Introduction
Comel-Netherton syndrome (C-NS) is an autosomal recessive disorder of the skin, hair and immune system first reported by Comel in 1949 and Netherton in 1958 (1, 2). This syndrome presents at or soon after birth with generalized erythroderma, scaling, and/or continuous peeling of the skin resembling nonbulloous congenital ichthyosiform erythroderma or peeling skin syndrome. In the neonatal period, 20% of the babies suffer from hypernatremic dehydration, electrolyte imbalances, perturbed thermoregulation, failure to thrive and recurrent infections which may result in neonatal demise [3–5]. The skin lesions are often pruritic, resemble atopic eczema, and show an unstable, undulating course. They are usually accompanied by hair shaft abnormalities that develop during early childhood and may result in diffuse alopecia. The hallmark of C-NS is trichorrhexis invaginata (bamboo hair), but other abnormalities, including pili torti (twisted hair) and trichorrhexis nodosa (hair of varying diameter) have been observed. Markedly elevated IgE levels, allergic reactions to food and common antigens, malnutrition, and increased susceptibility to skin, respiratory tract or systemic infections are also characteristic [6, 7].
There are nearly 150–160 cases of C-NS reported in the literature, its incidence might be 1/200.000 [6] due to challenging diagnostic problems during infancy and early childhood. This syndrome has overlapping features with atopic dermatitis and other recessive ichthyosis. Most patients with C-NS are sporadic cases however, there are reports of affected siblings and of consanguinity in about 10% of the families with C-NS which is common in autosomal recessive inheritance [8–10].
In this study, we present two consanguineous Turkish families (two sisters married with two brothers) of prenatal diagnosis of Netherton syndrome and successful intracytoplasmic sperm injection (ICSI) pregnancies using PGD and a review of literature. To our knowledge, this is the first report of PGD and ICSI in Comel-Netherton syndrome.
Cases
Two couples were referred to our clinic with infertility. Two sisters were married to two brothers, and they are consanguineous (first degree cousins). Both of the women had polycystic ovarian syndrome (PCOS), and the men had oligoasthenospermia.
Case 1
The first patient was 27 years-old G:3 P: 1 woman with PCOS. Woman who was weight 85 kg and tall 165 cm, had abnormal ratio of gonadotropins (LH/FSH ratio; 4,3). She was assessed as hirsutism according to Ferriman-Gallawey scoring system (score; 14). She had complaint of menstrual irregularity as oligohypomenorrhea for the menarche. Her husband had oligoasthenoteratospermia. Sperm count was 9 million/mm3 and 35% of sperms were motile and 4% of all sperms were had normal morphology according to Kruger’s strict criteria’s. The woman and her spouse had no systemic disease and any skin manifestaitions in their past medical and family histories.
After the second year of their marriage, they were referred to an IVF clinic. The first treatment cycle resulted with a pregnancy but, ended with missed abortion in the 6th gestational week. Genetic assessment was not made from termination material, because of couple did not agree. In the second treatment cycle, resulted with a pregnancy. Chorion villus sampling or amniocentesis was suggested but couples didn’t agree due to religion and economic problems. The fetus was born with Netherton syndrome and died because of infectious complications in the first month of life. Netherton syndrome was diagnosed on postmortem authopsy with karyotype analysis. Homozygous mutation was found in exon 4 as 238 insG and this mutation was confirmed by direct sequencing analysis with father and mother DNA samples of the parents. DNA samples were isolated from peripheral bloods and the mutation analysis was confirmed SPINK5 gene mutations on mother and father as 238 insG in exon 4. Genetic counseling was given and IVF with PGD was suggested to family for next pregnancy.
