Abstract
Significant advances in human functional brain imaging offer new opportunities for direct observation of the effects of nicotine, novel nicotinic agonists and nicotinic antagonists on human cognitive and behavioral performance. Careful research over the last decade has enabled investigators to explore the role of nicotinic systems on the functional neuroanatomy and neural circuitry of cognitive tasks in domains such as selective attention, working memory, episodic memory, cognitive control, and emotional processing. In addition, recent progress in understanding functional connectivity between brain regions utilized during cognitive and emotional processes offers new opportunities for examining drug effects on network-related activity. This review will critically summarize available nicotinic functional brain imaging studies focusing on the specific cognitive domains of attention, memory, behavioral control, and emotional processing. Generally speaking, nicotine appears to increase task-related activity in non-smokers and deprived smokers, but not active smokers. By contrast, nicotine or nicotinic stimulation decreases the activity of structures associated with the default mode network. These particular patterns of activation and/or deactivation may be useful for early drug development and may be an efficient and cost-effective method of screening potential nicotinic agents. Further studies will have to be done to clarify whether such activity changes correlate with cognitive or affective outcomes that are clinically relevant. The use of functional brain imaging will be a key tool for probing pathologic changes related to brain illness and for nicotinic drug development.
Keywords: nicotine, fMRI, mecamylamine, physostigmine, attention, memory, emotion
1. Introduction
Investigation of nicotinic cholinergic receptor function in the brain has traditionally relied on well-tested methods of behavioral pharmacology in animals and more recently humans [1–5]. These methods, especially with more recent technical improvements have been extraordinarily productive and have led to major advances in understanding of the role of nicotinic receptor systems on basic cognitive and behavioral systems [6]. However, the nature of nicotinic signaling systems and the limitations of the currently available pharmacologic agents (especially in humans) and models place constraints on the ability to discern how nicotinic modulation affects simple and more complex cognitive processes. Difficulties with acute versus chronic dosing in both animal and human models, especially with regard to studies of cognitive performance, along with the well-known constraints of studying smokers have led to uncertainties regarding to what extent nicotinic modulation is active in a variety of behavioral and cognitive domains. In addition, such limitations have presented significant obstacles to targeted drug development of nicotinic agents for the amelioration of cognitive and behavioral disorders. For example, it is difficult to model certain aspects of cognitive processes in rodents, especially in the area of episodic memory, executive or emotional processing. It is likely that the underlying task-related neural circuitry is significantly different between rodent and human models due to differences in neuroanatomy. This has led to challenges in utilizing animal models to predict the effects of human administration of nicotine or related compounds [7]. These challenges have contributed to problems translating basic and human-based findings into the development of strategies to preserve or enhance nicotinic functioning through drug development. Advances in human functional brain imaging offer new opportunities for direct observation of the neurobiological effects of nicotine, nicotinic antagonists, and novel nicotinic agonists on cognitive and behavioral performance.
Careful research over the last decade has enabled investigators to begin to explore the functional neuroanatomy and neural circuitry of numerous tasks in domains such as selective attention, working memory, episodic memory, behavioral inhibition and control, and emotional processing. As the cortical networks underlying cognitive operations are beginning to be understood, the opportunity to both acutely and chronically investigate how nicotinic modulation affects these cortical networks and their activity patterns becomes possible. Correlating the activity of these regions and networks with cognitive performance and accounting for variables such as age, gender, baseline performance, etc. will allow a much clearer understanding of both how important nicotinic modulation is to a particular cognitive task or domain as well as the potential for utilizing nicotinic agents to either stabilize or improve cognitive performance. It is now possible to see how utilizing drugs during functional brain imaging or so-called pharmacologic fMRI or PET may become an important tool in not only understanding how nicotinic systems modulate task-related cortical activity but such approaches may also be useful for initial drug development, examining whether a putative agent has significant activity on cognitive or behavioral domains relevant to potential target indications. The majority of such studies use an acute drug challenge before volunteers undergo a cognitive task in a magnetic resonance imaging (MRI) scanner. Functional MRI measurements basically capture blood oxygenation within brain regions via the so called BOLD (blood oxygen level dependent) contrast which was shown to be related to input and processing of neuronal activity within brain regions [8]. A comparison between drug and placebo then reveals the drug’s action on task-related brain activity. Note that the findings of such studies identify neurochemical modulation of brain activity that is induced by a specific task rather than excitation or inhibition of brain regions per se. In principle, designs of so called “pharmacological fMRI” studies do not differ from conventional fMRI experiments. The essential point in the data analysis is the group by condition interaction showing areas with significant differences in task-related activity between the drug and placebo. Plotting activity changes in these areas will provide further information on the modulatory action of the drug on a given cognitive process. This is illustrated for a nicotinic study of cognitive performance in Figure 1.
Figure 1.
Schematic illustration of two different group (placebo vs. nicotine) by condition (task vs. control) interactions in a pharmacological fMRI study.The upper part illustrates a nicotine-induced task related increase in neural activity in frontal cortex, as for example found in working memory or sustained attention paradigms. The lower part illustrates a nicotine-induced increase in deactivation in the posterior cingulate cortex. Such deactivations are usually found during task performance and are increased under nicotine.
In addition, recent progress in understanding functional and effective connectivity between brain regions utilized during cognitive and emotional processes offers new opportunities for examining drug effects on network-related activity. As activity patterns in cortical networks become better characterized, especially with regard to optimized cognitive or behavioral functioning, these patterns of activity may come to represent biomarkers that may be usable for investigating nicotinic function and/or predicting nicotinic drug effects on performance and behavior.
This review will evaluate available nicotinic functional brain imaging studies focusing on the specific cognitive domains of attention, memory, cognitive control, and emotional processing. While information on how nicotinic modulation affects some of these domains is limited or is just beginning to be studied in depth, the available literature allows preliminary conclusions and hypotheses to be developed on how nicotinic stimulation or blockade alters the cortical activity associated with certain cognitive operations. In addition, considerations of agonists and antagonists in terms of their use in functional imaging will be considered.
