Abstract
This review article discusses recent work on the melatonin-mediated circadian regulation and integration of molecular, dietary and metabolic signaling mechanisms involved in human breast cancer growth and the consequences of circadian disruption by exposure to light-at-night (LAN). The antiproliferative effects of the circadian melatonin signal are mediated through a major mechanism involving the activation of MT1 melatonin receptors expressed in human breast cancer cell lines and xenografts. In estrogen receptor (ERα+) human breast cancer cells, melatonin suppresses both ERα mRNA expression and estrogen-induced transcriptional activity of the ERα via MT1-induced activation of Gαi2 signaling and reduction of cAMP levels. Melatonin also regulates the transactivation of additional members of the steroid hormone/nuclear receptor super-family, enzymes involved in estrogen metabolism, expression/activation of telomerase and the expression of core clock and clock-related genes. The anti-invasive/anti-metastatic actions of melatonin involve the blockade of p38 phosphorylation and the expression of matrix metalloproteinases. Melatonin also inhibits the growth of human breast cancer xenografts via another critical pathway involving MT1-mediated suppression of cAMP leading to blockade of linoleic acid (LA) uptake and its metabolism to the mitogenic signaling molecule 13-hydroxyoctadecadienoic acid (13-HODE). Down-regulation of 13-HODE reduces the activation of growth factor pathways supporting cell proliferation and survival. Experimental evidence in rats and humans indicating that LAN-induced circadian disruption of the nocturnal melatonin signal activates human breast cancer growth, metabolism and signaling provides the strongest mechanistic support, thus far, for population and ecological studies demonstrating elevated breast cancer risk in night shift workers and other individuals increasingly exposed to LAN.
Keywords: Melatonin, Breast Cancer, Diet, Metabolism, Molecular Signaling, Circadian, Disruption
Introduction
The regular alternation in the light/dark cycle over each 24-hour period is the major synchronizer of the endogenous circadian pacemaker located in the suprachiasmatic nuclei (SCN) of the brain in all mammals including humans. The SCN, the neurons of which are most actively firing during the daytime, regulate a variety of hormonal, metabolic and behavioral responses so that internal physiological and metabolic processes are not only in synchrony with the external environment but are temporally coordinated and integrated with one another as well [1]. Environmental light is sensed by a small population of intrinsically photosensitive retinal ganglion cells in the retina that contain the blue light-sensitive photopigment melanopsin [2]. From there, information about the light/dark cycle is transmitted to the SCN via the retinohypothalamic tract. A polysynaptic output pathway from the SCN continues to transmit photoperiodic information ultimately to the pineal gland via the brainstem reticular formation, upper thoracic spinal cord and superior cervical ganglia of the sympathetic chain [3]. During nighttime, substantially reduced inhibitory neuronal activity in the SCN drives the pineal gland to produce high concentrations of the chronobiotic neurohormone melatonin, a potent anti-cancer indoleamine molecule. On the other hand, the pineal synthesis of melatonin during the daytime is almost negligible [4].
High nocturnal blood levels of melatonin are responsible for telling all the cells of the body, including cancer cells, that it is nighttime [5]. Changes in either the length of the day or the timing/phasing of light exposure can compromise SCN activity and/or the pineal gland production of melatonin, a phenomenon referred to as circadian disruption. Another aspect of circadian disruption relates to the ability of exposure of an organism to light at night (LAN) to suppress the amplitude of the nocturnal circadian melatonin signal [6]. The central biological timing mechanism provided by the SCN, including nocturnal melatonin production, has a profound impact on the development and growth of a variety of experimental malignancies in animal models of cancer [6-9]. Circadian disruption caused by either increasing the duration of daily light exposure [10, 11], chronically advancing the phasing of light exposure (chronic jet lag) [12] or LAN-induced suppression of the nocturnal circadian melatonin signal stimulates the development and growth of experimental tumors [10, 11]. Of all the forms of circadian disruption examined thus far, LAN-induced disruption of melatonin production appears to be the most potent promoter of oncogenesis [10, 11].
We now know that most, if not all, normal physiological and metabolic processes as well their pathological sequelae are temporally organized and vary predictably throughout the day and night to accommodate an organism’s need to anticipate and adapt to changes in its external environment. The daily rhythmic organization of human physiology and metabolism that is so characteristic of normal cells and tissues also persists during the earlier phases of oncogenesis in malignant neoplasms derived from these same structures. The temporal expression of a multitude of processes governing cancer initiation, growth, progression, invasion/metastasis is regulated by host circadian rhythmic outputs from the central circadian pacemaker in the SCN [13]. At the same time, a number of the very same core molecular clock genes and their respective proteins that operate the master clock in the SCN are coordinately expressed in a circadian manner not only in normal cells in the periphery but in cancer cells as well [14]. Currently, it is of great interest to cancer biologists that these core clock genes and proteins may be involved in cellular processes such as cell cycle traverse, cell proliferation, DNA damage/repair mechanisms, tumor suppressor activities, cell survival and apoptosis which themselves exhibit circadian rhythms [15]. Furthermore, it is clear that the central circadian system and perhaps even peripheral clock mechanisms are involved in the regulation of intermediary metabolism [16].
A major question in circadian cancer biology is how the central circadian pacemaker (i.e., SCN) transmits biological timing information to diverse and remote peripheral locations of cancer cells embedded within their microenvironment for the circadian organization of cell proliferation and tumor growth. Also, how does the central circadian timing system influence the regulatory interactions between signal transduction, transcriptional activity, intermediary metabolism and cell proliferation in tumors? Moreover, what role(s) do peripheral clock-controlled genes (CCGs) within cancer cells play in the daily organization of cancer cell proliferation and how are these peripheral circadian cancer clock mechanisms integrated and coordinated with central clock regulation of tumor growth processes? In this review article, we will focus on some of the most current work, primarily from our own laboratories, on the role of the nocturnal melatonin signal in the regulation of signal transduction, transcriptional activity, CCG gene expression, dietary/metabolic signaling and proliferation primarily in human breast cancer cells in vitro and in tissue-isolated human breast cancer xenografts in vivo. We will also discuss the consequences of circadian disruption of the melatonin signal by LAN on breast cancer growth processes in order to provide some initial answers to these questions posed above.
Melatonin inhibition of human breast cancer cell proliferation
Melatonin’s anticancer effects appear to be favorably disposed towards the suppression of cell proliferation. A physiological peak nighttime serum value of 1 nM in humans, significantly and directly suppresses the proliferation of both estrogen receptor α positive (ERα+) human breast cancer cell lines (i.e., MCF-7, T47D, ZR-75-1) and at least one estrogen receptor α negative (ERα−) (i.e., MDA-MB-468) cell line in vitro by delaying and slowing the progression of cells through the cell cycle [7, 17-19]. These growth-inhibitory actions of melatonin, however, exhibit a bell-shaped dose-response pattern with nocturnal physiological concentrations maximally inhibiting proliferation, while higher or lower concentrations exert little if any effect on ERα+ MCF-7 breast cancer cells [17]. Melatonin’s regulation of the cellular redox state and the maintenance of a reducing intracellular environment are critical for physiological melatonin’s antiproliferative effects to occur in ERα+ MCF-7 breast cancer cells [7]. With the exception of MDA-MB-468 cells, melatonin fails to inhibit the proliferation of most ERα− human breast cancer cell lines such as MDA-MB-231, MDA-MB-330 or BT-20 [19]. Interestingly, physiological as well as pharmacological levels of melatonin suppress the proliferation and growth of tissue-isolated ERα− and progesterone receptor negative (PR)- human breast cancer xenografts in nude rats via an MT1 receptor-mediated mechanism [20]. The antiproliferative actions of melatonin have been confirmed by numerous laboratories on human breast cancer cells in vitro as well as in other human cancer cell types (i.e., prostate, ovary, endometrium, liver, colon, placenta, bone, etc.) [7].
