Abstract
Colorectal cancer (CRC) screening rates have been low despite effectiveness of screening in reducing CRC mortality. This article outlines the theoretical background and development of an innovative, telephone-based risk communication designed to promote screening among individuals at increased risk for familial CRC. This ongoing intervention integrates the Extended Parallel Process Model of fear management and the motivational interviewing counselling style. Tailoring and implementation intentions are incorporated. The primary outcome is self-reported colonoscopy within nine months following intervention. If proven effective, the remote intervention could be broadly disseminated to individuals at increased familial CRC risk, especially those in geographically underserved areas.
Keywords: behavioural intervention theory, colorectal cancer screening, fear appeal, motivational interviewing, tailored intervention
Introduction
Overview
Colorectal cancer (CRC) is one of the most commonly occurring cancers and a leading cause of cancer deaths in many developed countries, including the United States (American Cancer Society (ACS), 2010a). A family history of CRC is one of the strongest risk factors for the disease. Inheritance is estimated to play a role in up to 25 per cent of CRC cases, and relatives of CRC patients may have a two- to eight-fold increased risk of the disease, depending on the family history (Kerber et al., 2005).
Colonoscopy may be the most effective procedure for reducing incidence, specifically via detection and removal of polyps, which are known CRC precursors. Guidelines recommend that individuals at intermediate to high familial risk begin colonoscopy at age 40 or 10 years younger than the earliest diagnosis in the family, and then repeat this screening test every three to five years (ACS, 2010b; National Comprehensive Cancer Network (NCCN), 2010). However, this population tends to underutilize screening colonoscopy (Ruthotto et al., 2007), perhaps because the majority of affected individuals, especially those in rural areas, do not receive adequate familial cancer risk assessment and risk-appropriate recommendations from their health care providers (Greiner et al., 2004; Overholser et al., 2009). Therefore, a risk communication strategy that effectively increases awareness of familial CRC risk and appropriate screening is needed. Research indicates that promoting CRC screening may call for an intervention that is motivational rather than solely educational (Costanza et al., 2007), while telephone counselling successfully overcomes geographic barriers in promoting CRC screening (Basch et al., 2006; Turner et al., 2008). Therefore, we have developed a tailored, motivation-based intervention to promote colonoscopy screening among people with familial CRC risk using telephone counselling and mailed printed materials. This article describes the conceptual background and rationale for integrating the Extended Parallel Process Model (EPPM) (Witte, 1992, 1998) and motivational interviewing (MI) (Miller and Rollnick, 2002) for guiding the intervention. To the best of our knowledge, the integration of these two prominent principles known to increase motivation for behaviour change has not been previously utilized, and thus creates a novel approach to CRC screening interventions.
The Family CARE project
The Family Colorectal Cancer Awareness and Risk Education (Family CARE) project is an ongoing randomized two-group trial. The project aims to test the efficacy of an intensive CRC risk communication consisting of tailored telephone-based CRC risk counselling, an educational brochure, a tailored follow-up letter and a tailored reminder postcard; and to compare it to a control arm utilizing the educational brochure only.
The Extended Parallel Process Model
Witte (1994: 114) has defined fear appeals as strategies that ‘attempt to arouse the emotion of fear by depicting a personally relevant and significant threat and then follows this description of the threat by outlining recommendations presented as feasible and effective in deterring the threat’. The EPPM posits that the key challenge for delivering effective risk communication messages is to help people manage their fear effectively. Fear is aroused if a disease (e.g. CRC) is perceived as personally relevant (high perceived susceptibility) and serious (high perceived severity). Arousal of fear motivates individuals to consider strategies that will help them manage a threat. According to the model, individuals who believe that the recommended action (e.g. getting colonoscopy) is effective in averting the threat (high response efficacy), and that they possess the ability to execute the behaviour (high self-efficacy), will manage the threat using a danger control process by confronting and endeavouring to prevent the health threat. However, if individuals do not believe that the recommended action will avert the threat (e.g. getting a colonoscopy does not work in reducing CRC risk) or if they have low self-efficacy, they are likely to engage in a fear control process through denial, avoidance and other psychological defence strategies instead of taking action to manage the health threat. Fear-arousing messages can also fail simply because they do not elicit fear, as is the case when the health threat is perceived as irrelevant and/or insignificant (e.g. when the individuals think they are too young to get CRC, do not know anyone who has developed CRC so do not believe the disease will happen to them or believe CRC is not serious because it can be prevented simply by getting polyps removed). Therefore, to change health behaviour in the desired direction with a fear management approach, a risk message should arouse fear by increasing people’s perceptions of the threat of a disease or condition prior to bolstering their efficacy perceptions in order to perform a recommended behaviour (Witte and Allen, 2000).
