Abstract
Purpose
To evaluate clinical outcomes of frozen-thawed embryo transfer cycles when one or two blastocysts are transferred.
Methods
Retrospective chart review
Results
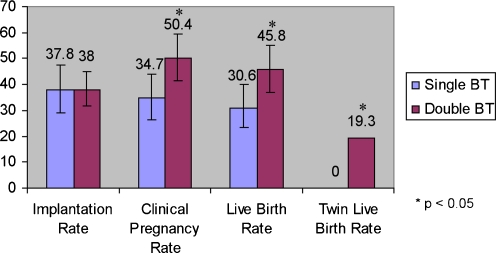
Two hundred forty-three frozen blastocyst transfer (FBT) cycles were analyzed. Clinical pregnancy rate (50.4% vs. 34.7%), live birth rate (45.8% vs. 30.6%), and twin live birth rate (19.3% vs. 0) were significantly higher in the double versus single FBT group, respectively (p < 0.05). Prior fresh cycle success with same-cohort embryos did not predict outcome of FBT cycle. When the fresh cycle was unsuccessful, there still was a significant increase in twinning when two frozen-thawed blastocysts were transferred.
Conclusions
Transferring two blastocysts during an FBT cycle resulted in higher live birth and twin live birth rates. Single FBT provided acceptable pregnancy rates for couples seeking to avoid a multiple pregnancy or for those having a single blastocyst stored. Interestingly, the outcome of fresh cycle with same-cohort embryos did not influence the outcome of frozen-thawed cycle.
Keywords: Frozen blastocyst transfer, Frozen cycle outcome, IVF, Multiple pregnancy, Number of embryos to transfer
Introduction
The development of extended embryo culture techniques has allowed IVF programs to offer patients the advantages of selecting the best embryo [1, 2]. Because of this, the practice of elective single embryo transfer (eSET) has become more acceptable during fresh cycles, with a great deal of evidence supporting eSET in good prognosis patients [3, 4]. The aim of transferring a smaller number of embryos is to lower the risks of multiple gestations and their associated obstetric and perinatal morbidity and mortality [5].
Due to concerns of decreased implantation potential of cryopreserved embryos [6] practices may hesitate to apply the same criteria to frozen cycles. According to the SART database, 15.2% of all ART cycles performed in 2008 were frozen-thawed embryo transfer cycles, and the success rate when using frozen embryos was lower than for fresh embryos for women in all age groups under the age of 41 (https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0; accessed on 12/2/2010).
Increasing experience and success with cryopreservation of embryos has prompted an interest in decreasing the number of embryos transferred during frozen cycles. As part of the fresh/frozen cycle combination, Thurin reported a 16.4% live birth rate in the single frozen ET group [3]. Berin et al. compared cycle outcomes when two or three embryos were transferred (multicellular and blastocysts, combined) and concluded that transferring two embryos resulted in the same live birth rate as three embryos but with a significantly decreased risk of multiples [7].
Programs may freeze embryos at the pronuclear or multicellular stage, but there has been an increasing interest in blastocyst cryopreservation. Only a few papers assess blastocyst stage transfers specifically, and that limited experience may suggest lower birth rates when transferring frozen blastocysts compared with fresh. Desai and Goldfarb conducted a retrospective analysis of 56 frozen transfers of a single blastocyst in 2005, with a goal of understanding the relationship between post-thaw morphology of blastocysts and pregnancy outcomes. They reported an 18% live birth rate following transfer of a single thawed blastocyst, noting that a more advanced blastocyst stage at the time of thaw was associated with improved cycle outcome [8]. This was a preliminary study, accounting for only age and infertility diagnosis as possible contributing factors, and was aimed at creating a model to help gauge effectiveness of cryopreservation program. Hydén-Granskog et al. reported on a larger group of patients, comparing single and double frozen embryo transfers, but the data was combined for different embryo stages at cryopreservation (day 2 and day 3 embryos, and blastocysts). These investigators reported an overall live birth rate of 19.2% vs. 25.7% following single vs. double SET in frozen cycles, respectively, but did not examine blastocyst transfers alone [9]. To our knowledge, the only other report addressing single blastocyst transfers in frozen cycles was a study by Yanaihara et al., which compared clinical outcomes of single vs. double frozen blastocyst transfers [10]. The live birth rates were not significantly different—29.1% with one blastocyst and 35.5% with two blastocysts, but a significantly higher twinning rate was seen when two blastocysts were transferred. This was a large study, with 562 included cycles, but there was limited information on important factors in patient background and fresh cycle characteristics. In addition, the data were not stratified by age or outcome of same-cohort fresh cycles, and analyses were not adjusted for repeat cycles in same patient.
