Abstract
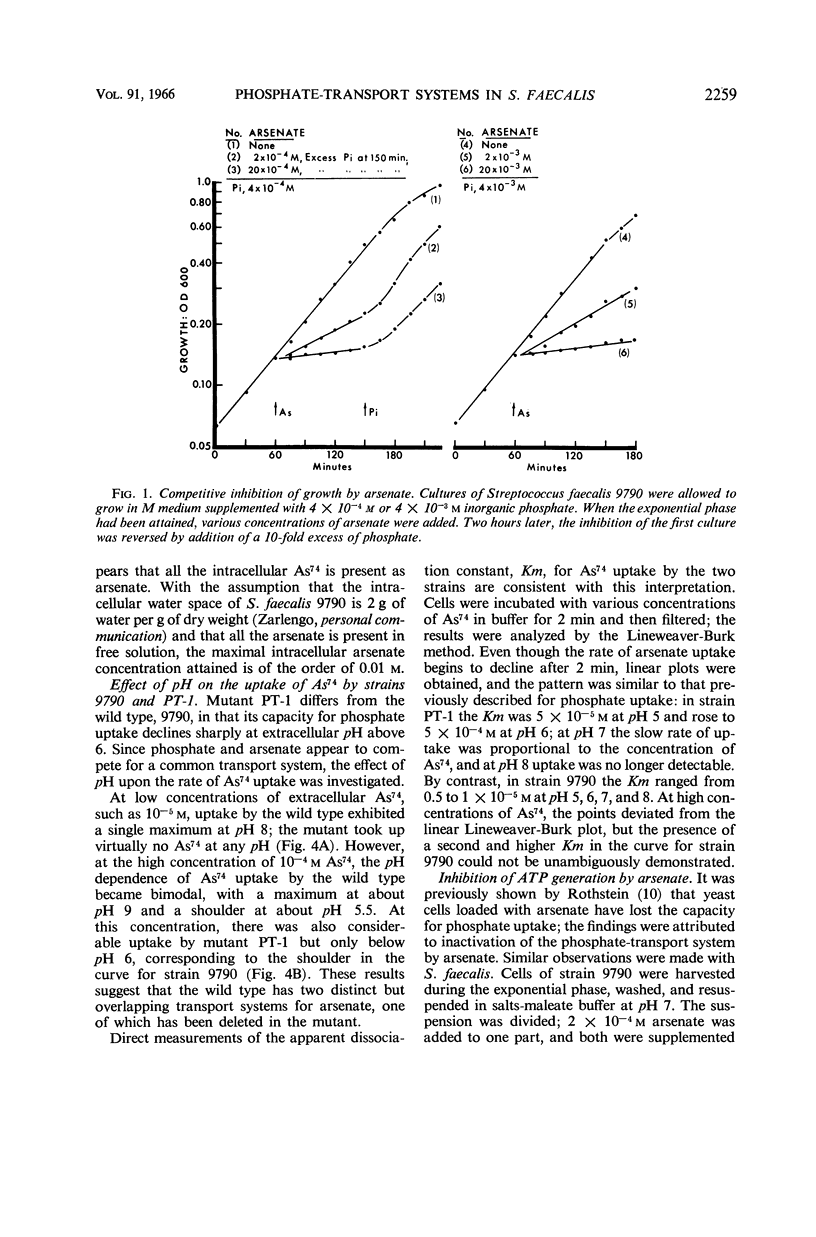
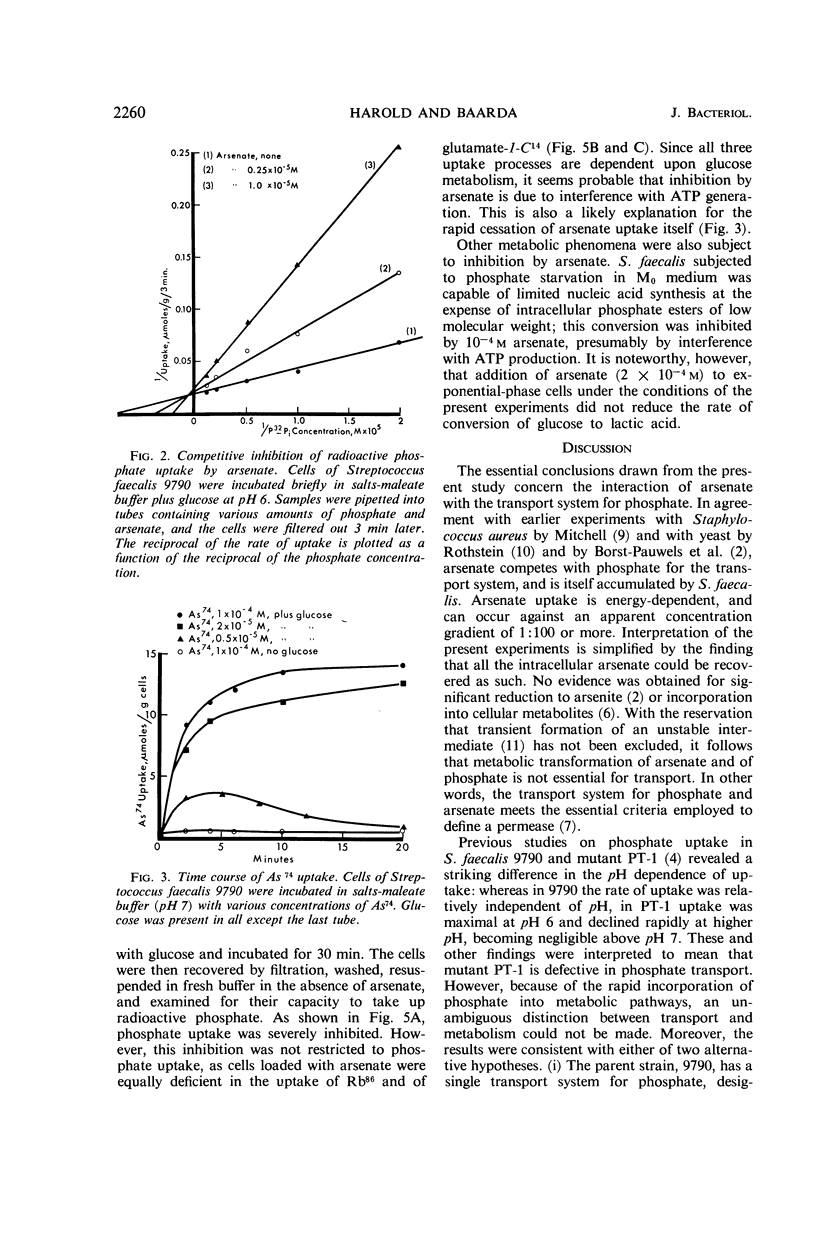
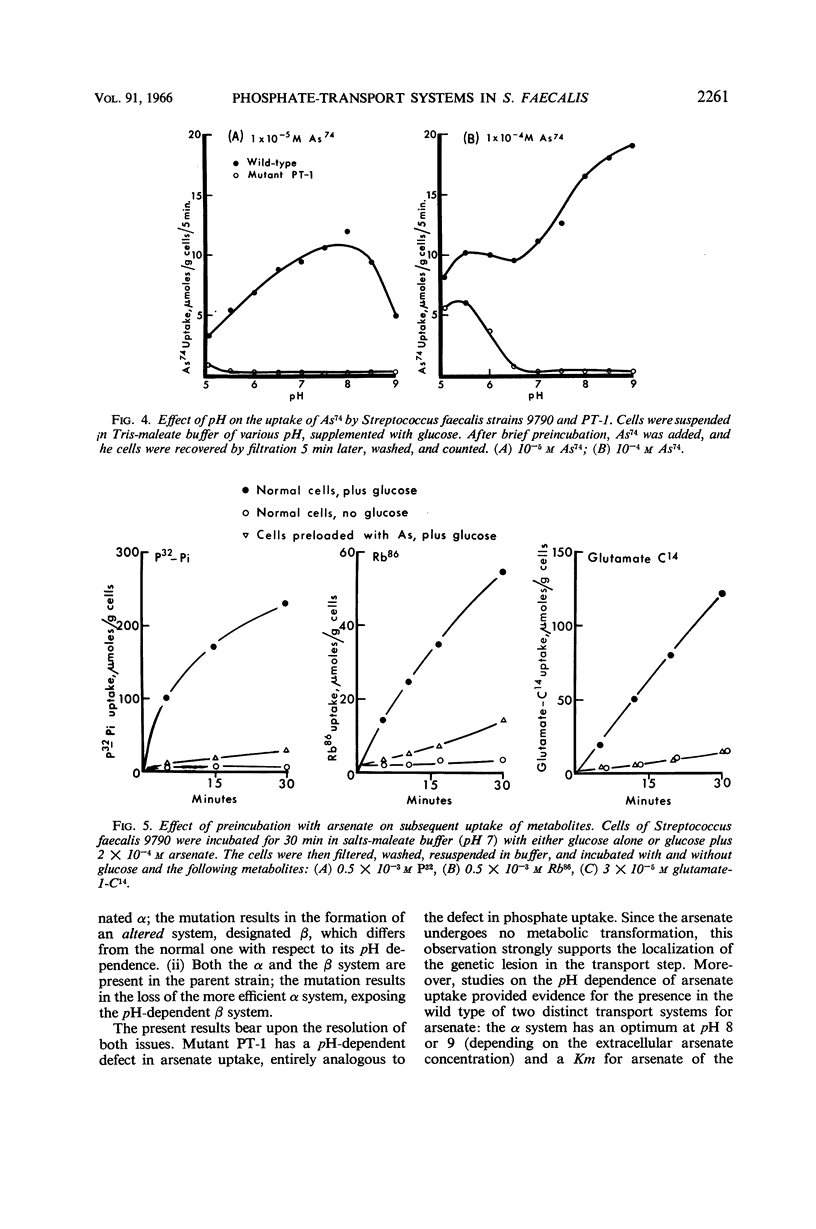
Harold, F. M. (National Jewish Hospital, Denver, Colo.), and J. R. Baarda. Interaction of arsenate with phosphate-transport systems in wild-type and mutant Streptococcus faecalis. J. Bacteriol. 91:2257–2262. 1966.—Arsenate competitively inhibits the growth of Streptococcus faecalis, primarily by competition with phosphate for a common transport system. Arsenate is itself accumulated by the cells; the uptake requires metabolic energy, and the intracellular arsenate level may reach 0.01 m. Cells loaded with arsenate have lost the capacity to take up radioactive glutamate, rubidium, phosphate, or arsenate itself, apparently by the uncoupling of adenosine triphosphate generation. The pH dependence of arsenate uptake is complex. At low concentrations of extracellular arsenate, uptake by the wild-type strain 9790 exhibits a single maximum about pH 8; mutant PT-1, previously shown to be defective in phosphate uptake, takes up essentially no arsenate. At high concentrations of arsenate, uptake by the wild type is bimodal with maxima at pH 5.5 and 