2. Attention
2.1 Visuospatial selective attention and sustained attention
Several attentional functions are modulated by the cholinergic agonist nicotine (for review see [1]). The neuroimaging literature has focused to a large extent on visuospatial selective attention and sustained attention. The first pharmacological fMRI study investigated the effects of 21 mg transdermal nicotine in a within-subject design in smokers using a rapid visual information processing task [9]. Behaviorally an increased number of hits was observed under nicotine, especially when nicotine was given in the second session. Neurally, nicotine enhanced BOLD activity in parietal cortex, thalamus, and the caudate nucleus. This suggests that improvement of attentional functions is due to an increase in neural activity in areas involved in attention [9]. However, the authors noted that when smokers were compared to nonsmokers they showed a hypoactivation in parietal cortex and caudate nucleus and subtle behavioral deficits. Several other studies have focused on the role of nicotine in visuospatial selective attention [10–12]. These studies were performed in non-smoking subjects using 2 mg of nicotine gum and compared neural activity in trials where cues either validly or invalidly indicated the most likely position of a subsequent target. A finding across all these studies was that BOLD activity in posterior parietal cortex, was reduced in invalidly cued trials, even though behavioral effects were often weak (Figure 2). Other brain areas showing activity decreases in some of these studies were located in middle frontal and superior and middle temporal regions. These apparently contradictory findings of increased and decreased parietal neural activity in attentional tasks under nicotine illustrate two points. First, it is difficult to compare drug effects in pharmacological fMRI studies across different tasks and contrasts. While Lawrence et al [9] showed that the parietal cortex is recruited when subjects had to attend and detect three number streams for 90 seconds as compared to detecting a single number, our own studies [10–12] showed that the parietal cortex is recruited when volunteers had to quickly shift their attention to a previously unattended location. In both instances, nicotine modulates parietal cortex activity but the cognitive processes and temporal aspects of these processes (tonic vs. phasic) underlying this parietal activity are different.
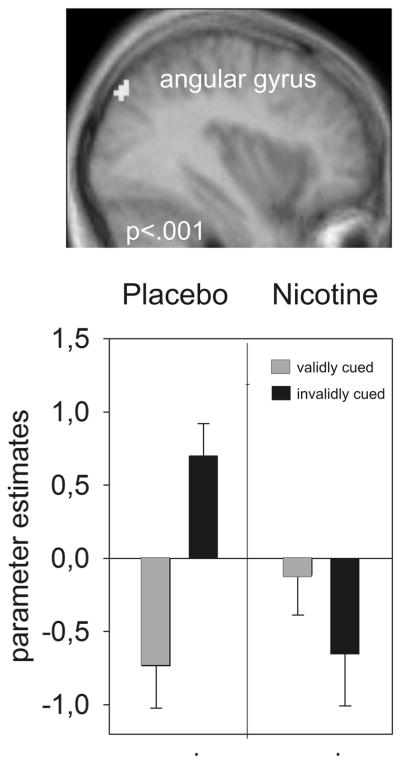
Figure 2.
Decreased neural activity under nicotine in a cued target detection task. A significant group by condition interaction was found in the right angular gyrus in posterior parietal cortex (n=13). The plot shows that the group by condition interaction was driven by a reduction of neural activity in invalidly cued trials under nicotine (Figure adapted from [10])
Even though a study by Hahn and colleagues [13] has shown that many of the reductions in neural activity after nicotine in frontal, temporal, visual and thalamic brain areas are observed even when different attentional processes are required, differences in task design, subject groups and cognitive processes examined between studies may nevertheless contribute to differences in the action of nicotine in one and the same brain region.
A second point which may contribute to increased and decreased parietal neural activity in attentional tasks under nicotine is related to whether nonsmokers or smokers serve as subjects. Nicotine, as with other drugs, may show effects that depend on baseline performance [14]. These effects resemble an inverted U-shape function where subjects who perform at optimum levels will show decreased performance after drug stimulation [3]. Indeed, many studies performed in healthy subjects show no or only weak effects of nicotine on cognitive performance. In contrast, subjects with suboptimal performance, such as patients with ADHD or Alzheimer’s disease are more likely to benefit from nicotine [3]. Baseline effects of cholinergic drugs are also reported on a neural level, at least for cholinesterase inhibitors. Bentley and colleagues [15] have shown opposing effects of physostigmine (which activates nicotinic-related circuits) on task-related neural activity across many brain regions in healthy individuals and patients with Alzheimer’s disease. A related finding is that of Warbrick et al. [16] who administered 1 mg of nicotine nasally and reported that reaction time benefits under nicotine correlated positively with nicotine-induced increases in BOLD signal (pooled over all active brain regions). The finding of mainly increased neural activity in smokers and mainly decreased neural activity in nonsmokers can be accommodated if smokers are conceptualized as cholinergically and/or attentionally “deficient”, perhaps due to smoking-induced changes in nicotinic receptor number and sensitivity [17]. Then, an increase of cholinergic function would increase attention-related neural activity as found by Lawrence et al. [9]. In contrast, the nonsmokers investigated with cued target detection tasks, whose cholinergic function (and parietal cortex activity) may have been close to the optimum showed decreases rather than increases of attention-related neural activity [10–12]. Human positron emission tomography receptor studies and structural MRI studies may shed further light into differential effects of nicotine in smokers and nonsmokers. For example, it has been shown that smokers have in many brain regions (apart from the thalamus) a higher density of nicotine receptors [18]. Further, structural MRI studies suggest that chronic tobacco use is correlated with prefrontal gray matter damage (e.g. [19, 20]). Note however, that there are also studies that did not find differences in nicotinic modulation of task-induced neural activity between smokers and nonsmokers [16, 21] or between smokers and patients with schizophrenia who show attentional deficits [22]. We discuss potential reasons for these apparently contradictory findings in section 5.1.
There have been few studies that have examined the effects of nicotinic blockade on attention-related brain activity. These studies utilized the antinicotinic drug mecamylamine to examine effects on attention and the corresponding brain activation. Mecamylamine is a noncompetitive nicotinic receptor antagonist that has specific effects at α4β2 nicotinic receptors and has been shown to impair a number of cognitive functions in a disease and dose-dependent way [23–25]. Thienel et al. [26] examined the effects of 15 mg of mecamylamine on the subcomponents of attention including alerting, orienting, and executive control using an attention network task (ANT). On the ANT task mecamylamine increased reaction time across all three attentional component processes compared to placebo. Mecamylamine had specific effects on brain activation related to orienting in that it reduced activation in the superior occipital gyrus, thalamus, parahippocampal gyrus and posterior cingulate. Effects of mecamylamine were also observed in the task comparisons examining executive functioning. Reduced activity for mecamylamine compared to placebo was observed in the gyrus rectus, precuneus and superior parietal area. Overall, mecamylamine reduced activity compared to placebo in brain regions specific to orienting and executive functioning in healthy younger men [26].