Melatonin receptor-mediated melatonin suppression of signal transduction and its impact on gene expression in human breast cancer cells
Several groups have demonstrated that melatonin binds to and activates MT1 and MT2 G protein-coupled receptors that, in turn, activate a number of G proteins including Gαi2, Gαi3, Gαq and Gα11 in a variety of tissues [21]. The activated MT1 receptor mediates the oncostatic actions of melatonin in ERα+ MCF-7 human breast cancer cells and is coupled to Gαi2, Gαi3, Gαq and Gα11 in this cell line. The growth-inhibitory effects of melatonin in breast cancer cells are reversed by non-selective melatonin MT1 and MT2 receptor antagonists while overexpression of the MT1 receptor in human breast cancer cells significantly enhances both the in vitro and in vivo inhibitory response of tumor cells to melatonin [22, 23]. Furthermore, confocal microscopic analysis reveals that the MT1 receptor is localized to the MCF-7 cell membrane and that some MT1 receptors colocalize with caveolin-1, a key protein in caveolae (lipid rafts) membrane-associated signaling platform [24]. Immunohistochemical analysis of 50 breast tumor biopsy specimens demonstrated a significant positive correlation between MT1 receptor and ERα expression [19]. Although MT2 receptors are not detectable in MCF-7 breast cancer cells in culture [25], they are expressed but are apparently non-functional in tissue-isolated ERα− MCF-7 human breast cancer xenografts [26].
Melatonin, at physiological nocturnal blood concentrations, modulates the transcriptional activity of ERα and other nuclear receptors in human breast cancer cells
That there is an important interplay between the melatonin and estrogen signaling pathways was first indicated by the fact that melatonin could suppress the estrogen-induced proliferation of human breast cancer cells in culture [27, 28]. Subsequently, melatonin was shown to not only suppress ERα mRNA expression [29] but estrogen-induced transcriptional activity of the ERα as well [25]. This latter effect down-regulates the expression of a number of mitogenic proteins and pathways including the anti-apoptotic protein Bcl-2, while inducing the expression of growth-inhibitory and apoptotic pathways including TGF-α and Bax [25]. The inhibitory action of melatonin on ERα transcriptional activity is mediated via the activation of Gαi2 signaling prompting a decrease in cAMP/PKA levels that culminate in decreased phosphorylation of the PKA sensitive S263 site on the ERα [26]. These findings that melatonin induces the modulation of ERα transcriptional activity were confirmed by another group who further reported that calmodulin (CaM) was also involved in this process [30], an observation that is consistent with the fact that the PKA pathway can impact Ca++/CaM activity in a number of tissues [31].
In addition to suppressing ERα transcriptional activity, melatonin, via different G proteins, can also modulate the transactivation of some other members of the steroid hormone/nuclear receptor super-family. For example, while melatonin reduces the transcriptional activity of the glucocorticoid receptor (GR), it augments the transcriptional activation of the retinoic acid receptor alpha (RARα) in human breast cancer cells in response to their ligands, via activation of Gαi2 and Gαq proteins, respectively [32]. In addition, melatonin decreases the transcriptional activity of the RA-related orphan receptor alpha (RORα) in human breast cancer cells [33]. Moreover, melatonin potentiates the transcriptional activity of the retinoic × receptor alpha (RARα) and the vitamin D3 receptor (VDR), in response to the specific ligands. It appears, however, that melatonin does not modulate all nuclear receptors inasmuch as neither stimulatory nor inhibitory effects of melatonin on ERβ transcriptional activity have been observed in either human breast cancer cells or in ERβ expressing HEK293 embryonic kidney cells [26]. It is still unclear whether melatonin’s modulatory effects on nuclear receptors, particularly ERα, are mediated via direct changes in receptor phosphorylation/dephosphorylation, regulation of coactivator (ie. CaM, SRC1, etc.) and/or coreppressor phosphorylation. Regardless of the exact mechanism(s), these data clearly demonstrate melatonin’s important impact on gene expression in human breast cancer cells by its action on specific signal transduction pathways.
Melatonin, at physiological nocturnal blood concentrations, acts as a selective estrogen enzyme modulator (SEEM) in the inhibition of human breast cancer growth
During the postmenopausal period, estrogens are locally synthesized in breast tissue from adrenal-derived androgenic precursors by the enzyme aromatase [34]. Aromatase is thought to play an important role in postmenopausal breast cancer by converting androstenedione to estrone/estradiol at sufficient levels to support preneoplastic changes and maintain high breast cancer levels of these estrogens in spite of very low or no circulating levels of estradiol. Other enzymes such as estrogen sulfatase (STS) and 17β-hydroxysteroid dehydrogenase (17β-HSD) convert less active estrogens into more potent forms while estrogen sulfotransferases (EST) catalyze estrogen into their less active sulfated forms. Melatonin at nocturnal physiological circulating concentrations inhibits aromatase, STS and 17β-HSD expression and activity in ER+ MCF-7 human breast cancer cells while at the same time stimulating EST. Furthermore, at pharmacological concentrations melatonin inhibits aromatase activity and in situ synthesis of estrogens in DMBA-induced rat mammary tumors [35].
The involvement of the MT1 receptor in inhibiting the aromatase pathway is supported by findings that transfection of the MT1 receptor in MCF-7 cells significantly decreases aromatase activity of the cells as compared with vector-transfected cells. The proliferation of estrogen-sensitive MCF-7 cells in an estradiol-free media but in the presence of testosterone is markedly inhibited by melatonin (1 nM) in those cells overexpressing the MT1 receptor than in vector transfected cells. Furthermore, MT1 receptor transfection alone induces a significant 55% inhibition of aromatase steady-state mRNA expression in comparison to vector-transfected MCF-7 cells while exposure of these cells to melatonin (1 nM) significantly down-regulates aromatase mRNA expression than in vector-transfected cells. Therefore, in addition to its role as a selective estrogen receptor modulator (SERM), melatonin also appears to fulfill the requirements as a SEEM; its functions as both a SERM and SEEM appear to be exerted, at least in part, through an MT1 melatonin receptor-mediated mechanism.