9; the uptake curve for mutant PT-1 corresponds to the shoulder in the curve for the wild type. The apparent dissociation constant for arsenate uptake by the wild type is approximately 10−5m from pH 5 to 9, whereas that for mutant PT-1 is about 5 × 10−5 M at pH 5 and rises rapidly with increasing pH. The results confirm the earlier conclusion that the lesion in mutant PT-1 resides in the transport of phosphate and arsenate. It is proposed that the wild type has two distinct transport systems, whereas the mutant has lost the one with alkaline pH optimum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. Reversible metabolic swelling of bacterial protoplasts. J Biol Chem. 1959 Feb;234(2):383–388. [PubMed] [Google Scholar]
- BORSTPAUWELS G. W., PETER J. K., JAGER S., WIJFEELS C. C. A STUDY OF THE ARSENATE UPTAKE BY YEAST CELLS COMPARED WITH PHOSPHATE UPTAKE. Biochim Biophys Acta. 1965 Jan 25;94:312–314. doi: 10.1016/0926-6585(65)90038-5. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L., ABRAMS A. A MUTANT OF STREPTOCOCCUS FAECALIS DEFECTIVE IN PHOSPHATE UPTAKE. J Biol Chem. 1965 Jul;240:3145–3153. [PubMed] [Google Scholar]