Because only a few studies have addressed single frozen blastocyst transfers, we wanted to examine our results with single and double blastocyst transfers, focusing on pregnancy outcomes. This inquiry was also prompted by the recent guidelines of the American Society of Reproductive Medicine, which recommend that the number of good quality thawed embryos transferred should not exceed the number that would have been transferred in a fresh cycle for each age group [11]. Our IVF unit elective SET program for fresh cycle blastocysts was established in 2004, and the favorable pregnancy rates [4] encouraged us to evaluate whether single ET in frozen cycles would be an efficacious and safe approach. Therefore, a comparison was performed to evaluate pregnancy outcomes in patients undergoing single frozen blastocyst transfer (FBT) with double frozen blastocyst transfer.
Materials and methods
Patient cycle selection
The study was approved by the Partners HealthCare Human Research Committee for medical record review. Two hundred ninety five consecutive FBT cycles from 1/1/2002 to 3/31/2008 at Massachusetts General Hospital were reviewed retrospectively. All cycles which involved the transfer of either one or two blastocysts were identified (n = 243).
Cryopreserved embryos were obtained from fresh cycles in which supernumerary embryos remained. Of note, those supernumerary embryos that reached an appropriate blastocyst stage on Day 5 or Day 6 of development were cryopreserved. Blastocysts were considered for cryopreservation when the blastocoel cavity filled the volume of the zona pellucida, an inner cell mass (ICM) was visible, and a cohesive, evenly distributed layer of healthy trophectoderm cells were demonstrated. This corresponded to ICM grading of 1 or 2 (with 1 being best and 4 being worst) and a trophectoderm grading of C or better (A best, D worst); modified Veeck grading [12].
Cycles in which two embryos transferred had resulted from different fresh cycles were excluded.
Stimulation and cryopreservation protocols
Three controlled ovarian hyperstimulation protocols were used during the fresh cycles resulting in the cryopreserved embryos : (1) low-dose luteal phase down-regulation protocol with GnRH agonist leuprolide acetate (Lupron; TAP Pharmaceuticals, North Chicago, IL USA), (2) a variable initiation antagonist protocol using ganirelix acetate (Antagon; Organon Inc., West Orange, NJ USA) daily once the lead follicle reached approximately 14 mm, or (3) flare protocol using leuprolide acetate in the follicular phase. All of the stimulation cycles were preceded by pre-treatment with oral contraceptive pills. Women <38 years old proceeded to Day 5 transfer if they had ≥4 embryos of at least six cells on Day 3. Women ≥38 years proceeded to Day 5 transfer if they had ≥6 embryos of high quality, with appropriate cell division from day 2 to day 3. Depending on their age, women had 1–4 blastocysts transferred on Day 5, and all supernumerary appropriate blastocysts were cryopreserved on Day 5 or Day 6.
The slow-freeze cryopreservation technique utilized a PBS-based medium with HSA protein supplementation. A modified Menezo two-step freeze protocol utilizing glycerol and sucrose as cryoprotectants was used for blastocyst cryopreservation (Menezo et al. 1992). First, a 10 min incubation was performed in 5% glycerol, followed by a 5 min incubation in a cryoprotectant solution of 10% glycerol and 0.2 mol/L sucrose solution. One to two blastocysts were loaded into Nunc cryovials which contained 0.3 mL of cryoprotectant solution (10% glycerol/0.2 mol/L sucrose). A controlled rate Planer freezer was used to cool the vials at a rate of −2°C/min until −7°C. After a 10 min hold period, the vials were seeded. They were further cooled at a rate of −0.3°C/min until −37°C. The cryovials were then plunged into liquid nitrogen as the final step in cryopreservation.
Blastocyst thawing was performed as follows. Cryovials were removed from the cryotank and kept at room temperature for 1 min. They were then immersed into a 30°C waterbath for 2 min, followed by a 4-step rehydration protocol. Embryos were first placed into a solution of 10% glycerol and 0.2 mol/L sucrose for 1 min, followed by 5% glycerol solution for 3 min, followed by 0.2 mol/L sucrose solution for 2 min, followed by rinsing in the PBS-based medium. The embryos were then cultured for either 24 h (if frozen on Day 5) or 4–5 h (if frozen on Day 6) in the 5% CO2 incubator to allow for re-expansion.