2.2 Default Mode Network
An often replicated finding in the human neuroimaging literature is an increase in neural activity in midline brain areas while people are at rest. Such activity increases were proposed to represent a default mode of brain function which is increased at rest and decreased during task performance [27]. Nicotinic imaging studies in smokers and nonsmokers have consistently found a reduction of such task-induced neural deactivations in default mode regions including anterior and posterior cingulate gyri, inferior parietal cortex, angular gyrus, and the medial frontal gyrus [13, 28–30], (see also Fig. 1 lower part for illustration). Similar results were recently found in the resting state [30]. One of these midline brain areas, the posterior cingulate cortex, also exhibited less deactivation in mildly deprived smokers as compared to nonsmokers which may indicate susceptibility to task irrelevant thoughts [30] Further, neural activity in brain areas of the default mode network were shown to contribute to the prediction of the behavioral effects of nicotine, suggesting that those subjects who showed difficulties in sustaining attention are the ones that most benefit from nicotine [31]. This latter approach that used multivariate analysis techniques may be a useful approach to predict drug effects. Interestingly, in a recent study of the α7 nicotinic receptor agonist 3-(2,4-Dimethoxybenzylidene)-anabaseine (DMXB-A) in non-smoking patients with schizophrenia, four weeks of dosing with the agonist produced reductions in default mode network activity that was at least partially related to CHRNA7 genotype [32]. These results suggest that nicotine effects on default mode network activity may be mediated at least in part through α7 nicotinic receptors. Furthermore, these results support the concept that nicotinic cholinergic stimulation improved attentional performance by decreasing the activity of default mode network structures and encouraging a shift from internal to external processing modes, permitting improved responsiveness to external cues [13]. These findings are further consistent with animal studies supporting the concept of cholinergic stimulation, particularly nicotinic stimulation, being responsible for a "mode shift" in terms of improved readiness to respond to external cues [6].
3. Memory
A meta-analysis of human psychopharmacological studies in smokers and nonsmokers suggests that nicotine improves accuracy in episodic memory only with short delays between encoding and recall. Working memory accuracy does not appear to be significantly modulated, however reaction times have been shown to be significantly improved under nicotine [1]. Although there are a few imaging studies which investigate the role of the cholinergic system on episodic memory [33, 34], the role of the nicotinic cholinergic system has mainly been investigated with respect to working memory. As for the above mentioned studies on nicotinic modulation of attention, results may depend upon whether nonsmokers or smokers serve as subjects [35]. Kumari and colleagues [36] used an n-back working memory task with numerals as stimuli. The n-back task is an attentionally demanding parametrically variable working memory task with loads varying from minimal (matching) to high (matching to item that occurs three items back). Nonsmokers received 12μg/kg subcutaneous nicotine and the results yielded a nicotine-induced increase in fronto-parietal activity which was strongest in the 1-back condition. In line with the studies mentioned above [13, 28–30] nicotine also induced a reduction in posterior cingulate activity under rest. The activity pattern resembles the findings of Lawrence et al. [9] using the rapid visual information processing task. Indeed, rapid visual information processing and n-back tasks bear several similarities in that both measure aspects of sustained attention and working memory (though to a different extent). Increases in dorsolateral and medial frontal cortex were also found in a region of interest analysis with the α4β2 partial agonist varenicline, however only in the most difficult 3-back condition [37]. Additionally a whole brain analysis revealed nicotine induced decreases of neural activity in inferior and superior frontal gyrus, posterior cingulate cortex and the parahippocampal gyrus among others. In contrast to the study of Kumari et al. [36], which showed strongest effects in the 1-back condition, deprived smokers served as subjects and the stimuli used for the n-back paradigm were complex geometric figures. A smoking-induced increase in dorsolateral prefrontal activity in deprived smokers that was specific to the most difficult 3-back condition was also reported by Xu and colleagues [38]. Notably, non-deprived smokers showed smoking-induced increases in the 1-back condition only, indicating that the status of deprivation is critical. The role of nicotine on switching between items in verbal working memory was investigated in a study by Sutherland et al. [39] who administered 21 mg transdermal nicotine to mildly deprived smokers. Even though nicotine yielded behavioral effects, no effects on neural activity related to task performance or switching were observed – one of the rare examples where neuroimaging was not found to be more sensitive than behavioral data. In summary, nicotinic stimulation during tasks of working memory seem to affect similar systems as those involved in sustained attention.
In two studies, Dumas and colleagues [40, 41] examined the effects of nicotinic blockade with 20 mg mecamylamine on brain activation during working memory and episodic memory tasks in healthy postmenopausal women not taking estrogen therapy. Nicotinic blockade with mecamylamine did not impair working memory performance on the n-back task, but did reduce frontal activation relative to placebo [40]. These findings are in line with the increases in frontal activation after nicotinic receptor stimulation with the administration of nicotine. Mecamylamine similarly did not impair recognition memory performance but did modulate brain regions involved in episodic memory using a continuous recognition memory task. Mecamylamine compared to placebo increased frontal and hippocampal activation during the encoding of new information into memory. Nicotinic blockade also resulted in decreased occipital activity compared to placebo during encoding [41]. During the retrieval phase of this task, nicotinic blockade resulted in increased activation in the inferior temporal gyrus, anterior hippocampus, occipital cortex, and the uncus (Figure 3). The use of a continuous recognition memory task allowed for the dissociation of encoding and retrieval processes during nicotinic system manipulation. Future studies should utilize tasks where the cognitive components of memory processes can be dissociated to more fully understand the role of nicotinic modulation.
Figure 3.

The effect of the nicotinic antagonist mecamylamine compared to placebo for retrieved words compared to encoded words in a sample (n = 6) of postmenopausal women not on estrogen (p < .01). Increased activation for mecamylamine compared to placebo in the inferior temporal gyrus, anterior hippocampus, occipital lobe, and the uncus. The use of a continuous recognition memory task allowed for the dissociation of encoding and retrieval processes during nicotinic system manipulation.