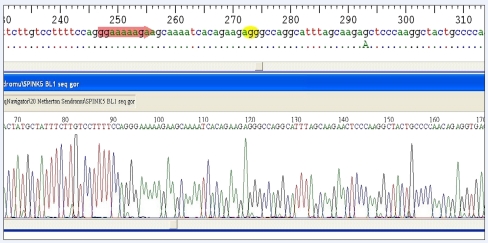
In the next treatment cycle, we planned prenatal genetic diagnosis (PGD). It was used long protocol with decapeptyl (triptorelin) and recombinant human FSH (Gonal-F; Serono International, Geneva, Switzerland) for controlled ovarian hyperstimulation. The estradiol levels were measured 3,500 nmol/L and ovarian picked up was made at the 10th day of cycle. Twenty-four oocytes were retrieved then 21 ICSI were conducted and 19 embryos were gained. There were 15 embryos on 3th day of fertilization and embryo biopsies were made from all embryos. According to PGD results, It was determined that four embryos had heterozygous mutation, four embryos had homozygous mutation and four embryos had homozygous normal karyotype (Figs. 1, 2, 3). In three of the embryos, there was no PCR amplification. Three embryos were transferred and one normal embryo was freezed. The woman gave birth to a healthy baby at 37.5th gestational weeks weighting 3,100 gr.
Fig. 1.
Heterozygous carrier embryo
Fig. 2.
Homozygous mutant embryo
Fig. 3.
Homozygous normal embryo
Case 2
The second patient was 25 years-old and G:3 P:1 woman with PCOS, she was sister of the first patient. Woman who was weight 78 kg and tall 167 cm, had hirsutism (Ferriman-Gallway score: 12). Her husband had oligoasthenoteratospermia according to Kruger’s strict criteria’s (sperm count; 13 million/mm3, normal morphology: 3% and motility 30%). After two miscarriages at first trimester, they were referred to an IVF clinic, and an ICSI pregnancy was resulted. In this pregnancy, amniocentesis was conducted on 16th week of pregnancy and it was determined as Netherton syndrome in genetic assessment in amniotic fluid. They did not accept termination of pregnancy because of religious reasons and the baby was borned. The baby deceased in the third month of life despite maximal intensive care intervention. The genetic counseling were given to family and PGD with IVF was suggested. The family applied to our IVF clinic for pregnancy after the 1 year later. We performed long protocol with GnRH agonist (Lucrin) and recombinant FSH (Gonal-F). 18 oocytes were retrieved then 17 ICSI were conducted and 17 embryos were gained. There were 13 embryos on 3th day of fertilization and embryo biopsies were made from all embryos.
The PGD results revealed five embryos had heterozygous mutation, four embryos had homozygous for the mutation and one embryo had homozygous normal. In three of the embryos, there was no PCR amplification. One normal and one heterozygous embryos were transferred on 5th day of fertilization. After 12 days of embryo transfer, beta hCG was measured as positive. The women gave birth to a healthy baby at 39th gestational weeks weighting 3,350 gr.
PGD technique
We performed blastomere biopsy in day 3 morning all embryos with Sage cooper surgical Quinn’s advantage™ medium with hepes (Ca+2-Mg+ free) and Nikon T300 inverted microscope embedded Hamilton Thorne Laser system. Two blastomeres were taken from each embryo [11]. The blastomeres were transferred into 0,2 ml PCR tubes with 1 μl of medium, covered with mineral oil and subjected to cell lysis. Cell lysis was performed by heating at 45°C for 15′, 96°C for 20′ after the addition of 5 μl of cell lysis buffer (Tween 20, Triton X-100, Prot.K, 10xPCR buffer) to each test tube. The PCR strategy consisted in an initial multiplex external amplification using of oligonucleotide primers, one specific for the regions encompassing IVS-3/exon 4/IVS-V of SPINK5 gene followed by nested PCR specific for this region. The primers sequence is listed in Table 1.
Table 1.