Melatonin, at nocturnal physiological blood concentrations, regulates telomerase activity and expression in human breast cancer
Telomerase is a specialized ribonucleoprotein DNA that elongates the repeats of short TG-rich sequences onto telomeres which constitute both ends of linear chromosomes in eukaryotic cells. Telomeres are progressively shortened during each cell cycle division in normal differentiated cells, in which telomerase activity is usually absent or low relative to many cancer cells, providing a signal for cell senescence [36]. In most cancer cells, however, telomerase is activated to avert this limiting step thus providing an additional mechanism for unrestricted proliferative capacity in these cells and malignant tumors through telomere elongation. The expression of TERT, the telomerase subunit that is the main determinant of enzyme activity tightly correlates with and is a surrogate for telomerase activation [37, 38]. At pharmacological concentrations administered in the drinking water, melatonin not only inhibits the growth of ERα+ MCF-7 human breast cancer xenografts in nude mice, but it also suppresses telomerase activity, as measured by the Telomerase Repeats Amplification Protocol (TRAP assay) in these tumors as compared with controls. Additionally, melatonin at a physiological concentration (1 nM) also reduces the steady state expression of both TERT mRNA and the TR subunit mRNA of telomerase in MCF-7 breast cancer cells while a pharmacological concentration (100 nM) causes a substantial reduction in estradiol-induced TERT mRNA expression in these cells as well [39, 40]. Thus, another important mechanism of melatonin’s oncostatic action at physiological and pharmacological concentrations in ERα+ human breast cancer cells may relate to its ability to suppress telomerase expression and activation, thus minimizing telomere elongation and setting these cells on a course toward apoptotic cell death.
Melatonin, at physiological nocturnal blood concentrations, inhibits human breast cancer cell invasion/metastasis
Early work on the antineoplastic effects of melatonin revealed that pinealectomy increased while pharmacological melatonin administration diminished the metastatic spread of solid tumors in rodent models of tumorigenesis [41, 42]. Subsequently, compared with numerous studies devoted to melatonin’s suppressive effects on human breast cancer cell proliferation, signal transduction and transcriptional regulation, only a minimal effort has been targeted toward the potential role of melatonin in breast cancer invasion and metastasis in vitro and in vivo. In one investigation in vitro, physiological concentrations of melatonin (1 nM) significantly reduced the invasive capacity of MCF-7 human breast cancer cells and blocked 17-β-estradiol (E2)-induced MCF-7 cell invasion while enhancing the expression of the adhesion proteins, E-cadherin and β1 integrin [43]. In a study examining the in vivo effect of melatonin on telomerase activity, melatonin treatment also lowered the incidence of metastases in nude mice implanted with MCF-7 xenografts, compared with control-treated mice [39] (see below).
In an effort to further assess and elucidate the potential anti-invasive/anti-metastatic actions of melatonin, we employed three different clones of MCF-7 cells with demonstrated high metastatic potential. These included the a) MCF-7/6 clone derived by serial passages in nude mice, b) MCF-7Her2.1 cells stably-transfected with and over expressing the Her2-neu/c-erbB2 construct, and c) MCF-7CXCR4 cells stably-transfected with and over expressing the CXCR4 cytokine G protein-coupled receptor. When expressed at high levels, both the Her2/neu (c-erbB2) and CXCR4 receptors are well known to increase the invasive/metastatic potential of breast cancer cells [44] and to even enhance metastatic homing to bone in the case of the CXCR4 receptor [45]. The invasive capacity of these clones were significantly greater than those of parental MCF-7 cells with MCF-7/6< MCF-7CXCR4<MCF-7Her2.1 cells. Exposure of MCF-7Her2.1 cells as well as the other two invasive clones to melatonin (1 or 10 nM) resulted in significant suppression (60-85% decrease) of cell invasion using a transwell assay system and matrigel covered inserts. The anti-invasive response to melatonin was enhanced by over-expression of the MT1 receptor and inhibited by administration of luzindole a non-selective MT1/MT2 receptor antagonist arguing for an MT1 receptor-mediated mechanism of melatonin’s anti-invasive action.
In many cases, invasion and metastasis in breast cancer cells are driven by enhanced activity of the p38 MAPK signaling pathway leading to elevated expression of the matrix metalloproteinases MMP2 and MMP9 [46]. For example, studies from our laboratory [47] have demonstrated that each of the metastatic MCF-7 clones describe above have enhanced expression of the phosphorylated forms of p38 and MMP2 and MMP9. Melatonin administration blocks p38 phosporylation and subsequently MMP2 and MMP9 expression, and in doing so, suppresses breast cancer cell invasion. Although we know these actions are MT1 receptor-mediated, we do not yet know the downstream signaling pathways that links the MT1 receptor to p38, however, we suspect that Rho may be a key factor in this process.
Melatonin regulation of circadian clock gene expression in human breast cancer cells
A connection between cancer development and the circadian cycle has been demonstrated in hormone-related cancers including breast and prostate cancers through the studies of pilots, flight attendants, and night shift workers who are more likely to have disrupted circadian cycles due to their abnormal work hours [6]. Approximately 15% - 20% of all mammalian genes are clock-controlled genes (CCGs), indicating extensive circadian gene regulation [48]. The “clock” mechanism that operates in both the SCN and peripheral cells (including cancer cells) consists of interacting positive and negative feedback loops that regulate the transcription of the clock genes, 12 of which have been identified [49]. The Clock and Bmal1 genes participate in a positive feedback loop, whereas period (Per) and cryptochrome (Cry) genes are involved in a negative feedback loop. A heterodimer of CLOCK and BMAL1 proteins not only rhythmically activates the transcription of clock genes Per1, Per 2, Per 3, Cry1, Cry2, and Rev-erbα, but also activates downstream CCGs. The rhythmic expression of BMAL1 is generated via its transcriptional activation by RORα and transcriptional repression by Rev-erbα [50]. We recently reported [51] that elevated expression of RORα1 can enhance BMAL1 transcription and expression in MCF-10A human breast epithelial cells and MCF-7 breast cancer cells and that melatonin blunts the transcriptional activation of the RORα1 blocking its induction of BMAL1 expression. Thus, melatonin, via its MT1 receptor, can directly suppress an important element of the clock mechanism in peripheral tissues, specifically breast epithelial and cancer cells.
Recent findings suggest that some CCGs function as tumor suppressors at the systemic, cellular, and molecular levels due to their involvement in cell proliferation, apoptosis (p53, Bax), cell cycle control (ChK2), and DNA damage response (p53, BRCA1 & 2, Ku70). A recent study showed that disruption of the Per2 gene is associated with tumor development in mice [52]. We have recently reported that expression of the clock protein (PER2) is lost in human breast cancer, while its reintroduction increases p53 expression and induces apoptosis [51].
The anti-aging protein, Silencing Information Regulator Two family member, SIRT1, has been linked with both the molecular circadian clock and cancer [53, 54]. SIRT1 is a NAD+-dependent class III histone deacetylase (HDAC) that impacts a broad spectrum of cellular processes including gene silencing, DNA 11 repair, apoptosis, cellular metabolism, cellular senescence, and aging. SIRT1 has been reported to bind directly to the CLOCK/BMAL1 heterodimer, promoting deacetylation and degradation of PER2 (a tumor suppressor that interacts with BRCA1). SIRT1 also suppresses the transcription or activity of a number of genes including p53, FOXOA1 & 3, and PGC-1α. This association involves suppression of DNA-repair enzymes including p53, BRCA1 and 2, and Ku70. Thus, SIRT1 may be a focal point through which the circadian clock may influence cancer development. Given that we have found that melatonin can regulate the expression of BMAL1, via modulation of RORα transcriptional activity, and that SIRT1 is associated with BMAL1, we asked whether melatonin is able to modulate the expression of SIRT1 in human breast cancer cells. SIRT1 is expressed at high levels in MCF-7 and MDA-MB- 231 human breast cancer cell lines as well as human umbilical vascular endothelial cells. Following transfection of an MT1-receptor expression construct into MCF-7 cells, treatment with melatonin (10 nM) dramatically suppressed SIRT1 expression in these cells [19].
