Abstract
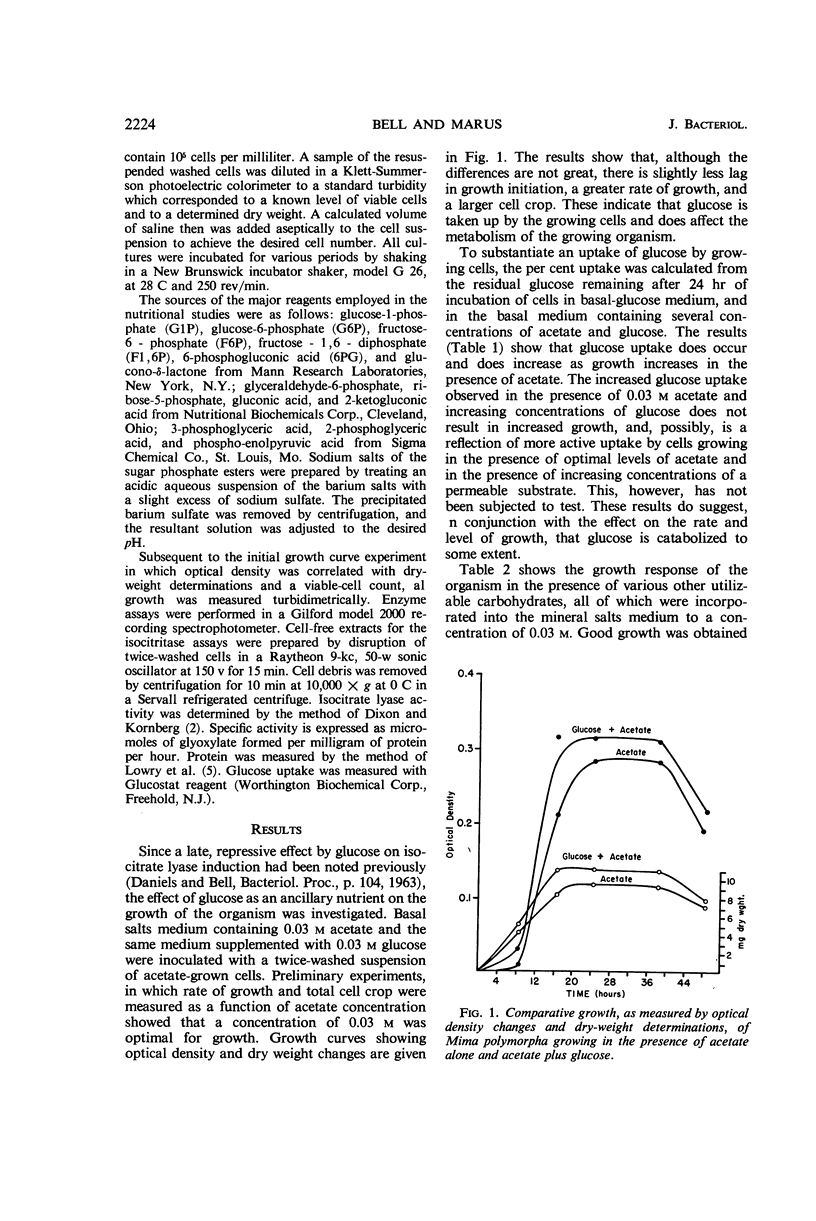
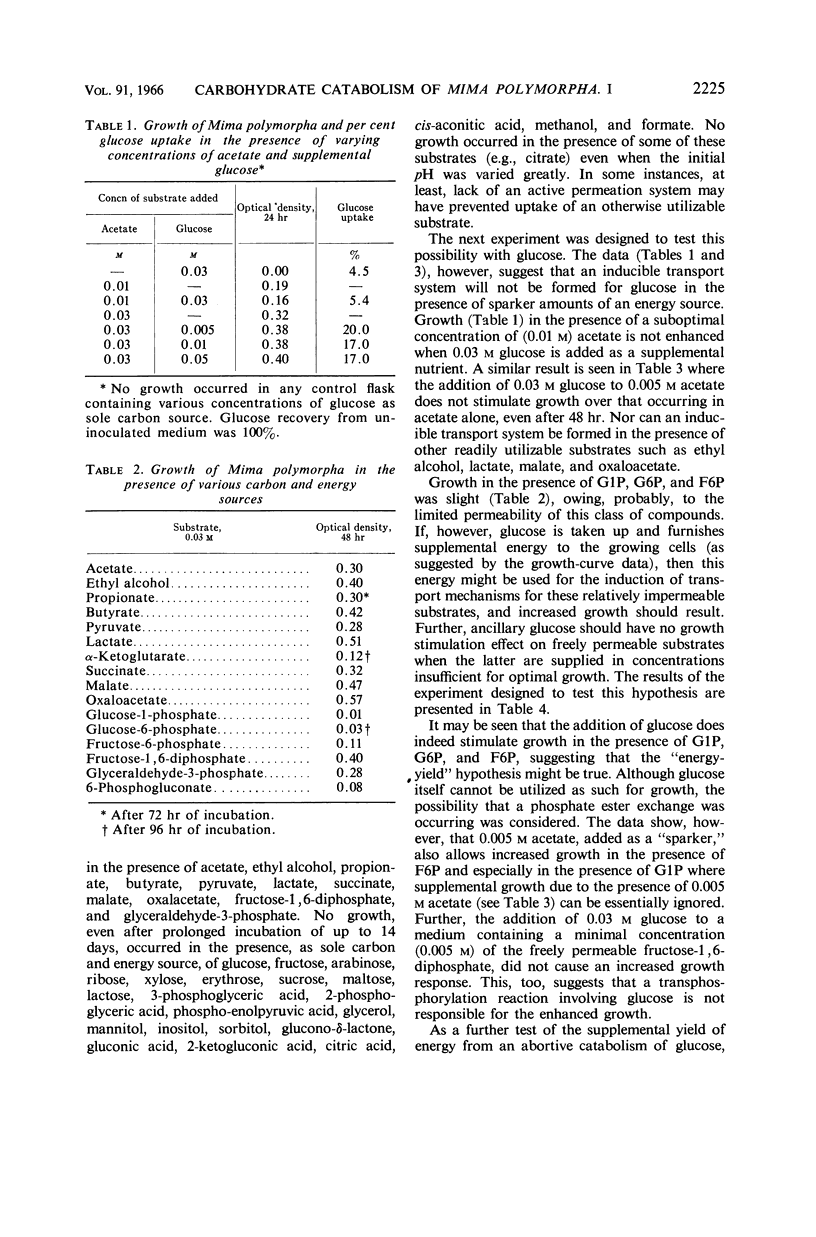
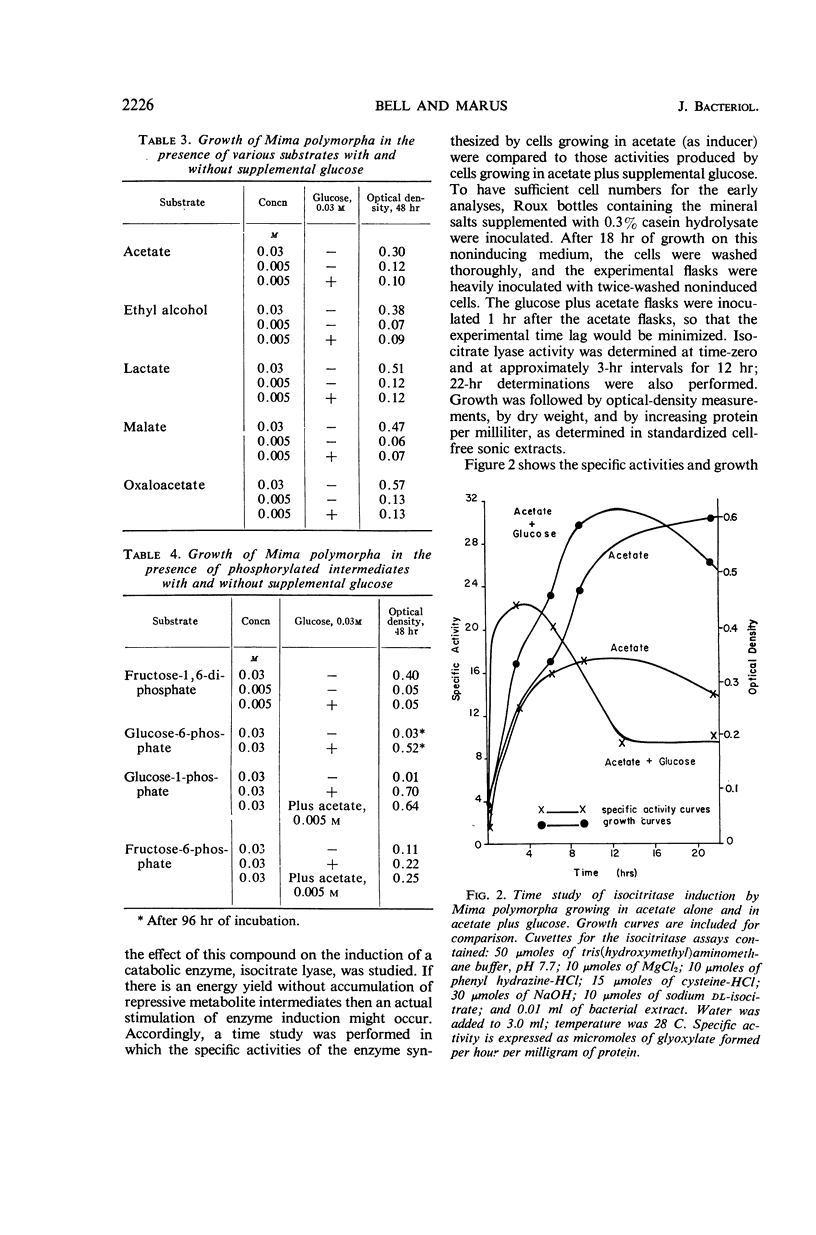
Bell, Emily J. (University of Cincinnati, Cincinnati, Ohio), and Adrienne Marus. Carbohydrate catabolism of Mima polymorpha. I. Supplemental energy from glucose added to a growth medium. J. Bacteriol. 91:2223–2228. 1966.—Mima polymorpha, unable to grow in the presence of nonphosphorylated sugars as sole source of carbon and energy, grows rapidly and well in the presence of acetate, ethyl alcohol, short-chained fatty acids, and permeable intermediates of the tricarboxylic acid cycle. Chemical evidence indicates, however, a limited uptake of glucose. Further, glucose, although incapable of supporting growth as the sole source of carbon and energy, does increase both the rate of growth and the total cell crop when added as an ancillary nutrient to cells growing in a mineral salts medium