Abstract
Although exposure to ionizing radiation (IR) can produce significant neurotoxicity, the mechanisms mediating this toxicity remain to be determined. Previous studies using neurons isolated from the central nervous system show that IR produces reactive oxygen species and oxidative DNA damage in those cells. Because the base excision DNA repair pathway repairs single-base modifications caused by ROS, we asked whether manipulating this pathway by altering APE1 expression would affect radiation-induced neurotoxicity. In cultures of adult hippocampal and sensory neurons, IR produces DNA damage as measured by phosphorylation of histone H2A.X and results in dose-dependent cell death. In isolated sensory neurons, we demonstrate for the first time that radiation decreases the capsaicin-evoked release of the neuropeptide CGRP. Reducing APE1 expression in cultured cells augments IR-induced neurotoxicity, whereas overexpressing APE1 is neuroprotective. Using lentiviral constructs with a neuronal specific promoter that selectively expresses APE1’s different functions in neurons, we show that selective expression of the DNA repair competent (redox inactive) APE1 constructs in sensory neurons resurrects cell survival and neuronal function, whereas use of DNA-repair deficient (redox active) constructs is not protective. Use of an APE1 redox-specific inhibitor, APX3330, also facilitates neuronal protection against IR-induced toxicity. These results demonstrate for the first time that the repair function of APE1 is required to protect both hippocampal and DRG neuronal cultures—specifically neuronal cells—from IR-induced damage, while the redox activity of APE1 does not appear to be involved.
Keywords: Neurotoxicity, Ref-1, radioprotection, DNA repair, peripheral neuropathy, chemobrain
1. Introduction
Ionizing radiation (IR) could affect the central nervous system (CNS) secondary to actions on non-neuronal targets such as disruption of the blood-brain barrier [1] or could have direct cytotoxic consequences to neurons [2, 3] and/or neurogenesis [4, 5]. Ionizing radiation (IR) can produce significant neurotoxicity, especially upon direct exposure to central nervous system tissues [6, 7]. Toxicities can range from fatigue to cognitive dysfunction [6, 8]. With high exposure, cell loss can occur in the brain and spinal cord [7].
Although many IR neurotoxicity studies focus on the CNS, the question remains whether radiation also causes significant toxicity to peripheral neurons (autonomic, motor, or sensory neurons). In the GI tract and bladder, IR alters levels of substance P and calcitonin gene-related peptide (CGRP), two neuropeptides found in small-diameter sensory neurons [9–11]. These peptides modulate intestinal injury after radiation: CGRP is protective, while SP contributes to the pathophysiology [12] in the gut, worsening early-onset radiation-induced toxicity [12]. Thus, radiation might directly affect sensory neurons, exacerbating radiation-induced injury in various organs.
The mechanisms by which IR produces neurotoxicity are undetermined. Using cultured neurons isolated from the central nervous system, several investigators have shown that IR exposure causes a dose-dependent increase in the production of reactive oxygen species (ROS) [13], increase in in DNA damage [13, 14], and apoptosis [13, 15]. Thus, it is plausible that, in situ, radiation-induced oxidative damage to DNA can subsequently alter neuronal function and cause cell death.
The base excision repair (BER) pathway is the main vehicle for repairing oxidative damage to DNA. It also has a prominent role in nondividing tissues [16–19]. A key component of BER, human apurinic/apyrimidinic (AP) endonuclease (APE1), is required for repair of single-base modifications, such as those caused by reactive ROS [16–19], including IR-induced ROS [20–22]. APE1 also functions as a major reduction-oxidation (redox) factor, which influences transcriptional regulation of gene expression [19, 20].
Because IR increases ROS and DNA damage in neurons [16, 17, 22], we asked whether modulating APE1’s expression could alter IR-induced neurotoxicity. As endpoints, we measured cell death, DNA damage, and IR’s ability to decrease the stimulated release of the neurotransmitter CGRP from sensory neurons. We demonstrated that APE1 is involved in protecting dorsal root ganglion (DRG) cells from IR’s killing effects and its ability to cause neuronal dysfunction. Using mutated APE1 proteins, we showed that the neuroprotective effect is mediated by APE1’s repair function. Additionally, a small-molecule inhibitor that blocks APE1’s redox function also enhances the molecule’s neuroprotective ability, but only in the presence of native APE1 and not with overexpression of an APE1 mutant with only redox activity. This latter finding suggests that blocking APE1 redox function can augment repair. Furthermore, these findings have important translational implications: using a small molecule to block IR’s deleterious effects during IR therapy would heighten the quality of life of cancer survivors [6, 23, 24].