Researchers have used the EPPM to guide numerous interventions to promote a wide range of health prevention behaviours, including cardiovascular disease (McKay et al., 2004), meningitis vaccinations promotion (Gore and Bracken, 2005) and hand-washing behaviour (Botta et al., 2008). For cancer prevention, the EPPM has been successful in promoting testicular self-examination (Morman, 2000) and skin cancer prevention (Cho and Salmon, 2006). However, there are no published data on the application of EPPM-based interventions in promoting CRC screening for average- or high-risk individuals. Only one intervention used fear-arousing messages regarding severity of CRC as part of an educational programme aimed at increasing CRC-related knowledge, but did not find effect of fear appeal (Makoul et al., 2009). Thus, our intervention is the first one to apply the fear management strategy proposed by the EPPM to a clinical CRC risk and screening intervention.
Motivational interviewing
In fear management communications, the delivery style is also important. Motivational interviewing (MI) is a client-centred, directive method for enhancing intrinsic motivation to change by exploring and resolving ambivalence (Miller and Rollnick, 2002). Instead of telling clients why or how to change their behaviour, counsellors use motivational strategies to elicit the client’s own thoughts or ideas around change. This ‘change talk’ can predict successful behaviour change (Amrhein et al., 2003). MI is comprised of a set of stylistic principles: (1) express empathy, making use of techniques such as respectful and reflective listening; (2) develop discrepancy, working to elicit the client’s own reasons for change; (3) roll with resistance, avoiding direct arguments that produce defensiveness; and (4) support self- efficacy, utilizing positive and supportive language that acknowledges freedom of choice in making decisions and planning for change.
A growing body of evidence demonstrates effectiveness of MI in motivating several types of health promoting behaviours (Britt et al., 2004; Lundahl and Burke, 2009; Martins and McNeil, 2009), including alcohol use (Vasilaki et al., 2006) and HIV-risk behaviour (Harding et al., 2001). However, the effectiveness of MI in promoting CRC screening is unclear. Manne et al. (2009) found that supplementing tailored print materials with telephone counselling that incorporated MI did not show greater efficacy than the print materials alone in promoting CRC screening among siblings of CRC cases. However, another recent study reported that telephone outreach interventions based on an integration of MI and the Transtheoretical Model (Prochaska and DiClemente, 1983; Prochaska et al., 1992) successfully increased CRC screening among individuals at average risk for CRC (Lasser et al., 2009). These mixed findings indicate the importance of clarifying the role of MI in theory-driven CRC screening promotion.
Integration of MI with fear management theory
While the content of our CRC risk communication relies heavily on the EPPM, the delivery style is based on MI. The contribution of MI is to encourage the client to engage and respond more positively to the risk information, rather than to view the client as a passive recipient of information. We have integrated MI into our intervention for several reasons. First, MI might reduce the chance of a defensive response after exposure to fear appeals. Defensive reaction is a primary concern when using fear appeals because such response usually leads to message rejection (Ditto and Lopez, 1992; Janis and Feshbach, 1953; Liberman and Chaiken, 1992). MI principles, namely, ‘roll with resistance’ and ‘support self-efficacy’ may be useful in reducing this defensiveness. ‘Roll with resistance’ means that rather than arguing with clients who show resistance to change, counsellors should accept this resistance as normal. They should strive to avoid confrontation, and instead encourage their clients to develop solutions to problems. Lessened confrontation may lead to lessened resistance. To support self-efficacy, counsellors may use MI to enhance client confidence in changing behaviour by emphasizing already available resources and knowledge, eliciting clients’ thoughts/plans to overcome the barrier(s), and affirming changes they have already made. The MI principle of self-efficacy enhancement is consistent with the EPPM, which highlights the importance of promoting self-efficacy in order to facilitate the danger control process.
