In the paper noted above, which was published in 2009, we reported safety information for patients on sodium oxybate (Xyrem®), including the numbers of patients with a prescription for sodium oxybate who had died from all causes (i.e., whether or not the deaths were considered related to treatment), from 2002 through March 30, 2008. The purpose of this letter is to present additional important safety data, to correct the previously reported numbers of deaths, and to provide updated information and analysis of these cases through May 31, 2011.
These data come from two main sources. The first is from ordinary pharmacovigilance reports to the company, its partners, regulatory agencies, or the literature. The second is from the controlled distribution system for this product, which includes a single central pharmacy, direct shipment of the drug to patients, and regular communication between patients and the central pharmacy. In addition to a report from a healthcare provider or a family member, the central pharmacy can learn about a patient death if a patient doesn't contact the central pharmacy for a routine refill, or if a drug shipment is not delivered to the patient and is returned by the shipping company, an inquiry is made by the pharmacy as to the reason. This allows the death of a patient to be recorded and tracked.
When the 2009 paper was written, we reported the data for all deaths that had been reported to the Drug Safety Department at Jazz Pharmaceuticals, Inc. However, we have recently discovered that there were an additional 82 deaths that occurred through March 30, 2008 (the cutoff date for the paper), that had not been properly recorded by the central pharmacy. Because these cases had not been reported to Jazz Pharmaceuticals Drug Safety, they were not included in the 2009 paper. We present here the corrected information including the newly identified cases.
The corrected total number of deaths from any cause in patients who had received sodium oxybate for the reporting period 2002 through March 30, 2008, is 112 (30 reported originally, plus 82 newly identified cases). In the 2009 paper, the mortality rate was calculated as the number of deaths per 100 treated patients. To provide a more precise rate, we calculated the mortality rate as patient-years of exposure, using both the original and the corrected numbers for this time period. Although this calculation includes the number of deaths worldwide in the numerator, the denominator (patient-years of exposure) is based on US numbers only, resulting in a potential slight overestimation of the rate. US-only exposure data were used because patient-years of exposure for Europe and Canada could not be obtained with the same precision as US data from the central pharmacy. Three out of 112 death cases are non-US cases. Based on this calculation, the mortality rates in patients worldwide who had received a prescription for sodium oxybate through March 30, 2008, are 0.18 for the original reported number, and 0.66 for the corrected number, which is higher than what was originally reported in 2009.
As part of ongoing pharmacovigilance activities, the collection of safety data has continued since the previous March 30, 2008, cutoff date for data included in the 2009 paper. We provide here an update on the number, rate, and causes of deaths through May 31, 2011.
Worldwide, there have been a total of 227 deaths in patients who received sodium oxybate via perscription from market introduction in 2002 through May 31, 2011. Cause of death was documented in 88 (39%) of the 227 cases. In an additional 37 (16%) cases, no cause of death was provided by the person or entity reporting the decedent's status; however a potential etiology was identified from medical history and/or follow-up information. In the remaining 102 (45%) cases, no etiological information was provided regarding any potential cause of death. Table 1 summarizes the information on causes of death in these cases.
Table 1.
Documented and suspected causes of death
| Cause (documented or suspected) of death | No. cases |
|---|---|
| Overdose (intentional, unintentional, suspected drug overdose with and without sodium oxybate) | 19 |
| Cardiac event | 17 |
| Malignancy | 15 |
| Suicide, other than due to drug overdose | 8 |
| Accidental death | 7 |
| Cerebrovascular accident | 6 |
| Respiratory event | 5 |
| Other known causes | |
| Diabetes | 2 |
| Natural cause | 2 |
| Urosepsis following Clostridium difficile infection | 1 |
| Worsening renal failure | 1 |
| Liver failure | 1 |
| Upper gastrointestinal hemorrhage postoperative complication | 1 |
| End-stage Parkinson disease | 1 |
| Underlying auto-immune disorder | 1 |
| Open heart surgery post-operative complication | 1 |
| No documented or suspected cause of death but information identified indicating a potential etiology | 37 |
| Unknown | 102 |
| Total | 227 |
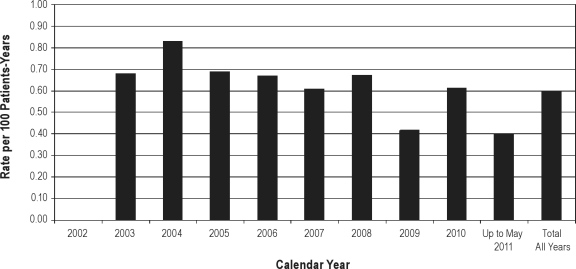
Among these 227 cases, 11 are non-US cases. The exposure for this time period in the US only was 37,992 patient-years. Using the same calculation (worldwide number of cases as numerator; US-only exposure numbers as denominator), the overall mortality rate for this period is 0.60 per 100 patient-years of exposure, consistent with the corrected rate for the period from 2002 through March 30, 2008. As Figure 1 shows, based on both corrected and subsequent data on reports of death from all causes, regardless of whether or not the cause was deemed to be related to sodium oxybate, the annual all-cause mortality rates have remained fairly stable over more than eight years of postmarketing experience.
Figure 1.
All-cause annual mortality rate by year from market introduction in 2002 through May 31, 2011
Disclosure Statement
The data was provided by Jazz Pharmaceuticals, Inc. (JPI). None of the non-JPI authors were paid for reviewing the data, for writing and reviewing the letter, and for making further suggestions for data analysis. Dr. Swick has received research funding from Jazz Pharmaceuticals/Orphan Medical, Takeda Pharmaceuticals, Merck, Cephalon, Sanofi-Aventis, Pfizer, Somaxon, GlaxoSmithKline, and Epix Pharmaceuticals. He has received funding as a consultant to Jazz Pharmaceuticals and Boehringer Ingelheim. He is on the Speakers' Bureaus of GlaxoSmithKline, Sepracor, Jazz Pharmaceuticals, Sanofi-Aventis, and Boehringer-Ingelheim. Dr. Thorpy is on the Speakers' bureaus for Cephalon Inc., and Jazz Pharmaceuticals. Dr. Benowitz has conducted clinical research for which medication was provided as a gift by Jazz Pharmaceuticals. Dr. Wang is an employee of Jazz Pharmaceuticals and owns stock and stock options in the company. Dr. Carter was an employee of Jazz Pharmaceuticals from 2007 – 2008 and has been an employee again since July 2011. He owns stock options of Jazz Pharmaceuticals.