Melatonin, at nocturnal physiological blood concentrations, suppresses human breast cancer growth activity in vivo via melatonin receptor-mediated inhibition of tumor signal transduction and metabolic activity
Another key pathway mediating melatonin’s cancer inhibitory action on tumor growth in vivo involves the essential omega-6 polyunsaturated fatty acid (PUFA), linoleic acid (LA). As the most prevalent PUFA in the Western diet, LA levels greatly exceed those required to prevent essential FA deficiency (i.e., 1% of total calories) [55]. Linoleic acid’s oncogenic effects are diverse and are related to its ability to upregulate the expression of genes and proteins involved in estrogen receptor ERα expression, cell cycle progression, G protein signaling, extracellular signal-regulated kinase (ERK1/2) and PI3 kinase/Akt/mTOR growth cascades while downregulating intercellular communication [11, 56]. As a potent oncogenic stimulus of both murine and human tumorigenesis, LA exerts actions on cancer cells that oppose virtually all of the oncostatic actions of melatonin cited above. In both tissue-isolated ERα (+ and −) human breast cancer xenografts, melatonin acts via melatonin receptors [57] to suppress tumor cAMP formation leading to a suppression of LA uptake and its metabolism to the mitogenic signaling molecule 13-hydroxyoctadecadenoic acid (13-HODE). Down-regulation of LA uptake and metabolism reduces the activation of the epidermal growth factor receptor (EGFR)/MEK/ERK1/2 pathway, which is important in cell proliferation and survival, culminating in tumor growth inhibition [20]. Another pathway that melatonin exerts a negative impact on is the Akt pathway also important for cell survival and proliferation. For example, the perfusion of tissue-isolated ERα− MCF-7 human breast cancer xenografts in situ with a physiological nocturnal concentration of melatonin (1 nM) results in a marked reduction in the activation of Akt as compared with a vehicle-perfused xenograft. The co-perfusion of a breast cancer xenograft with melatonin plus the non-selective MT1 and MT2 receptor antagonist S20928 prevents this effect, indicating that, like the effects of melatonin on the EGF/MEK/ERK1/2 pathway, this is a melatonin receptor-mediated response (unpublished results). The inability of the selective MT2 receptor antagonist, 4P-PDOT, to block this response argues for the involvement of a MT1 receptor-mediated mechanism. The fact that LA up-regulates whereas melatonin down-regulates transcriptional regulation of ERα in human breast cancer cells via a melatonin receptor-mediated inhibition of cAMP in these cells would potentially provide ample opportunity for cross-talk among these pathways.
Effects of circadian disruption of the nocturnal melatonin signal by LAN on the growth and metabolism of experimental human breast cancer
The risk of developing breast cancer is up to five times higher in industrialized nations than in underdeveloped countries. Overall, nearly 50% of breast cancers cannot be accounted for by conventional risk factors [58, 59]. Westernized nations have increasingly become 24-hour per day societies with greater numbers of people being exposed to more artificial light during the night both at home and particularly in the workplace [6, 60]. It has been postulated that light exposure at night may represent a unique risk factor for breast cancer in industrialized societies via its ability to suppress the nocturnal production of melatonin by the pineal gland [58, 59]. This hypothesis is based on studies showing that melatonin inhibits the development and growth of experimental models of breast cancer whereas either surgical removal of the pineal gland or exposure to constant light stimulates mammary tumorigenesis in rodents [7, 11]. This postulate is further strengthened by a number of epidemiological studies demonstrating that women working night shifts have a significantly elevated risk of breast cancer presumably due to their increased exposure to LAN [61-64]. In fact, the International Agency for Research on Cancer (IARC), an arm of the World Health Organization (WHO) has recently declared shift work at night, and ostensibly exposure to LAN, to be a probable carcinogen [65]. Indeed, subsequent epidemiological studies have confirmed that both pre- and postmenopausal, non-shift working women, in general, who have lower nocturnal levels of melatonin, possibly due to exposure to LAN, are at significantly greater risk for breast cancer than those with higher levels of melatonin at night [66, 67]. Interestingly, a significant positive association has now been reported between population LAN level and incidence rates of breast cancer for cities around the globe [68].
To explore the mechanistic link between exposure to LAN and breast cancer, we assessed the dose-response effects of LAN on the growth, LA uptake/metabolism to 13-HODE and signal transduction activity in tissue-isolated ERα− MCF-7 human breast cancer xenografts growing in nude female rats. Groups of rats were each exposed to different, increasing intensities (total dark, 0.02. 0.05, 0.06, 0.08 or 345 μW/cm2) of white, fluorescent polychromatic light during the dark phase of an alternating 12-hr light/12-hr dark cycle beginning two weeks before tumor implantation and continuing thereafter until the end of the tumor growth experiment. Following several weeks of exposure of rats to increasingly brighter light during the dark phase (from total darkness to bright light) there was a dose-dependent increase in the percent suppression of peak nocturnal serum melatonin levels. There was also an accompanying marked, dose-related increase in LA uptake, 13-HODE formation, DNA content, [3H]thymidine incorporation into DNA, tumor growth rates, ERK1/2 activation and cAMP levels as light intensity during the night increased and the nocturnal amplitude of blood melatonin levels decreased. Dark-phase serum corticosterone and estradiol levels were unaffected by increasing light intensities. Thus, the light intensity-dependent suppression of nocturnal circulating melatonin titers translated, in turn, to a light intensity-dependent stimulation of human breast cancer xenograft signal transduction activity, LA uptake/metabolism to 13-HODE, and growth/proliferative activity. An interesting aspect of this study was that dim light at night (0.08 μW/cm2 or 0.2 lux) which induced approximately a 65% suppression of the amplitude of the nocturnal melatonin signal in the blood resulted in a stimulation of tumor growth, signal transduction and metabolic activity that was nearly complete and equivalent to that observed in constant bright light-exposed tumor-bearing rats with complete melatonin suppression. This confirmed our previous findings on tumor growth and metabolism in tissue-isolated rat hepatoma 7288CTC in Buffalo rats exposed to either dim light at night or constant bright light [69, 70].
The stimulatory effects of dim light present during the entire night on rat hepatoma and human breast cancer xenograft growth demonstrated in our laboratory [20, 69, 70] were recently and independently corroborated with respect to the growth of 7,12-dimethylbenzanthracene (DMBA)-induced mammary carcinomas in female rats [71]. When tumors reached a predetermined size, rats were subjected to either LD12:12, bright LAN (300 lux), LD12:12 (300 lux) with a 30-min exposure to LAN near mid-dark phase, or to dim LAN (0.2 lux) during the entire 12-hour dark phase. Tumor growth rates were highest in rats exposed to dim LAN, and slowest in those remaining in LD12:12. Tumors also grew faster in rats exposed to bright LAN for 30 min and in those exposed to bright LAN as compared to the LD12:12 group. The onset of accelerated tumor growth occurred at week 4 for the dim LAN group, at week 6 for the pulsed LAN group, and at week 10 for the bright LAN group. A significant decrease in nighttime urinary excretion of 6-sulfatoxymelatonin was observed in all groups exposed to LAN. The light/dark normal difference in 6-sulfatoxymelatonin excretion was eliminated in both the dim LAN and bright LAN groups but not in the light pulsed LAN group. A marked increase in serum estradiol was observed in the dim LAN group only.