Second, MI may help strengthen motivation to change as a response to fear appeals. In addition to increasing fear-based motivation to obtain CRC screening (via enhanced perception of CRC health threat), MI may add greater motivation by eliciting an individual’s own fears and intrinsic reasons to prevent CRC (e.g. ‘I am afraid if I don’t get screened, I may have to undergo chemotherapy’).
MI also suggests strategies for counsellors to respond appropriately to the affective consequences of exposure to fear-arousing information. Risk communications that incorporate fear appeals attempt to elicit feelings of fear or concerns about threat. If counsellors do not address these negative emotions with appropriate support, the clients may feel rejected and hurt in addition to feeling fearful. The MI principle of ‘expressing empathy’ acknowledges this concern by encouraging counsellors to consider strongly the clients’ point of view. When clients feel they are understood, they may be more willing to express their thoughts and feelings about change. Therefore, rather than working to reduce fear and counteracting the intended effects of fear management strategies, MI would foster client willingness to share feelings and thoughts with counsellors.
Methods
Participant recruitment and randomization
The Family CARE study has been approved by the University of Utah Institutional Review Board. Registrars at the cancer registries of California, Colorado, Idaho, New Mexico and Utah, and the Rocky Mountain States sites of the Cancer Genetics Network, identify persons diagnosed with CRC between the ages of 40–59. Study staff members request that CRC cases or their next of kin, whom the registries and other sources also identify, provide contact information (name, date of birth and address) for their unaffected first-degree relatives (FDRs). Participant inclusion criteria include: (1) age 30–74; (2) having either one FDR diagnosed with CRC before age 60, or one FDR and an additional FDR or second-degree relative (SDR) diagnosed at any age; (3) no prior cancer diagnosis (except non-melanoma skin cancer); (4) no colonoscopy in the past five years; (5) awareness of CRC family history; (6) mental competence; and (7) ability to read and speak English fluently. Male and female relatives who meet these criteria receive a letter and recruitment brochure prior to telephone contact and eligibility screening.
After providing written informed consent, we randomly assign participants into either the intensive intervention or the control group by family unit. All participants complete a baseline survey, which includes a brief medical history, family CRC history, cancer screening experience, barriers to CRC screening and perception of CRC risk and severity, as well as response efficacy and self-efficacy regarding getting colonoscopy as derived from the EPPM. Responses constitute the basis for risk assessment and behaviour change counselling in the intensive intervention group. Participants complete follow-up surveys at one and nine months after the intervention. Participants with strong financial or other barriers to colonoscopy, as well as those who have not undergone colonoscopy by the nine-month follow-up, receive a recommendation to have a faecal occult blood test (FOBT), which is a secondary outcome of the study.
Interventions
Minimal intervention
Participants in the control (minimum intervention) group receive a targeted educational brochure. The brochure briefly defines CRC, describes the concept of elevated risk due to family history, explains the role of colonoscopy in prevention and encourages discussion with health care providers about CRC risk and screening. This brochure and examples of other study materials are available at: http://www.hci.utah.edu/research/programs~/familycare/figures.jsp.
Intensive CRC risk communication intervention
Within one month of the baseline survey, one of five certified cancer genetic counsellors conducts a 30–45-minute telephone counselling session with each participant in the intensive intervention group. Because tailored messages are generally more effective than generic messages in producing behavioural change (Manne et al., 2009; Noar et al., 2007), we individualize our telephone CRC risk counselling according to each participant’s perceptions of threat and efficacy as derived from the EPPM, and according to personal factors such as CRC screening history and knowledge and barriers to CRC screening. Prior to the telephone session, participants receive a four-page tailored visual aid in a sealed envelope for use during the phone call, along with the brochure. After the phone call, participants receive follow-up materials as described later.
Development of the risk communication telephone counselling intervention
Use of a study-specific manual assures that the counsellors adequately apply the elements of the EPPM and MI during the telephone counselling session. The manual provides strategies and a sequence for the session, rather than a script for counsellors to read to participants. Thus, while the telephone counselling is meant to be structured, the counsellors retain flexibility in applying MI counselling techniques throughout the session (the intervention manual can be viewed at http://www.hci.utah.edu/research/programs~/familycare/figures.jsp). The steps of the telephone counselling session are as follows.
