neuronal tissues could destabilize the upper airway innervation, musculature, and feedback control systems.
DHA status and OSA severity may be only coincidentally related, not causally. Since we found no difference in supplement use or oily fish intake across the spectrum of OSA severity, the reduced levels of DHA were probably not the result of a lower omega-3 FA intake (although our relatively crude assessment tool, the generally very low intakes and the small sample size might have prevented us from detecting such relations). Smoking was unrelated to OSA severity in our study but was inversely associated with DHA levels as we reported in another cohort.35 Increasing age was a risk factor for severe OSA in this analysis, and RBC omega-3 levels have also been related to age.36,37 However, age and omega-3 levels are directly related, and thus the inverse association between DHA levels and odds for severe OSA observed here was not confounded by age. Obesity is a risk factor for OSA,38 and obesity has been associated with reduced RBC omega-3 status.39 The latter relations are postulated to be secondary to increased peroxidation of RBC membrane FAs. It is thus possible that increased membrane FA oxidative damage could be a common thread tying together low omega-3 FA status, OSA, obesity, and CVD. However, in our study, the relations between the severity of OSA and RBC DHA levels were independent of body mass index (interaction p = 0.22), and therefore it seems unlikely that these relations were mediated by a common connection with obesity.
Partinen et al. found that untreated OSA patients were 4.9 times as likely to die from cardiovascular disease as those who had undergone tracheostomy.40 Others have reported that risk for death is 3 times as high in those with OSA as controls.41 Andreas et al. reported that over half of patients with coronary disease had OSA.42 Increased odds ratios for coronary disease were also seen in patients with OSA in the Sleep Heart Health Study.43 The underlying mechanisms are unclear at this time, but OSA is associated with hypertension,44,45 a prothrombotic state,46–48 and chronic inflammation.2 There is also an association of OSA with susceptibilities to cardiac arrhythmias.49–52
In conclusion, our study is the first to examine the relations between sleep disordered breathing and omega-3 FA levels. We found an independent, inverse relation between RBC DHA levels (and the Omega-3 Index) and the severity of OSA. It is tempting to hypothesize that the increased risk of CHD death associated with OSA could be at least partially explained by the lower tissue levels of omega-3 FAs in this patient population. Future studies should examine the possibility that dietary supplementation with omega-3 fatty acids might not only reduce risk for CHD, but could conceivably even improve OSA symptomatology.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Harris is the owner of a company that offers blood omega-3 testing (OmegaQuant, LLC, Sioux Falls, SD). The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
James Ladesich, M.D., was the Primary Investigator, the study coordinator, collected the data, wrote the first draft of the manuscript. James V. Pottala, M.S. performed the statistical analysis and assisted in manuscript preparation. Ann Romaker, M.D. was instrumental in obtaining funding for the trial, establishing the protocol and editing the manuscript. William Harris, Ph.D. assisted in preparing the grant application, designing the study, analyzing the blood samples, and editing the manuscript. This study was supported by a grant from the Saint Luke's Hospital Foundation, Kansas City, MO.
