Abstract
Multiple lines of evidence suggest a serotoninergic dysfunction in autism. The role of LMX1B in the development and maintenance of serotoninergic neurons is well known. In order to examine the role, if any, of LMX1B with autism pathophysiology, a trio-based SNP association study using 252 family samples from the AGRE was performed. Using pair-wise tagging method, 24 SNPs were selected from the HapMap data, based on their location and minor allele frequency. Two SNPs (rs10732392 and rs12336217) showed moderate association with autism with p values 0.018 and 0.022 respectively in transmission disequilibrium test. The haplotype AGCGTG also showed significant association (p = 0.008). Further, LMX1B mRNA expressions were studied in the postmortem brain tissues of autism subjects and healthy controls samples. LMX1B transcripts was found to be significantly lower in the anterior cingulate gyrus region of autism patients compared with controls (p = 0.049). Our study suggests a possible role of LMX1B in the pathophysiology of autism. Based on previous reports, it is likely to be mediated through a seretoninergic mechanism. This is the first report on the association of LMX1B with autism, though it should be viewed with some caution considering the modest associations we report.
Introduction
Autism and other developmental disabilities, clinically referred to as autism spectrum disorders (ASDs), are characterized by impairments in communication skills and social interaction, and the presence of repetitive stereotyped behaviors and interests. It is typically diagnosed by the age of three and has a prevalence rate of 60-70 per 10,000 children in broader diagnostic criteria as per the most recent estimates [1]. ASDs are considered to be among the most heritable of all psychiatric disorders. A recent largest population based twin study comprised of 10,895 twin pairs, reported 80% heritability for ASDs [2], confirming the previously reported heritability estimates [3], [4]. Linkage, candidate gene and whole genome association studies have suggested several genes and chromosomal regions associated with the disorder. However, none of these known causes individually account for more than 1–2% of the cases, and specific genetic mechanisms underlying the heritability of the disorder still remain largely cryptic. It was found that many different genetic changes in unrelated genes can cause indistinguishable ASD features; this genetic heterogeneity necessitate the need to look for more potential candidate genes associated with the disorder.
The LIM homeodomain transcription factor 1b (LMX1B) was initially characterized as a key regulator of the normal dorsoventral patterning in the developing limbs [5]. Several mutations reported in this gene have been found to lead to the pleiotropic phenotype, the nail platella syndrome [6]–[8]. Later, the role of Lmx1b in the development and maintenance of serotoninergic (5HTergic) neurons in the central nervous system (CNS) was reported, and thereafter, underlying mechanisms were studied in detail. Lmx1b knock-out mice were found to be lacking the entire central 5HTergic neurons [9], [10]. Further, it was shown that overexpression of Lmx1b enhances differentiation of mouse embryonic stem cells into 5HT neurons [11]. In addition to its role in the development of central 5HTeregic neurons, Lmx1b is also required for the normal biosynthesis of 5HT in adult brain, and possibly for the regulation of normal functions of 5HTergic neurons [12].
A role of 5HTergic system in the pathophysiology of autism was proposed based on following observations, a) hyperserotonemia in the whole blood cells and platelets of 25–50% of patients with autism [13], [14], b) depletion of tryptophan, the 5HT precursor, in ASD patients increased some stereotype behaviors associated with the disorder [15], c) treatment with selective serotonin reuptake inhibitors has shown to be effective in ameliorating the repetitive and/or compulsive behaviors in some autistic individuals [16] and d) recent neuroimaging studies have shown low levels of brain 5HT synthesis in autistic children [17] and reduction in serotonin transporter (SLC6A4) binding in different brain regions of both children and adults with the disorder [18], [19]. Compliant with these reports, several genetic association studies involving genes in the 5HT metabolism with a focus on the SLC6A4 were also attempted. While several SLC6A4 polymorphisms were shown to be associated with the disorder in some studies [20], [21], others failed to replicate the findings [22].
Taking together, these results provide compelling, though inconsistent evidence for the role of 5HTergic system in the pathophysiologic mechanism of ASDs. In view of the importance of LMX1B in the development of 5-HTergic neurons, it would be interesting to study its role in autism. Here we performed a trio-based study to examine the association of LMX1B with autism. We also assessed any alterations in the expression LMX1B in the postmortem brain samples of autism patients as compared to healthy controls.
