Abstract
A sensitive and precise LC-ESI-MS/MS method for determination of vandetanib (ZD6474) in human plasma and cerebrospinal fluid (CSF) using d4-ZD6474 as an internal standard (ISTD) was developed and validated. Sample preparation consisted of a simple liquid-liquid extraction with tert-butyl methyl ether containing 0.1% or 0.5% ammonium hydroxide. ZD6474 and ISTD were separated on a Kinetex C18 column (2.6 μ, 50 mm × 2.1 mm) at ambient temperature with an isocratic mobile phase (acetonitrile/10mM ammonium formate = 50/50, v/v, at pH 5.0) delivered at 0.11 mL/min. The retention time of both compounds was at 1.60 min in a runtime of three min. Detection was achieved by an API-3200 LC-MS/MS system, monitoring m/z 475.1/112.1 and m/z 479.1/116.2 for vandetanib and ISTD, respectively. The method was linear in the range of 0.25 to 50 ng/mL (R2 ≥ 0.990) for the CSF curve and from 1.0 to 3,000 ng/mL (R2 ≥ 0.992) for the plasma curve. The mean recovery for vandetanib was 80%. Within-day and between-day precisions were ≤ 8.8% and ≤ 5.9% for CSF and plasma, respectively. Within-day and between-day accuracies ranged from 95.0 to 98.5% for CSF, and from 104.0 to 108.5% for plasma. Analysis of plasma from six different sources showed no matrix effect for vandetanib (MF = 0.98, %CV ≤ 4.97, n = 6). This method was successfully applied to the analysis of pharmacokinetic samples from children with brain tumors treated with oral vandetanib.
Keywords: vandetanib (ZD6474), human plasma and cerebrospinal fluid (CSF), liquid-liquid extraction (LLE), liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS), lower limit of quantitation (LLOQ)
1. Introduction
Vandetanib (ZD6474), a novel orally available inhibitor of vascular endothelial growth factor receptor-2 (VEGFR-2) tyrosine kinase, has additional activity against epidermal growth factor receptor (EGFR) and RET tyrosine kinases [1,2]. Chronic daily oral administration of vandetanib has resulted in significant tumor growth inhibition in a range of histologically diverse human xenograft models. Well tolerated in adult phase I studies [3], it is currently in phase III clinical trials in several solid tumor types either alone or in combination with paclitaxel or pemetrexed.
In children, malignant brainstem tumors constitute ~20% of all brain tumors. Diffuse intrinsic pontine gliomas (DIPG) accounts for about 80% of these neoplasms. Despite clinical trials spanning over three decades, the outcome of children with DIPG remains dismal with long-term survival < 10%, and new drugs are desperately needed. Since the VEGFR-2 is the primary pro-angiogenic factor in high-grade gliomas, inhibition of this receptor represents a potentially promising target in the treatment of children with DIPG. Thus, vandetanib is under evaluation in combination with radiation therapy in clinical trials in children with DIPG. Key to these evaluations is an understanding of the plasma and CSF pharmacokinetics of vandetanib in children.
Currently only one analytical method for vandetanib has been reported in the literature, and this method was used to analyze vandetanib in murine plasma and tissue samples [4]. This method, which has a lower limit of quantitation (LLOQ) of 20 and 10 ng/ml in plasma and tissue, respectively was inadequate for use in our pediatric clinical pharmacokinetic study where small samples and low plasma concentrations were expected. Moreover, since we expected to obtain CSF samples from these patients, we needed a method that was sensitive enough to measure the extremely low vandetanib concentrations expected in the CSF. Thus, we developed and validated a rapid and sensitive liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) method to determine vandetanib concentrations in human plasma and CSF samples.