2. Materials and Methods
2.1 Materials
Tissue culture supplies were obtained from Invitrogen (Carlsbad, CA). Normocin came from InvivoGen (San Diego, CA). Nerve growth factor was purchased from Harlan Bioproducts for Science, Inc. (Indianapolis, IN). Poly-D-lysine, laminin, peripherin monoclonal antibodies, and routine chemicals came from Sigma-Aldrich Inc. (St. Louis, MO). Optiprep was obtained from NYCOMED PHARMA AS (Oslo, Norway). Neuroporter® (transfecting agent) was purchased from Gene Therapy Systems (San Diego, CA). Mouse monoclonal antihuman APE1 antibodies came from Novus Biologicals (Littleton, CO); anti-phospho-H2A.X antibodies were from Upstate Cell Signaling Solutions (Charlottesville, VA). Goat anti-mouse HRP conjugated IgG secondary antibody was from Zymed Laboratories Inc. (San Francisco, CA); actin antibodies were purchased from Thermo (Fremont CA). HA rat monoclonal antibodies were from Roche Applied Science (Mannhiem, Germany). Cy3-conjugated donkey anti-mouse IgG antibody, biotin-conjugated donkey anti-rabbit IgG, and Cy3-conjugated streptavidin were from Jackson ImmunoResearch (West Grove, PA). APX3330 was synthesized as previously described [25, 26]. The Animal Care and Use Committee at Indiana University School of Medicine, Indianapolis, IN, approved all procedures used in these studies.
2.2 Cell Cultures
Neuronal cultures were prepared from adult male (150–175 g) Sprague-Dawley rats (Harlan, Indianapolis, IN) as previously described [27, 28]. Briefly, hippocampal cells were dissociated using papain and mechanical agitation and separated by gradient centrifugation. The pellet of cells from a discontinuous gradient of Optiprep in L-15 media was washed then resuspended in 1ml of growth media consisting of Neurobasal Medium supplemented with 0.5 mM L-glutamine, 2% B-27 Supplement minus AO, 50 mg/ml Penicillin-Streptomycin, and 5 ng/ml of Basic Fibroblast Growth Factor (BFGF). Approximately 60,000 cells were plated onto poly-D-lysine and laminin coated plates and grown for 6–14 days in 5% CO2 at 37°C. Growth medium was changed every other day. For sensory neuronal culture, cells were dissociated using collagenase and mechanical dissociation. Approximately 30,000 DRG cells were plated into each well of 12- well culture plates or onto Lab-Tek chamber slides all precoated with poly-D-lysine and laminin. The sensory neurons were maintained in F-12 media supplemented with 10% horse serum, 2 mM glutamine, 100μg/ml normocin, 50 μg/ml penicillin, 50 μg/ml streptomycin, 50 μM 5-fluoro-2′-deoxyuridine, 150 μM uridine, and 30 ng/ml NGF in 3% CO2 at 37°C. Growth medium was changed every other day, and the cells were used after 11–13 days of culture.
2.3 Assay Methods
Sensory neuronal cultures grown on Lab-Tek microscope chamber slides were processed for immunofluorescence as previously published using a peripherin antibody (1:500) and HA antibody (1:500) [29]. Images were collected in red (peripherin), green (HA), and bright-field modes. A Zeiss LSM offline browser (R4.0: Carl Zeiss Inc.; Thornwood, NY) was used to determine co-localization of peripherin and HA. For trypan blue exculsion, equal volumes of 0.4% (w/v) Trypan blue to cell suspension were combined, mixed and scored under a phase contrast microscope. Dead cells were those that took up the trypan blue and stain blue, whereas the live cells had yellow nuclei.
For release studies, the sensory neuronal cultures were washed once with HEPES buffer consisting of (in mM) 25 HEPES, 135 NaCl, 3.5 KCl, 2.5 CaCl2, 1 MgCl2, 3.3 D-glucose, and 0.1% bovine serum albumin, pH 7.4 and maintained at 37°C. They were then incubated for successive 10 min intervals with 0.4 ml of HEPES buffer alone (to measure resting release), with buffer containing 30 nM capsaicin (to measure stimulated release), then with buffer alone (to measure recovery). After each incubation, the buffer was removed and the amount of CGRP in each sample was measured using radioimmunoassay as previously described [30]. After the release experiment, the cells in each well were scraped and sonicated in 0.4 M HCl and an aliquot taken to measure total CGRP content in the cultures using radioimmunoassay. Total content was calculated by adding the total amount released in all incubations to the amount measured in the cells. The release data was calculated as fmol released/well/10 min or as a % of the total peptide content in the cells [30]. Western blot analysis was performed as described previously [17, 28].
Neurons were transfected with siRNA to APE1 (APE1siRNA), or scramble siRNA (SCsiRNA) and were used as previously described [28].
2.4 Development of Viral Constructs
Adenoviral constructs containing 1) the CMV promoter, HA-tagged APE1, IRES, and enhanced green fluorescent protein (EGFP); or 2) CMV, IRES, and EGFP were developed as previously described [28]. DNA sequencing confirmed the constructs in the pLenti6-R4R2-V5 plasmid containing α CaM kinase II promoter (WT-, C65-, or 226+177-) APE1-IRES-EGFP. For adenoviral infection, adult neuronal cells were cultured as described in the absence or presence of siRNA treatment for 7 days, then exposed to adenoviruses (Ad5-IRES-eGFP and Ad5-HA-APE1-IRES-eGFP) at 30 pfu/cell for hippocampal cultures and 150 pfu/cell for sensory neuronal cultures. After 2 days, the virus was removed then cells were grown in normal media. For lentiviral infections, DRG cells were cultured 5 days before 150 pfu/cell of the lentivirus was added to the media. Two days later, the virus was removed; then cells were grown an additional 5 days in regular media. We previously demonstrated that APE1’s repair function is neuroprotective against oxidative DNA damage in hippocampal and sensory neuronal cultures [28]. In those studies and in the current work, we selectively reduced APE1 expression in the neuronal cultures with with siRNA to rat APE! mRNA and added back human APE1 transgenes that are not affected by the rat siRNA since the human APE1 homolog has a different nucleic acid sequence at the binding site [28].