Results
Single SNP TDT
Mendelian inheritance inconsistencies were not observed for any of the SNPs. For each SNP, >99% of the genotypes were scored; none of the SNPs showed deviation from HWE.
The results of TDT analysis are shown in Table 1. rs10732392 (p = 0.018; OR = 1.764; 95% CI for OR 1.095–2.842) and rs12336217 (p = 0.022; OR = 1.748; 95% CI for OR 1.076–2.841) showed significant associations with autism. However, these associations did not withstand the multiple testing correction. Overtransmission was observed for the minor allele A (62.82%) of rs10732392 and for minor allele G (62.67%) of rs12336217.
Table 1. Single SNP TDT results of LMX1B SNPs in 252 trio samples.
| Marker | db SNP ID | Genomic | Variation* | Location | Minor allele | T (%)‡ | p-value§ |
| Location | frequency† | ||||||
| SNP 1 | rs10732392 | 129396037 | G:A | Intron 2 | 0.078 | 48.92 | 0.018 |
| SNP 2 | rs10760444 | 129396434 | A:G | Intron 2 | 0.449 | 48.23 | 0.214 |
| SNP 3 | rs10448285 | 129397014 | C:T | Intron 2 | 0.376 | 50.64 | 0.601 |
| SNP 4 | rs12336217 | 129399870 | A:G | Intron 2 | 0.075 | 48.98 | 0.022 |
| SNP 5 | rs7858338 | 129406644 | T:C | Intron 2 | 0.26 | 51.61 | 0.085 |
| SNP 6 | rs11793373 | 129407543 | G:A | Intron 2 | 0.252 | 50.6 | 0.513 |
| SNP 7 | rs10819190 | 129408513 | G:A | Intron 2 | 0.414 | 49.56 | 0.739 |
| SNP 8 | rs6478750 | 129409198 | T:C | Intron 2 | 0.408 | 49.91 | 0.948 |
| SNP 9 | rs12555734 | 129411242 | C:A | Intron 2 | 0.24 | 51.25 | 0.16 |
| SNP 10 | rs13285227 | 129413298 | C:T | Intron 2 | 0.348 | 49.11 | 0.439 |
| SNP 11 | rs944103 | 129413490 | G:A | Intron 2 | 0.472 | 49.05 | 0.526 |
| SNP 12 | rs12555176 | 129414303 | G:T | Intron 2 | 0.074 | 50.11 | 0.809 |
| SNP 13 | rs7854658 | 129414938 | G:A | Intron 2 | 0.21 | 50.57 | 0.486 |
| SNP 14 | rs10987386 | 129416317 | C:T | Intron 2 | 0.191 | 49.5 | 0.519 |
| SNP 15 | rs12551234 | 129417809 | G:C | Intron 2 | 0.407 | 49.92 | 0.949 |
| SNP 16 | rs7853174 | 129419990 | G:A | Intron 2 | 0.394 | 49.04 | 0.452 |
| SNP 17 | rs10819194 | 129422023 | G:A | Intron 2 | 0.422 | 51.78 | 0.189 |
| SNP 18 | rs4322101 | 129428677 | A:G | Intron 2 | 0.416 | 51.19 | 0.37 |
| SNP 19 | rs7030919 | 129438872 | A:G | Intron 2 | 0.115 | 49.49 | 0.37 |
| SNP 20 | rs3737048 | 129458092 | G:T | Intron 6 | 0.107 | 50.39 | 0.474 |
| SNP 21 | rs10987413 | 129459438 | G:A | 3′ | 0.333 | 50.65 | 0.56 |
| SNP 22 | rs10760450 | 129459628 | C:T | 3′ | 0.21 | 50.58 | 0.475 |
| SNP 23 | rs10733682 | 129460914 | G:A | 3′ | 0.486 | 51.27 | 0.41 |
| SNP 24 | rs4083644 | 129461714 | C:T | 3′ | 0.28 | 49.93 | 0.943 |
T: Transmitted.
*Common allele is listed first.
†Based on the parental genotypes of 252 trios.
‡T% of common allele is listed, § Computed on the basis of likelihood ratio test; significant p-values (<0.05) are indicated in bold italics.