2. Experimental
2.1. Chemicals
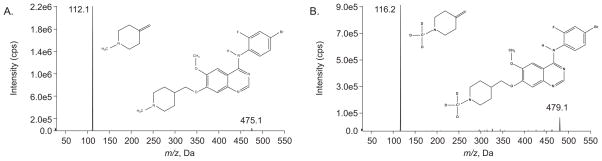
Vandetanib (ZD6474; > 99%, CAS No.: 443913-73-3) and its isotope [13C,d3]-ZD6474 as internal standard (ISTD) were supplied by AstraZeneca (Wilmington, Delaware, USA) (Fig. 1). HPLC grade acetonitrile was obtained from Burdick & Jackson (Muskegon, MI, USA), tert-Butyl methyl ether anhydrous (TBME; 99.8% purity), trifluoroacetic acid (TFA; ≥99% purity), and ammonium hydroxide (NH4OH; 28~30% ACS reagent grade) were purchased from Sigma- Aldrich. Ammonium formate (HCOONH4; 99% purity) was purchased from Fluka BioChemika (Buchs, Switzerland). Blank human plasma was obtained from Lifeblood (Memphis, TN, USA). Human CSF was purchased from Randox Laboratories Ltd (Antrim, United Kingdom). All water was distilled, deionized, and further purified via a Millipore Milli-Q UV plus and Ultra-Pure Water System (Tokyo, Japan). Other chemicals were purchased from standard sources and were of the highest quality available.
Figure 1.
Precursor mass spectra for vandetanib and its product (A) and [13C,d3]-vandetanib (ISTD) and its product (B) with positive ions.
2.2. Apparatus and conditions
2.2.1 Chromatographic conditions
The HPLC system consisted of a Shimadzu (Kyoto, Japan) system controller (SCL- 10AVP), pump (LC-20AD), autoinjector (SIL-20ACHT), and online degasser (DGU-14A). The chromatography of the analytes was performed at room temperature (25 °C) using a Phenomenex Kinetex C18 analytical column (50 mm × 2.1 mm, 2.6 μ; Torrance, CA, USA) preceded by a KrudKatcher ultra in-line filter, 0.5 μm. Analytes were eluted with an isocratic mobile phase (acetonitrile/10 mM HCOONH4 = 50/50, v/v, pH 5.0) at a flow rate of 0.11 mL/min for 3.0 min.
2.2.2. Mass spectrometric conditions
Detection was performed on an API-3200 LC MS/MS System (Toronto, Canada) equipped with a Turbo IonSpray® source (TIS; thermally and pneumatically assisted electrospray). The optimized conditions of MS/MS were as follows: ion spray source temperature at 450 °C, curtain (CUR) gas at 25, gas 1 (GS1) at 13.0, gas 2 (GS2) at 20.0, ionspray voltage (IS) at 4900 V, and collision-activated dissociation (CAD) at 10 units; declustering potential (DP) at 62 V, entrance potential (EP) at 5.5 V, collision energy (CE) at 32 V, and collision exit potential (CXP) at 4 V. The mass spectrometer was interfaced to a computer workstation running Analyst software (Version 1.5.1, Applied Biosystems, Foster City, CA) for data acquisition and processing. Data acquisition was performed at unit of both Q1 and Q3 resolution in positive multiple-reaction monitoring (MRM) mode, monitoring the transition of vandetanib protonated ion m/z 475.1 to product ion m/z 112.1, and of ISTD parent ion m/z 479.1 to the product ion m/z 116.2 (Fig. 1).
2.3. Sample preparation
2.3.1. Stock solutions
Stock solutions were prepared by dissolving vandetanib or ISTD into methanol to yield a concentration of 1.0 mg/mL. The stock solutions were stored at −80 °C (less than 5% change from nominal concentration over 6 months). The working stock solutions (vandetanib at concentrations of 5, 10, 40, 200, 500, and 1,000 ng/mL for CSF curve with ISTD at 100 ng/mL, and 20, 200, 1,000, 2,000, 10,000, and 20,000 ng/mL for plasma curve with ISTD at 2,000 ng/mL, respectively) were prepared by diluting the stock solution with 80% methanol water (v/v).
2.3.2. Calibration curve and quality controls
The calibration curve was made to quantitate low, medium, and high vandetanib levels in human CSF and plasma. Calibrators were made by adding working stock solutions of vandetanib and ISTD to blank CSF for the final vandetanib concentrations of 0.25, 0.50, 2.0, 10, 25, and 50 ng/mL with 5 ng/mL ISTD (CSF curve) or to blank human plasma for the final vandetanib concentrations of 1.0, 10, 50, 100, 500, 1,000, and 3,000 ng/mL with 100 ng/mL of ISTD (plasma curve). Separate quality control (QC) samples were prepared using the same methodology at vandetanib concentrations of 0.3, 8, and 40 ng/mL for the CSF curve, and 2.0, 200, and 2500 ng/mL for the plasma curve.