2.5 Ionizing Radiation Treatment
Cell cultures were irradiated using a Gammacell 40 Exactor Irradiator (Nordion International Inc). On the first day that the cells were exposed to IR, the media was changed and the culture plates transported to the irradiator at room temperature. Exposure times vary with dosing since the machine delivers 10 Gy/~16 min. In all experiments, control cultures were transported to the irradiator and kept in the room for the same time but not exposed to IR.
2.6 Data Analysis
Data were expressed as the mean ± the standard error of the mean (SEM) for at least 3 independent experiments from separate harvests. Statistical significance between groups (p < 0.05) was determined using ANOVA followed by the Tukey post-hoc test.
3. Results
3.1 Reducing APE1 expression augments radiation-induced neuronal cell death
We first confirmed findings of previous studies showing that IR produces oxidative DNA damage and apoptosis in neurons [31]. When we exposed neuronal cultures to increasing doses of radiation and examined viability 24 h later using trypan blue exclusion, we observed a dose-dependent cytotoxicity in sensory neuronal cultures and hippocampal cultures (Figures 1A, 1B). Survival of sensory neuronal cells cultured in media alone was 97 ± 2% after a 3-Gy dose and 83 ± 2% after a 10-Gy dose, respectively. After exposure to a 60-Gy dose, only 32 ± 3% of cells survived. Exposing hippocampal cultures to a 3- or 10-Gy dose resulted in 92 ± 2 % and 69 ±1% of cells excluding trypan blue, respectively; a 30-Gy dose reduced viability to 41 ± 2%.
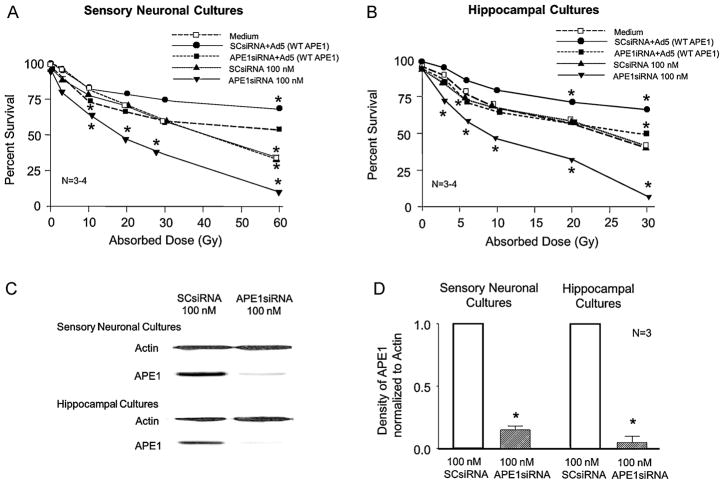
Figure 1. Reducing APE1 expression decreases cell viability in sensory neuronal and hippocampal cultures after IR; APE1 overexpression is protective.
A and B: Each point represents the mean % of cells surviving 24 h after exposure to IR as measured by trypan blue exclusion. Sensory neuronal cultures (A) or hippocampal cultures (B) were treated with medium alone (open squares), SCsiRNA (closed triangles), APE1siRNA (closed inverted triangles); then adenovirus containing the WT-APE1 construct (closed circles), or APE1siRNA then adenovirus containing the WT-APE1 construct (closed squares) as discussed in Methods. C: Representative Western blots showing APE1 and actin from cultures exposed to either SCsiRNA or APE1siRNA as indicated. D: The ordinate represents the density of APE1 bands from Western blots normalized to actin. Each column represents the mean ± SEM from 3 separate harvests of cells used in the experiments in the top figures and exposed to either SCsiRNA or APE1siRNA as indicated. An asterisk indicates a statistically significant reduction in Ape1 expression compared to values obtained on cultures exposed to SCsiRNA.
We next determined whether reducing APE1 expression in the cultures would alter IR’s toxic effects. Exposing neuronal cultures to SCsiRNA did not significantly alter the number of cells that excluded trypan blue in response to IR, compared to cells treated with medium alone (Figures 1A, 1B). In contrast, sensory and hippocampal cultures treated with APE1siRNA demonstrated enhanced cell death after IR. A 10-Gy dose of radiation significantly reduced cell viability from 83 ± 2% to 64 ± 2% in sensory neuronal cultures and from 69 ±1% to 46 ±1% in hippocampal cultures. Reduced APE1 expression resulted in survival of only 10 ± 1% of sensory neurons 24 h after exposure to a 60-Gy dose and 8 ± 2% of hippocampal cells after exposure to a 30-Gy dose. APE1 siRNA reduced hippocampal APE1 levels to 5 ± 5% of the level of control SCsiRNA; APE1 siRNA also reduced APE1 to 15 ± 3% in sensory neuronal cultures compared to cultures treated with SCsiRNA (Figures 1C and 1D). Scramble siRNA did not significantly alter APE1 expression compared to untreated cultures (data not shown).