Thus, the pattern of brain activation produced by nicotinic stimulation or blockade is dependent upon the task requirements, age, gender, and presumably the amount of engagement of nicotinic systems. Interestingly, only one of the nicotinic drug studies reviewed above used an approach that would have enabled dissociation of encoding, maintenance, or retrieval of working or episodic memory [41]. A study using the cholinesterase inhibitor physostigmine was able to show that cholinergic stimulation increases neural activity during encoding in sensory cortices and subsequently reduces retrieval demands in prefrontal cortices. Again, such approaches are especially valuable since they can dissociate cognitive processes which cannot be segregated behaviorally. The nicotinic antagonist mecamylamine produced reduced activation in frontal, parietal and occipital regions generally compared to placebo in attention and working memory tasks. However, a frontal and hippocampal increase was observed during the episodic memory task. Additional work is needed to disentangle the role of nicotinic systems in memory-related cognitive processes and more specifically the role of α4β2 as well as α7 receptors.
4. Cognitive Control and Emotion
4.1 Response Inhibition and Behavioral Control
There is increasing evidence that cholinergic system function, particularly nicotinic cholinergic system function, appear important for regulating cognitive control and/or response inhibition [42]. It has been hypothesized that dysfunction of the nicotinic cholinergic system contributes to impulsivity in disorders such as Attention Deficit/Hyperactivity Disorder (ADHD) via contributions to deficits in behavioral inhibition [43]. Behavioral studies have shown that acute nicotine normalizes response inhibition in both non-smoking adolescents and adults with ADHD [44, 45]. Studies using fMRI have defined neuroanatomical regions involved in inhibiting a pre-potent response including the right inferior frontal gyrus (IFG), pre-supplementary motor area (SMA) and basal ganglia [46–49]. A recent neuroimaging study in cigarette smokers found that baseline activation of these brain areas in smokers predicted success during a subsequent quit attempt [50]. Specifically, they found that smokers who activated greater areas of the response inhibition network were less likely to smoke in response to cravings than those who activated less at baseline. These findings support the validity of response inhibition, a laboratory measure of cognitive control, as an indicator for real world behavior change, and further demonstrate that the integrity of this neuroanatomical network predicts real-world inhibition of a behavior (smoking) in response to cravings. The authors further propose that this supports the possible diagnostic utility of neuroimaging data in smoking cessation [50].
While there are no pharmacological imaging studies of specific nicotinic agonists or antagonists on cognitive control in non-smokers, recent studies using the cholinergic drug rivastigmine in patients with multiple sclerosis demonstrate the utility of this approach. Impaired cognitive control has been documented very early in the disease process of MS [51]. Parry and colleagues conducted a pharmacological imaging study using the Stroop task (inhibition of word reading vs. color naming) following administration of rivastigmine, a cholinesterase inhibitor that increases both nicotinic and muscarinic signaling, and placebo. They demonstrated that rivastigmine normalized the pattern of brain activity associated with the conflict condition of the Stroop task in patients with MS [52]. Specifically, following acute rivastigmine administration patients with MS increase activity in the IFG to a level comparable to control subjects [52]. A second study by this group of investigators, using chronic treatment with rivastigmine, replicated this finding although the magnitude of the finding was smaller [53]. The authors suggest that rivastigmine may be amplifying the signal to noise ratio which is important in MS due to pathological changes such as demyelination and axon and synaptic loss [54, 55]. These results are consistent with nicotinic imaging studies of other cognitive operations including attention and memory where increased neural activation in task relevant areas is seen with nicotinic stimulation.
4.2 Eye Movements
Another approach to measuring response inhibition is evaluation of reflexive and volitional eye movements. Nicotine has been shown to reduce intrusive anticipatory eye movements (leading saccades) in the smooth pursuit eye movement task in both smoking and non-smoking patients with schizophrenia [56, 57]. In healthy volunteers nicotine improved performance in smokers (with abnormal baseline performance) and had no effect in non-smoking control subjects [57]. This finding has been further examined using fMRI in a group of schizophrenic subjects [58]. In this study, nicotine improved smooth pursuit eye movements, and this improvement was accompanied by decreased activation in the hippocampus suggesting nicotinic modulation of the overactivity normally seen in the hippocampus in schizophrenia [58, 59]. In addition, nicotine was associated with decreased activity in the parietal eye fields which may be related to improved inhibition of eye movements as this area is involved in the generation of automatic saccades which may be inhibited by nicotinic stimulation [58]. Further support for this hypothesis comes from a recent study examining the effects of chronic (three month) treatment with DMXB-A, a partial α7-nicotinic acetylcholine receptors agonist in non-smoking subjects with schizophrenia [60]. These investigators found similar effects of DMXB-A on brain activity to their previous nicotine study. Specifically, the 150 mg b.i.d. dosing was associated with reduced right hippocampal activity during the smooth pursuit eye movement task. Taken together there is strong evidence suggesting that nicotinic acetylcholine receptor system function modulates neural activity related to inhibition across a number of response modalities.
4.3 Modulation of Emotion
The use of functional neuroimaging to study nicotinic modulation of emotional processes is in its infancy. However, a strong evidence base supports the importance of cholinergic projections for modulating emotional processes. For example based on anatomical and animal studies, it has been suggested that nucleus basalis cholinergic fibers underlie stimulus evaluative processing in the amygdala which then impact the allocation of learning and attentional resources in the thalamus and cortex [61].
Interestingly, relatively little is known about how nicotinic stimulation or blockade affects the cortical activity associated with human emotional processing or emotional reactivity. However, further support for nicotinic cholinergic enhancement of neural processes related to emotion comes from a recent study examining the effects of acute nicotine on emotional processing in healthy non-smokers [62]. This study found that nicotine was associated with increased activity in response to unpleasant (but not pleasant) stimuli in the amygdala and other limbic and subcortical structures. The authors conclude that a single dose of nicotine can disrupt the integrity of the limbic circuitry that underlies emotional processing. These data are of particular relevance because a nicotinic antagonist has been studied as an augmentation treatment for depression [63, 64] and the S-enantiomer of mecamylamine is being studied in a phase-3 study for major depressive disorder [65, 66].
Cholinergic enhancement with physostigmine has been shown to produce changes in the neural processing of emotional faces and these changes depend on the task relevance of the stimulus [61]. For example, following physostigmine administration, anatomical regions known to be related to task-relevant emotional stimuli processing, including dorsolateral and medial prefrontal cortices, [67–69] showed increased activation in response to task relevant fearful faces. This evidence supports the hypothesis that the cholinergic stimulation differentially increases attention to emotionally task relevant stimuli, resulting in enhanced processing of these stimuli [70].