Patients preparing for frozen transfer cycles underwent programmed hormone replacement. This included a standard protocol of pituitary suppression with GnRH agonist leuprolide acetate (Lupron; TAP Pharmaceuticals, North Chicago, IL USA), followed by the use of increasing numbers of estradiol patches (Vivelle Dot; Novartis Pharmaceuticals, East Hanover, NJ USA). After 14–18 days of estrogen priming, daily progesterone supplementation was begun using a combination of progesterone 50 mg IM once a day and Endometrin 100 mg pv twice a day (Ferring Pharmaceuticals, Parsippany, NJ USA). Endometrial thickness was monitored prior to initiating progesterone, and if endometrial lining was less than 7 mm, estradiol 2 mg pv once to twice daily (Estrace; Warner Chilcott, Rockaway, NJ USA) was added for 5–7 days until adequate endometrial stripe development was seen. Embryos were thawed and transferred on ideal cycle day 20. If the transfer involved blastocysts which were frozen on different days, the Day 5 blastocyst was thawed the day prior to the transfer, and the Day 6 blastocyst was thawed on the day of the transfer. Confirmation of a successful implantation was obtained by detecting an elevated serum human chorionic gonadotropin (hCG) level (≥6 IU/L) 12 days after transfer. Serial transvaginal ultrasounds were performed beginning at 3 weeks after transfer to determine the number of gestational sacs, and thereafter until detection of fetal heart motion.
Statistics
The major variables analyzed included baseline patient characteristics, as well as fresh and frozen cycle characteristics. Main outcome variables included clinical pregnancy rate (defined as presence of fetal heart beat), live birth rate, and twin live birth rate. Continuous and categorical outcomes were analyzed using linear and logistic mixed models, respectively. Each model included a fixed effect identifying single vs. double frozen blastocyst transfer, and a random patient-specific intercept to control for covariance in outcomes of related cycles. Additional predictors of cycle outcome were included as fixed effects to test for associations with length of cryopreservation, use of donor eggs, fertilization using intra-cytoplasmic sperm injection (ICSI), prior live birth from a fresh cycle, and age group. Reported estimates are least-square means with Wald confidence intervals. Proportions were obtained by back-transformation of estimated log odds from the logistic models. A two-sided P value <0.05 was considered statistically significant. All analyses were performed using SAS (version 0.2, SAS Institute, Cary NC).
Results
A total of 243 frozen cycles in 203 patients were analyzed, including 26 donor egg cycles. Patient groups were similar in regards to their age, basal FSH and E2 values (Table 1). In addition, there were no differences in infertility diagnoses, the need for additional estrogen or endometrial thickness achieved in frozen cycle. The percentage of cycles involving ICSI technique were similar between the single FBT and double FBT groups (51.3% (95% CI 40.2–62.4) vs 41.1% (95% CI 31.0–52.1), p = 0.18, respectively). Donor oocyte was utilized in similar numbers of cycles, as well, among the two groups (11.4% (95% CI 6.1–20.3) in single FBT vs 13.8% (95% CI 8.0–22.8) in double FBT, p = 0.62). There were significantly more oocytes retrieved and more normally fertilized oocytes during the fresh cycle of those patients who underwent a double FBT. Because at least two embryos were thawed in the double FBT group, there were more cycles with at least one re-expanded blastocyst post-thaw (96.8% (95% CI 92–99) vs 81.6% (95% CI 73–88) for single FBT, p = 0.002), although similar numbers of cycles had high quality blastocysts when frozen. Importantly, significantly fewer embryos needed to be thawed in the single FBT group.
Table 1.