Session planning
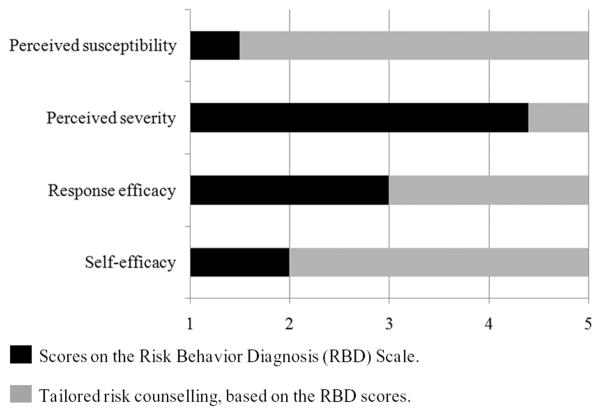
Before the telephone intervention, the counsellor reviews the participant’s responses to the baseline survey, especially responses to the 16-item Risk Behaviour Diagnosis (RBD) Scale (Witte et al., 1996; see Cheah, 2005 for construction of the scale) in order to assess participant perceptions about CRC susceptibility and severity, response efficacy and self-efficacy regarding colonoscopy. Scores from the RBD Scale determine the nature of tailored fear-arousing messages, counselling strategies and quantity of time spent in discussing each topic. Figure 1 provides an example in which RBD scores indicate low perceived susceptibility, high perceived severity, moderate response efficacy and low self-efficacy. In this example, the counsellor discusses perceived susceptibility extensively to convey accurately the participant’s elevated CRC risk due to family history, discusses perceived severity only briefly and spends a longer period of time addressing the participant’s perception of colonoscopy’s effectiveness. In addition to providing more (or less) material for each area, the counsellor uses MI techniques to draw out participant concerns and thoughts. Finally, the counsellor addresses screening barriers and ways to enhance participant confidence in his/her ability to obtain a colonoscopy. Throughout the session, the counsellor presents information in a neutral, non-judgemental style.
Figure 1.
An illustration of applying the EPPM to tailoring the CRC risk telephone counselling.
As part of the informed consent process, each specific participant gives written permission for the counsellor to audio-record all intervention calls.
Step 1: Introduction and building rapport
Using a warm, respectful attitude, the counsellor greets the participant and provides a basic orientation to the structure and direction of the 30–45-minute discussion. The participant confirms the availability of the visual aids and the privacy of the setting.
Step 2: Review family and medical history
The counsellor focuses on CRC during a brief review of the participant’s medical and family history. The visual aid includes a pedigree illustrating the participant’s family tree and all diagnoses of CRC and other cancers. The counsellor encourages the participant to share experiences regarding family members’ CRC diagnoses and treatment, as well as how CRC diagnoses in the family affect the participant’s CRC risk perception, for the purpose of increasing awareness of his/her personal risk for CRC.
Step 3: Intervention on perceived risk for CRC
To enhance CRC risk perception, the counsellor explains that a CRC-positive family history can increase an individual’s personal risk to double or triple the rate of the general population. The second visual aid presents this information verbally as well as in a graph format. For a participant who initially underestimates her/his CRC risk, the counsellor utilizes questions to elicit thoughts and feelings regarding that risk, as well as reflections to highlight areas of personal concern. For example, the counsellor might ask ‘How do your sister’s and mother’s diagnoses of colorectal cancer affect your risk?’ For a participant who estimates personal CRC risk accurately, the counsellor reflects and affirms the belief using MI techniques. For a participant who overestimates CRC risk, the counsellor asks for elaboration on reasons for the overestimate, provides information to correct the misconception and explains that the individual’s CRC risk is not too high to lose hope for prevention.
Step 4: Intervention on perceived severity of CRC
The counsellor provides the statistic that CRC is now the second leading cause of cancer death in the USA and causes approximately 50,000 deaths annually (ACS, 2010a). For a participant who has low or moderate baseline perception of severity, the counsellor encourages consideration of the negative consequences of getting CRC. The counsellor affirms the perspective of a participant who already has acknowledged the seriousness of CRC.