3.2 Overexpressing APE1 in neuronal cultures protects against radiation-induced cell death
Because reducing APE1 expression in neuronal cultures enhances radiation-induced cell death, we next determined whether overexpressing APE1 could be neuroprotective. Adenoviral constructs containing the CMV promoter, WT-APE1, IRES, and EGFP were used to infect sensory and hippocampal cultures pretreated with either SCsiRNA or APE1siRNA. Cells were exposed to siRNAs on Days 3–5, to virus on Day 7 (for 48 h), to IR on Day 11, and trypan exclusion measured 24 h after radiation. An adenoviral vector containing the EGFP construct was used as a control. Western blot analysis was performed on all cultures using an HA-antibody (data not shown) [28].
Overexpressing APE1 in neuronal cultures significantly attenuated IR’s ability to cause cell death (Figures 1A and 1B). For example, when sensory neurons treated with SCsiRNA were exposed to a 60-Gy dose of radiation, only 34 ± 2% of cells were viable after 24 h, but overexpressing APE1 after radiation increased cell viability to 69 ± 4%. In radiated cells where APE1 expression was reduced with siRNA treatment, the “add-back” of APE1 via adenoviral infection increased viability from 10 ± 1% to 53 ± 4%. Analogous results were observed in hippocampal cultures: 30 Gy of radiation reduced cell viability to 43 ± 2% and 8 ± 2% in SCsiRNA and APE1siRNA treated cells, respectively. Overexpressing APE1 increased viability to 66 ± 5 % in cultures treated with SCsiRNA and to 49 ± 2% in cells treated with APE1siRNA. Therefore, APE1 overexpression is neuroprotective in cells with reduced or normal complements of APE1. In the absence of IR, reducing or overexpressing APE1 did not alter cell viability (Figures 1A, 1B). Infection with the viral vector control did not significantly alter cell viability after radiation in cells treated with either SCsiRNA or APE1siRNA (not shown).
3.3 Effect of APE1 on DNA damage induced by ionizing radiation
To assess DNA double-strand breaks after IR, we measured histone H2A.X phosphorylation [32]. Neuronal cultures in the absence or presence of APE1 manipulations were exposed to significant but not maximal doses of IR that would affect cell viability: 30 Gy for sensory cells and 15 Gy for hippocampal cells (See Figure 1). Histone H2A.X phosphorylation was measured using Western blotting 0.5–3 h after IR exposure. In these experiments, pretreating cultures with APE1siRNA for 48 h significantly reduced the APE1 level to 15 ± 8% of the control sensory neuronal cultures and 10 ± 12% of the control in hippocampal cultures; SCsiRNA did not significantly alter APE1 levels.
The representative Western blot in Figure 2A and the summary data from 3 experiments in Figure 2B illustrate that irradiating sensory neuronal cultures treated with SCsiRNA (100 nM) increased the amount of phospho-H2A.X. However, reducing APE1 expression via APE1siRNA (100 nM) significantly increased H2A.X phosphorylation. Three h after radiation, the density of the phospho-H2A.X band (normalized to actin) in cultures exposed to SCsiRNA was 2.8 ± 1.0, whereas the band in cells exposed to APE1siRNA was 12 ± 0.9.
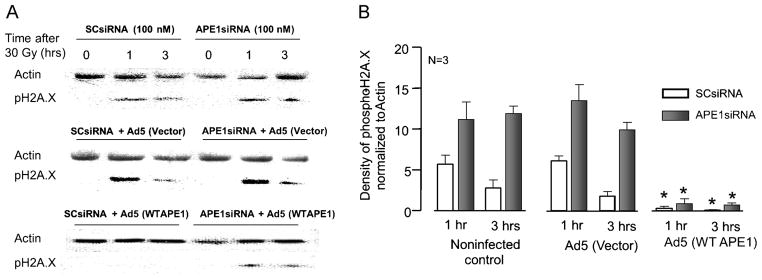
Figure 2. IR-induced H2A.X phosphorylation in cultured sensory neurons is augmented by reduced APE1 expression and blocked by APE1 overexpression.
A: Representative Western blots showing phospho-H2A.X and actin from cultures prior to 30 Gy of IR and 30 and 60 min afterward. Cultures were exposed to either SCsiRNA or APE1siRNA alone (top panel), siRNAs then the viral vector (middle panel), or siRNAs then Ad5 for WT APE1 (bottom panel). B: Densitometry of phospho-H2A.X expression normalized to actin from 3 independent experiments. Columns represent the mean ± SEM from cultures treated with SCsiRNA (open columns) or APE1siRNA (closed columns), with or without viral infection, as indicated 30 or 60 min after 30 Gy of IR.