REFERENCES
- 1.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 3.Nanas S, Sakellariou D, Kapsimalakou S, et al. Heart rate recovery and oxygen kinetics after exercise in obstructive sleep apnea syndrome. Clin Cardiol. 2010;33:46–51. doi: 10.1002/clc.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 5.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2009;205:538–43. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr. 2009;139:495–501. doi: 10.3945/jn.108.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen JH, Schmidt EB. Autonomic nervous system, heart rate variability and n-3 fatty acids. J Cardiovasc Med (Hagerstown) 2007;8(Suppl 1):S19–S22. doi: 10.2459/01.JCM.0000289276.10675.a1. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–52. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117:1130–7. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 10.Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 11.Siscovick DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–7. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 12.Colombo J, Kannass KN, Shaddy DJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–67. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 13.Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–85. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 14.Birch EE, Carlson SE, Hoffman DR, et al. The DIAMOND (DHA Intake and Measurement of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am J Clin Nutr. 2010;91:848–59. doi: 10.3945/ajcn.2009.28557. [DOI] [PubMed] [Google Scholar]
- 15.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 16.Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB, Lammi-Keefe CJ. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am J Clin Nutr. 2002;76:608–13. doi: 10.1093/ajcn/76.3.608. [DOI] [PubMed] [Google Scholar]
- 17.Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2010;14:287–97. doi: 10.1016/j.smrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. Am J Clin Nutr. 2007;86:1621–5. doi: 10.1093/ajcn/86.5.1621. [DOI] [PubMed] [Google Scholar]
- 19.Harris WS, von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 21.Harris WS, Mozaffarian D, Lefevre M, et al. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139:804S–819S. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, O'Keefe JH, Lavie CJ, Harris WS. Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat Rev Cardiol. 2009;6:753–8. doi: 10.1038/nrcardio.2009.188. [DOI] [PubMed] [Google Scholar]
- 23.Sekikawa A, Curb JD, Ueshima H, et al. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008;52:417–24. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 25.GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–30. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 27.Hibbeln JR, Ferguson TA, Blasbalg TL. Omega-3 fatty acid deficiencies in neurodevelopment, aggression and autonomic dysregulation: opportunities for intervention. Int Rev Psychiatry. 2006;18:107–18. doi: 10.1080/09540260600582967. [DOI] [PubMed] [Google Scholar]
- 28.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–12. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 29.Logan AC. Omega-3 fatty acids and major depression: a primer for the mental health professional. Lipids Health Dis. 2004;3:25–33. doi: 10.1186/1476-511X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146:105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 31.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 32.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 33.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–63. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Bazan NG. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;93:929–41. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- 35.Harris WS, Reid KJ, Sands SA, Spertus JA. Blood omega-3 and trans Fatty acids in middle-aged acute coronary syndrome patients. Am J Cardiol. 2007;99:154–8. doi: 10.1016/j.amjcard.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Block RC, Harris WS, Pottala JV. Determinants of blood cell omega-3 fatty acid content. Open Biomarkers J. 2008;1:1–6. doi: 10.2174/1875318300801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itomura M, Fujioka S, Hamazaki K, et al. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–5. [PubMed] [Google Scholar]
- 38.Shah N, Roux F. The relationship of obesity and obstructive sleep apnea. Clin Chest Med. 2009;30:455–65. doi: 10.1016/j.ccm.2009.05.012. vii. [DOI] [PubMed] [Google Scholar]
- 39.Cazzola R, Rondanelli M, Russo-Volpe S, Ferrari E, Cestaro B. Decreased membrane fluidity and altered susceptibility to peroxidation and lipid composition in overweight and obese female erythrocytes. J Lipid Res. 2004;45:1846–51. doi: 10.1194/jlr.M300509-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Partinen M. Epidemiology of obstructive sleep apnea syndrome. Curr Opin Pulm Med. 1995;1:482–7. doi: 10.1097/00063198-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep. 1995;18:149–57. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 42.Andreas S, Schulz R, Werner GS, Kreuzer H. Prevalence of obstructive sleep apnoea in patients with coronary artery disease. Coron Artery Dis. 1996;7:541–5. [PubMed] [Google Scholar]
- 43.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 44.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 45.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 46.Chin K, Ohi M, Kita H, et al. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–6. doi: 10.1164/ajrccm.153.6.8665063. [DOI] [PubMed] [Google Scholar]
- 47.Bokinsky G, Miller M, Ault K, Husband P, Mitchell J. Spontaneous platelet activation and aggregation during obstructive sleep apnea and its response to therapy with nasal continuous positive airway pressure. A preliminary investigation. Chest. 1995;108:625–30. doi: 10.1378/chest.108.3.625. [DOI] [PubMed] [Google Scholar]
- 48.Rangemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep. 1995;18:188–94. doi: 10.1093/sleep/18.3.188. [DOI] [PubMed] [Google Scholar]
- 49.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 50.Shepard JW, Jr., Garrison MW, Grither DA, Dolan GF. Relationship of ventricular ectopy to oxyhemoglobin desaturation in patients with obstructive sleep apnea. Chest. 1985;88:335–40. doi: 10.1378/chest.88.3.335. [DOI] [PubMed] [Google Scholar]
- 51.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 52.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]