Comparison of baseline and cycle characteristics
| Single FBT | Double FBT | p value | |
|---|---|---|---|
| Number of FBT cycles | 118 | 125 | |
| Mean age at freezing, years ± SEM (95% CI) | 32.7 ± 0.4 (32.0–33.5) | 31.8 ± 0.5 (31.1–32.6) | 0.05 |
| Basal FSH, U/mL ± SEM (95% CI) | 8.6 ± 0.9 (6.5–10.8) | 9.0 ± 0.9 (6.9–11.2) | 0.19 |
| Basal E2, pg/mL ± SEM (95% CI) | 43.5 ± 2.2 (38.7–48.3) | 44.4 ± 2.1 (39.5–49.2) | 0.18 |
| Live birth after fresh IVF cycle,% (95% CI) | 37.9 (28.3–48.4) | 24.8 (17.2–34.3) | 0.06 |
| # of oocytes retrieved during fresh cycle ± SEM (95% CI) | 12.8 ± 0.4 (11.9–13.7) | 14.1 ± 0.4 (13.2–15.0) | 0.04 |
| # of oocytes fertilized normally during fresh cycle ± SEM (95% CI) | 8.4 ± 0.3 (7.8–9.0) | 9.7 ± 0.4 (9.1–10.3) | 0.003 |
The outcomes of frozen-thawed embryo transfer cycles are detailed in Fig. 1. The clinical pregnancy and the live birth rates were significantly higher in the double FBT group. Importantly, twin live birth rate was also higher in this group. Of note, there were no monozygotic twins, triplets or ectopic pregnancies in the study population.
Fig. 1.
Outcomes of frozen blastocyst transfer cycles in all women
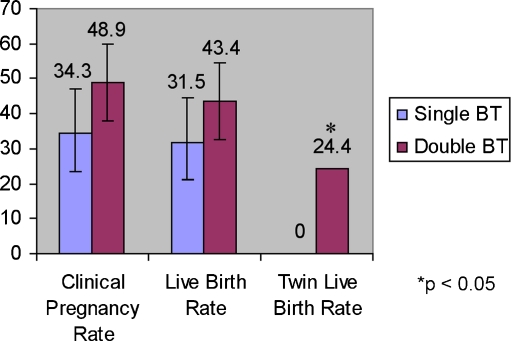
Cycles with double FBT were associated with lower rates of prior successful IVF (OR 0.43, 95% CI 0.24–0.79, p = 0.006). However, achieving a live birth in the fresh cycle from which the frozen embryos resulted was not associated with successful live birth in the frozen cycle (OR 1.06, 95% CI 0.6–1.9, p = 0.85). When the fresh cycle was not successful, double FBT was associated with higher clinical pregnancy, live birth, and significantly higher twinning rates among the same-cohort frozen cycles. (Fig. 2)
Fig. 2.
Outcomes of frozen blastocyst transfer cycles in women who did not achieve a live birth from fresh cycle
A particular group of interest included those patients who had a single blastocyst transferred in their fresh cycle and did not conceive. Subgroup analysis was performed for those patients in that group that returned for a same-cohort frozen cycle. 53.8% of these patients had a single FBT, with a resultant 28.6% (95% CI 13.2–52.1) live birth rate (singletons only). The remainder (46.2%) underwent a double FBT resulting in an overall live birth rate of 43.2% (95% CI 23.7–65.1), and a 25% twining rate. These findings were not significantly different (p = 0.31 for overall live birth rate, and p = 0.13 for twin live birth rate).
Use of a donated oocyte was not associated with probability of live birth (OR = 1.07, 95% CI 0.48 to 2.41, p = 0.87). There was no significant association between the use of ICSI technique and probability of live birth among frozen cycles (OR = 0.60, 95% CI 0.34 to 1.05, p = 0.07). More embryos were cryopreserved on Day 6 than Day 5, but the proportion of Day 5 vs. Day 6 embryos was not different in the two groups. Among double FBT cycles, odds of live birth were lower in our sample when only day 6 embryos were transferred, although the difference was not significant (OR 0.58, 95% CI 0.3–1.15, p = 0.11).
Stratifying women by age groups <35 years and ≥35 years revealed that there is no significant interaction between age and single vs. double FBT in predicting the likelihood of live birth (p = 0.22).
Discussion
There has recently been an effort to decrease the number of embryos transferred in frozen cycles, in order to reduce the incidence of multiple pregnancy, which remains the foremost complication of in vitro fertilization therapy. Since the success and experience with extended culture allowed for better selection methods, there has been increasing interest in assessing the efficacy of transferring one frozen-thawed blastocyst. To our knowledge, only three studies have examined outcomes associated with single frozen-thawed blastocyst transfer, and each study had its limitations. In the current study, we attempted to address some of these points, and examined clinical outcomes of one and two blastocyst transfers. Contrary to the findings by Yanaihara et al. [10], our results indicated that transferring two blastocysts in a frozen cycle leads to higher overall pregnancy and live birth rates. Important limitation of this study included a higher number of retrieved and fertilized oocytes, as well as a greater proportion of re-expanded embryos, in the double FBT group. However, chances of pregnancy with a single frozen blastocyst transfer remained acceptable, with a significantly lower risk of twin pregnancy. In addition, contrary to prior reports, our analyses demonstrated that fresh cycle outcome did not predict same-cohort frozen cycle outcomes.
