Abstract
Exposure to carcinogenic polycyclic aromatic hydrocarbons (PAHs) induces cytochrome P450 (CYP) 1A1 and 1B1 enzymes, which biotransform PAHs resulting in the formation of DNA adducts. We hypothesised that 2,3′,4,5′-tetramethoxystilbene (TMS), an analogue of resveratrol and a potent CYP1B1 inhibitor, may inhibit r7, t8, t9-trihydroxy-c-10-(N2deoxyguanosyl)-7,8,9,10-tetrahydro-benzo[a]pyrene (BPdG) adduct formation in cells exposed to benzo[a]pyrene (BP). To address this, MCF-7 cells were cultured for 96 h in the presence of 1 μM BP, 1 μM BP + 1 μM TMS or 1 μM BP + 4 μM TMS. Cells were assayed at 2–12 h intervals for: BPdG adducts by r7, t8-dihydroxy-t-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE)-DNA chemiluminescence immunoassay; CYP1A1 and 1B1 gene expression changes by relative real-time polymerase chain reaction; and CYP1A1/1B1 enzyme activity by ethoxyresorufin-O-deethylase (EROD) assay. Whereas maximal BPdG levels were similar for all exposure groups, the times at which the maxima were reached increased by 16 and 24 h with the addition of 1 and 4 μM TMS, respectively. The maximal expression of CYP1A1 and CYP1B1 occurred at 16, 24 and 48 h, but the maximal level for EROD-specific activity was reached at 24, 48 and 60 h, in cells exposed to 1 μM BP, 1 μM BP + 1 μM TMS or 1 μM BP + 4 μM TMS, respectively. The area under the curve from 4 to 96 h of exposure (AUC4–96 h) for BPdG adduct formation was not increased in the presence of TMS, but for CYP1A1 and CYP1B1 expression fold increase AUC4–96 h and EROD-specific activity AUC4–96 h, there were significant (P < 0.05) increases in the presence of 4 μM TMS. Therefore, during 96 h of exposure in MCF-7 cells, the combination of BP plus TMS caused a slowing of BP biotransformation, with an increase in CYP1A1 and CYP1B1 expression and EROD activity, and a slowing, but no change in magnitude of BPdG formation.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are present within the ambient atmosphere as a result of partial combustion of organic materials, and major sources of PAH exposure include vehicle exhaust, cigarette smoke and industrial emissions (1). In addition, humans are exposed to PAHs though consumption of charbroiled food in the diet (2–4). Of particular interest is the PAH benzo[a]pyrene (BP), which has long been known to induce tumours in laboratory animals (5) and has recently been declared a human carcinogen by the International Agency for Research on Cancer (http://en.wikipedia.org/wiki/List_of_IARC_Group_1_carcinogens) (6).
Once ingested or inhaled, BP is biotransformed into activated metabolites. As a ligand of the cytosolic aryl hydrocarbon receptor (AHR), BP binds to the receptor, translocates to the nucleus, dimerises with the AHR nuclear translocator and binds to xenobiotic response elements within DNA causing the transcription of downstream genes, including CYP1A1 and CYP1B1 (7–9). These two members of the CYP1 family contribute to the metabolism of BP into DNA-reactive species (10). In particular, CYP1A1 and CYP1B1 assist in the generation of r7, t8-dihydroxy-t-9,10-epoxy-7,8,9,10-tetrahydro-benzo[a]pyrene (BPDE), which binds preferentially to guanine residues within DNA to form several adducts, the most mutagenic of which is the stable r7, t8, t9-trihydroxy-c-10-(N2deoxyguanosyl)-7,8,9,10-tetrahydro-benzo[a]pyrene (BPdG) adduct (11,12). If DNA repair mechanisms are unable to remove these adducts, which are formed in many normal human tissues (13), mutations (5) and tumours may occur. Studies in experimental models have shown that DNA adduct formation initiates a complex sequence of events leading to tumour induction. In humans, using case–control study designs, investigators have found PAH–DNA adduct formation to be a risk factor for human colon cancer (14) and human lung cancer (15).
CYP1A1 and CYP1B1, which are expressed in hepatic and extrahepatic tissues, steroidogenic tissues and steroid-responsive tissues that include ovary, testis, adrenal gland, breast, uterus and prostate (16), have different expression profiles. CYP1A1 shows low expression in the absence of PAHs and is highly inducible upon exposure to PAHs (17). In contrast, CYP1B1 is constitutively expressed at relatively high levels and shows moderate inducibility after PAH exposures. CYP1A1 and CYP1B1 were reported to metabolise oestradiol to 2- and 4-catechol oestrogens, respectively. Further oxidation of particularly 4-hydroxy oestradiol to quinine, which reacts with DNA to form depurinating oestrogen–DNA adducts. is suspected to be involved in neoplastic transformation of breast cells (18–23). High CYP1B1 expression occurs in multiple human cancers, including breast, colon, lung, oesophagus, skin, lymph node, brain and testis (24), and its overexpression has been implicated in premalignant progression (25). Therefore, CYP1B1 is considered to be an universal tumour antigen and a potential target for cancer prevention (26–28). Indeed, in one study, a mouse model null for CYP1B1 was resistant to tumorigenesis induced by PAHs (29).
2,3′,4,5′-tetramethoxystilbene (TMS), an analogue of resveratrol, is considered to be a potential cancer preventive agent since it is a potent inhibitor of CYP1B1. In an Escherichia coli system, TMS was shown to competitively inhibit CYP1B1 with a 50-fold selectivity compared to CYP1A1 (30). In the human breast cancer cell line MCF-7, TMS effectively inhibited CYP1B1 expression and PAH-induced ethoxyresorufin-O-deethylase (EROD) activity (31,32) and reduced tumour volume of MCF-7 breast cancer cell xenografts by up to 53% (33). Since CYP1A1 and CYP1B1 play important roles in BP-induced DNA damage, we hypothesised that inhibition of the CYPs would inhibit formation of the BPdG adduct.