Similar results were observed in hippocampal cultures (Figure 3). Irradiating hippocampal cultures treated with SCsiRNA significantly increased the amount of phospho-H2A.X 30 and 60 min afterward and this was further increased by APE1 knockdown (Figure 3A; summary data in Figure 3B). These results demonstrate that neuronal cultures with reduced APE1 expression have increased double-strand breaks after IR treatment, as evidenced by H2A.X phosphorylation.
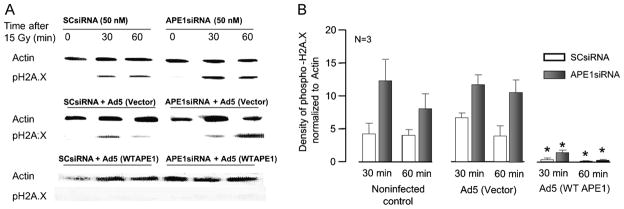
Figure 3. IR-induced H2A.X phosphorylation in cultures of hippocampal neurons is augmented by reduced APE1 expression and blocked by APE1 overexpression.
A: Representative Western blots showing phospho-H2A.X and actin from cultures prior to 15 Gy of IR and 30 and 60 min afterward. Cultures were exposed to either SCsiRNA or APE1siRNA alone (top panel), siRNAs then the viral vector (middle panel), or siRNAs then Ad5 for WT APE1 (bottom panel). B: Densitometric results of phospho-H2A.X expression, normalized to actin (3 independent experiments). Columns represent the mean ± SEM from cultures treated with SCsiRNA (open columns) or APE1siRNA (closed columns), with or without viral infection, as indicated 30 or 60 min after 15 Gy of IR.
Additional experiments were performed to overexpress APE1 in the neuronal cultures to determine if adding APE1 back into the cells provides protection against radiation-induced DNA damage. Exposure to the viral vector for 48 h two days after SCsiRNA or APE1siRNA increased H2A.X phosphorylation in a manner analogous to controls. Three h after radiation, the density of the phosphor-H2A.X band (normalized to actin) in sensory neuronal cultures exposed to SCsiRNA and adenoviral vector was 1.8 ± 0.6, whereas the band in cells exposed to APE1siRNA and vector was 10 ± 0.9 (Figure 2B, middle panel). For hippocampal cultures, 60 min after a 15-Gy dose of radiation the density of the phospho-H2A.X band (normalized to actin) in cultures exposed to SCsiRNA and adenoviral vector was 3.9 ± 1.6, whereas the band in cells exposed to APE1siRNA and vector was 11 ± 1.9.
Exposing neuronal cultures to an adenovirus containing WT APE1 dramatically attenuated the radiation-induced increase in phospho-H2A.X (Figures 2B, 3B). In sensory neuronal cultures exposed to APE1siRNA then Ad5-APE1, the density of phospho-H2A.X normalized to actin was 0.9 ± 0.6 and 0.7 ± 0.3 at 1 and 3 h after radiation, respectively. In hippocampal cultures exposed to APE1siRNA then Ad5-APE1, the density of phospho-H2A.X normalized to actin was 1.3 ± 0.4 and 0.2 ± 0.1 at 30 and 60 min after radiation, respectively. These results, taken with the cell viability data in Figure 1, support the notion that reduced APE1 expression in primary neuronal cultures augments IR cytotoxicity, while adding back the missing APE1 protein restores survival and reduces double-strand breaks, demonstrating that APE1 is intrinsically involved in neuronal cell survival.
3.4 Overexpressing APE1 using a neuronal-specific promoter protects sensory neurons against IR-induced cell death
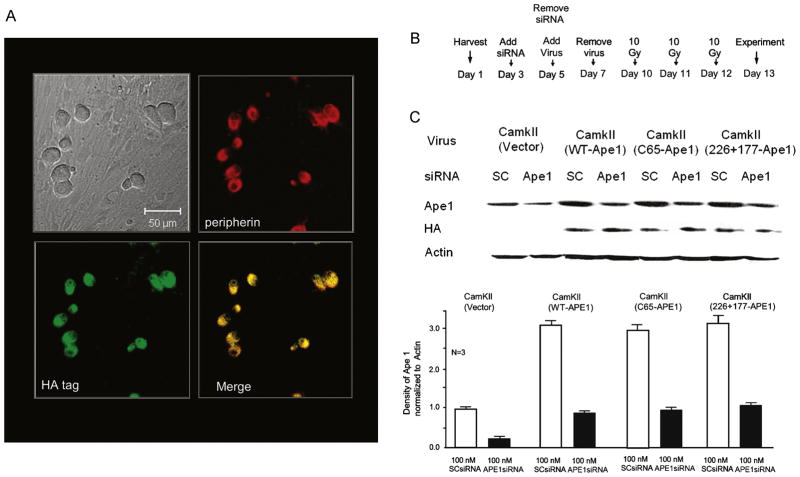
Although the aforementioned studies demonstrate that APE1 overexpression in neuronal cultures is neuroprotective, the use of adenoviral constructs with a CMV promoter does not reveal if APE1’s effects are in neurons themselves or in non-neuronal cells. To examine whether APE1 overexpression that is restricted to neurons is protective, we developed lentiviral constructs using a neuronal-specific promoter for α CaM kinase II [33]. Initially, we ascertained whether the expression of WT-APE1 would be restricted to neurons in culture using immunohistochemistry. We used double staining with neuronal markers peripherin [34] and HA-tagged APE1. Figure 4A shows that exposing sensory neurons to lentivirus for 24 h on Day 7 in culture and examining the cells at Day 12 demonstrated significant expression of HA-tagged APE1 in neurons but not in nonneuronal cells.
