Abstract
Intermittent vagus nerve stimulation can reduce the frequency of seizures in patients with refractory epilepsy. Stimulation of vagus nerve afferent fibers can also cause vocal cord dysfunction, laryngeal spasm, cough, dyspnea, nausea, and vomiting. Vagus nerve stimulation causes an increase in respiratory rate, decrease in respiratory amplitude, decrease in tidal volume, and decrease in oxygen saturation during periods of device activation. It usually does not cause an arousal, or a change in heart rate or blood pressure. Most patients have an increase in their apnea-hypopnea index (AHI). Patients with VNS can have central apneas, obstructive hypopneas, and obstructive apneas. These respiratory events can be reduced with changes in the vagus nerve stimulator operational parameters or with the use of CPAP. In summary, there are complex relationships between epilepsy and obstructive sleep apneas. In particular, patients with refractory epilepsy need assessment for undiagnosed and untreated obstructive sleep apnea before implantation of vagus nerve stimulator devices. Patients with vagus nerve stimulators often have an increase in apneic events after implantation, and these patients need screening for sleep apnea both before and after implantation.
Citation:
Parhizgar F; Nugent K; Raj R. Obstructive sleep apnea and respiratory complications associated with vagus nerve stimulators. J Clin Sleep Med 2011;7(4):401-407.
Keywords: Vagus nerve stimulator, sleep apnea, seizure disorder, dysphonia
Penry and Dean reported the use of vagus nerve stimulators (VNS) to control refractory epilepsy in 1990.1 Intermittent vagus nerve stimulation can reduce seizures by up to 50% in adult seizure patients and up to 90% in pediatric seizure patients. The FDA approved these devices for the treatment of refractory epilepsy in 1997 and for the treatment of refractory depression in 2005. VNS are currently under investigation for treatment of anxiety, obesity, bulimia, migraines, Alzheimer disease, chronic pain syndromes, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder.2
Vagus nerve stimulation can cause side effects involving the upper airway, lower respiratory tract, and upper gastrointestinal tract. These devices can decrease airflow, oxygen saturation, and respiratory amplitude during sleep.3 Most patients with VNS have an increase in their apnea-hypopnea index (AHI) after implantation.4 One-third of these patients develop mild obstructive sleep apnea post treatment,4,5 and a minority of patients develop severe obstructive sleep apnea secondary to VNS therapy.6 More than 50,000 patients have had VNS implants to date, and it is important for sleep medicine specialists to recognize the sleep disordered breathing and other respiratory complications related to VNS devices. We recently helped care for a patient who developed obstructive sleep apnea (OSA) after implantation of a VNS and then required discontinuation of the VNS. Our review of literature while attempting to answer some clinically relevant questions pertaining to this patient led to this review. This article focuses on six questions pertinent to the management of these patients and reviews the literature on sleep-related and respiratory complications associated with vagus nerve stimulators.
METHODS
We conducted a literature search with the PubMed database using combinations of text words: “vagal nerve stimulator” or “vagal nerve stimulation” and “respiratory complications,” “adverse effects,” or “sleep apnea.” We reviewed titles and abstracts to determine if the studies reported respiratory complications during vagal nerve stimulation. These complications included dyspnea, sleep apnea, pharyngitis, coughing, and voice alteration. We also reviewed abstracts for information on oxygen saturation, AHI, respiratory rate, and tidal volume in patients. From this group of selected titles and abstracts we reviewed the full-length articles. We limited our review to articles that documented specific data about their patients, such as total number of patients in the study, average age, and age range of patients. If the articles did not have this information, we excluded them from additional analysis. We also reviewed the reference lists from these articles and used the “related articles” algorithm in PubMed to identify additional articles.
This search narrowed the number of articles to 15 studies.3–17 In these studies, the number of patients included ranged from 1 to 60. Of these 15 articles, 4 involved only pediatric patients,8,12,13,17 7 involved only adult patients,4,5,10,11,14–16 and 4 studies involved both adult and pediatric patients.3,6,7,9 The patient ages ranged from 3 to70 years. If a study did not have the average patient age specifically stated in the article, we calculated the average patient age from the data published on individual patients in the study. We determined that there were 6 studies reporting VNS patients with obstructive sleep apnea4–7,15,17 and one study reporting a patient with central sleep apnea.14 AHI was reported in 8 studies4–7,10,14–16 and oxygen saturation was reported in 6.4–6,10,15,16
RESULTS
Question 1: How do vagus nerve stimulators work, and what are the usual settings?