Step 5: Intervention on response efficacy
The counsellor enhances response efficacy beliefs by stating that the earlier CRC is detected, the greater chance for five-year or longer survival. The third visual aid contains a table illustrating survival rates according to stage of diagnosis. The counsellor introduces the idea of the effectiveness of CRC prevention through colonoscopy screening. Depending on participant response efficacy level and knowledge about colonoscopy, the counsellor describes the procedure and its effectiveness in removing precancerous polyps. If applicable, the counsellor discusses procedure-related barriers to having colonoscopy, for example, bowel preparation and discomfort of the procedure (Jones et al., 2010). The counsellor also addresses questions, concerns or misconceptions about colonoscopy, and affirms accurate beliefs of the participant regarding efficacy of colonoscopy in preventing CRC. At this time, the counsellor provides specific information about colonoscopy screening guidelines (ACS, 2010b).
Step 6: Intervention on self-efficacy
The counsellor utilizes MI importance and confidence rulers (Miller and Rollnick, 2002) as an assessment of readiness to have a colonoscopy by asking the participant to rate the importance of and confidence in ability to undergo colonoscopy within the next six months on a 1–10 scale (1 being the lowest and 10 the highest importance/confidence). The counsellor then utilizes a series of open-ended questions to elicit the participant’s reasons for undergoing colonoscopy. Examples of counsellor questions include: ‘What are the things that make it important for you to get screened?’ and ‘What are some reasons that made you rate your confidence in getting a colonoscopy as three rather than as one?’ The counsellor explores those factors that the participant believes would increase colonoscopy priority and/or efficacy to get a colonoscopy. The counsellor also addresses the participant’s two most important barriers to getting a colonoscopy and resolves them as much as possible.
Step 7: Constructing an action plan
Depending on the participant’s readiness to get a colonoscopy at this point, the counsellor provides encouragement and assistance in creating a behavioural plan to schedule and follow through with colonoscopy. The fourth visual aid lists steps to obtaining a colonoscopy.
According to the self-regulation strategy of implementation intentions (Gollwitzer, 1993, 1999), people are more likely to carry out an intended action if they indicate when, where and how they will do so while formulating the plan. The counsellor applies implementation intention principles by encouraging the participant to formulate a plan (or a hypothetical plan if he/she is not ready or willing to make an actual plan) to obtain a colonoscopy, particularly to determine what his/her first step will be, as well as when and how he/she will execute the plan.
Step 8: Summary and closing
The counsellor provides a summary of the participant’s primary reasons for undergoing colonoscopy, as well as his/her action plan to do so. The counsellor also answers any questions the participant may have. For a participant with strong financial barriers to colonoscopy, the counsellor considers reinforcing FOBT, an alternative option that is not optimal but is better than no screening at all, and offers to provide the participant with a list of resources for free and/or reduced cost CRC screening. The counsellor thanks the participant for taking the time for the counselling session.
Post-session tailored follow-up letter
As additional reinforcement, a tailored summary letter is mailed to the participant immediately after the session. The letter adheres to the EPPM and describes the participant’s elevated familial CRC risk, severity of CRC, effectiveness of colonoscopy, resolution of the two most important barriers to undergoing colonoscopy discussed during the session and general guidance, and outlines the participant’s first steps towards obtaining a colonoscopy. The letter also includes a colonoscopy testimonial and a photo of a model whose age, gender and ethnicity are tailored to match those of each participant, as use of models has beneficial effects on promoting self-efficacy (Bandura, 1986). Because communication with health-care provider is an important predictor of colon cancer screening behaviours (Wee et al., 2005), with participant permission, we send a copy of the tailored letter to his/her primary care provider to encourage further discussion.
Tailored reminder postcard
Because patient reminders can be effective cues to action in enhancing adherence rates for CRC screening (Myers et al., 1991), two months after the telephone session, each participant receives a tailored reminder postcard reviewing the general and individualized first steps towards obtaining a colonoscopy.
Data collection
Study recruitment, randomization, interventions and follow-up assessments are ongoing. To date, we have recruited 330 individuals to the study with approximately equal numbers in each study group. Planned publications about the characteristics of the Family CARE study population and the intervention’s efficacy will follow upon completion of the study.
Counsellor training, supervision and fidelity evaluation
MI training
The five cancer genetic counsellors implementing the study intervention received 16 hours of training about basic MI and integration of MI elements into the fear appeal structure of the intervention from a member of the Motivational Interviewing Network of Trainers (SW). Prior to the beginning of the intervention, the counsellors conducted an average of 13 practice telephone counselling interventions and participated in 40 weekly or biweekly supervisory sessions. During these training sessions they reviewed practice interventions and received immediate feedback from the MI trainer and from a social psychologist (WP), both of whom focused on applying fear appeals and health behaviour change theories. Supervision remains ongoing during the implementation of the intervention in order to maintain adherence to the telephone counselling protocol and to the MI interpersonal skills of the counsellors.