Figure 4. Expression of APE1 and APE1 mutants in sensory neurons using lentivirus with the α CaM kinase II promoter.
A: Representative micrographs showing that expression of HA-tagged WT APE1 is restricted to sensory neurons 4 days after exposure to 150pfu/cell of lentivirus containing the α CaM kinase II promoter, HA-tagged WT-APE1, IRES, and EGFP. Top left panel: bright-field image. Top right panel: red staining for peripherin. Bottom left panel: green staining for the HA tag. Bottom right panel: co-localization of peripherin and HA, indicated by yellow color. B: Time line for experiments using siRNAs and lentiviral infection. C: Top portion: representative Western blots demonstrating APE1 expression, HA-tagged WT APE1, and APE1 mutants in cultures used in experiments illustrated in Figures 5 and 7. Cultures were exposed to either SCsiRNA or APE1siRNA, then to lentiviral constructs as indicated. Lower portion: bar graph summarizes the effects of various treatments to decrease or increase APE1 expression. Densitometric results from 3 independent experiments, normalized to actin. Columns represent the mean ± SEM from cultures treated with SCsiRNA (open columns) or APE1siRNA (shaded columns) with viral infection as indicated.
We also examined the expression of APE1 and HA-tagged WT-APE1, C65-APE1 (redox-deficient/DNA repair-proficient) [17, 28] and 226/177-APE1(redox-proficient/repair-deficient) [17] in the cultures we used in subsequent studies. For these studies, cells were exposed to siRNAs for 48 h starting Day 3 in culture and to viral constructs for 2 days starting on Day 5 (Figure 4B). Experiments were performed on Day 13, and expression was measured at the end of the experiments.
Neurons treated with the control virus containing the α CaM kinase II promoter and EGFP demonstrated APE1 expression, but no HA-tagged protein was detected (Figure 4C). However, APE1 expression increased with the APE1 constructs as it did in the HA-tagged APE1 constructs (Figure 4C). As in previous studies, exposing cells to APE1siRNA reduced APE1 expression; but the viral infection increased APE1 levels to control levels (Figure 4C). In cells treated with SCsiRNA, viral infection increased APE1 expression approximately three-fold (Figure 4C).
We next determined whether overexpression of APE1 in sensory neurons was protective when cultures were exposed to 10 Gy of radiation daily for three days and cell viability was measured 24 h after the last dose. The cell viability of irradiated sensory neuronal cultures treated with SCsiRNA and the lentiviral vector dropped to 71 ± 6 percent (Figure 5A). The siRNA and viral treatments in the absence of radiation did not result in significant cell death. Reducing APE1 expression with siRNA to 16 ± 4 % of controls (data not shown) significantly reduced cell viability after radiation: from 97 ± 4% in cells not exposed to radiation to 59 ± 4% in cells exposed to a dose of 10 Gy each day for 3 days (Figure 5B). Overexpressing WT-APE1 in sensory neurons attenuated IR’s cytotoxic effects: cell viability in neurons treated with SCsiRNA was 90 ± 4% (Figure 5A) and 87 ± 4 % in neurons treated with APE1siRNA (Figure 5B).
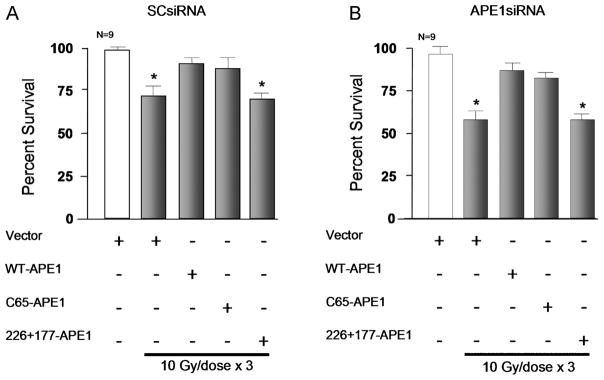
Figure 5. Overexpression of WT-APE1 and the redox mutant APE1 (C65), but not the repair mutant APE1 (226+177), attenuate radiation-induced cell death.
Columns represent the mean ± SEM of the % cells surviving 24 h after exposure to 3 doses of IR (10 Gy/dose) as measured by trypan blue exclusion. Sensory neuronal cultures were treated with SCsiRNA (A) or APE1siRNA (B) and viral constructs containing the α CaM kinase II promoter as indicated. Horizontal bars represent cells exposed to IR.