The vagus nerve is primarily a parasympathetic nerve composed of 20% afferent and 80% efferent fibers. The nerve leaves the medulla between the olivary nucleus and the inferior cerebellar peduncle and passes through the jugular foramen into the carotid sheath inserting itself between the internal carotid artery and the internal jugular vein. It then enters the neck, chest, and abdomen, innervating the viscera by contributing to the cardiac, esophageal, and pulmonary plexus. The afferent fibers originating in the jugular and nodosa ganglia enter the nucleus tractus solitarius and send projections to the areas of the cortex that are associated with seizure activity.
VNS are usually implanted by neurosurgeons or otolaryngologists in the operating room.3 The generator is placed subcutaneously in the left thoracic/anterior axillary region and connected via tunneled leads to the vagal electrodes.3 Two platinum helical bipolar electrodes and the anchor tether are wrapped around the left vagus nerve distal to the superior laryngeal nerve and superior and inferior cervical cardiac branches. The VNS delivers stimulation at set intervals, with adjustable factors, such as stimulation on-time, stimulation off-time, output current, frequency, and pulse width.18 The generator typically provides a 0.5-millisecond pulse repeated at 10-30 Hz for 30 sec, every 150 to 300 seconds.2 The current output is incrementally increased by 0.25 mA to 0.5 mA every 2 to 4 weeks according to the patient's response, until the optimum current output for stimulation is attained.18 A programming wand that communicates with the generator using radio frequency signals can be used to vary the output current, frequency, pulse width, and stimulation off and on time.2 Additionally, a 50 gauss bar magnet provided by the manufacturer can be used either to deliver a burst of vagal stimulation or to inhibit output, depending on whether the magnet is placed transiently or for a prolonged duration over the generator.2 The hand-held magnet can be used by patients to initiate the stimulator when an aura is felt or at seizure onset.18
Positron emission tomography studies have shown that VNS increases bilateral thalamic blood flow, which may lead to decreased seizure frequency and duration.19 Numerous animal studies with VNS support their utility in seizure control. Studies in cats have demonstrated that high-intensity vagus nerve stimulation desynchronizes cortical EEGs but low-intensity stimulation at the same frequency synchronizes the EEG.20 These studies show that VNS can desynchronize the interconnected cortical regions responsible for seizure activity. Additional studies in rats have demonstrated that VNS increases amino acid turnover and neuronal activity in certain areas of the brain, such as the locus ceruleus in the basolateral amygdala responsible for norepinephrine production. Experimental studies in rats show that decreased norepinephrine in the locus ceruleus decreases VNS effect on seizures.21 Recent studies have also shown VNS increase c-fos expression, which acutely activates the locus ceruleus and chronically activates the NTS, paraventricular nucleus of the hypothalamus, parabrachial nucleus, ventral bed nucleus of the stria terminalis, cingulated nucleus, and dorsal raphe nucleus.22 VNS may also increase GABA release, which stimulates the vagus nerve and decreases seizure activity. These effects likely form the basic mechanism(s) by which VNS reduce seizure activity.
Vagus nerve stimulators reduce the frequency of seizures > 50% in 35% to 45% of epileptic patients.18 Some trials have shown a mean or median reduction of 24% to 31% in seizure frequency in patients in the high-intensity VNS treatment groups. Patients in the low-intensity VNS treatment group had a 6% to 15% reduction in seizure frequency. These trials also show that seizure abortion occurs in 21% in the high VNS treatment group and in 9% in the low VNS group who used their hand-held magnet at seizure onset. VNS in pediatric patients reduce seizures by 50% to 90% at one year, and 74% at 36 months. Patients implanted younger than 12 years, patients with tuberous sclerosis, and Lennox-Gastaut patients may have better outcomes with VNS treatment.18
Question 2: Does intermittent vagus nerve stimulation cause adverse effects other than on sleep and respiration?
VNS side effects include voice alteration, hoarseness, sore throat, coughing, dyspnea, dyspepsia, nausea, vomiting, headache, and paresthesia. Voice alteration is the most common side effect and occurs in 2% to 88% of patients.18 Other respiratory side effects include coughing (6% to 45%), dyspnea (4% to 25%), and pharyngitis (25% to 35%) (Tables 1 and 2). Left vocal cord adduction caused by stimulation of the left recurrent laryngeal nerve is likely responsible for the laryngeal and respiratory symptoms that develop in these patients.18 More than half of the patients with VNS implants have intermittent or permanent vocal cord paresis.23,18 The left vagus has proportionally fewer cardiac efferent fibers, and placing the stimulator on this side potentially limits the arrhythmogenic effects of vagal stimulation. However, reversible bradyarrhythmias associated with VNS have been described,2 and patients with a history of bradyarrhythmias and asystole are at risk for more cardiac side effects.