Intervention fidelity
To ensure the fidelity of the fear appeal-guided intervention, two trained coders utilize a study-specific intervention checklist to assess the extent to which the counsellors complete the sequential steps of the counselling protocol. In addition, four independent coders trained in MI utilize the Motivational Interviewing Treatment Integrity (MITI) coding scheme (version 3.0) (Moyers et al., 2007) and the Behavior Change Counselling Index (BECCI) (Lane et al., 2005) to rate each counsellor’s MI competence, using 12 initial intervention tapes and a subsequent 15 per cent of tapes randomly selected from the rest of the intervention calls.
Strengths and potential limitations of the intervention
An important advantage of this multi-faceted intervention is its strong focus on behavioural theories of motivation. By integrating principles of EPPM and MI, both of which emphasize motivation to change, our CRC risk intervention is strongly motivational rather than primarily educational. Use of the EPPM allows us to focus specifically on why the participant has been unmotivated to change (e.g. because of low perceived risk, low self-efficacy). Tailoring enables us to intervene directly on each construct at an individual level, increasing chances for success in promoting behavioural change (Noar et al., 2007), while implementation intentions help increase the chance for translating intent to get a colonoscopy into actual behaviour (Gollwitzer and Sheeran, 2006).
In addition, this theory-guided behavioural intervention is the first to utilize the knowledge and expertise of cancer genetic counsellors in providing CRC risk assessments based on the pivotal role of family history. Previous studies relied on trained interventionists (Wahab et al., 2008), health educators (Manne et al., 2009) and telephone counsellors (Costanza et al., 2007). Using cancer genetic counsellors in CRC genetic risk assessment interventions may enhance education about the genetic implications of a cancer family history (Braithwaite et al., 2006), as they possess an extensive knowledge base regarding genetic influences on cancer risk and prevention.
We recognize some potential limitations of our behavioural intervention. Utilization of trained genetic counsellors from an academic setting, as well as the three individually tailored intervention tools (counselling call, follow-up letter and postcard), may limit the feasibility of export to other screening and prevention programmes. Nonetheless, the remote nature of the intervention may facilitate use of cancer genetic counsellors from a central intervention site, basic MI skills training is relatively straightforward for health educators and practitioners in real settings and a computer program could easily generate tailored materials to a large group of individuals. Regarding the role of the counsellors, our methodology does not allow us to determine the extent to which the cancer genetic counsellors’ expertise has contributed to effectiveness of the intervention. Use of other kinds of health practitioners and educators to implement the intervention may be more practical and cost-effective, but may or may not yield similar effects. If the intervention proves to be efficacious, it will be important for future studies to investigate the extent to which utilizing cancer genetic and other kinds of counsellors (e.g. health educators, lay health advisers) impacts efficacy of cancer screening intervention. Such studies would be useful before exporting the intervention into settings in which other kinds of interventionists may be more practical.
Acknowledgments
The Family CARE project is funded by the National Cancer Institute (1R01CA125194-03; Kinney, PI), Cancer Center Support Grant (5P30CA042014-20), and the Huntsman Cancer Foundation. The Family CARE project was also supported by the Utah Cancer Registry, which is funded by Contract # N01-PC-74400 from the National Cancer Institute’s SEER program with additional support from the Utah State Department of Health and the University of Utah.
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermission.nav
Competing Interests
None declared.
References
- American Cancer Society (ACS) Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010a. [Google Scholar]
- American Cancer Society (ACS) Detailed Guide: Colorectal Cancer. Can Colorectal Polyps and Cancer Be Found Early? 2010b Available at: http://www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_colon_and_rectum_cancer_be_found_early.asp?rnav=cri.
