Abstract
Background
Recent studies questioning the benefit of prostate specific antigen (PSA) screening have increased the need for evaluating factors contributing to variance in levels and their clinical relevance. An inverse relationship between body mass index (BMI) and PSA has been illustrated, however the clinical implications have not been specified. We performed a retrospective review of patients screened through our free screening clinic to delineate any relationship between PSA and BMI in an attempt to understand its possible clinical significance.
Methods
The authors retrospectively reviewed data collected in relation to PSA values and patient characteristics from a community outreach program supplying information and screening for prostate cancer between June of 2003 and August of 2009.
Results
Mean BMI of our patient population was 28.7m/kg2 (SD 5.4) and our mean PSA value was 1.28 (SD 1.77). Our data indicates a small, but statistically significant decrease in PSA for an increasing BMI with a 0.026 decrease in PSA for every unit increase in BMI.
Conclusions
Our study confirms the previously reported inverse relationship between PSA value and BMI. The significance of this finding and its impact on the value do not appear to indicate a rationale to change the accepted abnormal value in obese patients and should be used in the context of the clinical scenario and other PSA altering factors.
Keywords: prostate cancer, PSA, BMI, prostate cancer screening, PSA accepted normal value range
Introduction
Prostate cancer is the most common solid tumor in American men and the second leading cause of cancer-related mortality.1 Early detection has important implications for patient care and health care costs. Prostate-specific antigen (PSA) was introduced in the 1980s as a marker for identifying prostate cancer, and in 1992 the American Cancer Society recommended the use of PSA in routine screening for prostate cancer. Use of PSA for screening resulted in a significant increase in prostate cancer detection with an average annual increase in incidence of 16.4% between 1988 and 1992.2 Importantly, this increase in incidence has coincided with an increase in the proportion of newly diagnosed patients that have clinically localized disease to a current level of 80%.
While the detection of prostate cancer has become more common since the introduction of PSA, overall mortality remains high with an estimated 27,360 deaths in 2009, and relative survival of patients diagnosed with prostate cancer has changed little.2 Recent studies have questioned whether screening using PSA has resulted in decreased mortality from prostate cancer.3-6 The European Randomized Study of Screening for Prostate Cancer (ERSPC) noted a 20% reduction in rate of death related to prostate cancer, but cautioned against the risk of over diagnosis. This study also indicated that 1410 patients would have to be screened and 48 treated to prevent one prostate cancer related death.4 In the United States, the Prostate, Lung, Colon and Ovarian (PLCO) Cancer Screening Trial showed no difference in the rate of death between patients undergoing annual screening and those patients in usual care.3
One possible reason that screening for prostate cancer using PSA has not achieved reduced mortality is that PSA can be confounded by weight. Several studies have demonstrated an inverse relationship between PSA and body mass index (BMI).7-13 In one study, PSA decreased by 5.9% for every 5.2 kg/m2 increase in BMI.8 Since about 1/3 of American men are obese, if the relationship between BMI and PSA is indeed clinically significant, this could have large implications on the interpretation of PSA levels.14 This could explain why elevated BMI has been associated with an increased mortality and higher Gleason scores at diagnosis, although it is noted the association is inconsistent.15-17 The goal of this study was to evaluate the relationship between PSA level and BMI. We hypothesized that men with an increased BMI would have decreased PSA levels.
Materials and Methods
This was an observational cohort study using data collected by a community outreach program designed to increase awareness of prostate cancer and accessibility to prostate cancer screening in traditionally underserved populations. All procedures were approved by the local Institutional Review Board with written and informed consent obtained from all patients.
Subjects ≥40 years of age with a current PSA level, height and weight recorded were eligible for inclusion in this study. Subjects with a previous diagnosis of prostate cancer, previous resection of the prostate for benign conditions, endocrinologic conditions which could alter baseline PSA and previous or ongoing hormone modulating medications (e.g. saw palmetto, proscar, avodart and androderm) were excluded. The Siemens (Bayer) chemiluminescent method was used for PSA level determination. PSA levels ≥4.0 ng/ml were considered elevated. BMI was calculated as the weight in kilograms divided by the square of the height in meters.
The mean and standard deviation have been used as measures of central tendency and spread. Frequencies and percentages have been used to describe categorical variables. Subjects could have had more than one visit during the study period so a mixed effects linear model was used to estimate the effect of BMI on PSA. The model was adjusted for race and age. Analyses were conducted using SPSS v 17.0 (SPSS Inc., Chicago, IL)
Results
There were 1514 visits between June 2003 and August 2009. There were 206 visits excluded for patients with a history of prostate cancer, prostate surgery, previous use of hormone modulating medications or age <40 years. Height or weight was not assessed at 319 visits, PSA values were either missing or out of range for four visits, and race was unknown for two visits. These visits were also excluded, resulting in 983 visits by 767 unique patients being included in the analysis. Characteristics of the included and excluded subjects are detailed in Table 1.