Table 1.
Side effects associated with vagus nerve stimulators18
| Side Effects | Prevalence (%) |
|---|---|
| Dysphonia | 2-88 |
| Neck Pain | 6-100 |
| Other Pain | 7-30 |
| Coughing | 4-45 |
| Dyspnea | 2-25 |
| Pharyngitis | 25-35 |
| Dyspepsia | 4-18 |
| Dysphagia | 2-13 |
| Nausea | 4-20 |
| Vomiting | 2-18 |
| Headache | 2-24 |
| Paresthesia | 3-25 |
Table 2.
Studies reporting respiratory complications in patients with VNS
| Author | Study Size (#) | Average Age (years) | Age Range (years) | Dyspnea (%) | Pharyngitis (%) | Cough (%) | Voice Alteration (%) | Average O2 Sats (%) |
|---|---|---|---|---|---|---|---|---|
| Ghanem3 | 17 | 28.3 ± 16.6 | 9-64 | NA | NA | 24 | 18 | NA |
| Marzec4 | 16 | 34 ± 11.2 | 20-58 | NA | NA | NA | NA | 90 |
| Malow5 | 4 | 36 ± 15.5 | 20-57 | NA | NA | NA | NA | 86.8 |
| Hseih6 | 9 | 13.9 ± 4.5 | 6-20 | NA | NA | NA | NA | 96.7 |
| Murphy8 | 60 | 15 | 3.5-18 | NA | 0-13.3 | 13.7-25 | 13.3-21.7 | NA |
| Nakken9 | 47 | 34.4 | 12-70 | 4 | 23 | 19 | NA | NA |
| Holmes10 | 1 | 22 | NA | NA | NA | NA | NA | 96.6 |
| Banzett11 | 6 | NA | 26-51 | 67 | 83 | NA | NA | NA |
| Zaaimi12 | 10 | 11.6 | 7-18 | NA | NA | NA | NA | NA |
| Nagarajan13 | 8 | 11.4 ± 4.2 | 4-16 | NA | NA | NA | NA | NA |
| Ebben15 | 1 | 54 | NA | NA | NA | NA | NA | 67 |
| Gschliesser16 | 2 | 35.5 | 35-36 | NA | NA | NA | NA | 94.5 |
| Khurana17 | 26 | NA | 3-17 | NA | NA | 3.8 | NA | NA |
Question 3: Does intermittent vagus nerve stimulation change respiratory patterns?
Three case series11–13 with 6 to 10 subjects show that vagus nerve stimulation has the potential to change respiratory patterns during both wakefulness and sleep. Zaaimi et al. studied 10 children (average age 11.6 years) with VNS using polysomnography with EEG, ECG, thoracoabdominal movement, and nasal airflow measurements.12 VNS increased the respiratory rate, decreased respiratory amplitude, and slightly decreased oxygen saturation during sleep in these patients. VNS disturbed the coupling between the heart rate and respiratory rate in 6 children. These changes occurred only during periods of VNS activity and not during periods with device inactivation.12 Nagarajan et al. studied 8 pediatric patients with seizures (average age of 11.4 years) and recorded decreased tidal volumes and respiratory effort and increased respiratory rate during vagal nerve stimulation during sleep.13 Three of these patients also experienced a decrease in their oxygen saturation.13 Most of the children in these 2 studies had significant intellectual disability and used multiple seizure medications. Banzett et al. investigated the respiratory effects of VNS in 6 adult epileptic patients during the day.11 They found no systematic change in tidal volume, respiratory rate, heart rate, or blood pressure, but did find that VNS activation increased the end-expiratory volume by as much as 10% to 30% of the tidal volume in 4 patients who received higher VNS stimulation. This may be due to the A-fiber afferent stimulation.11
Question 4: Does intermittent vagus nerve stimulation increase the number of apneic or hypopneic events at night?