2.3.3. Human plasma and CSF sample preparation
Liquid-liquid extraction (LLE) was performed to prepare the CSF and plasma samples for this method. Briefly, 100 μL of CSF or plasma was spiked with ISTD (5.0 μL of 100 ng/mL for CSF or 2000 ng/mL for plasma) and placed in a 2 mL microcentrifuge screw cap tube with 1.5 mL of TBME containing 0.1% NH4OH for plasma or 0.5% NH4OH for acidified CSF sample. Both extraction solvents were prepared fresh daily. The sample mixture was vortex mixed for 15 sec for three times, and then centrifuged at 10,000 rpm for 5 minutes at 4°C. The top organic layer (~1.3 mL) was transferred to a new tube and dried over a stream of nitrogen for 15 minutes. The dried sample was reconstituted with 50 μL or 100 μL of 40% acetonitrile in 10mM HCOONH4 (pH 5.0) buffer for CSF or plasma samples, respectively. Each tube was vortex mixed for 30 seconds, the solution was transferred to an autoinjector vial, and 10μL or 20 μL of reconstituted plasma or CSF sample was injected onto the LC-MS/MS system, respectively.
2.3.4. Patient sample collection and storage
Whole blood samples were collected in sodium heparin tubes and immediately centrifuged at 10,000 rpm for 2 min to separate the plasma. CSF samples were first acidified by adding 3μL TFA in each 100 μL CSF sample, and then transferred into 2.0 mL screw-top tube. Both plasma and CSF were immediately frozen and stored in −80 °C until analysis.
2.4. Assay validation
2.4.1 Linearity and lower limit of quantitation
Two calibration curves in both human plasma and CSF were analyzed during the validation process. The linear regression of the ratio of vandetanib/ISTD peak areas was weighted by 1/x2. The coefficient of determination (R2) was used to evaluate the linearity of each calibration curve. The LLOQ was defined as the lowest concentration in each calibration curve that had both precision and accuracy within 20% and a signal/noise (S/N) ratio greater than 10.
2.4.2 Accuracy, precision, and recovery
The method developed for the measurement of vandetanib in human plasma and CSF was validated over five days by analysis of plasma and CSF quality control samples, and the within-day and between-day precision and accuracy for the method were determined. Recovery was assessed by preparing LLOQ, low, medium, and high quality control samples in triplicate in both plasma and CSF alongside neat samples of the same concentrations in 40% acetonitrile reconstitution solution. Both spiked CSF and plasma samples were extracted and analyzed to assess percent recovery in comparison to their neat samples.
2.4.3 Selectivity, carryover, and ion suppression
Selectivity of the assay was assessed by monitoring all selected ion transitions of vandetanib and ISTD after injection of blank human plasma from six different sources and blank human CSF from three different lots. Sample carryover was assessed by injecting neat vandetanib at 3,000 ng/ml and monitoring selected ion transitions through three blank wash samples (reconstitution solution). Ion suppression (matrix effect) was evaluated by calculating the matrix factor in six independent sources of plasma. The matrix factor is derived from a comparison of an extracted spiked sample (10 ng/mL) to a neat sample (10 ng/mL) [5].
2.4.4 Stability
The stability of vandetanib in human plasma (2.0 and 2500 ng/mL) and CSF (0.3 and 40 ng/mL) was assessed at room temperature (25 °C) or 4 °C for up to 24 h, and at −80 °C for up to 90 days. Blank human plasma and CSF were separately spiked with the indicated concentrations of vandetanib and left unextracted at 25 °C or 4 °C for 1, 12, and 24 h. Samples were extracted immediately after ISTD was added in at each scheduled time, and then vandetanib was quantitated. Long term stability of vandetanib in both plasma (2.0 and 2500 ng/mL) and CSF (0.3 and 40 ng/mL) at −80 °C was assessed. Unextracted spiked samples were stored at −80 °C for 7, 15, 40, 60, and 90 days. Samples were thawed, immediately extracted after adding ISTD, and then quantitated. Freeze-thaw stability was assessed by freezing unextracted spiked plasma sample as described above at −80 °C overnight, thawing at 25 °C, and extracting an aliquot, for a total of three freeze-thaw cycles. The freeze-thaw samples were analyzed in duplicate.