LD analysis
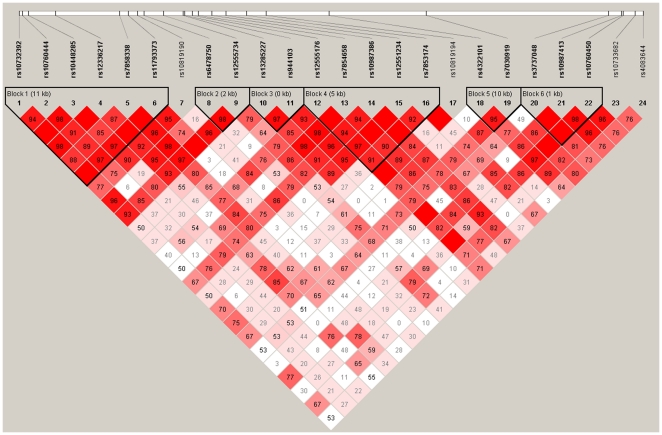
LD analysis based on D' values identified six distinct haploblocks across LMX1B gene. The first block consists of SNPs 01 to 06, the second block SNPs 08 and 09, the third block 10 and 11, fourth block 12 to 16, fifth block 18 and 19 and the sixth block included SNPs 20 to 22 (Figure 1).
Figure 1. Haploblock structure of LMX1B.
Six haplotype blocks were identified based on D' values calculated from 252 trios.
Haplotype TDT
The results of haplotype TDT is given in Table 2. Based on the LD structure of LMX1B, associations of haplotypes in the six haploblocks were analysed. The haplotype AGCGTG of the first block showed significant association with autism (p = 0.008).
Table 2. Haplotype associations of SNPs belonging to the six LD blocks of LMX1B, in 252 trios.
| Block | Haplotype* | Frequency | T(%) | Individual p- | Permutation p- | Block p- |
| value† | value‡ | value | ||||
| Block 1 (SNPs 01–06) | GGTATG | 0.355 | 51.67 | 0.6291 | 1 | |
| GACATA | 0.25 | 48.81 | 0.7487 | 1 | ||
| GACACG | 0.244 | 45.71 | 0.2568 | 0.994 | ||
| AGCGTG | 0.073 | 66.13 | 0.0079 | 0.114 | ||
| GACATG | 0.052 | 51.42 | 0.8461 | 1 | ||
| GGTACG | 0.014 | 30.77 | 0.1658 | 0.97 | 0.096 | |
| Block 2 (SNPs 08–09) | CC | 0.406 | 50.23 | 0.9432 | 1 | |
| TC | 0.353 | 54.03 | 0.2242 | 0.987 | ||
| TA | 0.239 | 44.23 | 0.1255 | 0.892 | 0.258 | |
| Block 3 (SNPs 10–11) | CG | 0.525 | 48.4 | 0.6123 | 1 | |
| TA | 0.345 | 52.71 | 0.4094 | 1 | ||
| CA | 0.126 | 48.79 | 0.8046 | 1 | 0.731 | |
| Block 4 (SNPs 12–16) | GGCGA | 0.379 | 53.41 | 0.3114 | 0.998 | |
| GGCGG | 0.209 | 45.31 | 0.2362 | 0.991 | ||
| GACCG | 0.201 | 48.99 | 0.8072 | 1 | ||
| GGTCG | 0.119 | 55.41 | 0.2624 | 0.994 | ||
| TGTCG | 0.071 | 48.81 | 0.8455 | 1 | 0.595 | |
| Block 5 (SNPs 18–19) | AA | 0.58 | 52.42 | 0.4476 | 1 | |
| GA | 0.304 | 47.25 | 0.1587 | 0.966 | ||
| GG | 0.112 | 53.61 | 0.4772 | 1 | 0.354 | |
| Block 6 (SNPs 20–22) | GGC | 0.35 | 55.39 | 0.111 | 0.868 | |
| GAC | 0.332 | 48.19 | 0.59 | 1 | ||
| GGT | 0.21 | 47.63 | 0.5365 | 1 | ||
| TGC | 0.107 | 46.45 | 0.4947 | 1 | 0.512 |
T: Transmitted / (Transmitted + Untransmitted).
‡10,000 permutations.
*All possible combinations of haplotypes with frequency >0.01 †Significant p-values (<0.05) are indicated in bold italics.