The aim of the present study was to evaluate TMS modulation of BP biotransformation and BPdG adduct formation in MCF-7 breast cancer cells exposed to BP for 96 h. We chose MCF-7 cells because they express both the oestrogen receptor α and the AHR. In addition, they have high CYP1B1 expression, high EROD activity and form high levels of BPdG adducts. In these experiments, rather than analyse at a single time point, we determined the effect of TMS at 2- to 12-h intervals during 4–96 h of BP exposure. In preliminary studies, BPdG adduct measurements in DNA from cells exposed to BP (1 μM) plus TMS (1.0 or 4.0 μM) for 12 and 24 h, showed inhibition of BPdG adduct formation at 12 h, but increased BPdG adduct formation at 24 h. In fact, the 24-h values were similar to those found in the group exposed to BP alone. This prompted us to evaluate the kinetics over time. For each parameter, BPdG adducts, CYP1A1 and CYP1B1 gene expression, and EROD activity, we calculated a value for area under the curve from 4 to 96 h of exposure (AUC4–96 h). An approach similar to this was effectively used in correlating adenoma induction and time-integrated DNA adduct level, calculated by integrating the area under the adduct persistence curves extrapolated to 240 days, in PAH exposed A/J mice (34). In our study, TMS exposure prolonged BP biotransformation. In cells exposed to BP plus TMS, the AUC4–96 h values showed enhanced CYP1A1 and CYP1B1 gene expression and increased CYP enzyme (EROD) activity but a similar level of BPdG adducts, compared to cells exposed to BP alone.
Materials and methods
Chemicals
TMS was purchased from Cayman Chemical (Ann Arbor, MI, USA) and BP was purchased from Sigma (St Louis, MO, USA). Both compounds were dissolved in dimethylsulfoxide (DMSO; Pierce, Thermo Fisher Scientific, Rockford, IL, USA) not exceeding a final concentration of 0.1% DMSO in all experiments. A solvent control containing only DMSO was included in all experiments.
Cell culture
MCF-7 cells [American Type Culture Collection (ATCC), Manassas, VA, USA] were cultured in Eagle’s Minimum Essential Medium (ATCC) supplemented with 10% foetal bovine serum (ATCC) and incubated at 37°C in 5% CO2. Cells were routinely subcultured at 60–70% confluency. Cells were also routinely tested for mycoplasma contamination using MycoAlert Mycoplasma Detection Kit (Lonza, Walkersville, MD, USA).
Cell viability
Cell viability was determined using the Cell Titer Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA). In brief, MCF-7 cells were seeded in 12-well plates (75 000 cells per well) in triplicate. Attached cells were exposed to 1 μM BP in the presence of 0, 1 or 4 μM TMS. At 4, 12, 24 or 72 h, RIPA Lysis Buffer (1x; Upstate Cell Signaling, Temecula, CA, USA) was added to lyse the cells. A diluted sample of homogeneous cell lysate was transferred in duplicate to a 96-well plate and combined with an equivalent volume of Cell Titer Glo Luminescence (Promega). Luminescence measured using the Tropix 717 Microplate Luminometer (Applied Biosystems, Foster City, CA, USA). To examine viability of cells exposed to BP alone, the 72-h treatment was repeated twice, and for each experiment cells were assayed in triplicate. The values are expressed as % of the DMSO-alone solvent control.
Cell exposures and DNA preparation
MCF-7 cells grown in T-75 flasks (500 000 cells per flask; Corning Life Sciences, Corning, NY, USA) were exposed to 1 μM BP in the presence of 0, 1 or 4 μM TMS. At 2, 4, 8, 12, 16, 24, 36, 48, 60, 72 and 96 h, cells were harvested for subsequent DNA or RNA extraction. For both DNA and RNA preparation, media was removed and cells were washed with phosphate-buffered saline (PBS). DNA was isolated using a non-organic procedure as per the manufacturer’s protocol (QIAamp DNA Blood Midi Kit; Qiagen, Valencia, CA, USA) with slight modifications. After adding lysis buffer, cell lysates were incubated (20 min at 37°C) with RNase A (2 mg; Sigma) followed by incubation (30 min at 70°C) with Proteinase K (120 mAU; Sigma). DNA was isolated and suspended in 0.01 M Tris pH 7.4, 0.001 M EDTA (TE buffer). The quality of DNA was determined by ultraviolet (UV) absorbance at 260 nm using the NanoDrop 1000 spectrophotometer (NanoDrop, Wilmington, DE, USA), while DNA quantity was determined by SYBR Green I nucleic acid stain (Molecular Probes, Eugene, OR, USA) measuring fluorescence (with excitation at 492 nm and emission at 526 nm) using a microplate reader (Infinite M200; Tecan, Durham, NC, USA).