Eight case reports and small case series totaling 36 patients (Table 3)4–7,10,14–17 show that VNS increases the AHI, which usually improves after discontinuation of the VNS. Marzec et al. studied 16 adult patients with VNS to determine if there was a change in AHI and oxygen saturation during sleep with VNS activation.4 The average baseline AHI in these patients was 1.9; this increased to 4.2 after activation, and 5 patients had an AHI > 5. Esophageal pressure monitoring in one patient in this series demonstrated that these events were primarily obstructive in nature. These patients also had decreased airflow and decreased but persistent respiratory effort during periods of VNS activation, possibly secondary to decreased airway dimensions. The average oxygen saturation for these patients during inactivated VNS periods was 92.2%; this decreased to 90% during activated periods. CPAP eliminated the respiratory events in one patient.4
Table 3.
Studies reporting sleep disordered breathing in patients with VNS
| Author | Study Size (#) | Average Age (years) | Age Range (years) | AHI (average) | OSA (%) | Central Sleep Apnea (%) |
|---|---|---|---|---|---|---|
| Marzec4 | 16 | 34 ± 11.2 | 20-58 | 4.18 ± 3.7 | 12.5 | NA |
| Malow5 | 4 | 36 ± 15.5 | 20-57 | 21.2 ± 6.2 | 25 | NA |
| Hseih6 | 9 | 13.9 ± 4.5 | 6-20 | 14.4 ± 19.4 | 100 | NA |
| Malow7 | 2 | 15 ± 1.4 | 14-16 | 6.2 ± 2.8 | 100 | NA |
| Holmes10 | 1 | 22 | NA | 12.4 | NA | NA |
| Papacostas14 | 1 | 23 | NA | 9.7 | NA | 100 |
| Ebben15 | 1 | 54 | NA | 82.3 | 100 | NA |
| Gschliesser16 | 2 | 35.5 | 35-36 | 4.9 ± 4.3 | NA | NA |
| Khurana17 | 26 | NA | 3-17 | NA | 30.8 | NA |
Hsieh et al. studied 9 epileptic pediatric patients (13.9 ± 4.6 years old) with VNS using polysomnography during sleep.6 Sleep efficiency was reduced, and sleep latency and REM latency were increased. The average AHI was 14.4 ± 19.4, and the arousal index was 31.8 ± 39.4. The mean oxygen saturation was 96.7% ± 1.1%, and they had 10 ± 9 desaturation episodes per hour.6 Eight patients had snoring or loud breathing during sleep. Six patients had an AHI ≥ 5; these patients had 8.3 ± 11.3 central apneas/h, 73.3 ± 61.5 obstructive hypopneas/h, and 18.8 ± 26.9 obstructive apneas/h. One patient had a high event rate when supine (180/h), and one had a high event rate when in REM sleep (15/h). The mean BMI in these patients was 30.0 ± 13.2, but there was no correlation between the respiratory event rate and BMI. This study reports the highest AHI observed in these patients and demonstrates that children can have clinically significant OSA associated with VNS therapy.6 One of these patients was found to have an AHI of 37 after VNS placement.6 Repeat polysomnography on this patient with the VNS turned off showed that her AHI had decreased from 37 to 10.6
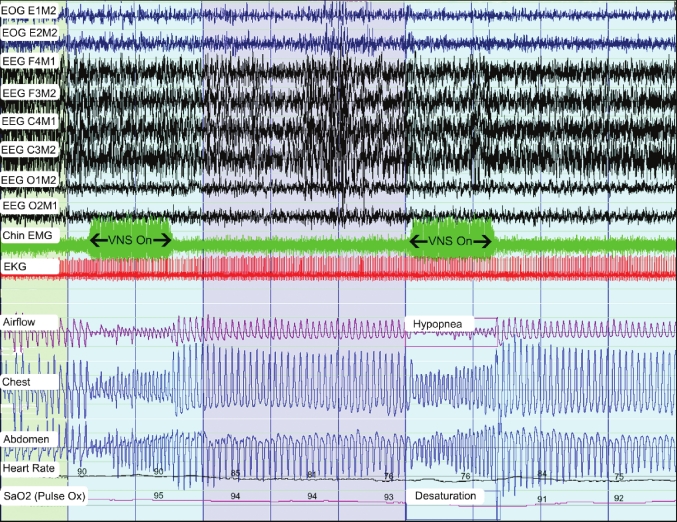
VNS implantation consistently decreases airflow in most patients4,5 and could cause apneas in some patients (Figure 1). This change in airflow and the observation of stimulation related vocal cord paresis or supraglottic spasm in some patients suggest that direct laryngeal muscle stimulation related to VNS contributes to sleep disordered breathing events in these patients.3 Bernards reported a case of intermittent laryngeal obstruction during general anesthesia with a laryngeal mask coinciding with VNS activation.24 This patient had no daytime symptoms; this suggests that patients who compensate for the laryngeal dysfunction during the day may be unable to when under anesthesia.24 Similar events could occur in patients with VNS who are asymptomatic during the daytime but develop obstructive apnea or hypopnea during the night because sleep related laryngeal muscle relaxation prevents compensation for laryngeal dysfunction resulting from VNS.