LMX1B expression in the postmortem brains
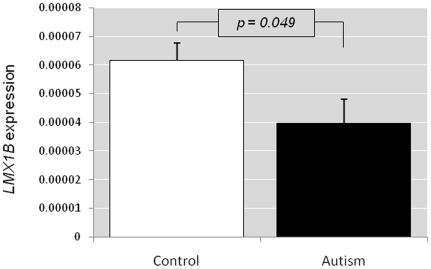
No significant difference in age, sex and postmortem intervals was observed between autism and control groups in all the brain regions (ACG, MC and THL). There was a significant difference in LMX1B expression between the autism and control group in the ACG (p = 0.049) (Figure 2). Expression was significantly lower in autism groups with a fold change of (2−△△CT) 0.43. No LMX1B expression could be detected in the other two brain regions (MC and TH).
Figure 2. LMX1B expression in the brain.
LMX1B expression in the anterior cingulate gyrus region of the brain of autism patients compared to that of control samples.
Discussion
In this study, we examined the association of the transcription factor gene LMX1B with autism in Caucasian population. In the trio-based study, we found nominal associations for two SNPs (rs10732392 and rs12336217) and a haplotype with autism. To the best of our knowledge, this is the first study which reported an association between LMX1B and autism; a previous study reported the association between LMX1B and schizophrenia [23], which is also a neurodevelopmental disorder. Both the SNPs which are found to be associated with the disorder are located in the introns (intron 2) and may lack any direct functional importance. We also found that the LMX1B mRNA expression in general, is rather low in adult brain; detected only in ACG. However, LMX1B mRNAs were found to be significantly lower in the ACG of autistic brains than the similar regions of control brain tissues.
Multiple lines of evidence suggested a serotoninergic dysfunction in many patients with autism, although the results are still inconclusive. Involvement of several transcription factors are reported in the 5HTergic differentiation. In mammalian CNS, a sequential activation of transcription factors in the hindbrain, starting with the regulation of the expression of Nkx2-2 by the Shh signaling pathway, has been proposed [9]. It was observed that 5HT neurons are absent in the mice lacking Nkx2-2 [24]. It occupies the highest hierarchical position in the genetic cascade that involved in the development of 5HT neurons. Another transcription factor Pet1, expressed in the post mitotic 5HT neurons was reported to be the terminal differentiation factor, which acts in the final step of the transcriptional cascade that establishes the final identity of 5HT neurons. Mice lacking Pet1 had 70–80% fewer 5-HT neurons than normal mice. The Lmx1b ablation does not affect the expression Nkx2.2 and Shh [9], [25] putting these factors upstream of Lmx1b. However, during development, Lmx1b precedes pet1, and Lmx1b knock-out mice showed loss of Pet1 expression [10]. In vivo, Pet1 expression was increased in neurons overexpressing Lmx1b [11]. Thus, Lmx1b has been proposed as an essential link between Nkx2.2 and Pet1 in the genetic cascade that controls the early specification and terminal differentiation of 5HTergic neurons in the hindbrain. Lmx1b expression was shown to be the rate limiting step in this cascade of events for specifying the 5HT phenotype [11]. Further, Lmx1b, together with Pet1, is also involved in the serotonin metabolism as it controls a set of molecules essential for the serotonin synthesis (TPH2), vesicular transport (VMAT2) and reuptake after synaptic release (SLC6A4) in the developing as well as adult brain [10], [12].
ACG region plays important role in the pathophysiology of autism as shown by previous reports [26], [27]. Our positron emission tomography studies had shown that a reduction in SLC6A4 binding in the cingulate cortices is associated with an impairment of social cognition in autistic subjects [19]. The present finding of reduced LMX1B expression in the ACG of autism group, therefore, could have some deleterious effects on the serotonergic system, given the role of LMX1B in the differentiation of 5HT neurons in developing brain, and in the maintenance of 5HT system in adult brain.
In conclusion, we report a possible association of the transcription factor LMX1B with autism pathogenesis. However, our results should be interpreted with some caution, given the limitations in sample size of postmortem brain samples and the modest associations we found in genetic and gene expression studies.
Materials and Methods
Subjects
DNA samples from trio families recruited to the Autism Genetic Resource Exchange [28] were used for the single nucleotide polymorphism (SNP) association study. We selected 252 trios families with male offspring scored for autism. Only Caucasians (white) were selected and non-idiopathic autism cases were excluded.