Primer sequences for single cell PCR analysis
| Primer | Base Sequence |
|---|---|
| SPINK5 external 4F | ACCATGTTCGAGATATTTTTCAATGT |
| SPINK5 external 4R | CCTCCTAAGTTGTTGGCTTCAAAAC |
| SPINK5 internal 4F | TGCTACCAATTTTGACATGCCAG |
| SPINK5 internal 4R | TCAAAAGTAAACAGGTTGGCTCCA |
After cell lysis, and neutralization, 1,5 mM MgCl2, 200 μM of each dNTP, 2.5 U TaqPolymerase (Fermentas), 10 pmol of each outer primer, were added to each tube, in a total volume of 50 μl. The first round PCR employed a 95°C denaturation temperature in the first ten cycles as a means to reduce ADO [12], followed by subsequent denaturation temperature of 94°C in 25 remaining cycles. Each round of PCR was preceded by an initial 4′ denaturation step at 94°C and followed by a final extension step of 10′ at 72°C. Extension temperature was 60°C.
For the second round of DNA amplification, 2 μl of the primary PCR reaction product was added to another tube containing 5 μl of 10X PCR Buffer II (500 mM KCl, 100 mM Tris HCl, pH 8,3—Applied Biosystems, Foster City, CA), 1,5 mM MgCl2, 200 μM of each dNTP, 2.5 U TaqPolymerase (Fermentas), 10 pmol of each inner primer, in a total volume of 50 μl, and the tubes were cycled as follows: initial denaturation step 1′ at 94°C for 1 cycle; denaturation 30″ at 94°C, annealing 1′ at 60°C and extension 1′ at 72°C, for 35 cycles; final extension step of 10′ at 72°C, 1 cycle.
Mutation analysis was carried out using automated sequence analysis method. Microcon 100 (Amicon, Beverly, MA) purified PCR products were sequenced by direct cycle sequencing using fluorescent-labelled dideoxy terminators (ET-Terminator Cycle Sequencing Ready Reaction Kit—Amersham) and run on the MegaBase 1000 Genetic Analyzer (General Electric). The sequences obtained were than compared with wild type controls using Sequence Navigator® Software (GE-MegaBase) for mutation analysis. Each identified mutation was confirmed by sequencing of the opposite strand.
Discussion
Until recently, C-NS could not possible to perform diagnosis before the pregnancy. In contrast to a number of other inherited skin disorders, ultra-structural examination of fetal skin biopsies, for defective cornification of the epidermis is not reliable [13]. Fetal skin keratinization begins after 24th week whereas in utero fetal skin biopsy is generally performed between 19 and 22 weeks’ gestation [14–16].
Preimplantation genetic testing is an early form of prenatal diagnosis, where genetic defects in embryos created in vitro are analyzed before implantation in the uterus [17]. This offers couples at risk of a genetic disease the chance to have an unaffected child, without facing termination of pregnancy. To date, many studies have addressed the impact of preimplantation genetic screening in different groups of patients, however, its effectiveness has not been consistently proven [18]. Despite this, according to the recent European Society of Human Reproduction and Embryology (ESHRE) PGD consortium data collection, the number of PGD cycles performed for aneuploidy screening, pregnancies, and babies reported annually have increased considerably [19]. Polymerase chain reaction and fluorescence in situ hybridization are the two common techniques employed on a single or two cells obtained via embryo biopsy. The couple who seek in vitro fertilization may screen their embryos for aneuploidy and the couple at risk for a monogenic disorder but averse to abortion of the affected fetuses after prenatal diagnosis, are likely to be the best candidates to undergo this procedure [20].
The recent identification of the defective gene in C-NS made DNA-based prenatal diagnosis possible [21]. SPINK5 (serin protease inhibitor Kazal-type 5) encodes the Kazal-type serin protease inhibitor domains. LECT1 is thought to be involved in the regulation of proteolysis in skin barrier formation and immunity and this protein is highly expressed in thymus and mucous epithelia [22]. After the first report of mutations in C-NS [19], more than 20 SPINK5 mutations have been identified and reported [23, 24].The recent discovery of SPINK5 on chromosomal region 5q31 as the gene responsible for C-NS now enables causative mutations to be identified in these families. However, SPINK5 comprises 33 exons transcribed into a 3.7 kb coding mRNA, and is not amenable to quick mutation screening. In addition, the majority of SPINK5 identified so far are single base changes that have been identified in almost every exon of the gene. Thus the detection of SPINK5 mutations requires the screening of the entire coding sequence and splice sites of the gene, using a highly sensitive mutation detection technique such as denaturing high performance liquid chromatography [25].