Determination of BPdG adducts by BPDE–DNA chemiluminescence immunoassay
For the DNA adduct studies, cells were exposed as described above on two separate occasions and monitored during 96 h. For each experiment, DNA samples were assayed for BPdG adducts on three separate occasions using the BPDE–DNA chemiluminescence immunoassay (CIA), as described previously (35). In brief, opaque 96-well high-binding plates (Greiner Bio-one, Longwood, FL, USA) were coated at room temperature with 100 pg of sonicated BPDE–DNA (modified to 0.33%) or calf thymus DNA, in 0.1 ml of Reacti-Bind DNA coating solution (Pierce, Thermo Fisher Scientific). Plates were stored at −20°C until further use. For BPDE–DNA CIA analysis, plates were warmed to room temperature and washed three times with PBS containing 0.05% Tween 20 (PBST) and 0.02% sodium azide (pH 7.4) using an automated plate washer (Ultrawash Plus; Dynex, Chantilly, VA, USA). Plates were blocked with casein (0.25%; Applied Biosystems) in PBST at 37°C for 90 min to reduce non-specific binding. Sample DNA (10–35 ng) or standard BPDE–DNA was sonicated (30 sec at 20% amplitude using an Ultrasonic Processor, Sonics & Materials, Inc., Newtown, CT, USA) and mixed with an equal volume of BPDE–DNA antiserum (rabbit # 31, bleed 08/16/78) diluted 1:3 000 000 in PBST containing casein (0.25%). Standard BPDE–DNA (modified to 1.0 BPdG/106 nucleotides) in calf-thymus DNA was serially diluted so that each well contained an equal quantity of DNA but varying amounts of BPdG adduct (0–16 fmol/well). After adding samples and standards to wells, plates were incubated at 37°C for 90 min followed by washing with PBST and incubation with biotinylated anti-rabbit antibody (1:2500; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in PBST containing casein (0.25%) at room temperature for 60 min. After washing, plates were incubated with streptavidin alkaline phosphatase (1:5000; Avidix-AP; Applied Biosystems) in PBST containing casein (0.25%) at room temperature for 60 min. Plates were subsequently washed eight times with PBST, once with distilled water and three times with Tris buffer (20 mM Tris and 1 mM MgCl2, pH 9.5) before addition of CDP Star with Emerald II solution (Applied Biosystems). After overnight incubation at 4°C, plates were brought to room temperature and luminescence was read using a Tropix 717 Microplate Luminometer (Applied Biosystems). For each assay, a single 5–35 ng DNA sample was incubated in three experimental wells and one control well. The standard curve 50% inhibition was at 0.3 ± 0.02 fmol (mean ± SE, n = 9), and the lower limit of detection for 20 μg DNA/well was 1.5 adducts/109 nucleotides.
RNA and complementary DNA preparation
Samples of MCF-7 cells, exposed as described above, were subjected to RNA isolation by RNAqueous-4PCR (Ambion, Austin, TX, USA) including DNase treatment of samples. The quality and quantity of RNA were determined by UV absorbance at 260 nm (Nanodrop). RNA samples were reverse-transcribed into complementary DNA (cDNA) using RETROscript (Ambion) utilising random decamers. The quantity of cDNA was determined by Quant-iT OliGreen ssDNA nucleic acid stain (Molecular Probes) measuring fluorescence (with excitation at 480 nm and emission at 520 nm) using a Tecan Infinite M200 microplate reader.
Relative real-time polymerase chain reaction for CYP1A1 and CYP1B1 expression
Real-time polymerase chain reaction (RT-PCR) was performed with approximately 25–40 ng of diluted cDNA in the presence of iQ SYBR Green Supermix (1x; Bio-Rad, Hercules, CA, USA) and 330 nM gene-specific primers using the iQ 5 Real-Time PCR Detection System (Bio-Rad). The sequence of the custom primers were: CYP1A1 forward, 5′-TCTGGCCTCTGTCATCTTCTGT-3′; CYP1A1 reverse, 5′-ATGGCCCTGGTGGATTCTTC-3′; CYP1B1 forward, 5′-ATGTCCTGGCCTTCCTTTATGA-3′; CYP1B1 reverse, 5′-AGACAGAGGTGTTGGCAGTG-3′; GAPDH forward, 5′-AACGTGTCAGTGGTGGACCT-3′ and GAPDH reverse, 5′-GCCTGCTTCACCACCTTCTT-3′. After an initial 1-min denaturation step, a three-step PCR was performed including: 10 sec at 95°C, 20 sec at 60°C and 20 sec at 75°C, for 40 cycles. Cycle threshold values were normalised to the housekeeping gene GAPDH, while the fold change was calculated using the 2−∆∆Ct method. This 96-h experiment was performed twice, and samples from all the time points of each experiment were assayed twice.
EROD assay
EROD assay was used to measure CYP1A1 and 1B1 enzyme-specific activities combined. In brief, MCF-7 cells were seeded in 12-well plates (75 000 cells per well) in triplicate. Attached cells were exposed to 1 μM BP in the presence or absence of 1 or 4 μM TMS or an equivalent volume of DMSO (solvent control). At 2, 4, 8, 12, 16, 24, 36, 48, 60, 72 or 96 h, media was removed and the plate was washed twice with PBS to stop the incubation. EROD solution [5 μM ethoxyresorufin (Sigma) and 1.5 mM salicyclamide (Sigma) dissolved in PBS] was added to each well containing cells and incubated at 37°C for 20 min. After incubation, 100 μl of EROD solution from each well was plated in duplicate into a white 96-well low-binding plate (Greiner Bio-One) using 5 μM resorufin as a standard. Conversion of ethoxyresorufin to resorufin was measured using a microplate reader (Infinite M200; Tecan) with excitation and emission wavelengths of 560 and 592 nm, respectively. Subsequently, remaining EROD solution was removed from wells and 1× RIPA Lysis Buffer (Upstate Cell Signaling) was added. After sonication (20 sec at 20% amplitude using a Sonifier Cell Disruptor; Heat Systems-Ultrasonics, Inc., Plainview, NY, USA), protein was quantitated using BCA Protein Assay Kit (Pierce, Thermo Fisher Scientific) utilising a bovine serum albumin standard, while absorbance was read on the Tecan Infinite M200 microplate reader at 562 nm. Overall, EROD activity was expressed as picomoles resorufin per minute per microgram protein.
AUC values and statistical comparisons
In order to compare the total BPdG adducts formed during a 92-h period of exposure (4–96 h), we have expressed the data as AUC4–96 h for BPdG adducts/106 nucleotides. AUC values were calculated using the Trapezoidal rule. The hourly rate for BPdG AUC was expressed as BPdG adducts/106 nucleotides × h. Similar calculations and designations were used to express AUC4–96 h for fold increase in induction of CYP1A1 or CYP1B1expression, compared to the unexposed solvent control, and AUC4–96 h for picomoles resorufin per minute per microgram protein for the EROD assay. Statistical comparisons among the different treatment groups were performed using the Mann–Whitney rank sum test and the Student’s t-test.