Brain samples
Frozen postmortem brain tissues from autistic patients and controls were provided by the Autism Tissue Program (ATP; Princeton, NJ; http://www.autismtissueprogram.org) and Harvard Brain Tissue Research Center (HBTRC; Belmont, MA; http://www.brainbank.mclean.org/). Tissues were obtained from three brain regions important in cognitive and behavior processing namely a) anterior cingulate gyrus (ACG- 8 autism and 13 controls), b) motor cortex (MC- 7 autism and 8 controls), and c) thalamus (THL-8 autism and 9 controls). The demographic features of the samples are described in Table 3.
Table 3. Postmortem brain tissue information.
| Sample IDa | Diagnosis | Age (years) | Gender | PMI (hours) | Race | Cause of death | Brain regionsb |
| UMB 818 | Control | 27 | M | 10 | Caucasian | Multiple injuries | ACG |
| UMB 1065 | Control | 15 | M | 12 | Caucasian | Multiple injuries | ACG, THL |
| UMB 1297 | Control | 15 | M | 16 | African American | Multiple injuries | ACG, MC, THL |
| UMB 1407 | Control | 9 | F | 20 | African American | Asthma | ACG, MC, THL |
| UMB 1541 | Control | 20 | F | 19 | Caucasian | Head injuries | ACG, MC, THL |
| UMB 1649 | Control | 20 | M | 22 | Hispanic | Multiple injuries | ACG, MC, THL |
| UMB 1708 | Control | 8 | F | 20 | African American | Asphyxia, multiple injuries | ACG, MC, THL |
| UMB 1790 | Control | 13 | M | 18 | Caucasian | Multiple injuries | ACG |
| UMB 1793 | Control | 11 | M | 19 | African American | Drowning | ACG, MC, THL |
| UMB 1860 | Control | 8 | M | 5 | Caucasian | Cardiac Arrhythmia | ACG |
| UMB 4543 | Control | 28 | M | 13 | Caucasian | Multiple injuries | ACG, MC, THL |
| UMB 4638 | Control | 15 | F | 5 | Caucasian | Chest injuries | ACG |
| UMB 4722 | Control | 14 | M | 16 | Caucasian | Multiple injuries | ACG, MC, THL |
| UMB 797 | Autism | 9 | M | 13 | Caucasian | Drowning | ACG, THL |
| UMB 1638 | Autism | 20 | F | 50 | Caucasian | Seizure | ACG, MC, THL |
| UMB 4231 | Autism | 8 | M | 12 | African American | Drowning | ACG, MC, THL |
| UMB 4721 | Autism | 8 | M | 16 | African American | Drowning | ACG, MC, THL |
| UMB 4899 | Autism | 14 | M | 9 | Caucasian | Drowning | ACG, MC, THL |
| B 5000 | Autism | 27 | M | 8.3 | NA | NA | ACG, MC, THL |
| B 6294 | Autism | 16 | M | NA | NA | NA | ACG, MC, THL |
| B 6640 | Autism | 29 | F | 17.83 | NA | NA | ACG, MC, THL |
Autism Tissue Program (ATP) identifier.
Brain regions for which, each sample was available.
M: Male; F: Female, PMI: Postmortem interval, ACG: Anterior cingulate gyrus; MC: Motor cortex; THL: Thalamus; NA: Not available.
Selection of SNPs
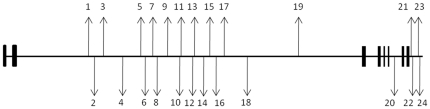
LMX1B, located in 9q33.3 (129,376,748 – 129,463,311), is 86.56kb in size and consists of eight exons. The genomic structure is based on the UCSC (http://www.genome.ucsc.edu) assembly of the human genome. SNPs for the association studies were selected using the information from international HapMap project (http://www.hapmap.org) and National Centre for Biotechnology Information (NCBI dbSNP: http://www.ncbi.nlm.nih.gov/SNP). On the basis of their genomic locations and minor allele frequencies (MAF >0.1), 24 SNPs were selected (Figure 3; Table 1), using the pair-wise tagging option of Haploview.v4.1 (http://www.broad.mit.edu/mpg/haploview).
Figure 3. Genomic structure of LMX1B gene.
Locations of SNPs selected for the association study, based on the HapMap data on Caucasian population, are denoted by arrows. Exons are indicated by boxes.
Genotyping
Assay-on-demand/Assay-by-design SNP genotyping products (ABI, Foster City, CA, USA) were used to score SNPs, based on the TaqMan assay method [29]. Genotypes were determined in ABI PRISM 7900HT Sequence Detection System (SDS) (Applied Biosystems), and analyzed using SDS v2.0 (ABI).