The clinical course often begins at birth. Infants with C-NS are born with congenital erythroderma (generalized erythema and scaling) or these skin findings can develop within the first few weeks postpartum. The extent of these findings varies greatly. At the most severe end of the spectrum, infants can be born with collodion membrane. These infants with more severe cases can also have an associated failure to thrive and hypernatremic dehydration, likely secondary to excess fluid loss from a defective skin barrier [26]. Rarely, infants can develop bronchopneumonia or sepsis. Nails and teeth are generally normal. The initial erythroderma usually evolves into ichthyosis linearis circumflexa (ILC) over time. ILC is not present at all times in patients, and may change with seasonal climate changes. Patients also have features suggestive of atopic dermatitis with erythema and lichenification at flexural creases and diffuse generalized xerosis. An extreme erythroderma can be observed with infection or excitement. Other clinical features include mental deficiency, neurological deficits (either seizure disorders or spastic diplegia), delayed growth and body development, short stature, recurrent infections (skin, eye, upper or lower respiratory tract), hypogammaglobulinemia or hypergammaglobulinemia [27].
Laboratory tests for C-NS are not as critical as evaluation of the hair shaft abnormality under light microscopy. Aminoaciduria occurs in a minority of the patients, with some question if this occurs secondary to use of topical or systemic steroids [23]. A peripheral eosinophilia is commonly found [28]. The serum IgE level is often elevated, sometimes to extreme levels [29]. Positive skin tests or RAST responses to environmental and/or food allergens are commonly observed [28]. Skin biopsy specimens are generally nonspecific and not helpful for the diagnosis. Histopathology often reveals changes resembling psoriasis with acanthosis, hypergranulosis, and occasionally spongiosis progressing to microvesiculation [29]. The hair shaft abnormality crucial for diagnosis of C-NS is the finding of trichorrhexis invaginata (bamboo hairs and ball and socket deformity) under light microscopy secondary to a defect of keratinization of the hair cortex [28]. Trichorrhexis nodosa and pili torti also occur [30].
Many treatments have been attempted in patients with C-NS. Topical corticosteroids are inconsistently successful. Low dose oral corticosteroids, etretinate and psoralen ultraviolet A therapy was also used. Topical tacrolimus will likely achieve minimal benefit, and may be contraindicated for patients with extensive skin involvement with concern for systemic absorption [22, 31].
There are some articles about prenatal diagnosis of C-NS with amniocentesis or chorionic villus sampling in literature [21, 32, 33]. This study is first report of ICSI pregnancy with PGD in C-NS. This is important especially in countries in which consanguineous pregnancies are often seen and autosomal recessive disorders are prevelant. IVF with PGD can be a appropriate treatment option for patients who have diagnosed any genetic diseases and gene disorders.
Footnotes
Capsule We show that prenatal diagnosis of Netherton syndrome is possible with PGD.