In contrast, when these investigators examined task irrelevant emotional stimuli they found a different pattern of cholinergically mediated neural activation. Specifically, physostigmine was associated with increased activation in orbitofrontal and parietal cortices in response to task irrelevant fearful faces [70]. The authors note that these anatomical regions are related to processing the affective value of background stimuli [68] and suggest that cholinergic enhancement of this process may improve efficiency of attentional engagement to emotional stimuli depending on whether the stimuli are task relevant or irrelevant [70].
These initial studies provide intriguing evidence for nicotinic cholinergic modulation of neural processing of emotional stimuli, particularly negative stimuli. The mechanism for this may be through increased efficiency of limbic-frontal functional connections resulting in greater allocation of attentional resources to negative stimuli in the presence of enhanced nicotinic cholinergic function. Future investigations will be looking more carefully at how nicotinic stimulation or nicotinic blockade affects both limbic structures that generate negative emotion as well as prefrontal structures involved in the modulation and control of limbic circuitry.
5. Discussion
5.1 Advantages and Disadvantages of Nicotinic Functional Imaging
The findings reviewed here show that neuroimaging data can contribute over and above the available behavioral data to the understanding of how alterations, either pharmacologically or pathologically, of nicotinic function change specific cognitive processes. One of the major advantages of functional brain imaging in humans, particularly with fMRI, is the ability to investigate many brain areas at once. In contrast intra- and extracellular recordings in animals can only be achieved in a limited set of brain regions within one experiment. fMRI data analyses can be either done at the level of whole brain analyses that are agnostic to the particular structures potentially involved in the effects of the drug or can be restricted to region of interest (ROI) analyses to examine hypothesized structures. Furthermore, connectivity analyses and multivariate methods allow the assessment of drug effects on cortical networks and allow the examination of how nicotinic modulation of particular cortical structures and networks is relevant to particular cognitive operations. Drug modulation of different processes within the same task (e.g. encoding of information, maintenance, and retrieval during episodic memory) can be individually assessed using event-related fMRI techniques. Utilizing traditional psychopharmacology methods and behavioral analysis is insufficient to disentangle these components of episodic memory. Correlating brain activity, performance, and drug treatment allows the parsing of nicotinic effects on particular components of cognitive operations with a precision that was not heretofore available in humans. It is also possible that fMRI is a more sensitive measure of potential drug effects than behavioral or cognitive data alone as fMRI can reveal changes in cortical activity or in the allocation of cortical resources even in the absence of obvious performance changes. This could indicate alterations in neural compensation which may be influenced by nicotinic drug treatment [71]. This may convey advantages for nicotinic drug discovery both in terms of being able to precisely understand the potential for a particular agent to alter task-related cortical activity as well as potential changes in default mode network activity which may correlate with improved task performance.
However, there are some potential limitations and problems with fMRI and other similar functional brain imaging techniques which also apply to nicotinic imaging studies reviewed above. Subtle differences in task design and experimental implementation may produce significant differences in results. Furthermore, differences in data analytic approaches (univariate versus multivariate analyses, whole brain versus ROI-based analyses, etc.) may lead to difficulties in clearly identifying drug treatment effects and will affect the consistency of results. Implementation of fMRI procedures in multiple sites may be challenging with different magnets that have subtle but nonetheless important potential performance differences. Even for multi-site structural imaging studies, considerable work has to be done to ensure quality control and contrast similarities between different machines and investigators. Finally, obvious differences in task design and subject groups, similar to non-imaging behavioral pharmacology, will have significant effects on nicotinic drug studies. As the literature on nicotinic effects on brain activity is relatively small, the effects of age, sex, smoking status, and psychiatric disorder are only just beginning to be worked out.
As fMRI applies to nicotinic agents, the complexities of smoking status, nicotine agonist/antagonist exposure, test design, and experimental variables makes simple or sweeping statements about nicotinic effects on cognitive task-related cortical activity impossible. Nonetheless, the available data suggest that nicotinic stimulation appears to have several broad effects which may differ depending on whether the subjects used in the studies were smokers, deprived smokers, or non-smokers and whether the areas examined are task-related or related to resting state activity. Generally speaking, nicotine appears to increase task-related activity in deprived smokers, but not active smokers. Nicotine also appears to increase task-related activity in non-smokers. By contrast, nicotine or nicotinic stimulation decreases the activity of structures associated with the default mode network.
5.2 Approaches to Detect Individual Drug Responses in Patients
As mentioned above, many drugs show differential effects depending on baseline performance. fMRI studies investigating patterns of task-related or spontaneous neural activity prior to drug administration may bear important information to classify subjects into potential responders or non-responders [31, 72]. Multivariate fMRI data analyses that take the spatial pattern of neural activity into account may be especially promising for further classification of individuals [73]. Further, information obtained from structural MRI scans may help with decisions regarding appropriate drug treatment. There is, for example, preliminary evidence in a small number of patients with hemispatial neglect, that only those patients with an intact temporoparietal cortex benefit from nicotine[74]. Except in smokers [19, 20], there has been relatively little investigation on the effects of nicotine or other nicotinic agents on brain structural measures such as white matter integrity, grey matter density, or volumetric imaging.
Another potentially promising line of research are pharmacogenetic fMRI studies performed with nicotinic drugs. For example a study of the dopaminergic system compellingly demonstrates an interaction of COMT genotype and neural and behavioral responses to amphetamine [75]. Tregallas and colleagues [32] have demonstrated a connection between changes in default mode network activity following the α7 nicotinic agonist DMXB-A and a polymorphism in CHRNA7, the α7- nicotinic acetylcholine receptor subunit gene, that was previously found to be associated with schizophrenia. As genes for nicotinic receptors and genes for related compounds that interact with those receptors have been linked to both diseases and normal development [76, 77], it is likely that future research may continue to exploit the relationship between disease genetics and nicotinic drug responses.