- Jung C., Rothstein A. Arsenate uptake and release in relation to the inhibition of transport and glycolysis in yeast. Biochem Pharmacol. 1965 Jul;14(7):1093–1112. doi: 10.1016/0006-2952(65)90039-0. [DOI] [PubMed] [Google Scholar]
- Kay E. R. Incorporation of radioarsenate into proteins and nucleic acids of the Ehrlich Lettré ascites carcinoma in vitro. Nature. 1965 Apr 24;206(982):371–373. doi: 10.1038/206371a0. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Transport of phosphate across the osmotic barrier of Micrococcus pyogenes; specificity and kinetics. J Gen Microbiol. 1954 Aug;11(1):73–82. doi: 10.1099/00221287-11-1-73. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN A. Interactions of arsenate with the phosphate-transporting system of yeast. J Gen Physiol. 1963 May;46:1075–1085. doi: 10.1085/jgp.46.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLOCUM D. H., VARNER J. E. Transfer of O18 in arsenolysis reactions. J Biol Chem. 1960 Feb;235:492–495. [PubMed] [Google Scholar]
- TOENNIES G., SHOCKMAN G. D. Quantitative amino acid assimilation in homofermentative metabolism. Arch Biochem Biophys. 1953 Aug;45(2):447–458. doi: 10.1016/s0003-9861(53)80021-4. [DOI] [PubMed] [Google Scholar]