References
- 1.Comel M. Ichthyosis linearis circumflexa. Dermatologica. 1949;98:133–136. doi: 10.1159/000257290. [DOI] [PubMed] [Google Scholar]
- 2.Netherton EW. A unique case of trichorrhexis nodosa-bamboo hairs. Arch Dermatol. 1958;78:483–487. doi: 10.1001/archderm.1958.01560100059009. [DOI] [PubMed] [Google Scholar]
- 3.Jones SK, Thomason LM, Surbrugg SK, Weston WL. Neonatal hypernatraemia in two siblings with Netherton’s syndrome. Br J Dermatol. 1986;114(6):741–743. doi: 10.1111/j.1365-2133.1986.tb04885.x. [DOI] [PubMed] [Google Scholar]
- 4.Hausser I, Anton-Lamprecht I, Hartschuh W, Petzoldt D. Netherton’s syndrome: ultrastructure of the active lesion under retinoid therapy. Arch Dermatol Res. 1989;281(3):165–172. doi: 10.1007/BF00456387. [DOI] [PubMed] [Google Scholar]
- 5.Smith DL, Smith JG, Wong SW, deShazo RD. Netherton’s syndrome. Br J Dermatol. 1995;133(1):153–154. doi: 10.1111/j.1365-2133.1995.tb02520.x. [DOI] [PubMed] [Google Scholar]
- 6.Bitoun E, Chavanas S, Irvine AD, et al. Netherton syndrome; disease expression and spectrum of SPINK5 mutations in 21 families. J Int Dermatol. 2002;118:352–361. doi: 10.1046/j.1523-1747.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 7.Sybert VP. Netherton syndrome. In: Sybert VP, editor. Genetic skin disorders. New York: Oxford University Press; 1997. pp. 31–34. [Google Scholar]
- 8.Dupré A, Bonafé JL, Carrère S. Comel’s linear circumflex ichthyosis and Netherton’s syndrome. General conceptions based on study of 4 cases. Ann Dermatol Vénéréol. 1978;105(1):49–54. [PubMed] [Google Scholar]
- 9.Caputo R, Vanotti P, Bertani E. Netherton’s syndrome in two adult brothers. Arch Dermatol. 1984;120(2):220–222. doi: 10.1001/archderm.120.2.220. [DOI] [PubMed] [Google Scholar]
- 10.Kassis V, Nielsen JM, Klem-Thomsen H, Dahl-Christensen J, Wadskov S. Familial Netherton’s disease. Cutis. 1986;38(3):175–178. [PubMed] [Google Scholar]
- 11.Fasouliotis SJ, Schenker JG. Preimplantation genetic diagnosis principles and ethics. Hum Reprod. 1998;13(8):2238–2245. doi: 10.1093/humrep/13.8.2238. [DOI] [PubMed] [Google Scholar]
- 12.Ray PF, Handyside AH. PCR from single cells for preimplantation diagnosis. Methods Mol Med. 1996;5:245–258. doi: 10.1385/0-89603-346-5:245. [DOI] [PubMed] [Google Scholar]
- 13.Hausser I, Anton-Lamprecht I. Severe congenital generalized exfoliative erythroderma in newborns and infants: a possible sign of Netherton syndrome. Pediatr Dermatol. 1996;13(3):183–199. doi: 10.1111/j.1525-1470.1996.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 14.Elias S, Esterly NB. Prenatal diagnosis of hereditary skin disorders. Clin Obstet Gynecol. 1981;24(4):1069–1087. doi: 10.1097/00003081-198112000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Eady RA. Fetoscopy and fetal skin biopsy for prenatal diagnosis of genetic skin disorders. Semin Dermatol. 1988;7(1):2–8. [PubMed] [Google Scholar]
- 16.Holbrook KA, Smith LT, Elias S. Prenatal diagnosis of genetic skin disease using fetal skin biopsy samples. Arch Dermatol. 1993;129(11):1437–1454. doi: 10.1001/archderm.129.11.1437. [DOI] [PubMed] [Google Scholar]
- 17.Sermon K. Current concepts in Preimplantation genetic diagnosis (PGD): a molecular biologist’s view. Hum Reprod Update. 2002;22:312–318. doi: 10.1093/humupd/8.1.11. [DOI] [PubMed] [Google Scholar]
- 18.Shahine LK, Cedars MI. Preimplantation genetic diagnosis does not increase pregnancy rates in patients at risk for aneuploidy. Fertil Steril. 2006;85:51–56. doi: 10.1016/j.fertnstert.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 19.Goossens V, Harton G, Moutou C, Traeger-Synodinos J, Rij M, Harper JC. ESHRE PGD Consortium data collection IX: cycles from January to December 2006 with pregnancy follow-up to October 2007. Hum Reprod. 2009;24:1786–1810. doi: 10.1093/humrep/dep059. [DOI] [PubMed] [Google Scholar]