Additional experimental studies from our laboratory uncovered important new relationships between circadian biology, the endogenous nocturnal melatonin signal, and its suppression by LAN, relative to human breast cancer risk [20]. The signal transduction, metabolic and proliferative activity of human breast cancer tissue, growing in nude rats, perfused in situ with blood collected during completely dark nights from premenopausal female subjects (i.e., high melatonin), is markedly reduced as compared to when the tissue is perfused with daytime blood (i.e., low melatonin). Subsequent exposure of the subjects to bright (2800 lux), polychromatic white fluorescent light during the night completely extinguishes the tumor inhibitory activity of their blood. That this effect was achieved via LAN-induced melatonin suppression is supported by the fact that addition of a physiological nocturnal concentration of melatonin (500 pM) to the low melatonin blood collected from light-treated subjects restored the tumor inhibitory activity that was observed in the melatonin-rich blood collected at night during total darkness. Therefore, melatonin is the first soluble, nocturnal anticancer signal to be identified in humans that directly links the central circadian clock with some of the important mechanisms regulating breast carcinogenesis. These findings also provide the first definitive nexus between the exposure of healthy premenopausal female human subjects to bright, white, polychromatic LAN and the enhancement of human breast oncogenesis via circadian disruption (i.e., suppression) of the nocturnal, oncostatic melatonin signal. The suppression of circadian melatonin production by ocular exposure to bright white light at night, leading to augmented nocturnal tumor uptake of dietary LA and its conversion to mitogenically active 13-HODE, can now be afforded serious consideration as a potentially new risk factor for human breast cancer. As alluded to above, subsequent epidemiological studies have confirmed that both pre- and postmenopausal women, in general, who have lower nocturnal levels of melatonin are at significantly greater risk for breast cancer than those with higher levels of melatonin at night [66, 67].
Circadian rhythms of cancer fatty acid uptake and metabolism, their disruption by dim LAN in vivo and the impact on growth activity in tissue-isolated human breast cancer xenografts
Initial work from our laboratory with the highly melatonin-sensitive tissue-isolated Morris rat hepatoma 7288CTC revealed that this tumor exhibits prominent circadian rhythms in LA uptake and 13-HODE production [11, 72]. For example, tumor LA (and total FA) uptake and the formation of 13-HODE occurs during the mid-light phase of a 12L:12D light/dark cycle when melatonin levels are extremely low in the blood of tumor-bearing male Buffalo rats. On the other hand, during the mid-dark phase, tumor LA uptake and 13-HODE production plummet to undetectable levels coincident with peak circulating titers of melatonin. The rhythms of daytime elevation and nocturnal diminution in tumor fatty acid uptake and metabolism are eliminated in animals lacking a nocturnal circadian melatonin signal due to removal of the pineal gland, resulting in high rates of LA uptake, metabolism to 13-HODE during both the light and dark phases and consequently, significantly higher rates of tumor growth compared with sham-operated animals. These findings indicate that the circadian rhythms of LA uptake and metabolism to 13-HODE in tissue-isolated rat hepatoma 7288CTC under light/dark entrained conditions are not endogenous tumor rhythms but rather, oscillations driven directly by and dependent upon the nocturnal circadian melatonin signal emanating from the pineal gland.
In more recent work, we have examined whether tissue-isolated ERα− MCF-7 human breast cancer xenografts grown in female nude rats, like rat hepatomas described above, also exhibit circadian rhythms in LA uptake, metabolism to 13-HODE and proliferative activity and, if so, whether those rhythms would be disrupted by exposure of tumor-bearing rats to dim LAN (0.2 lux; 0.08 μW/cm2) at night [73]. Prominent oscillations in the tumor fatty acid uptake and metabolism occur with peak LA and TFA uptake and production of 13-HODE during the light phase 2 hrs prior to lights off and a nadir in those parameters during the dark phase 2 hrs before lights on. Similar fluctuations were observed in the proliferative activity of these human breast cancer xenografts with peak incorporation of [3H]thymidine into DNA also during the light phase 2 hrs prior to lights off and lowest thymidine incorporation during the dark phase 2 hrs prior to lights on [73]. The high daytime and low nighttime levels of tumor proliferative activity, LA uptake and metabolism to 13-HODE were 180 degrees out of phase with the plasma melatonin rhythm in the host rats (unpublished results). Exposure of tumor-bearing rats to dim LAN resulted in an almost complete suppression of the nocturnal circadian melatonin signal as observed in previous studies [20] and a complete elimination of the tumor rhythms of LA uptake and metabolism and proliferative activity (unpublished results).
Taken together, these preliminary findings demonstrate for the first time that tissue-isolated human breast cancer xenografts exhibit prominent daily rhythms of LA uptake and metabolism and proliferative activity indicating that tumor metabolism is a very dynamic, homeokinetic process. The notion that these rhythms in tumor LA metabolism and proliferative activity are, in fact, circadian rhythms driven by the nocturnal circadian melatonin signal is supported by the findings that they are negated when nocturnal melatonin production is nearly totally suppressed by exposure to dim LAN (unpublished results).
Summary and Conclusions
The oncostatic actions of nighttime circulating concentrations of melatonin are mediated through activation of MT1 receptors expressed by ERα+ and some ERα− human breast cancer cell lines and xenografts. In ERα+ human breast cancer cell cells, melatonin suppresses both ERα mRNA expression and estrogen-induced transcriptional activity of the ERα via the activation of Gαi2 signaling, thus lowering cAMP/PKA levels and reducing the phosphorylation of a PKA-sensitive site on the ERα. Melatonin also modulates the transactivation of additional members of the steroid hormone/nuclear receptor super-family including the GR, RARα, RORα and VDR. As a SEEM, melatonin, at nocturnal physiological circulating concentrations, inhibits both the expression and activity of aromatase, STS and 17β-HSD in ERα+ MCF-7 human breast cancer cells while at the same time stimulating EST through interaction with the MT1 melatonin receptor. Another important mechanism of melatonin’s oncostatic action on human breast cancer cells at physiological concentrations is based on its ability to suppress telomerase expression and activation to subvert telomere elongation resulting in decreased cell survival.
In the realm of invasion and metastasis, physiological concentrations of melatonin reduce the invasive capacity of MCF-7 human breast cancer cells and block E2-induced MCF-7 cell invasion while enhancing the expression of the adhesion proteins, E-cadherin and β1 integrin in vitro. The anti-invasive/anti-metastatic actions of physiological nocturnal melatonin concentrations extend to other more highly invasive clones of MCF-7 cells via an MT1 melatonin receptor-mediated mechanism involving the blockade of p38 phosporylation and downstream expression of specific matrix metalloproteinases. An additional new feature of melatonin’s molecular regulation of breast cancer cell proliferation relates to its ability to regulate the expression of core clock genes such as BMAL1 as well as associated CCGs such as SIRT1.