2.5. Application of method to patient pharmacokinetic samples
Serial samples for pharmacokinetic studies of vandetanib were obtained as part of a clinical phase I study of pediatric patients with DIPG. Whole blood was collected at the following times: pre-dose, and 1, 2, 4, 8, and 24 h after oral vandetanib administration. Blood samples (1.0 mL) were collected in tubes containing heparin (BD, Franklin Lakes, NJ, USA) and immediately centrifuged at 10,000 rpm for 2 min to separate the plasma. Plasma samples were stored at −80 °C until analysis. Samples were extracted and quantitated using the described method.
3. Results and discussion
3.1. Sample collection
During our method development studies, we noted vandetanib adsorbed to collection tubes used to collect CSF but not those used to collect plasma. In our preliminary studies, we observed that CSF concentration values were 0.57 ± 0.03 of expected values, whereas plasma concentration values were 0.99 ± 0.03 of expected values. Plasma samples were less likely to exhibit binding to tube surfaces due to the extensive plasma protein binding observed with vandetanib (>90% protein binding). Because the extensive binding of vandetanib observed in the CSF collection tubes, we evaluated approaches to mitigate this binding. By adding TFA to the CSF collection tubes at a final concentration of 3% (i.e., 3 μl TFA in 100 μl CSF sample) we were able to prevent the binding of vandetanib to the collection tubes. However, the TFA slightly acidified the CSF sample leading to decreased extraction efficiency. Thus, we slightly altered our LLE procedure for CSF samples to achieve similar extraction efficiency by using a more basic liquid extraction solvent (i.e., TBME with 0.5% NH4OH) compared to that used with plasma samples (i.e., TBME with 0.1% NH4OH).
3.2. Chromatography
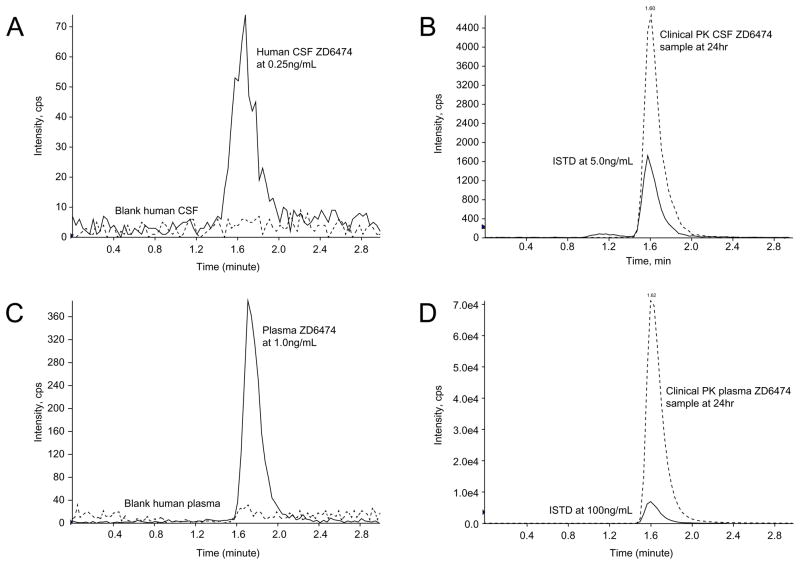
A Kinetex C18 column (2.6 μ, 50 mm × 2.1 mm) was chosen for separation because of better performance including peak shape, peak intensity, and retention time at ambient temperature. After evaluating many different combinations of organic solvents and aqueous buffers, the mobile phase (MP) was acetonitrile/10 mM HCOONH4 at pH 5.0 (50/50 = v/v). This MP gave the maximal peak intensity and best peak shape as well as a short retention time with a flow rate of 0.11 mL/min (Fig. 2). The retention time of both vandetanib and ISTD was approximately 1.60 min in both human plasma and CSF (Fig. 2B and D).
Figure 2.
LC-MS/MS chromatogram of both human CSF and plasma samples including blank, LLOQ (panels A and C), and samples from a representative pharmacokinetic study after liquid-liquid extraction (panels B and D).