Most of the studies mentioned above used univariate data analysis methods which assess drug induced changes in neural activity in each and every voxel on its own. Multivariate data analysis approaches which take the spatial pattern of neural activity of many voxels into account are more sensitive than univariate approaches [78]. These approaches may not only be used to classify individuals but also to reveal differences in neural profile of different drugs. A recent study which used a multivariate pattern classification analysis has thus shown differential neural actions of amphetamine and atomoxetine which, though having different neurochemical mechanisms, produce similar clinical effects in ADHD [79]. Future studies may try to use these multivariate techniques to reveal subtle differences between different nicotinic receptor agonists in terms of their effects on cognitive operations.
Recently there is growing interest in analyzing BOLD activity while subjects are at rest. Such approaches gauge spontaneous neural activity in the low frequency range and bear several advantages for studying brain networks in patients [80]. Since drugs do not only affect task-related neural responses but also underlying spontaneous activity, resting state analyses are also of high interest for pharmacological fMRI studies. There have been a few studies on the effects of nicotine on resting state connectivity. Tanabe and colleagues [30] have shown that nicotine reduced neural activity in one resting state network, the so-called default mode network, and increased activity in the extra-striate resting state network in non smokers. Improvements in nicotine withdrawal symptoms in abstinent smokers were shown to be associated with an increase in inverse coupling between this network and a usually anti-correlated network, the executive network [81]. Others have focused their analyses of acute effects of nicotine on cingulate cortex connectivity and shown enhanced functional resting state connectivity of the cingulate cortex with several fronto-parietal brain areas [82]. In contrast, the severity of nicotine addiction was related to the strength of anterior cingulate-striatal resting state functional connectivity, a circuit that may be at least partly genetically modulated [82, 83].
6. Summary
The maturation of techniques in functional brain imaging and the widespread adoption of standardized methods for fMRI offer an opportunity for a much clearer understanding of the role of nicotinic receptor systems in cognitive and emotional functioning as well as the exploration of the effects of nicotinic agents on cognitive and emotional processes. While the range of cognitive tasks that have been explored with nicotinic agents is relatively small, being restricted mostly to attentional and working memory tasks, and to a lesser extent episodic memory and emotional control or inhibition tasks, the available data suggest that nicotinic stimulation tends to deactivate midline cortical structures involved in resting state activity such as the default mode network and mostly activate task-related structures. This deactivationactivation pattern appears to be in the service of reorienting cortical systems towards external versus internal processing and/or preparing cortical systems to receive external input. Some of the previously noted effects such as the deactivation of default mode network structures may serve as a biomarker for the effects of nicotinic agents, particularly if such activities correlate with cognitive or behavioral performance. These particular patterns of activation and/or deactivation may thus be useful for early drug development and may be an efficient and cost-effective method of screening potential nicotinic agents. Further studies will have to be done to clarify whether such activity changes correlate with cognitive or affective outcomes that are clinically relevant. Correlations between cortical activity changes seen with nicotinic agents and genetic markers are only in its infancy although certain positive findings suggest that this may be a fruitful area for pharmacogenomics and so-called imaging genomics.
There is already been significant functional brain imaging work examining the relationship between smoking-related behaviors, craving for cigarettes, and regional brain activity. While mostly relevant to cigarette smoking and potential addiction related behaviors, such approaches may be useful to help more clearly understand the roles of nicotinic systems on emotional and addictive processes.
The effects of nicotinic agents on structural brain imaging remains an area for further development. Potential areas of focus include chronic effects of nicotine or nicotinic agents on measures of structural integrity such as gray matter density using voxelbased morphometry, white matter integrity utilizing diffusion tensor imaging, and blood flow using arterial spin labeling. Correlation and/or direct comparison of functional imaging data with the extensive literature on nicotinic effects on electrophysiologic measures of brain function such as EEG and ERP will need to be accomplished as well as correlations with receptor-based imaging utilizing positron emission tomography (PET).
Acknowledgments
The preparation of this work was supported by R01 021476 (PN), K01 AG030380 (JD), K23 MH079216 (AP), DFG TH766/6-1 (CT), NCRR- 00109 and DoE SC 0001753. The authors would like to thank Amanda Kutz and Ashley Pfaff for assistance with the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–69. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin ED, Bradley AD, Addy N, Sigurani N. Hippocampal a7 and a4b2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–65. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- 3.Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Current Opinion in Pharmacology. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Rusted JM, Sawyer R, Jones C, Trawley SL, Marchant NL. Positive effects of nicotine on cognition: the deployment of attention for prospective memory. Psychopharmacology (Berl) 2009;202:93–102. doi: 10.1007/s00213-008-1320-7. [DOI] [PubMed] [Google Scholar]
- 5.Stolerman IP, Naylor CG, Mesdaghinia A, Morris HV. The duration of nicotineinduced attentional enhancement in the five-choice serial reaction time task: lack of long-lasting cognitive improvement. Behav Pharmacol. 2009;20:742–54. doi: 10.1097/FBP.0b013e328333b290. [DOI] [PubMed] [Google Scholar]
- 6.Hasselmo ME, Sarter M. Modes and Models of Forebrain Cholinergic Neuromodulation of Cognition. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–39. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis NK. The neural basis of the blood–oxygen–level–dependent functional magnetic resonance imaging signal. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2002;357:1003–37. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–48. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- 10.Vossel S, Thiel CM, Fink GR. Behavioral and Neural Effects of Nicotine on Visuospatial Attentional Reorienting in Non-Smoking Subjects. Neuropsychopharmacology. 2007;33:731–8. doi: 10.1038/sj.npp.1301469. [DOI] [PubMed] [Google Scholar]