Statistical Analysis
PedCheck v1.1 (http://www.watson.hgen.pitt.edu) was used to identify and eliminate all Mendelian inheritance inconsistencies in the trio genotype data. SNPs were tested for Hardy–Weinberg Equilibrium (HWE) using Haploview. SNP associations were examined by transmission disequilibrium test (TDT), using the TDTPHASE option of UNPHASED v2.403 (http://portal.litbio.org); expectation maximization (EM) algorithm was used to resolve uncertain haplotypes, to infer missing genotypes and to provide maximum-likelihood estimation of frequencies.
A linkage disequilibrium (LD) plot was constructed using the D' values. Pair-wise LD values between SNPs were estimated using Haploview. Subsequently, associations of haplotypes (frequency >0.01) belonging to the various haploblocks of LMX1B were also examined using Haploview.
Extraction of RNA from brain tissues
The brain tissues were homogenized by ultrasonication and total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), in accordance with the manufacturer's protocol. The RNA samples were further purified using RNeasy Micro Kit (QIAGEN GmbH, Hilden, Germany), following the manufacturer's instructions. The quantity (absorbance at 260 nm) and quality (ratio of absorbance at 260 nm and 280 nm) of RNA were estimated with a NanoDrop ND-1000 Spectrophotometer (Scrum, Tokyo, Japan).
Quantitative real-time reverse transcriptase PCR (qRT-PCR)
ImProm-II Reverse Transcription System (Promega, Madison, WI, USA) was used to synthesize first-strand cDNA from the total RNA according to the manufacturer's protocol.
RT-PCR primers for LMX1B (NM_001174146.1) (F-cctttgagcaagtaaggataatgaatg, R-gggactgaatttcccagcaa) and endogenous reference GAPDH (NM_002046.3) (F-atcagcaatgcctcctgcac, R-tggcatggactgtggtcatg) were designed using primer express v2.0 (Applied Biosystems). SYBR Green qRT-PCR assays were performed using QuantiTect SYBR Green PCR kit (Qiagen). All the reactions were performed in triplicate, in the ABI PRISM 7900HT Sequence Detection System. CT values, which reflect the mRNA expression levels, were determined. LMX1B CT of each sample was normalized to the corresponding CT for the internal control by calculating △CT (△CT = Target gene CT – GAPDH CT) to obtain the relative mRNA expression of the target gene. Quantification of the gene expression was performed by calculating △△CT (△△CT = △CT of the autistic group - △CT of the control group).The fold change in gene expression between the two groups was determined by calculating 2−△△CT.
Statistical analysis
For the gene expression studies, statistical calculations were performed using PSAW statistics 18.0 software (IBM-SPSS, Tokyo, Japan). The difference in age and postmortem interval between autistic and control groups was examined by t-test. The chi-square test was used to examine the sex distribution; alteration in gene expression between the two groups was analyzed by Mann-Whitney U-test.
Acknowledgments
We thank Dr. Jane Pickett, Director of Brain Resources and Data, Autism Tissue Program, for facilitating brain tissue collection. Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, Maryland. Tissue samples were also provided by the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R 24 MH 068855. We thank Ms. Tae Takahashi for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to Dr. K. Nakamura. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fombonne E, Quirke S, Hagen A. Prevalence and interpretation of recent trends in rates of pervasive developmental disorders. Mcgill J Med. 2009;12:73. [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, Anckarsater H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 3.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 4.Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RL, Tabin CJ. Molecular models for vertebrate limb development. Cell. 1997;90:979–990. doi: 10.1016/s0092-8674(00)80364-5. [DOI] [PubMed] [Google Scholar]
- 6.Knoers NV, Bongers EM, van Beersum SE, Lommen EJ, van Bokhoven H, et al. Nail-patella syndrome: identification of mutations in the LMX1B gene in Dutch families. J Am Soc Nephrol. 2000;11:1762–1766. doi: 10.1681/ASN.V1191762. [DOI] [PubMed] [Google Scholar]
- 7.Milla E, Hernan I, Gamundi MJ, Martinez-Gimeno M, Carballo M. Novel LMX1B mutation in familial nail-patella syndrome with variable expression of open angle glaucoma. Mol Vis. 2007;13:639–648. [PMC free article] [PubMed] [Google Scholar]