There are several factors to consider with regards to the results obtained in this study. The group which underwent a single FBT was slightly older, had less oocytes retrieved and less oocytes fertilized when compared to the double FBT group, contributing to the inherent difference between these groups. However, despite the relative lower oocyte yield and fertilization rate, this group still had a respectable pregnancy and live birth outcomes. This further supports our conclusions that single FBT may be an acceptable option for some couples.
In this study, we showed that achieving a live birth in the same-cohort fresh cycle did not predict the outcome of frozen cycle. This finding is in contrast to other studies which showed that successful fresh cycle was a positive predictor of frozen cycle success [14, 15]. The notion of homogeneity of embryo cohort has been cited as a possible explanation for the positive predictive effect, but no concrete evidence for this has been presented. Additionally, this retrospective study found that if two embryos were transferred in the group which did not conceive during their fresh cycle, there was a non-significant increase in the pregnancy and live birth rates, and the rate of twinning remained significantly elevated. This finding suggests that failing to conceive in the fresh cycle did not diminish risks of multiple pregnancy when more embryos were transferred in the frozen cycle, which may affect decision-making by the physician and the patient in regards to number of embryos to transfer. A study by Berin et al. [7] confirms the lack of positive predictive effect of successful fresh cycle, as well as the increase in multiple pregnancies if more embryos are transferred. In this patient population, however, there may have been other factors responsible for the lack of association between fresh and frozen cycle outcome. This effect could have been observed if more patients who failed fresh single blastocyst transfer opted to receive a DBT, whereas those who succeeded with the prior fresh cycle were more inclined to request an SBT or to have only one blastocyst in storage, with an associated lower pregnancy rate.
Another important group of patients to consider are those that did not conceive after one blastocyst was transferred in their fresh cycle. There may be a tendency on both the part of the patient and physician to consider transferring more embryos during the frozen cycle for these patients. In fact, we did see a higher live birth rate when two frozen-thawed blastocysts were transferred after failing a single fresh blastocyst transfer, with a quarter of these patients delivering twins. However, this finding did not reach statistical significance possibly due to the small number of patients in this situation, with only 11/41 cycles resulting in a live birth in the single FBT group, vs. 12/29 cycles in the double FBT group.
In large databases for the Centers for Disease Control and Prevention, frozen embryo transfer pregnancy rates are reported to be lower than for fresh embryo transfers (http://apps.nccd.cdc.gov/art/NationalSummaryReport.aspx), and thus physicians hesitate transferring a single embryo during the frozen cycle. An indirect marker assessing the implantation potential of each embryo is re-expansion after the thawing procedure. It has been reported that when an embryo undergoes fast re-expansion prior to transfer, the rate of clinical pregnancy and implantation is doubled compared to non-re-expanded embryos [13]. In the double FBT group, there were more embryos thawed, hence a greater likelihood that at least one embryo would be re-expanded. In the single FBT group, a great majority of embryos were also re-expanded. One caveat to observing re-expansion is that the embryos are cryopreserved at either day 5 or day 6 of embryonic development, depending on when they reach the adequate blastocyst stage. Accordingly, day 5 embryos are thawed 1 day prior to transfer, whereas day 6 embryos are thawed on the day of the transfer. Presumably, day 5 embryos have a longer period of time during which re-expansion can be observed.
Interestingly, the origin of oocyte (donor vs. self) was not predictive of live birth. This seems counter-intuitive, since an embryo derived from a donor egg is expected to be more robust, owing to the likely favorable genetics. However, it is possible that the ability to develop to blastocyst stage confers a more favorable outcome a priori. It has been shown that in donor cycles, blastocyst transfers result in better implantation and pregnancy rates compared to cleavage stage embryos [16]. Similarly, being able to select best embryo when it has been cultured to the blastocyst stage during fresh cycles in general infertile population, has improved pregnancy outcomes [17]. Therefore, it is not surprising that whether or not the embryo was derived from a donor egg was not predictive of outcome. Our finding was consistent with a report by Tang et al. [18], confirming a lack of outcome differences between donor and non-donor frozen cycles.