A major pathway by which melatonin inhibits the growth of both tissue-isolated ERα (+ and −) human breast cancer xenografts involves melatonin action MT1 melatonin receptors to suppress tumor cAMP leading to a blockade of LA uptake and its metabolism to the mitogenic signaling 13-HODE. The down-regulation of LA uptake and metabolism to 13-HODE reduces the activation EGFR/MEK/ERK1/2 and Akt pathways, which are major contributors to the support of cancer cell proliferation and survival. The exposure of rats bearing tissue-isolated ERα− human breast cancer xenografts to increasingly brighter light intensities during the dark phase of a 12L:12D light/dark cycle caused a dose-dependent decrease in peak nocturnal serum melatonin levels with accompanying dose-related increases in signal transduction activity, LA uptake and metabolism to 13-HODE and ultimately tumor growth.
A unique experimental approach to examining the effects of LAN on humans in relation to breast cancer growth involves the direct perfusion of tissue-isolated ERα + or − human breast cancer xenografts growing in situ in nude rats with blood collected from human female subjects during either the day, night or following exposure to LAN. Melatonin-depleted daytime blood maintained high levels of tumor signal transduction, LA metabolic and growth activity while melatonin-replete blood collected during the night provoked a marked reduction in these parameters; however, subsequent exposure of subjects to bright, polychromatic white fluorescent LAN completely abrogated the circadian anticancer signal conferred on nighttime blood by elevated concentrations of melatonin. The circadian nature of the nocturnal melatonin anticancer signal is further corroborated by the demonstration that tissue-isolated human breast cancer xenografts grown in nude rats exhibit prominent daily rhythms of high LA uptake/metabolism and proliferative activity during the day followed very low activity during the night that are eliminated when nocturnal melatonin production is nearly totally suppressed by exposure of the animals to dim LAN.
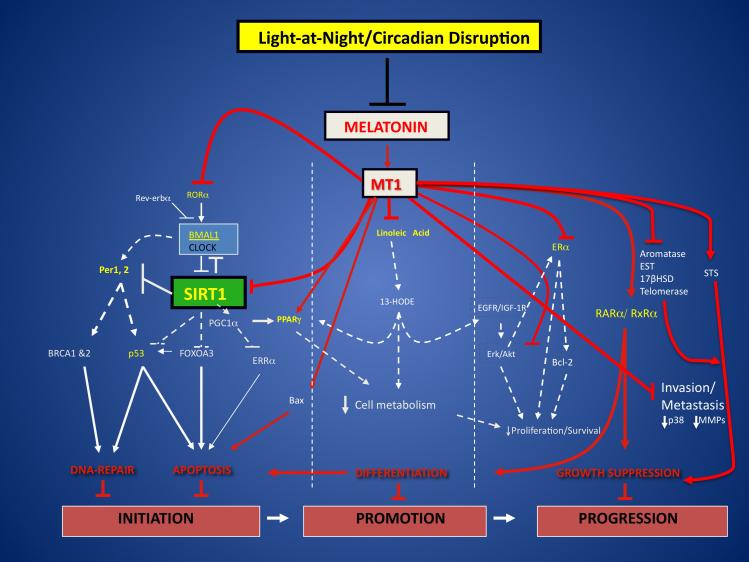
The experimental findings reviewed and summarized here provide sound evidence at multiple levels that there are a variety of molecular endocrine, dietary and metabolic signaling mechanisms that take advantage of a variety of opportunities for interaction and cross-talk that not only sustain, but further exacerbate human breast cancer cell proliferation/survival, invasion and metastasis. These mechanisms are temporally-organized, integrated and coordinated not only by the central circadian pacemaker in the SCN, but perhaps also by the activity of peripheral clocks in breast cancer cells themselves. Whether the circadian nature of tumor molecular, dietary/metabolic signaling and growth is ultimately the result of an interplay and balance between the host’s systemic timing mechanism and the breast cancer cell’s local molecular clock operations is unknown. Nevertheless, as the most stable and reliable output of the SCN, the nocturnal, circadian melatonin signal from the pineal gland, thus far, represents the first soluble anticancer factor to be identified in humans that directly links the central circadian clock with some of the most important mechanisms regulating breast carcinogenesis. It is apparent that the circadian melatonin signal is an essential systemic host factor for temporally coordinating breast cancer cell proliferation/survival, and perhaps invasion and metastasis, by gating these mechanisms during the night and putting breast cancer cells to “sleep”. The suppression of the circadian melatonin signal by LAN, in turn, “wakes-up” breast cancer cells leading to an enhancement of human breast oncogenesis via a disruption in its circadian organization. Moreover, uninterrupted darkness itself may serve as a protective mechanism against the development and growth of breast cancer [10, 11](Fig. 1).
Figure 1.
Impact of the nocturnal circadian melatonin signal, via the MT1 receptor, and its disruption by LAN on molecular/endocrine and dietary/metabolic regulatory mechanisms governing breast cancer initiation, growth promotion and progression. Abbreviations used are: 13-HODE (13-hydroxyoctadecadienoic acid), 17βHSD (17β-hydroxysteroid dehydrogenase), Akt (Serine/Threonine Protein Kinase), Bax (Bcl-2-Associated × Protein), BMAL1 (Brain and Muscle Aryl Hydrocarbon Receptor Nuclear Translocator – Like 1), BRCA 1 & 2 (Breast Cancer 1 & 2), CLOCK (Circadian Locomotor Output Cycles Kaput), EGFR (Epidermal Growth Factor Receptor), ERα (Estrogen Receptor Alpha), Erk (Extracellular Signal-Regulated Kinase), ERRα (Estrogen Related Receptor Alpha), EST (Estrogen Sulfotransferase), IGF-1R (Insulin-like Growth Factor-1 Receptor), MMP (Matrix Metalloproteinase), MT1 (Melatonin Receptor 1), Per 1, 2 (Period 1, 2), PGC1α (Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha), PPARγ (Peroxisome Proliferator-Activated Receptor Protein Gamma), RARα (Retinoic Acid Receptor Alpha), Rev-erbα (aka NR1D1, Nuclear Receptor Subfamily 1, group D, member 1), RORα (RAR-related Orphan Receptor Alpha), R×Rα (Retinoid × Receptor Alpha), SIRT1 [Sirtuin (Silent Mating Type Regulation 2 Homolog) 1], STS (Estrogen Sulfatase). Figure adapted and modified from reference [19].
Knowledge of melatonin-based circadian coordination and gating of breast cancer molecular/endocrine and dietary/metabolic regulatory mechanisms provides an unprecedented opportunity for a more rational approach to aiming both old and newly-emerging breast cancer therapies not only at the most promising mechanistic targets but, perhaps more importantly, at the time of day or night when these targets are most vulnerable to intervention. Such a circadian-optimized approach to breast cancer therapeutics has the potential to increase tumor treatment efficacy while simultaneously reducing toxicity to the host. On the other hand, the consequences of LAN-induced disruption of the nocturnal melatonin signal may include not only an increase in breast cancer risk in the general population, but also a loss of an important circadian signal to normal and breast cancer cells that could compromise the ability of clinicians to employ circadian-based breast cancer targeted preventative and therapeutic strategies mentioned above.
Acknowledgements
The work by the authors presented in this article was generously supported by National Institutes of Health grants R01CA85408 (DEB) and R01CA054152 (SMH), Army Department Of Defense grant (SMH), National Institute of Environmental Health Sciences R21ES11659 (DEB), Edwin W. Pauley Foundation (DEB) and Grants for Laboratory Animal Science (GLAS) (RTD).