Results
Cell viability
To assess survival of MCF-7 cells exposed to 1 μM BP, 1 μM BP + 1 μM TMS and 1 μM BP + 4 μM TMS, cells were incubated for up to 72 h without a media change. Luminescence units from exposed cells, expressed as a percentage of luminescence units from solvent (DMSO)-treated cells at the same time intervals, are shown in Table I. In all exposure groups, cell viability remained >90% for the first 24 h, but by 72 h, cell survival dropped to 60–70%. This decrease may have been exacerbated by the absence of a media change. However, the cytotoxicity observed was essentially due to the presence of BP, as addition of TMS did not alter cell survival.
Table I.
| Hours of exposure | 1 μM BP | 1 μM BP + 1 μM TMS | 1 μM BP + 4 μM TMS |
| 4 | 92.6 ± 3.32 | 104.8 ± 2.73 | 100.9 ± 1.63 |
| 12 | 103.3 ± 3.20 | 106.8 ± 1.84 | 100.5 ± 2.61 |
| 24 | 98.7 ± 4.34 | 105.8 ± 1.62 | 105.4 ± 1.76 |
| 72 | 58.2 ± 7.74 | 71.0 ± 1.33 | 68.2 ± 5.27 |
Percent viability values, based on luminescence, for all three groups at each time point were not significantly different. TMS alone caused no reduction in cell viability (data not shown).
Cells were exposed on two separate occasions (Experiment 1 and Experiment 2) and for each experiment, triplicates were measured twice.
BPdG adduct formation
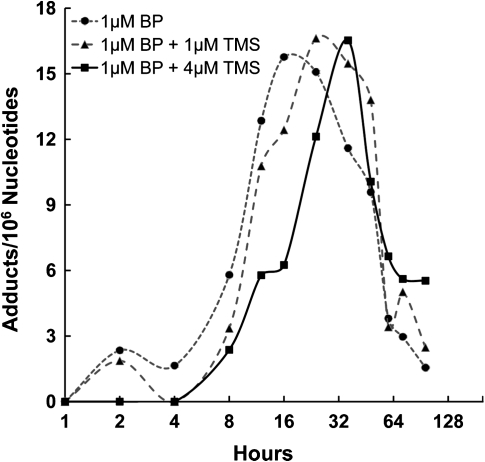
Levels of BPdG adducts were assessed by BPDE–DNA CIA, which quantifies unknown samples by comparison to a known BPDE–DNA standard curve. For the adduct analysis, the 96-h exposure was performed twice on separate occasions. In cells exposed to 1 μM BP alone, the BPdG levels reached a maximum level at 16 h with a value of 15.7 adducts/106 nucleotides (Figure 1). The maxima were 15.9 and 16.6 BPdG adducts/106 nucleotides at 24 and 48 h, respectively, for the groups exposed to 1 μM BP + 1 μM TMS and 1 μM BP + 4 μM TMS, respectively (Figure 1). To measure the AUC4–96 h and quantify the total DNA adduct burden, we calculated AUC4–96 h values as BPdG adducts/106 nucleotides (Table II). The AUC4–96 h values for cells exposed to BP plus TMS were similar to the value for cells exposed to BP alone (P = 0.180 by Mann–Whitney rank sum test for 1 μM TMS and P = 0.249 by Student’s t-test for 4 μM TMS). Therefore, the major consequence of TMS exposure on BPdG levels appeared to be a lengthening of the time at which the maximal adduct levels were reached.
Fig. 1.
BPdG profiles (adducts/106 nucleotides) shown here (Experiment 1) in MCF-7 cells exposed during 96 h to 1 μM BP (closed circles), 1 μM BP + 1 μM TMS (filled triangles) or 1 μM BP + 4 μM TMS (filled squares). These profiles are similar to those found in Experiment 2. The data represent average of two replicate exposures per experiment with three assay replicates for each exposure. Inter-experiment variation, inter-replicate variation and intra-assay variation was ∼20, 15 and 5%, respectively. Maximal adduct values, though similar for all treatment groups, were reached at 16, 24 and 48 h for cells exposed to1 μM BP, 1 μM BP + 1 μM TMS and 1 μM BP + 4 μM TMS, respectively.
Table II.
Average AUC4–96 h for BPdG levels (adducts/106 nucleotides)
| Exposure groups | % of BP alone | BPdG adducts/106 nucleotides AUC4–96 h (mean ± range, n=2)a | BPdG AUC adducts/106 nucleotides/h |
| 1 μM BP | 100 | 578 ± 116 | 6.28 |
| 1 μM BP+1 μM TMS | 117 | 678 ± 167 | 7.37 |
| 1 μM BP+4 μM TMS | 115 | 664 ± 89 | 7.22 |
AUC4–96 h for BPdG adducts/106 nucleotides determined in two experiments; there were no statistically significant differences among the three experimental groups (Mann–Whitney rank sum test).
CYP1A1 and CYP1B1 gene expression
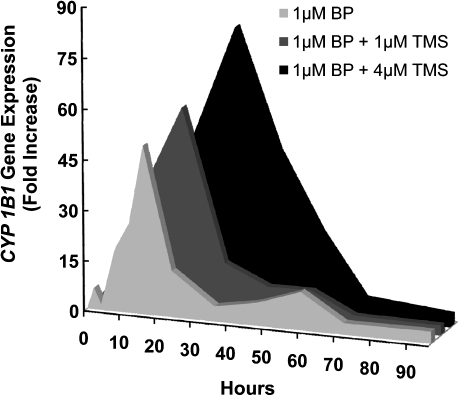
The CYP1A1 and CYP1B1 enzymes are considered to play a major role in the activation of BP to metabolites that bind to DNA. In these experiments, we used relative RT-PCR to evaluate CYP1A1 and CYP1B1 gene expression in two 96-h experiments performed as outlined above (Tables III and IV; Figures 2 and 3). Values shown are fold increase in expression compared to solvent control. The maximal fold increase values for CYP1A1 gene expression were 758, 1713 and 2995 for BP alone, BP + 1 μM TMS and BP + 4 μM TMS, respectively, with corresponding peak times of 16, 24 and 36 h, respectively (Figure 2). For CYP1A1 expression, we calculated the AUC4–96 h values to determine the magnitude of the CYP1A1 gene expression change (Table III). The data showed a delay in reaching the maximal fold increase, and a significant increase in CYP1A1 fold change AUC4–96 h for cells exposed to 1 μM BP + 4 μM TMS, compared to cells exposed to BP alone (P = 0.004) (Table III).