- 8.Marini M, Bocciardi R, Gimelli S, Di Duca M, Divizia MT, et al. A spectrum of LMX1B mutations in Nail-Patella syndrome: new point mutations, deletion, and evidence of mosaicism in unaffected parents. Genet Med. 2010;12:431–439. doi: 10.1097/GIM.0b013e3181e21afa. [DOI] [PubMed] [Google Scholar]
- 9.Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, et al. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- 10.Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, et al. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolmazon V, Alenina N, Markossian S, Mancip J, van de Vrede Y, et al. Forced expression of LIM homeodomain transcription factor 1b enhances differentiation of mouse embryonic stem cells into serotonergic neurons. Stem Cells Dev. 2011;20:301–311. doi: 10.1089/scd.2010.0224. [DOI] [PubMed] [Google Scholar]
- 12.Song NN, Xiu JB, Huang Y, Chen JY, Zhang L, et al. Adult raphe-specific deletion of Lmx1b leads to central serotonin deficiency. PLoS One. 2011;6:e15998. doi: 10.1371/journal.pone.0015998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook EH, Leventhal BL. The serotonin system in autism. Curr Opin Pediatr. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GM, Gutknecht L, Cohen DJ, Brailly-Tabard S, Cohen JH, et al. Serotonin transporter promoter variants in autism: functional effects and relationship to platelet hyperserotonemia. Mol Psychiatry. 2002;7:831–836. doi: 10.1038/sj.mp.4001099. [DOI] [PubMed] [Google Scholar]
- 15.McDougle CJ, Naylor ST, Cohen DJ, Aghajanian GK, Heninger GR, et al. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch Gen Psychiatry. 1996;53:993–1000. doi: 10.1001/archpsyc.1996.01830110029004. [DOI] [PubMed] [Google Scholar]
- 16.Kolevzon A, Mathewson KA, Hollander E. Selective serotonin reuptake inhibitors in autism: a review of efficacy and tolerability. J Clin Psychiatry. 2006;67:407–414. doi: 10.4088/jcp.v67n0311. [DOI] [PubMed] [Google Scholar]
- 17.Chandana SR, Behen ME, Juhasz C, Muzik O, Rothermel RD, et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23:171–182. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 2008;50:593–597. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67:59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- 20.McCauley JL, Olson LM, Dowd M, Amin T, Steele A, et al. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid-compulsive subset of autism. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:104–112. doi: 10.1002/ajmg.b.20151. [DOI] [PubMed] [Google Scholar]
- 21.Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, et al. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramoz N, Reichert JG, Corwin TE, Smith CJ, Silverman JM, et al. Lack of evidence for association of the serotonin transporter gene SLC6A4 with autism. Biol Psychiatry. 2006;60:186–191. doi: 10.1016/j.biopsych.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Bergman O, Westberg L, Nilsson LG, Adolfsson R, Eriksson E. Preliminary evidence that polymorphisms in dopamine-related transcription factors LMX1A, LMX1B and PITX3 are associated with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1094–1097. doi: 10.1016/j.pnpbp.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Pattyn A, Vallstedt A, Dias JM, Sander M, Ericson J. Complementary roles for Nkx6 and Nkx2 class proteins in the establishment of motoneuron identity in the hindbrain. Development. 2003;130:4149–4159. doi: 10.1242/dev.00641. [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, Chen CL, Luo P, Tan M, Qiu M, et al. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haznedar MM, Buchsbaum MS, Metzger M, Solimando A, Spiegel-Cohen J, et al. Anterior cingulate gyrus volume and glucose metabolism in autistic disorder. Am J Psychiatry. 1997;154:1047–1050. doi: 10.1176/ajp.154.8.1047. [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, et al. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123(Pt9):1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- 28.Geschwind DH, Sowinski J, Lord C, Iversen P, Shestack J, et al. The autism genetic resource exchange: a resource for the study of autism and related neuropsychiatric conditions. Am J Hum Genet. 2001;69:463–466. doi: 10.1086/321292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranade K, Chang MS, Ting CT, Pei D, Hsiao CF, et al. High-throughput genotyping with single nucleotide polymorphisms. Genome Res. 2001;11:1262–1268. doi: 10.1101/gr.157801. [DOI] [PMC free article] [PubMed] [Google Scholar]