Table 1.
Characteristics of included and excluded visits*
| Included visits (N=983) |
Excluded visits (N=521) |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Number | Mean/Frequency | SD / % | Number | Mean/Frequency | SD / % | |
| Age at PSA (years) | N=983 | 55.42 | 9.71 | N=527 | 53.35 | 14.75 |
|
| ||||||
| African American | 684 | 69.6 | 385 | 80.0 | ||
|
| ||||||
| Height (ft) | N=983 | 5.8 | 0.3 | N=135 | 5.9 | 0.3 |
| Weight (lbs) | N=983 | 200 | 39 | N=138 | 201 | 41 |
| BMI | N=983 | 28.68 | 5.16 | N=135 | 28.64 | 5.40 |
|
| ||||||
| PSA | N=983 | 1.39 | 1.96 | N=524 | 1.64 | 2.93 |
Data are given as mean and standard deviation or frequency and percent.
Of the 767 patients with at least one visit meeting inclusion criteria, the mean age at first PSA was 54 (SD 9) years and 72% were African-American. Mean BMI was 28.7kg/m2 (SD 5.4); 78% were overweight or obese (BMI ≥ 25). Mean PSA was 1.28 (SD 1.77) ng/ml; 44 patients had at least one PSA level over 4.0 ng/ml.
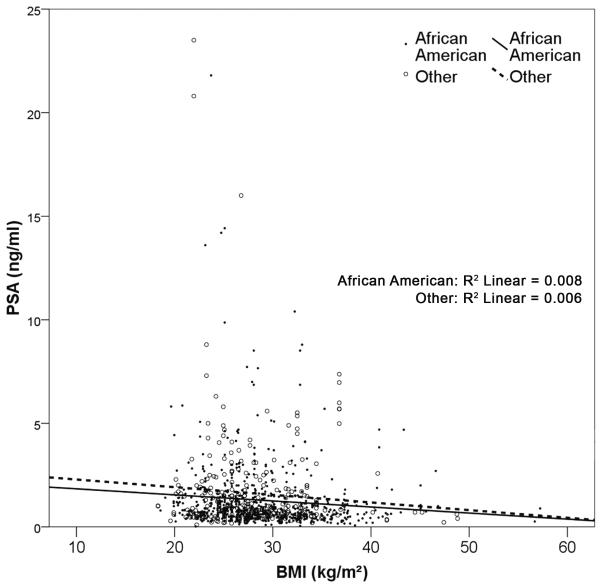
There was a small, but statistically significant trend towards decreasing PSA for an increasing BMI (Figure 1); for every unit increase in BMI, PSA decreased by 0.026ng/ml. In addition, for every unit increase in age, PSA increased by 0.034 ng/ml (Table 2). These values illustrated in the table serve to provide estimations of the deduction or contribution on PSA value that obesity and age provide, respectively.
Figure 1.
Association between BMI and PSA
Table 2.
Parameter estimates
| Parameter | Estimate* | 95% confidence interval | p-value |
|---|---|---|---|
| BMI | −0.026 | (−0.050 to − 0.002) | 0.031 |
| Age | 0.034 | (0.021 to − 0.047) | <0.001 |
| African-American v Other | −0.200 | (−0.465 to − 0.066) | 0.140 |
Estimation of shift in PSA value for every unit increase or presence of indicated parameter.
Discussion
Consistent with previous research, this study suggests there is a negative association between BMI and PSA levels. The degree of association is similar to that identified in other studies, and also to studies showing associations between PSA and lean and fat mass ratios and waist circumference.7-10 Clinical utility of this finding has yet to be demonstrated and our data show that it is likely to be of limited relevance for application to routine screening; assuming a linear relationship, the BMI would have to change by 40 kg/m2 to result in a change in PSA levels of 1 ng/ml. While the individual associations are small, in combination they might attain clinical relevance and might be considered when evaluating the need for further testing (e.g. biopsy) for patients with PSA values close to the common normal limit of 4.0ng/ml. However, we do not believe the associations observed in our study warrant a reassessment of this normal value.