3.3. Mass spectrometry
Detection was achieved by an API-3200 LC/MS/MS system at unit (Q1) and unit (Q3) resolution in positive multiple reaction monitoring (MRM) mode. All parameters related to MS detection were optimized to achieve the best signal response of each selected ion. The full-scan MS1 and MS2 mass spectra were obtained by infusion of neat vandetanib and ISTD. The precursor scans of vandetanib and ISTD were demonstrated in Fig. 1A and 1B, respectively. The monitored parent/product ion transitions of vandetanib (Fig. 1A) and ISTD (Fig. 1B) were m/z 475.1→112.1 and 479.1→116.2, respectively.
3.4. Precision, accuracy and recovery
To assess within-day and between-day precision and accuracy, we evaluated the validation parameters for vandetanib (Table 1). Variability was reported as relative standard deviation (%RSD) and accuracy was expressed as percentage error (%Error) in Table 1. Within-day and between-day precision for plasma vandetanib was ≤ 5.9% and accuracies ranged from 104.0 to 108.0%. For CSF vandetanib, precisions were ≤ 8.8% and accuracies ranged from 95.0 to 98.5%. The average recovery of both plasma and CSF was > 80%, and was similar in low, medium, and high concentration samples.
Table 1.
Validation parameters of vandetanib in human plasma and CSF
| Vandetanib (ng/mL) | Within-day (n=6) | Between-day (n=5) | |||
|---|---|---|---|---|---|
| %RSD | %Error | %RSD | %Error | ||
| Plasma | 1.0 | 3.4 | 8.5 | 5.9 | 5.6 |
| 2.0 | 4.4 | 6.8 | 5.6 | 4.0 | |
| 200 | 1.9 | 7.7 | 4.6 | 4.6 | |
| 2500 | 4.0 | 8.0 | 4.6 | 7.9 | |
| CSF | 0.25 | 2.6 | −2.0 | 3.1 | −2.5 |
| 0.30 | 0.8 | −1.8 | 1.9 | −1.5 | |
| 8.0 | 1.2 | −2.9 | 1.5 | −2.5 | |
| 200 | 8.8 | −3.6 | 6.2 | −5.0 | |
3.5. Linearity and lower limit of quantitation
The vandetanib calibration curves were linear from 1.0 to 3,000 ng/mL in plasma and from 0.25 to 50 ng/mL in CSF, with the correlation coefficients (R2) greater than 0.992 for both curves. The LLOQ for plasma was 1.0 ng/mL (S/N = 29, %CV = 3.4, n = 6) and for CSF was 0.25 ng/mL (S/N = 15, %CV = 2.6, n = 6), both of which were used as the lowest calibrator in the curve (Fig. 2A and C).
3.6. Selectivity, ion suppression, and carryover
Selectivity of the assay was assessed by monitoring all selected ion transitions using blank human plasma from six different individuals (n = 6) and blank human CSF from three different lots (n = 3). No co-eluting peak at or around the retention time of both vandetanib and ISTD (1.60 min) were observed. No significant ion suppression (matrix effects) was noted (MF = 0.98; %CV = 4.97, n = 6).
During method development, sample carryover was evaluated by assessing the responses of analyte from blank samples after injection of the highest standard (i.e., 3000 ng/ml vandetanib). Unexpectedly a signal representing ~80% of the LLOQ was noted in the blank sample after the high standard, which strongly indicated carryover. This finding was noted even though washing solutions of 40 to 70% acetonitrile or methanol in water were regularly used. After extensive follow-up, the source of the carryover was identified as the autosampler needle. To reduce this needle carryover effect, several washing solutions were tested. No carryover effect was observed when TFA 0.1% in 50% acetonitrile water (v/v) was used. Thus, the acidified needle washing solution was used during the method validation and sample analysis phase.
3.7. Stability
Spiked human samples were aliquotted in duplicate and stored separately at 4 °C, 25 °C, and −80 °C. Internal standard was added to samples prior to the sample preparation at each scheduled time and the stability for each concentration was determined. At 4 °C and 25 °C, vandetanib was stable for up to 24 hours in human plasma and CSF (< 10% change; see Table 2a). Vandetanib plasma and CSF samples were stable (< 10% change) for at least 90 days at −80 °C (Table 2b) and through three freeze-thaw cycles (< 4% change; Table 2c).