Table III.
Fold increase AUC4–96 h for CYP1A1 gene expression compared to solvent control
| Exposure groups | % of BP alone | CYP1A1 Expression fold increase vs unexposed cells AUC4–96 h (mean ± range, n=2)a | CYP1A1 Expression AUC fold increase/h |
| 1 μM BP | 100 | 6257 ± 2604 | 68.0 |
| 1 μM BP + 1 μM TMS | 277 | 17416 ± 11141 | 189.3 |
| 1 μM BP + 4 μM TMS | 1038 | 58972 ± 15394a | 641.0 |
CYP1A1 AUC4–96 h values from cells exposed to BP + 1 μM TMS were not different from cells exposed to BP alone (P = 0.343 by Mann–Whitney rank sum test). However, CYP1A1 AUC4–96 h values from cells exposed to BP + 4 μM TMS were significantly different from cells exposed to BP alone (P = 0.004 by Mann–Whitney rank sum test).
Table IV.
Fold increase AUC4-96h for CYP1B1 gene expression compared to solvent control
| Exposure groups | % of BP alone | CYP1B1 Expression fold increase vs unexposed cells AUC4–96 h (mean ± range, n=2)a | CYP1B1 Expression AUC fold increase/h |
| 1 μM BP | 100 | 876 ± 137 | 9.5 |
| 1 μM BP + 1 μM TMS | 137 | 1214 ± 298 | 13.2 |
| 1 μM BP + 4 μM TMS | 311 | 2634 ± 19a | 28.8 |
CYP1B1 AUC4–96 h values from cells exposed to BP + 1 μM TMS were not different from cells exposed to BP alone (P = 0.343 by Mann–Whitney rank sum test). However, CYP1B1 AUC4–96 h values from cells exposed to BP + 4 μM TMS were significantly different from cells exposed to BP alone (P = 0.029 by Mann–Whitney rank sum test).
Fig. 2.
CYP1A1 expression (fold increase compared to solvent control) profiles shown here (Experiment 1) represent MCF-7 cells exposed during 96 h to 1 μM BP (light gray), 1 μM BP + 1 μM TMS (dark gray) or 1 μM BP + 4 μM TMS (black). These profiles are similar to those found in Experiment 2. The group exposed to 1 μM BP + 4 μM TMS had a significantly higher (P = 0.004) CYP1A1 AUC4–96 h fold change, compared to the group exposed only to 1 μM BP. Maximal CYP1A1 expression (fold increase) was reached at 16, 24 and 48 h for 1 μM BP, 1 μM BP + 1 μM TMS and 1 μM BP + 4 μM TMS, respectively.
Fig. 3.
CYP1B1 expression (fold increase compared to solvent control) profiles shown here (Experiment 1) in MCF-7 cells exposed during 96 h to 1 μM BP (light grey), 1 μM BP + 1 μM TMS (dark gray) or 1 μM BP + 4 μM TMS (black). Values are similar to those found in Experiment 2. The group exposed to 1 μM BP + 4 μM TMS had a significantly higher (P = 0.029) CYP1B1 AUC4–96 h fold change compared to the group exposed to 1 μM BP. Maximal CYP1B1 expression (fold increase) was reached at 16, 24 and 48 h for 1 μM BP, 1 μM BP + 1 μM TMS and 1 μM BP + 4 μM TMS, respectively.
CYP1B1 gene expression was also up-regulated upon exposure to BP. Fold increase values for maximal CYP1B1 gene expression were 50, 60 and 83 for 1 μM BP, 1 μM BP + 1 μM TMS and 1 μM BP + 4 μM TMS, respectively, and occurred at 16, 24 and 36 h, respectively (Figure 3). Mean CYP1B1 gene expression fold change AUC4–96 h values were 876, 1214 and 2635 for BP alone, BP + 1 μM TMS and BP + 4 μM TMS, respectively (Table IV), and were significantly increased in cells exposed to BP + 4 μM TMS in comparison to BP alone (P = 0.029). Therefore, TMS exposure, in combination with BP, increased overall CYP1A1 and CYP1B1 gene expression in a dose-dependent manner.
EROD enzyme assay for CYP1A1 and CYP1B1 activity
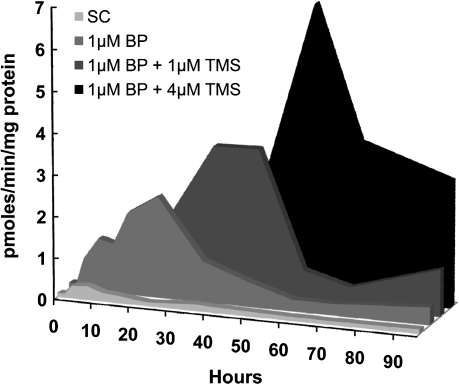
The EROD assay was used to link CYP1A1 and CYP1B1 gene expression to CYP1A1/CYP1B1 enzyme activity. The EROD assay measures the two enzyme activities together, and data were expressed as picomoles resorufin per minute per microgram protein. As expected, BP exposure induced EROD activity in MCF-7 cells, when compared to solvent-exposed cells (Figure 4, Table V). The addition of TMS delayed BP-induced EROD activity, with maximal values found at 24, 48 and 60 h for BP alone, BP + 1 μM TMS and BP + 4 μM TMS, respectively. The maximal values were higher in the presence of TMS, showing 2.36, 5.08 and 9.15 pmol resorufin/min/mg protein, for BP alone, BP + 1 μM TMS and BP + 4 μM TMS, respectively (Figure 4). AUC4–96 h values were 81.3, 151.9 and 268.5 pmol resorufin/min/mg protein for BP alone, BP + 1 μM TMS and BP + 4 μM TMS, respectively, with significantly increased values in both TMS groups, compared to the group exposed to BP alone (P < 0.05 for both groups) (Table V). Overall, TMS exposure increased and delayed BP-induced EROD activity, even beyond the delays observed for BPdG formation and CYP1A1 and CYP1B1 gene expression.