- 20.Adiga SK, Kalthur G, Kumar P, Girisha KM. Preimplantation diagnosis of genetic diseases. J Postgrad Med. 2010;56(4):317–20. doi: 10.4103/0022-3859.70943. [DOI] [PubMed] [Google Scholar]
- 21.Chavanas S, Garner C, Bodemer C, Ali M, Teillac DH, Wilkinson J, et al. Localization of the Netherton syndrome gene to chromosome 5q32, by linkage analysis and homozygosity mapping. Am J Hum Genet. 2000;66(3):914–921. doi: 10.1086/302824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mägert HJ, Ständker L, Kreutzmann P, Zucht HD, Reinecke M, Sommerhoff CP, et al. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J Biol Chem. 1999;274(31):21499–21502. doi: 10.1074/jbc.274.31.21499. [DOI] [PubMed] [Google Scholar]
- 23.Ishida-Yamamoto A, Deraison C, Bonnart C, Bitoun E, Robinson R, O’Brien TJ, et al. LEKTI is localized in lamellar granules, separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J Invest Dermatol. 2005;124(2):360–366. doi: 10.1111/j.0022-202X.2004.23583.x. [DOI] [PubMed] [Google Scholar]
- 24.Bitoun E, Micheloni A, Lamant L, Bonnart C, Tartaglia-Polcini A, Cobbold C, et al. LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in Netherton syndrome. Hum Mol Genet. 2003;12(19):2417–2430. doi: 10.1093/hmg/ddg247. [DOI] [PubMed] [Google Scholar]
- 25.Bitoun E, Bodemer C, Amiel J, Prost Y, Stoll C, Calvas P, et al. Prenatal diagnosis of a lethal form of Netherton syndrome by SPINK5 mutation analysis. Prenat Diagn. 2002;22:121–126. doi: 10.1002/pd.247. [DOI] [PubMed] [Google Scholar]
- 26.Sun JD, Linden KG. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45(6):693–697. doi: 10.1111/j.1365-4632.2005.02637.x. [DOI] [PubMed] [Google Scholar]
- 27.Greene SL, Muller SA. Netherton’s syndrome. Report of a case and review of the literature. J Am Acad Dermatol. 1985;13:329–337. doi: 10.1016/S0190-9622(85)70170-3. [DOI] [PubMed] [Google Scholar]
- 28.Smith DL, Smith JG, Wong SW, deShazo RD. Netherton’s syndrome: a syndrome of elevated IgE and characteristic skin and hair findings. J Allergy Clin Immunol. 1995;95:116–123. doi: 10.1016/S0091-6749(95)70159-1. [DOI] [PubMed] [Google Scholar]
- 29.Dawber RPR. Clinical aspects of hair disorders. Dermatol Clin. 1996;14:753–772. doi: 10.1016/0738-081X(95)00117-X. [DOI] [PubMed] [Google Scholar]
- 30.Altman J, Stround J. Netherton’s syndrome and ichthyosis linearis circumflexa. Arch Dermatol. 1969;100:550–558. doi: 10.1001/archderm.100.5.550. [DOI] [PubMed] [Google Scholar]
- 31.Saif GB, Al-Khenaizan S. Netherton syndrome: successful use of topical tacrolimus and pimecrolimus in four siblings. Int J Dermatol. 2007;46(3):290–294. doi: 10.1111/j.1365-4632.2006.02956.x. [DOI] [PubMed] [Google Scholar]
- 32.Müller FB, Hausser I, Berg D, Casper C, Maiwald R, Jung A, et al. Genetic analysis of a severe case of Netherton syndrome and application for prenatal testing. Br J Dermatol. 2002;146(3):495–499. doi: 10.1046/j.1365-2133.2002.04625.x. [DOI] [PubMed] [Google Scholar]
- 33.Sprecher E, Chavanas S, DiGiovanna JJ, Amin S, Nielsen K, Prendiville JS, et al. The spectrum of pathogenic mutations in SPINK5 in 19 families with Netherton syndrome: implications for mutation detection and first case of prenatal diagnosis. J Invest Dermatol. 2001;117(2):179–187. doi: 10.1046/j.1523-1747.2001.01389.x. [DOI] [PubMed] [Google Scholar]