Table 2a.
Stability of vandetanib in human plasma and CSF at 25 °C and 4 °C.
| Vandetanib (ng/mL) | % Difference from control | |||
|---|---|---|---|---|
| 1 h | 12h | 24h | ||
| 25°C | ||||
| Plasma | 2.0 | 0.0 | −1.5 | −4.5 |
| 2500 | −0.4 | 4.4 | −0.4 | |
| CSF | 0.3 | −10.0 | −10.0 | −8.0 |
| 40 | −4.7 | −1.0 | −1.7 | |
| 4°C | ||||
| Plasma | 2.0 | 0.0 | −7.5 | −5.5 |
| 2500 | −0.4 | −7.6 | −2.0 | |
| CSF | 0.3 | −8.3 | −8.7 | −5.3 |
| 40 | −1.0 | −1.3 | −4.7 | |
Table 2b.
Stability of vandetanib in human plasma and CSF at −80 C.
| Vandetanib (ng/mL) | % Difference from control | |||||
|---|---|---|---|---|---|---|
| 7 day | 15 day | 30 day | 40 day | 60 day | 90 day | |
| Plasma | ||||||
| 2.0 | 1.9 | 4.7 | −1.6 | 4.0 | −4.8 | 0.7 |
| 2500 | 2.7 | 0.7 | 3.3 | 0.6 | 6.6 | −6.8 |
| CSF | ||||||
| 0.30 | −3.3 | 6.7 | 5.3 | 2.7 | −2.6 | 4.5 |
| 40 | 9.2 | 8.4 | 7.8 | 8.6 | −3.0 | 5.2 |
Table 2c.
Stability of vandetanib in human plasma and CSF through 3 freeze-thaw cycles.
| Vandetanib (ng/mL) | % Difference from control | ||
|---|---|---|---|
| 1st cycle | 2nd cycle | 3rd cycle | |
| Plasma | |||
| 2.0 | −3.4 | 0.9 | 2.7 |
| 2500 | 1.3 | −3.5 | −1.9 |
| CSF | |||
| 0.25 | 7.7 | 0.5 | 4.5 |
| 40 | 0.8 | 0.7 | 2.2 |
3.8. Application of method to clinical pharmacokinetic study
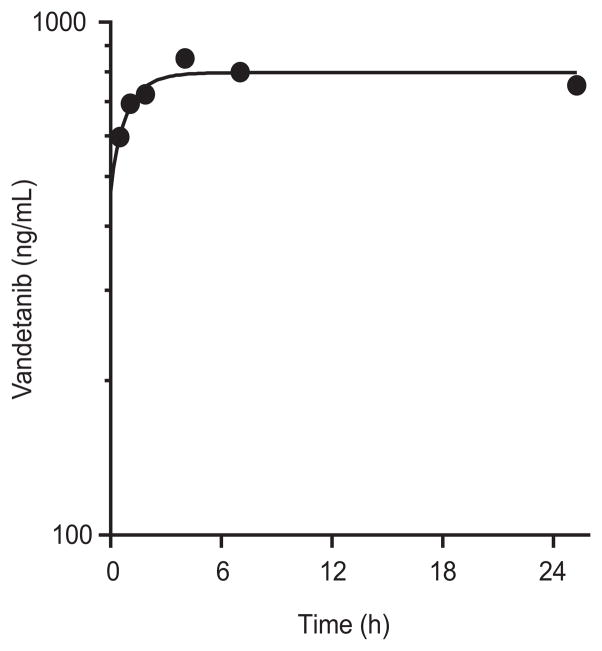
To show the applicability of the method, we analyzed plasma samples from a pediatric patient enrolled on a Phase I clinical trial of vandetanib. After oral vandetanib administration, serial whole blood samples were collected over 24 hours. After liquid-liquid extraction, samples were reconstituted and then analyzed by the method described in this report. A representative plasma concentration-time profile for vandetanib after oral administration is depicted in Figure 3.
Figure 3.
Representative vandetanib plasma concentration-time profile from a pediatric patient after a single vandetanib oral dose (110 mg/m2).