Figure 1.
A 300-second representative epoch from a patient with a vagus nerve stimulator
Chin EMG (green) shows artifacts corresponding to the VNS activation (labeled “VNS On”). VNS activation corresponds to an increase in respiratory rate, decrease in airflow, decrease in chest and abdominal excursion, and a variable decrease in oxygen saturation.
Several other possibilities could explain the development of sleep disorders secondary to vagal nerve stimulation. Since the vagus nerve is connected to the brainstem respiratory control center and relays information from baroreceptors, chemoreceptors, and stretch receptors, it may influence breathing patterns during sleep.5 Neurons in the medial pontine reticular formation form connections via efferents and afferents to the pontomedullary nuclei which regulate breathing and upper airway musculature, and cholinergic agonists in the medial pontine reticular formation can affect the breathing frequency, minute ventilation, and tidal volume. In addition, the vagus nerve innervates the upper airway musculature by efferents originating in the dorsal motor nucleus and nucleus ambiguous, and its activation could close the pharynx resulting in obstructive sleep apnea.5
Vagus nerve stimulation can also cause central apneas.14 Papacostas et al. studied an adult patient who developed central sleep apnea (AHI 9.7) after implantation of a VNS.14 Her apnea events decreased in severity and frequency when her vagal nerve stimulator parameters were decreased.14 This case report along with the studies by Marzec et al. demonstrating that the crescendos in esophageal pressures in their patients with VNS-induced OSA were not consistently time-locked to the start of VNS activation,4 and Hsieh's report6 on 6 patients who had both central and obstructive apneas suggest that the mechanism of sleep disordered breathing induced by VNS is complex and needs more study.
These studies report information on small heterogeneous patient populations. There are significant differences in age and respiratory event rates. In addition, the respiratory events included both obstructive and central apneas. The explanation for these complications likely depends on the patient's body habitus, cranial morphology, preexisting OSA, seizure medications, and differential effects of vagal stimulation on efferent output to upper airway muscles and central respiratory control centers. Therefore, while case reports and small series show that VNS increases AHI in most patients and that it can be clinically significant in some patients, we do not know how often this happens (prevalence) in the overall patient population, what proportion of patients develop clinically significant OSA secondary to VNS, and if there are any risk factors specific to this patient populations apart from risk factors for OSA in general, which put them at a risk for developing OSA after VNS placement. Larger prospective studies in the future can answer these questions.
Question 5: Does sleep apnea impact seizure control in patients with VNS?
There are limited published data that address this question in patients with VNS. Studies in patients with seizures (but no VNS) and OSA suggest that apnea treatment may reduce seizure frequency, but these studies are underpowered to demonstrate the exact effect of OSA therapy in patients with sleep apnea syndrome and a seizure disorder. These studies do confirm that these patients frequently have OSA. Sleep and sleep disorders have complex association and interactions with epilepsy.25–27 Over 80% of children with epilepsy have snoring, apneas/hypopneas, and periodic limb movements,28 and 30% of patient with drug resistant seizures have sleep apnea.29 Malow et al. studied the effect of treating OSA in 2 epileptic patients with VNS to determine if this decreased seizure activity in these patients.7 These 2 pediatric patients (ages 14 and 16 years) had a baseline AHIs of 3.8 and 8.6, respectively. The respiratory events during VNS activity resolved with CPAP. However, this therapy had no effect on the seizure activity. In addition, one of the patients could not tolerate the CPAP, and the other used it sporadically.7 Therefore, CPAP can treat respiratory complications associated with VNS but did not reduce seizure activity in this small study. Malow et al. also randomized 35 subjects with seizures and OSA to sham vs. therapeutic CPAP for 10 weeks.30 Reduction in seizures ≥ 50% was observed in 28% of subjects in the therapeutic group compared to 15% of those in the sham group, but this result was not statistically significant (p = 0.40).30 Vaughn et al. reported a resolution of seizures or a significant improvement in seizure control in 4 of 10 patients with obstructive sleep apnea and seizures, after treatment with CPAP or positional therapy.31 Response in the other 6 patients in that series was variable.31 Khurana et al. reported that tonsillectomy and adenoidectomy reduced seizure frequency by ≥ 50% in children with mild to moderate OSA after placement of a VNS.17 Presumably reducing the number of apneas and hypopneas improved seizure control in this small group of patients.17 In addition, a major treatment issue in these patients is CPAP compliance, especially in the pediatric population. More prospective studies involving a larger number of patients are needed to determine whether VNS-induced apnea influences seizure control and/or quality of life in patients with epilepsy.