which contains 0.03 m acetate as the carbon and energy source. A yield of energy from an abortive catabolism of glucose is hypothesized. In addition to the enhancement of growth rate and total cell crop, this hypothesis is supported by the facts that: (i) transport systems for the slightly permeable phosphorylated hexoses appear to be induced when glucose is incorporated into a medium capable of supporting growth and (ii) the rate of induction and the total activity of an inducible enzyme, isocitrate lyase E.C. 4.1.3.1., are markedly increased in the presence of supplemental glucose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marus A., Bell E. J. Carbohydrate catabolism of Mima polymorpha. II. Abortive catabolism of glucose. J Bacteriol. 1966 Jun;91(6):2229–2236. doi: 10.1128/jb.91.6.2229-2236.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C. Mutant of Aerobacter aerogenes lacking glucose repression. J Bacteriol. 1960 Oct;80:536–543. doi: 10.1128/jb.80.4.536-543.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES H. C., AJL S. Occurrence and function of isocitritase and malate synthetase in bacteria. J Bacteriol. 1960 Mar;79:341–345. doi: 10.1128/jb.79.3.341-345.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS I. Z., WOLFFE E. L. Utilization of labeled fructose-6-phosphate and fructose-1,6-diphosphate by Escherichia coli. Arch Biochem Biophys. 1951 Aug;33(1):165–166. doi: 10.1016/0003-9861(51)90090-2. [DOI] [PubMed] [Google Scholar]
- ROCKWOOD S. W., BELL E. J., REEVES H. C. ISOCITRITASE LEVELS OF MEMBERS OF THE TRIBE MIMEAE. J Bacteriol. 1963 Apr;85:945–946. doi: 10.1128/jb.85.4.945-946.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH R. A., GUNSALUS I. C. Distribution and formation of isocitritase. Nature. 1955 Apr 30;175(4461):774–775. doi: 10.1038/175774b0. [DOI] [PubMed] [Google Scholar]