In our data, there was a higher odds of live birth in double FBT group, across all age groups, and particularly in the younger age category. Not surprisingly, all twins were also born to patients in that group. This is in agreement with other reports, which cite age as an important predictor of cycle outcomes [13, 19]. Data suggest that negative predictors of IVF outcome, such as elevated basal FSH level, may not apply to women who meet criteria for extended culture, and have embryos that reach the blastocyst stage [20]. This may explain why there was only a weak interaction between age and number of embryos transferred in predicting live birth in our cohort.
Limitations of the study include its retrospective design, difference in the two groups of patients with regards to the number of oocytes retrieved and fertilized, and relatively small numbers of patients in certain subgroups. Women who did not have a live birth following an elective fresh single embryo transfer represent a challenging group, and having more patients in that category would allow for more significant conclusions to guide the physician in counseling the patient. One aspect that might be further considered when evaluating the study is that the single FBT group consisted of those who had a single cryopreserved blastocyst remaining and those who chose to use only one despite multiple cryopreserved blastocysts available. However, the fact that our analysis found that age and outcome of prior fresh cycle did not predict frozen cycle success, may suggest that each blastocyst has a similar chance of implantation, regardless of the cohort of embryos from which it resulted.
A selection bias inherently exists when the number of embryos to transfer is studied in a retrospective fashion. Patients who have had a successful IVF pregnancy or other children are perhaps more likely to request a single blastocyst transfer to minimize the risks of twins. In addition, this cohort maybe in a more favorable category a priori since they’ve been able to conceive in the past. The complex decision-making which goes into determining the number that both the clinician and patient feel comfortable with takes into account previous obstetrical history and social and economic situation of the family involved, and is thus difficult to study in isolation.
In conclusion, transferring two blastocysts during a frozen cycle provided higher rates of clinical pregnancy and live birth, as well as an increased the risk of twin pregnancy. However, transferring one blastocyst resulted in a live birth rate of 30.6%, with no twin deliveries in our dataset. This number is similar to the U.S. national average live birth rate of 35.6% in the most favorable age category with an average of 2.2 embryos transferred (https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0; accessed on 12/2/2010), with the inherent risk of multiple pregnancy. While live birth rates were better with double FBT in the population studied, single FBT may offer acceptable pregnancy rates for selected patient groups, such as couples seeking to avoid a multiple pregnancy or those having a single embryo stored. Another significant finding in this study was that the outcome of the fresh cycle did not predict frozen cycle outcome. Although this may be contrary to popular belief, this point is worthy of further exploration in larger studies. Finally, further prospective reports are needed to determine best prognosis patient groups for single blastocyst transfer in frozen-thawed embryo transfer cycles.
Footnotes
Capsule
Transferring one frozen-thawed blastocyst resulted in acceptable success rates, with higher overall live birth and twinning rates when transferring two, regardless of fresh cycle outcome.
Contributor Information
Inna Berin, Phone: +1-646-3015300, Email: inna_berin@yahoo.com.