Question 6: How should patients with vagus nerve stimulators and obstructive sleep apnea be managed?
Treatment options for these patients include changing the VNS parameters, positive airway pressure therapy, or even discontinuing the VNS device; the choice of therapy needs to be individualized. VNS operational parameters can influence the AHI in patients with a VNS. A cycling time of 300 sec4 is a common but not universal setting for most vagus nerve stimulators. Since most obstructive events are related to vagal stimulation in these patients and more severe sleep disordered breathing is associated with shorter cycling times,4,16 increasing the cycling time to 300 sec in patients with shorter cycling times will likely improve the sleep disordered breathing in these patients. Stimulation frequency also affects the severity of airflow obstruction and can be adjusted in patients with obstructive sleep apnea/hypopnea secondary to VNS. Airflow obstruction and obstructive events related to vagal nerve stimulation are highest at a device setting of 30 Hz, are less at 20 Hz, and are virtually nonexistent at 10 Hz.5 Both lowering the frequency and increasing the cycle time have been recommended by the manufacturer to prevent worsening of OSA during VNS therapy. Although it is not recommended by the manufacturer, in addition to reprogramming the VNS, temporarily deactivating the VNS using the magnet could be considered during a portion of the diagnostic study to estimate the severity of any underlying sleep disordered breathing that a patient has independent of the VNS. Additionally, in patients with severe sleep disordered breathing related to VNS in whom other therapeutic options are either ineffective or not tolerated, taping the magnet over the stimulator during sleep may be an alternative to deactivating the stimulator completely. The authors are unaware of any published literature on the effectiveness of the VNS when it is on only for a portion of the day, but it seems reasonable to speculate that vagal nerve stimulation for approximately 16 h in a 24-h period is more effective than no stimulation at all. More research is needed to address the question of adequacy of seizure control in these patients in whom the VNS device is switched off during the night only to prevent OSA. VNS-related sleep disordered breathing events also depend on patient related factors. The AHI is often higher in the supine position, and positional therapy could be used as an adjunct in these patients.4 Marzec et al. and Hsieh et al. reported that CPAP was effective in treating sleep disordered breathing events in 2 patients with VNS,4,6 but Ebben et al. noted that CPAP titration with the device turned on was difficult and that CPAP did not eliminate all sleep disordered breathing events.15 The AHI of 82.3 for that patient on a diagnostic polysomnography dropped to 0.4 with CPAP titration with VNS off, as compared to a final AHI of 13 with titration being performed with the VNS on.15 The patient reported by Hsieh et al. had an AHI of 37 after VNS placement; this AHI dropped to 10 by just switching off the VNS.6 CPAP therapy was effective in this patient, and the AHI after CPAP titration even with the VNS turned on was 0.8.6 Abnormal sleep architecture, which likely contributes to daytime symptoms in patients with sleep disordered breathing related to VNS devices, can improve or normalize after switching off the VNS.6
Management decisions in patients with refractory seizures who are candidates for VNS implantation are complex. The Malow study demonstrated that patients with refractory seizures (defined by ≥ 2 per month) had a high likelihood of having obstructive sleep apnea (33%, 13 of 39 patients), and were likely to score high on the sleep apnea scale of the Sleep Disorders Questionnaire.29 These and other authors acknowledged poor compliance and tolerance of CPAP in these patients,4,29 a problem that is not unique to this group but could pose challenges in pediatric patients with developmental problems. Therefore, VNS candidates should be screened for obstructive sleep apnea. Patients with poor sleep hygiene should receive counseling; patients with a positive screening test should undergo a more formal evaluation for obstructive sleep apnea. Patients with sleep apnea should be treated with CPAP and other approaches used in OSA, and seizure frequency may decrease. Patients with no OSA or treated OSA and refractory seizures then become candidates for VNS therapy but need close clinical follow-up post implantation to determine whether they have clinically important side effects. Respiratory symptoms can be potentially be treated by changing device parameters, adjusting the frequency and cycle time, or adding CPAP for obstructive apneas. Severe side effects might require discontinuation of VNS therapy. However, since VNS devices are often implanted in patients with refractory epilepsy as the last treatment option, most patients would find this a difficult decision. Ultimately, patients must decide if the VNS seizure control is worth the possible accompanying side effects.