Fig. 4.
EROD activity (picomoles resorufin per minute per milligram protein) profiles shown here (Experiment 1) in MCF-7 cells exposed during 96 h to: solvent control (light gray), 1 μM BP (medium gray), 1 μM BP + 1 μM TMS (dark gray) or 1 μM BP + 4 μM TMS (black). Profiles are similar to those found in Experiment 2. EROD activity in cells exposed to BP plus TMS was significantly higher than EROD activity in cells exposed to BP alone (P = 0.004 for BP versus BP + 1 μM TMS; P = 0.002 for BP versus BP + 4 μM TMS). Maximal EROD activity levels were reached at 24, 48 and 60 h for 1 μM BP, 1 μM BP + 1 μM TMS and 1 μM BP + 4 μM TMS, respectively.
Table V.
Values for EROD activity as picomoles per minute per microgram protein AUC4–96 h
| Exposure groups | % of BP alone | pmol/min/mg protein AUC4–96 h (mean ± range, n = 2) | EROD pmol/min/mg protein per hour |
| Solvent control | 12.3 ± 5.5 | 0.13 | |
| 1 μM BP | 100 | 81.3 ± 17.2 | 0.88 |
| 1 μM BP + 1 μM TMS | 156 | 151.9 ± 42.4a | 1.65 |
| 1 μM BP + 4 μM TMS | 276 | 268.4 ± 90.4a | 2.92 |
EROD-specific activity expressed as picomoles per minute per milligram protein. AUC4–96 h in cells exposed to BP plus TMS was significantly higher compared to EROD activity in cells exposed to BP alone (P = 0.004 for BP versus BP +1 μM TMS and P = 0.002 for BP versus BP + 4 μM TMS by Mann–Whitney rank sum test).
Discussion
Because the activities of the CYP1A1 and CYP1B1 enzymes are considered essential for BPdG adduct formation and TMS is a potent CYP1B1 inhibitor, we hypothesised that addition of TMS to the culture media of cells exposed to BP would result in a reduced number of BPdG adducts. While some investigators have addressed the effect of TMS on PAH–DNA adduct formation in cells exposed for 24 h or less (32,36), we hypothesised that following events for 96 h of exposure would be more informative. In these studies, during 96 h of exposure to 0, 1 and 4 μM TMS in the presence of 1 μM BP, we found maximal BPdG adduct formation at 16, 24 and 48 h, respectively, showing that TMS delays the activation of BP. A similar delay was found in time required to reach maximal CYP1A1 and CYP1B1 gene expression, and maximal EROD activity was delayed even further. In the presence of TMS, we observed a slowing of the biotransformation processes, as well as higher AUC4–96 h values for CYP1A1 and CYP1B1 gene expression and EROD activity, compared to cells exposed to BP alone.
Because EROD activity measures the combined CYP1A1/CYP1B1 enzyme activities and TMS slowed the induction of BP-induced EROD activity, we were unable to distinguish whether or not TMS selectively altered either CYP1A1 or CYP1B1. Furthermore, the cells exposed to both BP and TMS showed very similar effects for CYP1A1 and CYP1B1 gene expression. Therefore, it was not possible to ascertain the role of either enzyme in modulation of BPdG levels.
The CYP1B1 enzyme is an important component of both oestrogen and PAH metabolism. In humans, TMS is being considered for chemoprevention of oestradiol-mediated carcinogenesis because it is a competitive inhibitor of CYP1B1, with a 50-fold selectivity for CYP1B1 compared to CYP1A1 (30). In MCF-7 human breast cancer cells, TMS effectively inhibited CYP1B1 expression and PAH-induced EROD activity (30,32). Short incubations (<1 h) with TMS inhibited 4-hydroxylation of oestradiol in CYP1B1-expressing E.coli membranes (30). In addition, TMS reduced tumour volume of MCF-7 breast cancer cell xenografts grown in mice, by up to 53% (33).
The unanticipated delay in BP activation found in MCF-7 cells exposed to BP plus TMS could be due to various phenomena, many of which may be addressed experimentally. Since TMS is a competitive inhibitor of CYP1B1, the TMS delay may be attributed to competition between BP and TMS for conversion by CYP1B1. A previous study showed that incubation of TMS with bicistronic CYP1B1 membranes and NADPH produced a single metabolite at a very slow rate (<0.1/min) (30). Therefore, the slow metabolism of TMS by CYP1B1 may delay activation of BP. In addition, resveratrol, a TMS analogue, is an AHR antagonist and suppresses dioxin-responsive element-binding activity (37,38). Therefore, TMS may also inhibit AHR-dependent activity (31) by competing with BP for binding to the AHR. Additionally, the slow metabolism of TMS may allow BP intermediates to remain stable for a longer period of time, and some BP metabolites, such as benzo[a]pyrene-7,8-dione, bind the AHR and induce AHR-dependent gene expression (39). It is important to recognise that other CYPs and enzyme cascades may be involved in BP and TMS metabolism, and further studies are required to elucidate the impact of TMS upon formation of individual BP metabolites.
Though TMS is a potent competitive inhibitor of CYP enzymes at the enzymatic level, control incubations performed for 24 h with TMS (1.0 or 4.0 μM) alone in MCF-7 cells did not result in significant differences in cell viability or the cellular EROD activity (data not shown). However, TMS exposure (24 h) significantly induced CYP gene expression. CYP1A1 was induced by 780-fold (1.0 μM TMS) and 360-fold (4.0 μM TMS), and CYP1B1 was induced by 3-fold (1.0 μM TMS) and 2.5-fold (4.0 μM TMS), whereas BP (1.0 μM, 24 h) alone induced CYP1A1 by 181-fold and CYP1B1 by 4-fold. Since our focus is to understand the modulation of BPdG adduct formation by TMS, we did not perform the time-kinetic AUC experiments with TMS alone.