- 11.Thiel CM, Fink GR. Effects of the cholinergic agonist nicotine on reorienting of visual spatial attention and top-down attentional control. Neuroscience. 2008;152:381–90. doi: 10.1016/j.neuroscience.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 12.Giessing C, Thiel CM, Rosler F, Fink GR. The modulatory effects of nicotine on parietal cortex activity in a cued target detection task depend on cue reliability. Neuroscience. 2006;137:853–64. doi: 10.1016/j.neuroscience.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Hahn B, Ross TJ, Wolkenberg FA, Shakleya DM, Huestis MA, Stein EA. Performance effects of nicotine during selective attention, divided attention, and simple stimulus detection: an fMRI study. Cereb Cortex. 2009;19:1990–2000. doi: 10.1093/cercor/bhn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins KA. Baseline-dependency of nicotine effects: a review. Behavioural Pharmacology. 1999;10:597–615. doi: 10.1097/00008877-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer'd disease and health. Brain. 2008;131:409–24. doi: 10.1093/brain/awm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warbrick T, Mobascher A, Brinkmeyer J, Musso F, Stoecker T, Shah N, et al. Direction and magnitude of nicotine effects on the fMRI BOLD response are related to nicotine effects on behavioral performance. Psychopharmacology. 2011:1–12. doi: 10.1007/s00213-010-2145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, et al. Effect of Smoking History on [3H]Nicotine Binding in Human Postmortem Brain. Journal of Pharmacology and Experimental Therapeutics. 1997;282:7–13. [PubMed] [Google Scholar]
- 18.Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, et al. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F-FA-85380. J Nucl Med. 2008;49:1628–35. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn S, Schubert F, Gallinat J. Reduced thickness of medial orbitofrontal cortex in smokers. Biol Psychiatry. 2010;68:1061–5. doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–8. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, et al. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology. 1998;136:179–89. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- 22.Hong LE, Schroeder M, Ross TJ, Buchholz B, Salmeron BJ, Wonodi I, et al. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophr Bull. 2011;37:416–25. doi: 10.1093/schbul/sbp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newhouse PA, Potter A, Corwin J, Lenox R. Age-related effects of the nicotinic antagonist mecamylamine on cognition and behavior. Neuropsychopharmacology. 1994;10:93–107. doi: 10.1038/npp.1994.11. [DOI] [PubMed] [Google Scholar]
- 24.Newhouse PA, Potter A, Corwin J, Lenox R. Acute nicotinic blockade produces cognitive impairment in normal humans. Psychopharmacology. 1992;108:480–4. doi: 10.1007/BF02247425. [DOI] [PubMed] [Google Scholar]
- 25.Newhouse PA, Potter A, Lenox R. The effects of nicotinic agents on human cognition: Possible therapeutic applications in Alzheimer's and Parkinson's diseases. Medicinal Chemistry Research. 1993;2:628–42. [Google Scholar]
- 26.Thienel R, Voss B, Kellerman T, Reske M, Halfter S, Sheldrick AJ, et al. Nicotinic antagonist effects on functional attention networks. International Journal of Psychopharmacology. 2009;12:1295–305. doi: 10.1017/S1461145709990551. [DOI] [PubMed] [Google Scholar]
- 27.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine Enhances Visuospatial Attention by Deactivating Areas of the Resting Brain Default Network. 2007. pp. 3477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ettinger U, Williams SC, Patel D, Michel TM, Nwaigwe A, Caceres A, et al. Effects of acute nicotine on brain function in healthy smokers and nonsmokers: estimation of inter-individual response heterogeneity. Neuroimage. 2009;45:549–61. doi: 10.1016/j.neuroimage.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe J, Nyberg E, Martin L, Martin J, Cordes D, Kronberg E, et al. Nicotine effects on default mode network during resting state. Psychopharmacology. 2011:1–9. doi: 10.1007/s00213-011-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giessing C, Fink GR, Rösler F, Thiel CM. fMRI Data Predict Individual Differences of Behavioral Effects of Nicotine: A Partial Least Square Analysis. Journal of Cognitive Neuroscience. 2007;19:658–70. doi: 10.1162/jocn.2007.19.4.658. [DOI] [PubMed] [Google Scholar]
- 32.Tregellas JR, Tanabe J, Rojas DC, Shatti S, Olincy A, Johnson L, et al. Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biological Psychiatry. 2011;69:7–11. doi: 10.1016/j.biopsych.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozzali M, MacPherson SE, Dolan RJ, Shallice T. Left prefrontal cortex control of novel occurrences during recollection: a psychopharmacological study using scopolamine and event-related fMRI. Neuroimage. 2006;33:286–95. doi: 10.1016/j.neuroimage.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Kukolja J, Thiel CM, Fink GR. Cholinergic stimulation enhances neural activity associated with encoding but reduces neural activity associated with retrieval in humans. J Neurosci. 2009;29:8119–28. doi: 10.1523/JNEUROSCI.0203-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, et al. Effect of nicotine on brain activation during performance of a working memory task. Proceedings of the National Academy of Sciences. 2001;98:4728–33. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumari V, Gray JA, Ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, et al. Cognitive effects of nicotine in humans: an fMRI study. NeuroImage. 2003;19:1002–13. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- 37.Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, et al. Effects of the [alpha]4[beta]2 Partial Agonist Varenicline on Brain Activity and Working Memory in Abstinent Smokers. Biological Psychiatry. 67:715–21. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Xu K, Ernst M, Goldman D. Imaging Genomics Applied to Anxiety, Stress Response, and Resiliency. Neuroinformatics. 2006;4:51–64. doi: 10.1385/NI:4:1:51. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland M, Ross T, Shakleya D, Huestis M, Stein E. Chronic smoking, but not acute nicotine administration, modulates neural correlates of working memory. Psychopharmacology. 2011;213:29–42. doi: 10.1007/s00213-010-2013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumas JA, Saykin AJ, McDonald BC, McAllister TW, Hynes ML, Newhouse PA. Nicotinic versus muscarinic blockade alters verbal working memoryrelated brain activity in older women. American Journal of Geriatric Psychiatry. 2008;16:272–82. doi: 10.1097/JGP.0b013e3181602a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumas JA, McDonald BC, Saykin AJ, McAllister TW, Hynes ML, West JD, et al. Cholinergic modulation of hippocampal activity during episodic memory encoding in postmenopausal women: a pilot study. Menopause. 2010;17:852–9. doi: 10.1097/gme.0b013e3181e04db9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potter AS, Newhouse PA, Bucci DJ. Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behavioral Brain Research. 2006;175:201–11. doi: 10.1016/j.bbr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Potter AS, Newhouse PA, Bucci DJ. Central nicotine cholinergic systems: A role in the cognitive dysfunction in Attention-Deficit/Hyperactivity Disorder? Behavioural Brain Research. 2006;175:201–11. doi: 10.1016/j.bbr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav. 2008;88:407–17. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2004;176:182–94. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- 46.Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–4. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–83. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122 ( Pt 5):981–91. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- 49.Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 50.Berkman ET, Falk EB, Lieberman MD. In the Trenches of Real-World Self- Control: Neural Correlates of Breaking the Link Between Craving and Smoking. Psychol Sci. 2011 doi: 10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Audoin B, Reuter F, Duong MVA, Malikova I, Confort-Gouny S, Cherif AA, et al. Efficiency of cognitive control recruitment in the very early stage of multiple sclerosis: a one-year fMRI follow-up study. Multiple Sclerosis. 2008;14:786–92. doi: 10.1177/1352458508089360. [DOI] [PubMed] [Google Scholar]