Sarah T. McLellan, Phone: +1-617-7268868, FAX: +1-617-7248882
Thomas L. Toth, Phone: +1-617-7268868, FAX: +1-617-7248882
Diane L. Wright, Phone: +1-617-7268868, FAX: +1-617-7248882
References
- 1.Mangalraj AM, Muthukumar K, Aleyamma T, Kamath MS, George K. Blastocyst stage transfer vs cleavage stage embryo transfer. J Hum Reprod Sci. 2009;2:23–26. doi: 10.4103/0974-1208.51339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reh A, Fino E, Krey L, Berkeley A, Noyes N, Grifo J. Optimizing embryo selection with day 5 transfer. Fertil Steril. 2010;93:609–615. doi: 10.1016/j.fertnstert.2009.02.070. [DOI] [PubMed] [Google Scholar]
- 3.Thurin A, Hausken J, Hillensjo T, Jablonowska B, Pinborg A, Strandell A, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392–2402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- 4.Styer AK, Wright DL, Wolkovich AM, Veiga C, Toth TL. Single-blastocyst transfer decreases twin gestation without affecting pregnancy outcome. Fertil Steril. 2008;89:1702–1708. doi: 10.1016/j.fertnstert.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Bergh T, Ericson A, Hillensjo T, Nygren KG, Wennerholm UB. Deliveries and children born after in-vitro fertilisation in Sweden 1982–95: a retrospective cohort study. Lancet. 1999;354:1579–1585. doi: 10.1016/S0140-6736(99)04345-7. [DOI] [PubMed] [Google Scholar]
- 6.Kolibianakis EM, Zikopoulos K, Devroey P. Implantation potential and clinical impact of cryopreservation—a review. Placenta. 2003;24(Suppl B):S27–S33. doi: 10.1016/S0143-4004(03)00133-4. [DOI] [PubMed] [Google Scholar]
- 7.Berin I, Engmann LL, Benadiva CA, Schmidt DW, Nulsen JC, Maier DB. Transfer of two versus three embryos in women less than 40 years old undergoing frozen transfer cycles. Fertil Steril. 2010;93:355–359. doi: 10.1016/j.fertnstert.2009.01.101. [DOI] [PubMed] [Google Scholar]
- 8.Desai N, Goldfarb J. Examination of frozen cycles with replacement of a single thawed blastocyst. Reprod Biomed Online. 2005;11:349–354. doi: 10.1016/S1472-6483(10)60843-7. [DOI] [PubMed] [Google Scholar]
- 9.Hyden-Granskog C, Unkila-Kallio L, Halttunen M, Tiitinen A. Single embryo transfer is an option in frozen embryo transfer. Hum Reprod. 2005;20:2935–2938. doi: 10.1093/humrep/dei133. [DOI] [PubMed] [Google Scholar]
- 10.Yanaihara A, Yorimitsu T, Motoyama H, Ohara M, Kawamura T. Clinical outcome of frozen blastocyst transfer: single vs. double transfer. J Assist Reprod Genet. 2008;25:531–534. doi: 10.1007/s10815-008-9275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519. doi: 10.1016/j.fertnstert.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 12.Veeck L, Saninovic N. Human blastocysts in vitro. In: The encyclopedia of visual medicine series: an atlas of human blastocysts. New York: The Parthenon Publishing Group; 2003, p. 118.
- 13.Shu Y, Watt J, Gebhardt J, Dasig J, Appling J, Behr B. The value of fast blastocoele re-expansion in the selection of a viable thawed blastocyst for transfer. Fertil Steril. 2009;91:401–406. doi: 10.1016/j.fertnstert.2007.11.083. [DOI] [PubMed] [Google Scholar]
- 14.Wang JX, Yap YY, Matthews CD. Frozen-thawed embryo transfer: influence of clinical factors on implantation rate and risk of multiple conception. Hum Reprod. 2001;16:2316–2319. doi: 10.1093/humrep/16.11.2316. [DOI] [PubMed] [Google Scholar]
- 15.El-Toukhy T, Khalaf Y, Al-Darazi K, O’Mahony F, Wharf E, Taylor A, et al. Cryo-thawed embryos obtained from conception cycles have double the implantation and pregnancy potential of those from unsuccessful cycles. Hum Reprod. 2003;18:1313–1318. doi: 10.1093/humrep/deg235. [DOI] [PubMed] [Google Scholar]
- 16.Schoolcraft WB, Gardner DK. Blastocyst culture and transfer increases the efficiency of oocyte donation. Fertil Steril. 2000;74:482–486. doi: 10.1016/S0015-0282(00)00685-3. [DOI] [PubMed] [Google Scholar]
- 17.Papanikolaou EG, Camus M, Kolibianakis EM, Landuyt L, Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–1146. doi: 10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- 18.Tang R, Catt J, Howlett D. Towards defining parameters for a successful single embryo transfer in frozen cycles. Hum Reprod. 2006;21:1179–1183. doi: 10.1093/humrep/dei490. [DOI] [PubMed] [Google Scholar]
- 19.Salumets A, Suikkari AM, Makinen S, Karro H, Roos A, Tuuri T. Frozen embryo transfers: implications of clinical and embryological factors on the pregnancy outcome. Hum Reprod. 2006;21:2368–2374. doi: 10.1093/humrep/del151. [DOI] [PubMed] [Google Scholar]
- 20.Thum MY, Kalu E, Abdalla H. Elevated basal FSH and embryo quality: lessons from extended culture embryos: raised FSH and blastocyst quality. J Assist Reprod Genet. 2009;26:313–318. doi: 10.1007/s10815-009-9313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]