Potential use of TMS in the human population may enhance the risk of steroid-induced carcinogenesis and/or PAH-induced carcinogenesis in steroid-responsive tissues of humans who are constantly exposed to high levels of environmental PAHs. Here, TMS failed to inhibit BPdG adduct formation and did slow the rate of BP metabolism, which likely increased the half-life of BP and its metabolites and may have contributed to the overall increase in CYP gene expression and enzyme activity. However, despite significant increases in CYP gene expression and enzyme activity, the AUC4–96 h for BPdG adduct formation was not significantly increased. It is possible that this is due to a threshold effect from the fixed cellular concentration of BP (1.0 μM).
The results presented here are, to some extent, directly contrary to a previously published study (31), which also employed MCF-7 cells. There are, however, substantial differences in the two study designs. Our study did not involve 2, 3, 7, 8-tetrachloridibenzo-p-dioxin as the activating agent, and we exposed cells simultaneously, not sequentially, to the BP and the TMS. In addition, our cells were mostly confluent at the time of exposure, and it is not clear that that was the case with the study by Chun et al. (31). More confluent cells would likely have sustained less toxicity to TMS alone. In our study, there was some toxicity at the 72-h point but that appeared to be largely due to the presence of the BP (Table I). It is unclear to what extent the different methods for measuring cell viability, used in the two studies, may have influenced the cytotoxicity numbers. Finally, the time courses of the two experiments were quite different, as Chun et al. (31) followed events for 48 h, and our study was carried out to 96 h. There may be other reasons for the discrepancies observed, but these are some of the most obvious ones.
In conclusion, our results demonstrate that during chronic exposure to BP, the formation of DNA damage was not attenuated by simultaneous addition of TMS. The fact that our studies with BP were carried out for 96 h and DNA adduct formation was not reduced, points to the possibility that in humans the use of TMS as a potential chemopreventive agent should be approached with caution.
Funding
Intramural program of the Center for Cancer Research, National Cancer Institute, National Institute of Health
Acknowledgments
Conflict of interest statement: None declared.
References
- 1.Phillips DH. Fifty years of benzo(a)pyrene. Nature. 1983;303:468–472. doi: 10.1038/303468a0. [DOI] [PubMed] [Google Scholar]
- 2.Rothman N, Poirier MC, Baser ME, Hansen JA, Gentile C, Bowman ED, Strickland PT. Formation of polycyclic aromatic hydrocarbon-DNA adducts in peripheral white blood cells during consumption of charcoal- broiled beef. Carcinogenesis. 1990;11:1241–1243. doi: 10.1093/carcin/11.7.1241. [DOI] [PubMed] [Google Scholar]
- 3.Rothman N, Correa-Villasenor A, Ford DP, Poirier MC, Haas R, Hansen JA, O'Toole T, Strickland PT. Contribution of occupation and diet to white blood cell polycyclic aromatic hydrocarbon-DNA adducts in wildland firefighters. Cancer Epidemiol. Biomarkers Prev. 1993;2:341–348. [PubMed] [Google Scholar]
- 4.Roth MJ, Strickland KL, Wang GQ, Rothman N, Greenberg A, Dawsey SM. High levels of carcinogenic polycyclic aromatic hydrocarbons present within food from Linxian, China may contribute to that region's high incidence of oesophageal cancer. Eur. J. Cancer. 1998;34:757–758. doi: 10.1016/s0959-8049(97)10071-5. [DOI] [PubMed] [Google Scholar]
- 5.Boysen G, Hecht SS. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutat. Res. 2003;543:17–30. doi: 10.1016/s1383-5742(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 6.IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010;92:1–853. [PMC free article] [PubMed] [Google Scholar]
- 7.Nebert DW, Puga A, Vasiliou V. Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann. N. Y. Acad. Sci. 1993;685:624–640. doi: 10.1111/j.1749-6632.1993.tb35928.x. [DOI] [PubMed] [Google Scholar]
- 8.Denison MS, Whitlock JP., Jr. Xenobiotic-inducible transcription of cytochrome P450 genes. J. Biol. Chem. 1995;270:18175–18178. doi: 10.1074/jbc.270.31.18175. [DOI] [PubMed] [Google Scholar]
- 9.Hankinson O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 10.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada T, Gillam EM, Oda Y, Tsumura F, Sutter TR, Guengerich FP, Inoue K. Metabolism of benzo[a]pyrene to trans-7,8-dihydroxy-7, 8-dihydrobenzo[a]pyrene by recombinant human cytochrome P450 1B1 and purified liver epoxide hydrolase. Chem. Res. Toxicol. 1999;12:623–629. doi: 10.1021/tx990028s. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein IB, Jeffrey AM, Jennette KW, Blobstein SH, Harvey RG, Harris C, Autrup H, Kasai H, Nakanishi K. Benzo(a)pyrene diol epoxides as intermediates in nucleic acid binding in vitro and in vivo. Science. 1976;193:592–595. doi: 10.1126/science.959820. [DOI] [PubMed] [Google Scholar]
- 13.Gyorffy E, Anna L, Kovacs K, Rudnai P, Schoket B. Correlation between biomarkers of human exposure to genotoxins with focus on carcinogen-DNA adducts. Mutagenesis. 2008;23:1–18. doi: 10.1093/mutage/gem043. [DOI] [PubMed] [Google Scholar]
- 14.Gunter MJ, Divi RL, Kulldorff M, et al. Leukocyte polycyclic aromatic hydrocarbon-DNA adduct formation and colorectal adenoma 1. Carcinogenesis. 2007;28:1426–1429. doi: 10.1093/carcin/bgm022. [DOI] [PubMed] [Google Scholar]