- Amrhein PC, Miller WR, Yahne CE, Palmer M, Fulcher L. Client commitment language during motivational interviewing predicts drug use outcomes. Journal of Consulting and Clinical Psychology. 2003;71(5):862–878. doi: 10.1037/0022-006X.71.5.862. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Foundation of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- Basch CE, Wolf RL, Brouse CH, et al. Telephone outreach to increase colorectal cancer screening in an urban minority population. American Journal of Public Health. 2006;96(12):2246–2253. doi: 10.2105/AJPH.2005.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta RA, Dunker K, Fenson-Hood K, Maltarich S, McDonald L. Using a relevant threat, EPPM and interpersonal communication to change hand-washing behaviours on campus. Journal of Communication in Healthcare. 2008;1(4):373–381. [Google Scholar]
- Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: A systematic review and meta-analysis. Family Cancer. 2006;5(1):61–75. doi: 10.1007/s10689-005-2577-1. [DOI] [PubMed] [Google Scholar]
- Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: A review. Patient Education and Counseling. 2004;53(2):147–155. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Cheah WH. Condom attitudes and risk perceptions: A test of the extended parallel processing model. Journal of Intercultural Communication Research. 2005;34(4):213–232. [Google Scholar]
- Cho H, Salmon CT. Fear appeals for individuals in different stages of change: Intended and unintended effects and implications on public health campaigns. Health Communication. 2006;20(1):91–99. doi: 10.1207/s15327027hc2001_9. [DOI] [PubMed] [Google Scholar]
- Costanza ME, Luckmann R, Stoddard AM, et al. Using tailored telephone counseling to accelerate the adoption of colorectal cancer screening. Cancer Detection and Prevention. 2007;31(3):191–198. doi: 10.1016/j.cdp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Ditto PH, Lopez DF. Motivated skepticism: Use of differential decision criteria for preferred and nonpreferred conclusions. Journal of Personality and Social Psychology. 1992;63(4):568–584. [Google Scholar]
- Gollwitzer PM. Goal achievement: The role of intentions. European Review of Social Psychology. 1993;4:141–185. [Google Scholar]
- Gollwitzer PM. Implementation intentions: Strong effects of simple plans. American Psychologist. 1999;54(7):493–503. [Google Scholar]
- Gollwitzer PM, Sheeran P. Implementation intentions and goal achievement: A meta-analysis of effects and processes. Advances in Experimental Social Psychology. 2006;38:69–119. [Google Scholar]
- Gore TD, Bracken CC. Testing the theoretical design of a health risk message: Reexamining the major tenets of the Extended Parallel Process Model. Health Education & Behavior. 2005;32(1):27–41. doi: 10.1177/1090198104266901. [DOI] [PubMed] [Google Scholar]
- Greiner KA, Engelman KK, Hall MA, Ellerbeck EF. Barriers to colorectal cancer screening in rural primary care. Preventive Medicine. 2004;38(3):269–277. doi: 10.1016/j.ypmed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Harding R, Dockrell MJD, Dockrell J, Corrigan N. Motivational interviewing for HIV risk reduction among gay men in commercial and public sex settings. AIDS Care. 2001;13(4):493–501. doi: 10.1080/09540120120058021. [DOI] [PubMed] [Google Scholar]
- Janis IL, Feshbach S. Effect of fear-arousing communications. Journal of Abnormal and Social Psychology. 1953;48(1):78–92. doi: 10.1037/h0060732. [DOI] [PubMed] [Google Scholar]
- Jones RM, Woolf SH, Cunningham TD, et al. The relative importance of patient-reported barriers to colorectal cancer screening. American Journal of Preventive Medicine. 2010;38(5):499–507. doi: 10.1016/j.amepre.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber RA, Neklason DW, Samowitz WS, Burt RW. Frequency of familial colon cancer and hereditary nonpolyposis colorectal cancer (Lynch syndrome) in a large population database. Familial Cancer. 2005;4(3):239–244. doi: 10.1007/s10689-005-0657-x. [DOI] [PubMed] [Google Scholar]
- Lane C, Huws-Thomas M, Hood K, Rollnick S, Edwards K, Robling M. Measuring adaptations of motivational interviewing: The development and validation of the behavior change counseling index (BECCI) Patient Education and Counseling. 2005;56(2):166–173. doi: 10.1016/j.pec.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lasser KE, Murillo J, Medlin E, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: Results of a pilot study. BMC Family Practice. 2009;10:37. doi: 10.1186/1471-2296-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman A, Chaiken S. Defensive processing of personally relevant health messages. Personality and Social Psychology Bulletin. 1992;18(6):669–679. [Google Scholar]
- Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: A practice-friendly review of four meta-analyses. Journal of Clinical Psychology. 2009;65(11):1232–1245. doi: 10.1002/jclp.20638. [DOI] [PubMed] [Google Scholar]
- Makoul G, Cameron KA, Baker DW, et al. A multimedia patient education program on colorectal cancer screening increases knowledge and willingness to consider screening among Hispanic/Latino patients. Patient Education and Counseling. 2009;76(2):220–226. doi: 10.1016/j.pec.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Manne SL, Coups EJ, Markowitz A, et al. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Annals of Behavioral Medicine. 2009;37(2):207–217. doi: 10.1007/s12160-009-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RK, McNeil DW. Review of Motivational Interviewing in promoting health behaviors. Clinical Psychology Review. 2009;29(4):283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- McKay DL, Berkowitz JM, Blumberg JB, Goldberg JP. Communicating cardiovascular disease risk due to elevated homocysteine levels: Using the EPPM to develop print materials. Health Education & Behavior. 2004;31(3):355–371. doi: 10.1177/1090198104263353. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. New York: Guilford Press; 2002. Facilitating change; pp. 24–26. [Google Scholar]
- Morman MT. The influence of fear appeals, message design, and masculinity on men’s motivation to perform the testicular self-exam. Journal of Applied Communication Research. 2000;28(2):91–116. [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Miller WR, Ernst D. Motivational Interviewing Treatment Integrity 3.0 (MITI 3.0) 2007 Available at: http://casaa.unm.edu/download/miti3.pdf.
- Myers RE, Ross EA, Wolf TA, Balshem A, Jepson C, Millner L. Behavioral interventions to increase adherence in colorectal cancer screening. Medical Care. 1991;29(10):1039–1050. doi: 10.1097/00005650-199110000-00009. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Colorectal Cancer Screening. V.2.2011. 2010 doi: 10.6004/jnccn.2010.0003. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychological Bulletin. 2007;133(4):673–693. doi: 10.1037/0033-2909.133.4.673. [DOI] [PubMed] [Google Scholar]
- Overholser L, Zittleman L, Kempe A, et al. Use of colon cancer testing in rural Colorado primary care practices. Journal of General Internal Medicine. 2009;24(10):1095–1100. doi: 10.1007/s11606-009-1063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behavior. American Psychologist. 1992;47(9):1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- Ruthotto F, Papendorf F, Wegener G, et al. Participation in screening colonoscopy in first-degree relatives from patients with colorectal cancer. Annals of Oncology. 2007;18(9):1518–1522. doi: 10.1093/annonc/mdm200. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Weiner M, Berry SD, Lillie K, Fosnocht K, Hollenbeak CS. Overcoming poor attendance to first scheduled colonoscopy: A randomized trial of peer coach or brochure support. Journal of General Internal Medicine. 2008;23(1):58–63. doi: 10.1007/s11606-007-0445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: A meta-analytic review. Alcohol & Alcoholism. 2006;41(3):328–335. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- Wahab S, Menon U, Szalacha L. Motivational interviewing and colorectal cancer screening: A peek from the inside out. Patient Education and Counseling. 2008;72(2):210–217. doi: 10.1016/j.pec.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: The role of patient factors and physician counseling. Preventive Medicine. 2005;41(1):23–29. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Witte K. Putting the fear back into fear appeals: The extended parallel process model. Communication Monographs. 1992;59(4):329–349. [Google Scholar]
- Witte K. Fear control and danger control: A test of the extended parallel process model (EPPM) Communication Monographs. 1994;61(2):113–134. [Google Scholar]
- Witte K. Fear as motivator, fear as inhibitor: Using the extended parallel process model to explain fear appeal successes and failures. In: Andersen PA, Guerrero LK, editors. Handbook of Communication and Emotion: Research, Theory, Applications, and Contexts. San Diego, CA: Academic Press; 1998. pp. 423–450. [Google Scholar]
- Witte K, Allen M. A meta-analysis of fear appeals: Implications for effective public health campaigns. Health Education & Behavior. 2000;27(5):591–615. doi: 10.1177/109019810002700506. [DOI] [PubMed] [Google Scholar]
- Witte K, Cameron KA, McKeon JK, Berkowitz JM. Predicting risk behaviors: Development and validation of a diagnostic scale. Journal of Health Communication. 1996;1(4):317–341. doi: 10.1080/108107396127988. [DOI] [PubMed] [Google Scholar]