- 52.Parry AM, Scott RB, Palace J, Smith S, Matthews PM. Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain. 2003;126:2750–60. doi: 10.1093/brain/awg284. [DOI] [PubMed] [Google Scholar]
- 53.Cader S, Palace J, Matthews PM. Cholinergic agonism alters cognitive processing and enhances brain functional connectivity in patients with multiple sclerosis. J Psychopharmacol. 2009;23:686–96. doi: 10.1177/0269881108093271. [DOI] [PubMed] [Google Scholar]
- 54.Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol. 2000;47:391–5. [PubMed] [Google Scholar]
- 55.Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006;67:960–7. doi: 10.1212/01.wnl.0000237551.26858.39. [DOI] [PubMed] [Google Scholar]
- 56.Olincy A, Johnson LL, Ross RG. Differential effects of cigarette smoking on performance of a smooth pursuit and a saccadic eye movement task in schizophrenia. Psychiatry Res. 2003;117:223–36. doi: 10.1016/s0165-1781(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 57.Avila MT, Sherr JD, Hong E, Myers CS, Thaker GK. Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology. 2003;28:2184–91. doi: 10.1038/sj.npp.1300265. [DOI] [PubMed] [Google Scholar]
- 58.Tregellas JR, Tanabe JL, Martin LF, Freedman R. FMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. The American journal of psychiatry. 2005;162:391–3. doi: 10.1176/appi.ajp.162.2.391. [DOI] [PubMed] [Google Scholar]
- 59.Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. The American journal of psychiatry. 2004;161:315–21. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- 60.Tregellas JR, Olincy A, Johnson L, Tanabe J, Shatti S, Martin LF, et al. Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:938–42. doi: 10.1038/npp.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Effects of attention and emotion on repetition priming and their modulation by cholinergic enhancement. J Neurophysiol. 2003;90:1171–81. doi: 10.1152/jn.00776.2002. [DOI] [PubMed] [Google Scholar]
- 62.Kobiella A, Ulshofer DE, Vollmert C, Vollstadt-Klein S, Buhler M, Esslinger C, et al. Nicotine increases neural response to unpleasant stimuli and anxiety in non-smokers. Addiction biology. 2011;16:285–95. doi: 10.1111/j.1369-1600.2010.00237.x. [DOI] [PubMed] [Google Scholar]
- 63.George TPMDF, Sacco KAP, Vessicchio JCL, Weinberger AHP, Shytle RDP. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitorrefractory major depressive disorder: a preliminary study. Journal of Clinical Psychopharmacology June. 2008;28:340–4. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- 64.Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotine acetylcholine receptors as targets for antidepressants. Molecular Psychiatry. 2002;7:525–35. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 65.Lippiello PM, Beaver JS, Gatto GJ, James JW, Jordan KG, Traina VM, et al. TC-5214 (S-(+)-Mecamylamine): A Neuronal Nicotinic Receptor Modulator with Antidepressant Activity. CNS Neuroscience & Therapeutics. 2008;14:266–77. doi: 10.1111/j.1755-5949.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindsley CW. (S)-(+)-Mecamylamine (TC-5214): A Neuronal Nicotinic Receptor Modulator Enters Phase III Trials as an Adjunct Treatment for Major Depressive Disorder (MDD) ACS Chemical Neuroscience. 2010;1:530–1. doi: 10.1021/cn100070s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–41. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 68.Armony JL, Dolan RJ. Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia. 2002;40:817–26. doi: 10.1016/s0028-3932(01)00178-6. [DOI] [PubMed] [Google Scholar]
- 69.Perlstein WM, Elbert T, Stenger VA. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1736–41. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. NeuroImage. 2003;20:58–70. doi: 10.1016/s1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- 71.Dumas JA, Newhouse PA. The cholinergic hypothesis of cognitive aging revisited again: Cholinergic functional compensation. Pharmacology, Biochemistry & Behavior. 2011 doi: 10.1016/j.pbb.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Honey GD, Corlett PR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK, et al. Individual differences in psychotic effects of ketamine are predicted by brain function measured under placebo. J Neurosci. 2008;28:6295–303. doi: 10.1523/JNEUROSCI.0910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu CH, Mourao-Miranda J, Costafreda SG, Khanna A, Marquand AF, Williams SC, et al. Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biol Psychiatry. 2008;63:656–62. doi: 10.1016/j.biopsych.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 74.Vossel S, Kukolja J, Thimm M, Thiel C, Fink G. The effect of nicotine on visuospatial attention in chronic spatial neglect depends upon lesion location. J Psychopharmacol. 2010;24:1357–65. doi: 10.1177/0269881109105397. [DOI] [PubMed] [Google Scholar]
- 75.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Todd RD, Lobos EA, Sun LW, Neuman RJ. Mutational Analysis of the nicotinic acetylcholine receptor alpha-4 subunit gene in attention deficit/hyperactivity disorder:evidence for association of an intronic polymorphism with attention problems. Molecular Psychiatry. 2003;8:103–8. doi: 10.1038/sj.mp.4001257. [DOI] [PubMed] [Google Scholar]
- 77.Hruska M, Keefe J, Wert D, Tekinay AB, Hulce JJ, Ibañez-Tallon I, et al. Prostate Stem Cell Antigen Is an Endogenous lynx1-Like Prototoxin That Antagonizes α7-Containing Nicotinic Receptors and Prevents Programmed Cell Death of Parasympathetic Neurons. The Journal of Neuroscience. 2009;29:14847–54. doi: 10.1523/JNEUROSCI.2271-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haynes J-D, Rees G. Decoding mental states from brain activity in humans. Nat Rev Neurosci. 2006;7:523–34. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- 79.Marquand AF, De Simoni S, O'Daly OG, Williams SC, Mourao-Miranda J, Mehta MA. Pattern Classification of Working Memory Networks Reveals Differential Effects of Methylphenidate, Atomoxetine, and Placebo in Healthy Volunteers. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 81.Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–9. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- 82.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, et al. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–41. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107:13509–14. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]