Although these data indicate that overexpressing WT-APE1 specifically in sensory neurons attenuates IR-induced cytotoxicity, the question remained whether the repair or redox function of APE1 mediates the protective effects. To determine this, we selectively overexpressed either the repair-competent APE1 mutation (C65-APE1) or the redox-competent mutation (226+177-APE1), following the protocol shown in Figure 4B. In both cases these mutations also had an HA tag to allow confirmation of over expression (see Figure 4C). Overexpressing C65-APE1 in sensory neurons mimicked the effects we observed with WT-APE1. With expression of the repair-competent mutation, viability in cultures treated with SCsiRNA after three daily doses of 10 Gy was 88 ± 6% (Figure 5A), whereas viability of cells treated with APE1siRNA was 83 ± 3% (Figure 5B). This compares favorably to 71 ± 6 and a 59 ± 4% viability of cells in cultures treated with the lentiviral vector and SCsiRNA or APE1siRNA, respectively. In neurons overexpressing 226+177-APE1, radiation reduced cell viability of cultures treated with SCsiRNA to 68 ± 5% (Figure 5A). Cultures treated with APE1siRNA demonstrated 58 ± 3% viability (Figure 5B) which was not significantly different from vector-treated cultures after exposure to IR. These results strongly support the notion that APE1’s DNA repair component—not its redox component—provides neuroprotection from IR.
3.5 Effect of APE1 on radiation-induced changes in release of iCGRP from sensory neurons
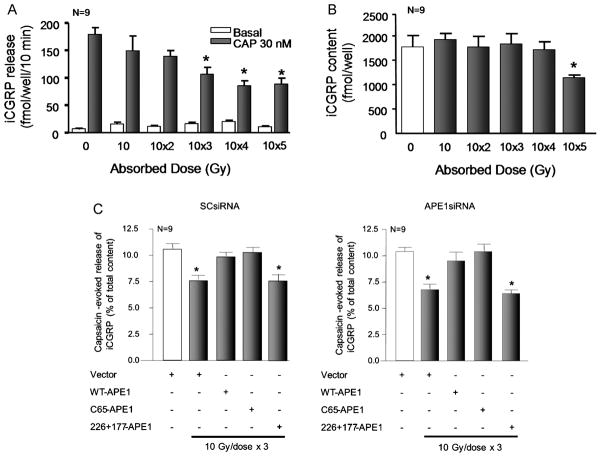
To characterize the effects of IR and APE1 on an endpoint of sensory neuronal function, we examined resting and capsaicin-evoked release of the neuropeptide CGRP. For these studies, we initially determined the effects of exposing neuronal cultures to single or multiple doses of 10 Gy of IR on the content and release of iCGRP. Figures 6A and 6B show that, 24 h after exposing sensory neurons to either one or two once-daily doses of 10 Gy there was no significant effect on resting or capsaicin-evoked iCGRP release (as measured in fmol released/well of cells/10 min) or on the total content of CGRP in the cultures. In contrast, exposing the cultures to 10 Gy/day for 3 or 4 days significantly reduced the capsaicin-evoked iCGRP release (Figure 6A). In a similar manner, the total content of iCGRP was not affected by up to 4 doses of 10 Gy given once per day (Figure 6B). Exposing cultures to 5 doses considerably reduced iCGRP content. IR had no appreciable effect on resting release of iCGRP from sensory neurons (Figure 6A). Based on these results and the fact that multiple doses of radiation are used in therapy, we used 10 GY given daily for 3 days in subsequent studies and examined release 24 h after the last dose.
Figure 6. Overexpression of WT-APE1 and the redox mutant APE1 (C65), but not the repair mutant APE1 (226+177) attenuates radiation-induced decreases in iCGRP release from sensory neurons.
A: Columns represent the mean ± SEM of iCGRP release in fmol/well/min for sensory neurons in culture, either not exposed to radiation or exposed to 10 Gy/day for 1 to 5 days. Twenty-four hours after the last IR dose, wells of cells were exposed for 10 min to HEPES alone (basal; open columns), or HEPES in the presence of 30 nM capsaicin (solid columns). B: Columns represent the mean ± SEM of iCGRP content in fmol/well for sensory neurons in culture from the release experiments in Figure A. C: Columns are the mean ± SEM of the release of iCGRP evoked by 30 nM capsaicin as the % of total iCGRP content over a 10-min period. Open columns are cultures not exposed to ionizing radiation; filled columns are cells exposed to 10 Gy/day for 3 days. Cultures were treated with SCsiRNA (left panel) or APE1siRNA (right panel) and viral constructs.