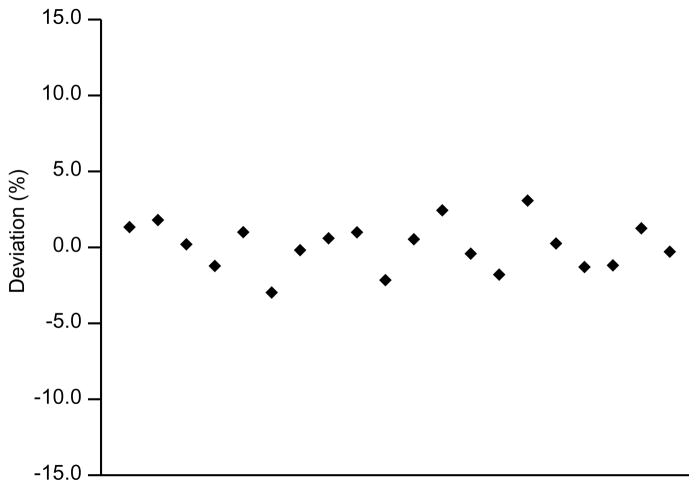
3.9. Re-analysis of incurred samples
In recognition that calibrators and QC samples used to validate bioanalytical methods may not be representative of incurred samples [6], confirmatory re-analysis of incurred samples has been performed to further validate the reproducibility of bioanalytical method [7]. Thus, 20 patient samples (2 samples per patient) were analyzed in duplicate in one day. The deviation from the second to the first measurement was then calculated and expressed as a percentage. As shown in Fig. 4, less than ±5% change was noted. This is well below the classical “2-3-15” rule, which required at least two-thirds of the replicate measurements are less than ±15% from original value.
Figure 4.
Incurred sample re-analysis: deviation of the replicate from the original analysis.
4. Conclusion
We report here a sensitive and rapid LC-MS/MS method for quantitation of vandetanib in human plasma and CSF samples. This analytical method was developed and validated for clinical pharmacokinetic studies of vandetanib in children, and it shows sufficient precision and accuracy for application in those studies. The lower limit of quantitation was 0.25 ng/ml (a 20-fold improvement in vandetanib plasma LLOQ was achieved under our assay condition compared to previous published work), which was sufficient for our application to human CSF samples. The rapid separation within 3 min using a narrow-bore short column and a low flow rate allows for a short assay runtime and reduces solvent costs as well as minimizes environmental impact of toxic solvents. Finally, we have successfully applied this LC–MS/MS method by measuring vandetanib in both human plasma and CSF from a clinical pharmacokinetic study of children treated with vandetanib.
Acknowledgments
This research was supported by NIH Awards P01 CA 23099, Cancer Center Support (CORE) Grant CA 21765, and by ALSAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ciardiello F, Caputo R, Damiano V, Troiani T, Vitagliano D, Carlomagno F, Veneziani BM, Fontanini G, Bianco AR, Tortora G. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:1546. [PubMed] [Google Scholar]
- 2.Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, Graham GA, Hughes GD, Thomas AP, Stokes ES, Curry B, Richmond GH, Wadsworth PF, Bigley AL, Hennequin LF. Cancer Res. 2002;62:4645. [PubMed] [Google Scholar]
- 3.Holden SN, Eckhardt SG, Basser R, de Boer R, Rischin D, Green M, Rosenthal MA, Wheeler C, Barge A, Hurwitz HI. Ann Oncol. 2005;16:1391. doi: 10.1093/annonc/mdi247. [DOI] [PubMed] [Google Scholar]
- 4.Zirrolli JA, Bradshaw EL, Long ME, Gustafson DL. J Pharm Biomed Anal. 2005;39:705. doi: 10.1016/j.jpba.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Kim MSJH, Lee JA, Pyo D, Yoon H-R, Lee HJ, Lee KR. Chromatographia. 2004;60:335. [Google Scholar]
- 6.Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, Shah VP, Skelly JP, Swann PG, Weiner R. Pharm Res. 2007;24:1962. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- 7.Rocci ML, Jr, Devanarayan V, Haughey DB, Jardieu P. AAPS J. 2007;9:E336. doi: 10.1208/aapsj0903040. [DOI] [PMC free article] [PubMed] [Google Scholar]