CONCLUSION
VNS cause an increase in respiratory rate, decrease in respiratory amplitude, decrease in tidal volume, and decrease in oxygen saturation during periods of activation. They usually do not cause an arousal or a change in heart rate or blood pressure. VNS can cause sleep apnea or exacerbate sleep apnea in patients with a preexisting diagnosis. These devices are used in developmentally abnormal children and in patients receiving CNS active drugs that could influence respiratory drive and upper airway patency during sleep. Therefore, these patients may be at risk for apnea without the usual risk factors for sleep apnea. If VNS therapy is approved for other indications in adults, VNS may be frequently used in patients with the usual risk factors for apnea. Routine screening for sleep disturbances is indicated in these patients before and after implantation, and we need more formal studies post implantation for side effects. Management decisions in patients with VNS-related side effects are potentially complex and require input from the patient, the family, the neurologist, and a sleep specialist.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31(Suppl 2):S40–3. doi: 10.1111/j.1528-1157.1990.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 2.Hatton KW, McLarney JT, Pittman T, Fahy BG. Vagal nerve stimulation: overview and implications for anesthesiologists. Anesth Analg. 2006;103:1241–9. doi: 10.1213/01.ane.0000244532.71743.c6. [DOI] [PubMed] [Google Scholar]
- 3.Ghanem T, Early SV. Vagal nerve stimulator implantation: an otolaryngologist's perspective. Otolaryngol Head Neck Surg. 2006;135:46–51. doi: 10.1016/j.otohns.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Marzec M, Edwards J, Sagher O, Fromes G, Malow BA. Effects of vagus nerve stimulation on sleep-related breathing in epilepsy patients. Epilepsia. 2003;44:930–5. doi: 10.1046/j.1528-1157.2003.56202.x. [DOI] [PubMed] [Google Scholar]
- 5.Malow B.A., Edwards J., Marzec M., Sagher O., Fromes G. Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology. 2000;55:1450–4. doi: 10.1212/wnl.55.10.1450. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh T, Chen M, McAfee A, Kifle Y. Sleep-related breathing disorder in children with vagal nerve stimulators. Pediatr Neurol. 2008;38:99–103. doi: 10.1016/j.pediatrneurol.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Malow BA, Weatherwax KJ, Chervin RD, et al. Identification and treatment of obstructive sleep apnea in adults and children with epilepsy: a prospective pilot study. Sleep Med. 2003;4:509–15. doi: 10.1016/j.sleep.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Murphy JV. Left vagal nerve stimulation in children with medically refractory epilepsy. The Pediatric VNS Study Group. J Pediatr. 1999;134:563–6. doi: 10.1016/s0022-3476(99)70241-6. [DOI] [PubMed] [Google Scholar]
- 9.Nakken KO, Henriksen O, Roste GK, Lossius R. Vagal nerve stimulation--the Norwegian experience. Seizure. 2003;12:37–41. doi: 10.1016/s1059131102001383. [DOI] [PubMed] [Google Scholar]
- 10.Holmes MD, Chang M, Kapur V. Sleep apnea and excessive daytime somnolence induced by vagal nerve stimulation. Neurology. 2003;61:1126–9. doi: 10.1212/01.wnl.0000086812.62554.06. [DOI] [PubMed] [Google Scholar]
- 11.Banzett RB, Guz A, Paydarfar D, Shea SA, Schachter SC, Lansing RW. Cardiorespiratory variables and sensation during stimulation of the left vagus in patients with epilepsy. Epilepsy Res. 1999;35:1–11. doi: 10.1016/s0920-1211(98)00126-0. [DOI] [PubMed] [Google Scholar]
- 12.Zaaimi B, Grebe R, Berquin P, Wallois F. Vagus nerve stimulation induces changes in respiratory sinus arrhythmia of epileptic children during sleep. Epilepsia. 2009;50:2473–80. doi: 10.1111/j.1528-1167.2009.02190.x. [DOI] [PubMed] [Google Scholar]