References
- 1.HASTINGS MH, REDDY AB, MAYWOOD ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 2.BERSON DM, DUNN FA, TAKAO M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 3.TECLEMARIAM-MESBAH R, TER HORST GJ, POSTEMA F, et al. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J Comp Neurol. 1999;406:171–182. [PubMed] [Google Scholar]
- 4.CLAUSTRAT B, BRUN J, CHAZOT G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 5.REITER RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153–158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 6.STEVENS RG, BLASK DE, BRAINARD GC, et al. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115:1357–1362. doi: 10.1289/ehp.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BLASK DE, SAUER LA, DAUCHY RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2:113–132. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 8.SANCHEZ-BARCELO EJ, COS S, MEDIAVILLA D, et al. Melatonin-estrogen interactions in breast cancer. J Pineal Res. 2005;38:217–222. doi: 10.1111/j.1600-079X.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 9.WITT-ENDERBY PA, RADIO NM, DOCTOR JS, et al. Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J Pineal Res. 2006;41:297–305. doi: 10.1111/j.1600-079X.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 10.BLASK DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–264. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 11.BLASK DE, DAUCHY RT, SAUER LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179–188. doi: 10.1385/ENDO:27:2:179. [DOI] [PubMed] [Google Scholar]
- 12.FILIPSKI E, DELAUNAY F, KING VM, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 13.HRUSHESKY WJ. The temporal organization of life: the impact of multi-frequency non-linear biologic time structure upon the host-cancer balance. Jpn J Clin Oncol. 2000;30:529–533. doi: 10.1093/jjco/hyd134. [DOI] [PubMed] [Google Scholar]
- 14.CANAPLE L, KAKIZAWA T, LAUDET V. The days and nights of cancer cells. Cancer Res. 2003;63:7545–7552. [PubMed] [Google Scholar]
- 15.YOU S, WOOD PA, XIONG Y, et al. Daily coordination of cancer growth and circadian clock gene expression. Breast Cancer Res Treat. 2005;91:47–60. doi: 10.1007/s10549-004-6603-z. [DOI] [PubMed] [Google Scholar]
- 16.KOHSAKA A, BASS J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 17.HILL SM, BLASK DE. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1988;48:6121–6126. [PubMed] [Google Scholar]
- 18.COS S, BLASK DE, LEMUS-WILSON A, et al. Effects of melatonin on the cell cycle kinetics and “estrogen-rescue” of MCF-7 human breast cancer cells in culture. J Pineal Res. 1991;10:36–42. doi: 10.1111/j.1600-079x.1991.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 19.HILL SM, FRASCH T, XIANG S, et al. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Ther. 2009;8:337–346. doi: 10.1177/1534735409353332. [DOI] [PubMed] [Google Scholar]
- 20.BLASK DE, BRAINARD GC, DAUCHY RT, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65:11174–11184. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- 21.BRYDON L, ROKA F, PETIT L, et al. Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3), and G(q/11) proteins. Mol Endocrinol. 1999;13:2025–2038. doi: 10.1210/mend.13.12.0390. [DOI] [PubMed] [Google Scholar]
- 22.COLLINS A, YUAN L, KIEFER TL, et al. Overexpression of the MT1 melatonin receptor in MCF-7 human breast cancer cells inhibits mammary tumor formation in nude mice. Cancer Lett. 2003;189:49–57. doi: 10.1016/s0304-3835(02)00502-5. [DOI] [PubMed] [Google Scholar]
- 23.YUAN L, COLLINS AR, DAI J, et al. MT(1) melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol Cell Endocrinol. 2002;192:147–156. doi: 10.1016/s0303-7207(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 24.LAI L, YUAN L, CHENG Q, et al. Alteration of the MT1 melatonin receptor gene and its expression in primary human breast tumors and breast cancer cell lines. Breast Cancer Res Treat. 2009;118:293–305. doi: 10.1007/s10549-008-0220-1. [DOI] [PubMed] [Google Scholar]
- 25.RAM PT, KIEFER T, SILVERMAN M, et al. Estrogen receptor transactivation in MCF-7 breast cancer cells by melatonin and growth factors. Mol Cell Endocrinol. 1998;141:53–64. doi: 10.1016/s0303-7207(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 26.LAI L, YUAN L, CHEN Q, et al. The Galphai and Galphaq proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation. J Pineal Res. 2008;45:476–488. doi: 10.1111/j.1600-079X.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GIRGERT R, BARTSCH C, HILL SM, et al. Tracking the elusive antiestrogenic effect of melatonin: a new methodological approach. Neuro Endocrinol Lett. 2003;24:440–444. [PubMed] [Google Scholar]
- 28.GIRGERT R, HANF V, EMONS G, et al. Membrane-bound melatonin receptor MT1 down-regulates estrogen responsive genes in breast cancer cells. J Pineal Res. 2009;47:23–31. doi: 10.1111/j.1600-079X.2009.00684.x. [DOI] [PubMed] [Google Scholar]
- 29.MOLIS TM, SPRIGGS LL, HILL SM. Modulation of estrogen receptor mRNA expression by melatonin in MCF-7 human breast cancer cells. Mol Endocrinol. 1994;8:1681–1690. doi: 10.1210/mend.8.12.7708056. [DOI] [PubMed] [Google Scholar]
- 30.DEL RIO B, PEDRERO JMGARCIA, MARTINEZ-CAMPA C, et al. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. J Biol Chem. 2004;279:38294–38302. doi: 10.1074/jbc.M403140200. [DOI] [PubMed] [Google Scholar]
- 31.DAI J, INSCHO EW, YUAN L, et al. Modulation of intracellular calcium and calmodulin by melatonin in MCF-7 human breast cancer cells. J Pineal Res. 2002;32:112–119. doi: 10.1034/j.1600-079x.2002.1844.x. [DOI] [PubMed] [Google Scholar]
- 32.KIEFER TL, LAI L, YUAN L, et al. Differential regulation of estrogen receptor alpha, glucocorticoid receptor and retinoic acid receptor alpha transcriptional activity by melatonin is mediated via different G proteins. J Pineal Res. 2005;38:231–239. doi: 10.1111/j.1600-079X.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 33.DAI J, RAM PT, YUAN L, et al. Transcriptional repression of RORalpha activity in human breast cancer cells by melatonin. Mol Cell Endocrinol. 2001;176:111–120. doi: 10.1016/s0303-7207(01)00449-x. [DOI] [PubMed] [Google Scholar]
- 34.COS S, GONZALEZ A, MARTINEZ-CAMPA C, et al. Estrogen-signaling pathway: a link between breast cancer and melatonin oncostatic actions. Cancer Detect Prev. 2006;30:118–128. doi: 10.1016/j.cdp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 35.COS S, GONZALEZ A, GUEZMES A, et al. Melatonin inhibits the growth of DMBA-induced mammary tumors by decreasing the local biosynthesis of estrogens through the modulation of aromatase activity. Int J Cancer. 2006;118:274–278. doi: 10.1002/ijc.21401. [DOI] [PubMed] [Google Scholar]
- 36.FELDSER DM, HACKETT JA, GREIDER CW. Telomere dysfunction and the initiation of genome instability. Nat Rev Cancer. 2003;3:623–627. doi: 10.1038/nrc1142. [DOI] [PubMed] [Google Scholar]