- 15.Tang D, Santella RM, Blackwood AM, Young TL, Mayer J, Jaretzki A, Grantham S, Tsai WY, Perera FP. A molecular epidemiological case-control study of lung cancer. Cancer Epidemiol. Biomarkers Prev. 1995;4:341–346. [PubMed] [Google Scholar]
- 16.Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- 17.Kimura S, Gonzalez FJ, Nebert DW. Tissue-specific expression of the mouse dioxin-inducible P(1)450 and P(3)450 genes: differential transcriptional activation and mRNA stability in liver and extrahepatic tissues. Mol. Cell. Biol. 1986;6:1471–1477. doi: 10.1128/mcb.6.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6:75–91. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalieri EL, Rogan EG. A unifying mechanism in the initiation of cancer and other diseases by catechol quinones. Ann. N. Y. Acad. Sci. 2004;1028:247–257. doi: 10.1196/annals.1322.029. [DOI] [PubMed] [Google Scholar]
- 20.Cavalieri EL, Rogan EG, Chakravarti D. Initiation of cancer and other diseases by catechol ortho-quinones: a unifying mechanism. Cell. Mol. Life Sci. 2002;59:665–681. doi: 10.1007/s00018-002-8456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavalieri EL, Stack DE, Devanesan PD, et al. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl Acad. Sci. U S A. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu F, Zahid M, Saeed M, Cavalieri EL, Rogan EG. Estrogen metabolism and formation of estrogen-DNA adducts in estradiol-treated MCF-10F cells. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction and catechol-O-methyltransferase inhibition. J. Steroid Biochem. Mol. Biol. 2007;105:150–158. doi: 10.1016/j.jsbmb.2006.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu F, Zahid M, Wang C, Saeed M, Cavalieri EL, Rogan EG. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. (Phila) 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray GI, Taylor MC, McFadyen MC, McKay JA, Greenlee WF, Burke MD, Melvin WT. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997;57:3026–3031. [PubMed] [Google Scholar]
- 25.Carnell DM, Smith RE, Daley FM, Barber PR, Hoskin PJ, Wilson GD, Murray GI, Everett SA. Target validation of cytochrome P450 CYP1B1 in prostate carcinoma with protein expression in associated hyperplastic and premalignant tissue. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:500–509. doi: 10.1016/j.ijrobp.2003.09.064. [DOI] [PubMed] [Google Scholar]
- 26.Chun YJ, Kim S. Discovery of cytochrome P450 1B1 inhibitors as new promising anti-cancer agents. Med. Res. Rev. 2003;23:657–668. doi: 10.1002/med.10050. [DOI] [PubMed] [Google Scholar]
- 27.McFadyen MC, Murray GI. Cytochrome P450 1B1: a novel anticancer therapeutic target. Future Oncol. 2005;1:259–263. doi: 10.1517/14796694.1.2.259. [DOI] [PubMed] [Google Scholar]
- 28.Bruno RD, Njar VC. Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development. Bioorg. Med. Chem. 2007;15:5047–5060. doi: 10.1016/j.bmc.2007.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buters JT, Doehmer J, Gonzalez FJ. Cytochrome P450-null mice. Drug Metab. Rev. 1999;31:437–447. doi: 10.1081/dmr-100101929. [DOI] [PubMed] [Google Scholar]
- 30.Chun YJ, Kim S, Kim D, Lee SK, Guengerich FP. A new selective and potent inhibitor of human cytochrome P450 1B1 and its application to antimutagenesis. Cancer Res. 2001;61:8164–8170. [PubMed] [Google Scholar]
- 31.Chun YJ, Lee SK, Kim MY. Modulation of human cytochrome P450 1B1 expression by 2,4,3',5'-tetramethoxystilbene. Drug Metab. Dispos. 2005;33:1771–1776. doi: 10.1124/dmd.105.006502. [DOI] [PubMed] [Google Scholar]
- 32.Mahadevan B, Luch A, Atkin J, Haynes M, Nguyen T, Baird WM. Inhibition of human cytochrome p450 1b1 further clarifies its role in the activation of dibenzo[a, l]pyrene in cells in culture. J. Biochem. Mol. Toxicol. 2007;21:101–109. doi: 10.1002/jbt.20168. [DOI] [PubMed] [Google Scholar]
- 33.Park H, Aiyar SE, Fan P, et al. Effects of tetramethoxystilbene on hormone-resistant breast cancer cells: biological and biochemical mechanisms of action. Cancer Res. 2007;67:5717–5726. doi: 10.1158/0008-5472.CAN-07-0056. [DOI] [PubMed] [Google Scholar]
- 34.Ross JA, Nelson GB, Wilson KH, Rabinowitz JR, Galati A, Stoner GD, Nesnow S, Mass MJ. Adenomas induced by polycyclic aromatic hydrocarbons in strain A/J mouse lung correlate with time-integrated DNA adduct levels. Cancer Res. 1995;55:1039–1044. [PubMed] [Google Scholar]
- 35.Divi RL, Beland FA, Fu PP, et al. Highly sensitive chemiluminescence immunoassay for benzo[a]pyrene-DNA adducts: validation by comparison with other methods, and use in human biomonitoring. Carcinogenesis. 2002;23:2043–2049. doi: 10.1093/carcin/23.12.2043. [DOI] [PubMed] [Google Scholar]
- 36.Ruan Q, Gelhaus SL, Penning TM, Harvey RG, Blair IA. Aldo-keto reductase- and cytochrome P450-dependent formation of benzo[a]pyrene-derived DNA adducts in human bronchoalveolar cells. Chem. Res. Toxicol. 2007;20:424–431. doi: 10.1021/tx060180b. [DOI] [PubMed] [Google Scholar]
- 37.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol. Pharmacol. 1999;56:784–790. [PubMed] [Google Scholar]
- 38.Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ, Kim DH, Kang KS, Cho MH, Surh YJ. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25:2005–2013. doi: 10.1093/carcin/bgh183. [DOI] [PubMed] [Google Scholar]
- 39.Burczynski ME, Penning TM. Genotoxic polycyclic aromatic hydrocarbon ortho-quinones generated by aldo-keto reductases induce CYP1A1 via nuclear translocation of the aryl hydrocarbon receptor. Cancer Res. 2000;60:908–915. [PubMed] [Google Scholar]