- 13.Nagarajan L, Walsh P, Gregory P, Stick S, Maul J, Ghosh S. Respiratory pattern changes in sleep in children on vagal nerve stimulation for refractory epilepsy. Can J Neurol Sci. 2003;30:224–7. doi: 10.1017/s0317167100002638. [DOI] [PubMed] [Google Scholar]
- 14.Papacostas SS, Myrianthopoulou P, Dietis A, Papathanasiou ES. Induction of central-type sleep apnea by vagus nerve stimulation. Electromyogr Clin Neurophysiol. 2007;47:61–3. [PubMed] [Google Scholar]
- 15.Ebben MR, Sethi NK, Conte M, Pollak CP, Labar D. Vagus nerve stimulation, sleep apnea, and CPAP titration. J Clin Sleep Med. 2008;4:471–3. [PMC free article] [PubMed] [Google Scholar]
- 16.Gschliesser V, Hogl B, Frauscher B, Brandauer E, Poewe W, Luef G. Mode of vagus nerve stimulation differentially affects sleep related breathing in patients with epilepsy. Seizure. 2009;18:339–342. doi: 10.1016/j.seizure.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Khurana DS, Reumann M, Hobdell EF, et al. Vagus nerve stimulation in children with refractory epilepsy: unusual complications and relationship to sleep-disordered breathing. Childs Nerv Syst. 2007;23:1309–12. doi: 10.1007/s00381-007-0404-8. [DOI] [PubMed] [Google Scholar]
- 18.Milby AH, Halpern CH, Baltuch GH. Vagus nerve stimulation in the treatment of refractory epilepsy. Neurotherapeutics. 2009;6:228–37. doi: 10.1016/j.nurt.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry TR, Votaw JR, Pennell PB, et al. Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology. 1999;52:1166–73. doi: 10.1212/wnl.52.6.1166. [DOI] [PubMed] [Google Scholar]
- 20.Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39:677–86. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 21.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–14. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by Vagal nerve stimulation. Neuropsychopharmacology. 2008;33:1884–95. doi: 10.1038/sj.npp.1301570. [DOI] [PubMed] [Google Scholar]
- 23.Zalvan C, Sulica L, Wolf S, Cohen J, Gonzalez-Yanes O, Blitzer A. Laryngopharyngeal dysfunction from the implant vagal nerve stimulator. Laryngoscope. 2003;113:221–5. doi: 10.1097/00005537-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Bernards CM. An unusual cause of airway obstruction during general anesthesia with a laryngeal mask airway. Anesthesiology. 2004;100:1017–8. doi: 10.1097/00000542-200404000-00037. [DOI] [PubMed] [Google Scholar]
- 25.Malow BA. The interaction between sleep and epilepsy. Epilepsia. 2007;48(Suppl 9):36–8. doi: 10.1111/j.1528-1167.2007.01400.x. [DOI] [PubMed] [Google Scholar]
- 26.Mendez M, Radtke RA. Interactions between sleep and epilepsy. J Clin Neurophysiol. 2001;18:106–27. doi: 10.1097/00004691-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Manni R, Terzaghi M. Comorbidity between epilepsy and sleep disorders. Epilepsy Res. 2010;90:171–7. doi: 10.1016/j.eplepsyres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Kaleyias J, Cruz M, Goraya JS, et al. Spectrum of polysomnographic abnormalities in children with epilepsy. Pediatr Neurol. 2008;39:170–6. doi: 10.1016/j.pediatrneurol.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Malow BA, Levy K, Maturen K, Bowes R. Obstructive sleep apnea is common in medically refractory epilepsy patients. Neurology. 2000;55:1002–7. doi: 10.1212/wnl.55.7.1002. [DOI] [PubMed] [Google Scholar]
- 30.Malow BA, Foldvary-Schaefer N, Vaughn BV, et al. Treating obstructive sleep apnea in adults with epilepsy: a randomized pilot trial. Neurology. 2008;71:572–7. doi: 10.1212/01.wnl.0000323927.13250.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughn BV, D'Cruz OF, Beach R, Messenheimer JA. Improvement of epileptic seizure control with treatment of obstructive sleep apnoea. Seizure. 1996;5:73–8. doi: 10.1016/s1059-1311(96)80066-5. [DOI] [PubMed] [Google Scholar]