- 37.HESELMEYER-HADDAD K, JANZ V, CASTLE PE, et al. Detection of genomic amplification of the human telomerase gene (TERC) in cytologic specimens as a genetic test for the diagnosis of cervical dysplasia. Am J Pathol. 2003;163:1405–1416. doi: 10.1016/S0002-9440(10)63498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.YASUI W, TAHARA H, TAHARA E, et al. Expression of telomerase catalytic component, telomerase reverse transcriptase, in human gastric carcinomas. Jpn J Cancer Res. 1998;89:1099–1103. doi: 10.1111/j.1349-7006.1998.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LEON-BLANCO MM, GUERRERO JM, REITER RJ, et al. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res. 2003;35:204–211. doi: 10.1034/j.1600-079x.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 40.LEON-BLANCO MM, GUERRERO JM, REITER RJ, et al. RNA expression of human telomerase subunits TR and TERT is differentially affected by melatonin receptor agonists in the MCF-7 tumor cell line. Cancer Lett. 2004;216:73–80. doi: 10.1016/j.canlet.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 41.LAPIN V. Pineal gland and malignancy. Osterr Z Onkol. 1976;3:51–60. [PubMed] [Google Scholar]
- 42.BLASK DE. The pineal: an oncostatic gland? In: Reiter Rj., editor. The Pineal Gland. Raven Press; New York: 1984. pp. 253–284. [Google Scholar]
- 43.MAO L, YUAN L, SLAKEY LM, et al. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res. 2010;12:R107. doi: 10.1186/bcr2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.TAN M, YAO J, YU D. Overexpression of the c-erbB-2 gene enhanced intrinsic metastasis potential in human breast cancer cells without increasing their transformation abilities. Cancer Res. 1997;57:1199–1205. [PubMed] [Google Scholar]
- 45.SCHMID BC, RUDAS M, REZNICZEK GA, et al. CXCR4 is expressed in ductal carcinoma in situ of the breast and in atypical ductal hyperplasia. Breast Cancer Res Treat. 2004;84:247–250. doi: 10.1023/B:BREA.0000019962.18922.87. [DOI] [PubMed] [Google Scholar]
- 46.KIM MS, LEE EJ, KIM HR, et al. p38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res. 2003;63:5454–5461. [PubMed] [Google Scholar]
- 47.MAO L, HILL SM. Inhibition of cell proliferation and blockade of cell invasion by melatonin in human breast cancer cells mediated through multiple signaling pathways. 96th Annual Mtg American Association for Cancer Research; Anaheim, CA. 2005. [Google Scholar]
- 48.CAILOTTO C, LEI J, VAN DER VLIET J, et al. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PLoS One. 2009;4:e5650. doi: 10.1371/journal.pone.0005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.KENNAWAY DJ, OWENS JA, VOULTSIOS A, et al. Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1528–1537. doi: 10.1152/ajpregu.00018.2007. [DOI] [PubMed] [Google Scholar]
- 50.GUILLAUMOND F, DARDENTE H, GIGUERE V, et al. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 51.XIANG S, COFFELT SB, MAO L, et al. Period-2: a tumor suppressor gene in breast cancer. J Circadian Rhythms. 2008;6:4. doi: 10.1186/1740-3391-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.FU L, PELICANO H, LIU J, et al. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 53.NAKAHATA Y, KALUZOVA M, GRIMALDI B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LIU T, LIU PY, MARSHALL GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–1705. doi: 10.1158/0008-5472.CAN-08-3365. [DOI] [PubMed] [Google Scholar]
- 55.SAUER LA, DAUCHY RT, BLASK DE. Polyunsaturated fatty acids, melatonin, and cancer prevention. Biochem Pharmacol. 2001;61:1455–1462. doi: 10.1016/s0006-2952(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 56.REYES N, REYES I, TIWARI R, et al. Effect of linoleic acid on proliferation and gene expression in the breast cancer cell line T47D. Cancer Lett. 2004;209:25–35. doi: 10.1016/j.canlet.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 57.BLASK DE, DAUCHY RT, SAUER LA, et al. Oral melatonin supplementation in rats and a human subject suppresses the growth activity of steroid receptor negative human breast cancer xenografts in female nude rats via an MT1 receptor-mediated suppression of signal tranduction and linoleic acid uptake and metabolism. AACR Meeting Abstracts.2005. p. 1358. [Google Scholar]
- 58.STEVENS RG. Electric power use and breast cancer: a hypothesis. Am J Epidemiol. 1987;125:556–561. doi: 10.1093/oxfordjournals.aje.a114569. [DOI] [PubMed] [Google Scholar]
- 59.STEVENS R, LONDON SJ. Breast Cancer. In: Stevens Rg, Le Anderson Bw., editors. The Melatonin Hypothesis: Breast Cancer and the Use of Electric Power. Battelle Press; Columbus: 1997. pp. 9–24. [Google Scholar]
- 60.STEVENS RG, REA MS. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control. 2001;12:279–287. doi: 10.1023/a:1011237000609. [DOI] [PubMed] [Google Scholar]
- 61.STEVENS RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol. 2009;38:963–970. doi: 10.1093/ije/dyp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.HANSEN J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12:74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 63.DAVIS S, MIRICK DK, STEVENS RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 64.SCHERNHAMMER ES, LADEN F, SPEIZER FE, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 65.STRAIF K, BAAN R, GROSSE Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 66.SCHERNHAMMER ES, HANKINSON SE. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst. 2005;97:1084–7. doi: 10.1093/jnci/dji190. [DOI] [PubMed] [Google Scholar]
- 67.SCHERNHAMMER ES, BERRINO F, KROGH V, et al. Urinary 6-sulfatoxymelatonin levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2008;100:898–905. doi: 10.1093/jnci/djn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.KLOOG I, STEVENS RG, HAIM A, et al. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control. 2010;21:2059–2068. doi: 10.1007/s10552-010-9624-4. [DOI] [PubMed] [Google Scholar]
- 69.DAUCHY RT, SAUER LA, BLASK DE, et al. Light contamination during the dark phase in “photoperiodically controlled” animal rooms: effect on tumor growth and metabolism in rats. Lab Anim Sci. 1997;47:511–518. [PubMed] [Google Scholar]
- 70.DAUCHY RT, BLASK DE, SAUER LA, et al. Dim light during darkness stimulates tumor progression by enhancing tumor fatty acid uptake and metabolism. Cancer Lett. 1999;144:131–136. doi: 10.1016/s0304-3835(99)00207-4. [DOI] [PubMed] [Google Scholar]
- 71.COS S, MEDIAVILLA D, MARTINEZ-CAMPA C, et al. Exposure to light-at-night increases the growth of DMBA-induced mammary adenocarcinomas in rats. Cancer Lett. 2006;235:266–271. doi: 10.1016/j.canlet.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 72.BLASK DE, SAUER LA, DAUCHY RT, et al. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor-mediated signal transduction events. Cancer Res. 1999;59:4693–4701. [PubMed] [Google Scholar]
- 73.BLASK DE, DAUCHY RT, BRAINARD GC, et al. Circadian stage-dependent inhibition of human breast cancer metabolism and growth by the nocturnal melatonin signal: consequences of its disruption by light at night in rats and women. Integr Cancer Ther. 2009;8:347–353. doi: 10.1177/1534735409352320. [DOI] [PubMed] [Google Scholar]