There are inherent limitations recognized in our study. As this is a research population obtained through a community outreach program, we have a specific subset of patients which may not be representative of the population at large. This would clearly preclude any outcomes-based analysis. However, we feel that this subset still serves to assess an association between the clinical parameter of BMI and the currently accepted screening standard of care, PSA. There are additional risk factors for prostate cancer which can be prospectively or retrospectively analyzed in a screening population including: free PSA, PSA velocity, fat distribution, lean and fat mass ratios, palpable prostate abnormalities and serum testosterone levels. Given the natural limitations of our study population as a screening population with limited funding, our analysis captures the stated aim of an association between BMI and PSA. This association has been attributed to different factors which include alteration in androgen levels due to obesity as well as hemodilution10-11. The relative risk of prostate cancer due to these biochemical changes is not clearly defined and PSA serves as an intermediary to these potential effects.
As debate continues as to the utility of using PSA levels to screen for prostate cancer,3-6 our study suggest that the association between BMI and PSA level is of insufficient magnitude to broadly impact the sensitivity or specificity of PSA levels to prostate cancer. The overall benefit of prostate screening under the currently recommended parameters remains to be determined.
Conclusion
Our study found a quantitative association between PSA and BMI. The magnitude of effect does not appear to justify changing the accepted limit of normal for obese persons, although the decision to conduct further testing might be informed when considering obesity in combination with other factors known to impact PSA levels.
Acknowledgments
Sources of support: The prostate screening clinic which is the source of data in this article is funded by corporate sponsorship including Western Southern Life, American Financial Corporation and the American Cancer Society. This publication was also supported by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 1UL1RR026314-01.
Footnotes
Financial disclosure: The authors have no commercial association or other arrangement that might pose or imply a conflict of interest in connection with the submitted paper.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Cancer Statistics Working Group . United States Cancer Statistics: 1999–2005 Incidence and Mortality Web-based Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Atlanta: 2009. Available at: www.cdc.gov/uscs. [Google Scholar]
- 2.Surveillance Epidemiology, and End Results (SEER) Program [accessed January 15, 2010]; Released April 2009, based on the November 2008 submission. http://www.seer.cancer.gov.
- 3.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder FH, Hugosson J, Roobol MJ, et al. ERSPC Investigators Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 5.Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery E, Barry MJ. Screening, treatment, and prostate cancer mortality in the Seattle area and Connecticut. J Gen Intern Med. 2008;23(11):1809–1814. doi: 10.1007/s11606-008-0785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esserman L, Yiweh S, Thompson I. Rethinking Screening for Breast Cancer and Prostate Cancer. JAMA. 2009;302:1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 7.Culp S, Porter M. The effect of obesity and lower serum prostate-specific antigen levels on prostate-cancer screening results in American men. BJUI. 2009;104(10):1457–1461. doi: 10.1111/j.1464-410X.2009.08646.x. [DOI] [PubMed] [Google Scholar]
- 8.Werny DM, Thompson T, Saraiya M, et al. Obesity is negatively associated with prostate-specific antigen in U.S. men, 2001-2004. Cancer Epidemiol Biomarkers Prev. 2007;16:70–76. doi: 10.1158/1055-9965.EPI-06-0588. [DOI] [PubMed] [Google Scholar]
- 9.Rundle A, Richards C, Neugut A. Body composition, abdominal fat distribution, and prostate-specific antigen test results. Cancer Epidemiol Biomarkers Prev. 2009;18(1):331–336. doi: 10.1158/1055-9965.EPI-08-0247. [DOI] [PubMed] [Google Scholar]
- 10.Muller H, Raum E, Rothenbacher D, Stegmeier C, Brenner H. Association of diabetes and body mass index with levels of prostate-specific antigen: Implications for correction of prostate-specific antigen cutoff values? Cancer Epidemiol Biomarkers Prev. 2009;18(5):1350–1356. doi: 10.1158/1055-9965.EPI-08-0794. [DOI] [PubMed] [Google Scholar]
- 11.Bañez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298(19):2275–2280. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 12.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103(5):1092–1095. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 13.Han JH, Chang IH, Ahn SH, et al. Association between serum prostate-specific antigen level, liver function tests and lipid profile in healthy men. BJUI. 2008;102:1097–1101. doi: 10.1111/j.1464-410X.2008.07774.x. [DOI] [PubMed] [Google Scholar]
- 14.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 15.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 16.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in med with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–445. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 17.Bañez LL, Sun L, Trock BJ, et al. Body mass index and prostate specific antigen as predictors of adverse pathology and biochemical recurrence after prostatectomy. J Urol. 2009;182(2):491–496. doi: 10.1016/j.juro.2009.04.007. [DOI] [PubMed] [Google Scholar]