Exposing sensory neurons that were pretreated with SCsiRNA (which did not reduce APE1 expression) and the lentiviral vector to 3 doses of 10 GY resulted in a significant reduction in capsaicin-evoked release of iCGRP from 10.6 ± 0.5 % of total iCGRP content for controls to 7.7 ± 0.4 % of total iCGRP content (figure 6C). This inhibition in release by IR is analogous to that observed in untreated cells (figure 6A), indicating that SCsiRNA and control viral infection do not affect iCGRP release. When cells were pretreated with APE1siRNA which reduced the expression of APE1 by approximately 90%, IR significantly reduced capsaicin-evoked release to 6.8 ± 0.5 % of total iCGRP content (figure 6C). We next determined if increasing the expression of APE1 or APE1 mutants in sensory neurons could reverse the effects of IR on iCGRP release. As in previous experiments, cultures were first treated with siRNAs, infected with viral vectors using the neuronal specific promoter ∝CaM kinase II, then exposed to three doses of 10 Gy and release of iCGRP measured. When cells treated with SCsiRNA or APE1siRNA were infected with the virus containing WT-APE1 or the C65-APE1, the ability of IR to reduce capsaicin-evoked peptide release was blocked. For example, in cells exposed to Ape1siRNA, viral vectors containing the WT-APE1 or C65-APE1 constructs and radiation, the capsaicin-evoked release was 9.5 ± 0.8 and 10.4 ± 0.8 % of total content, respectively. In contrast, overexpressing the repair deficient/redox competent mutant of APE1, 226+177A-APE1 had no effect on the ability of radiation to reduce capsaicin-evoked release of iCGRP. In cells treated with SCsiRNA release was 7.8 ± 0.3 % of total content (Figure 6C, left panel), while in cells exposed to APE1siRNA release was 6.4 ± 0.4 % of total content (Figure 6B). Together the results support the notion that the repair component of APE1 and not the redox function reverse the functional toxicity produced by IR.
3.6 APX3330 affects IR-induced cell death and inhibition of iCGRP release
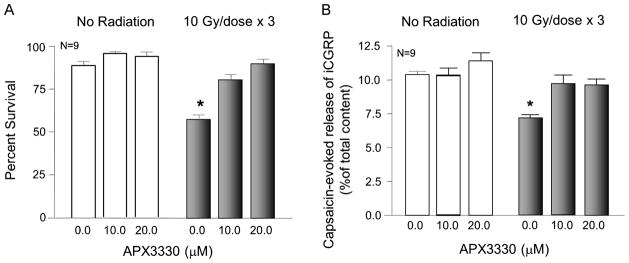
To substantiate that APE1’s redox function is not critical for protecting sensory neurons from IR-induced toxicity, we determined how the APE1 redox-specific inhibitor APX3330 [25, 26, 35] affected radiation-induced neurotoxicity. APX3330 was added to the media 3 days prior to the first IR exposure and maintained throughout the 3 days of radiation treatment (10 Gy/day). Cell viability or release studies were performed 24 h after the last dose of radiation. Consistent with earlier studies (Figure 5) when neuronal cultures were exposed to 3 doses of radiation, cell viability was significantly reduced, to 57 ± 2 % (Figure 7A). However, cultures treated with 10 μM APX3330 demonstrated cell viability of 81 ± 3%. Exposing cultures to 20 μM APX3330 effectively blocked IR’s effects. Neither concentration of APX3330 had any effect on cell viability in cultures that were not exposed to radiation.
Figure 7. APX3330 attenuates IR-induced cell death and decreases in iCGRP release from sensory neurons.
A: Columns represent the mean ± SEM of % cells surviving 24 h with no exposure to radiation (open columns) or after exposure to 3 doses of 10 Gy of IR (shaded columns) as measured by trypan blue exclusion. B: Columns show the mean ± SEM of the release of iCGRP evoked by 30 nM capsaicin as the % of total iCGRP content over a 10-min period. Open columns are cultures not exposed to IR; filled columns are cells exposed to 10 Gy/day for 3 days. Cultures were treated with APX3330 as indicated for three days before and throughout IR exposure.
Release studies also were performed 24 h after the last dose of radiation. As observed in previous experiments, 3 doses of 10 Gy significantly reduced capsaicin-evoked iCGRP release from 10.6 ± 0.3 % of total iCGRP content in the cultures to 7.1 ± 0.4 % of total content (Figure 7B). Treating sensory neurons in culture with either 10 μM or 20 μM APX3330 completely blocked the effects of radiation on peptide release but did not alter capsaicin-evoked release in control cells (Figure 7B).
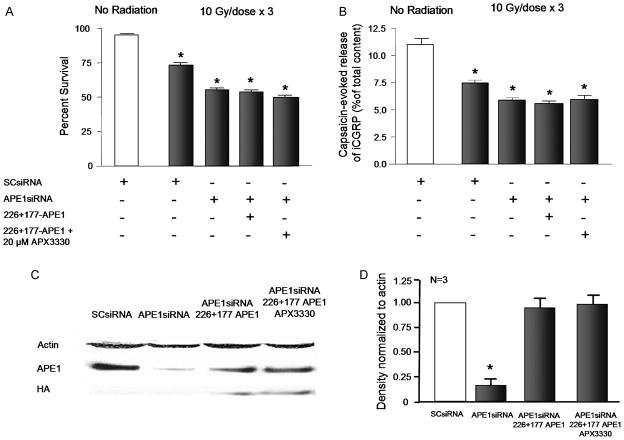
Because APX3330 reversed the neurotoxicity induced by IR, we asked whether the effect would occur through blocking the redox actions of APE1. Based on the data above using mutant APE1 constructs, it appears that the repair component of APE1 is critical for neuroprotection. Thus, we tested whether APX3330 would be protective under conditions of reduced expression of native APE1 coupled with overexpression of the repair deficient/redox competent mutant (266-177-APE1). In these experiments, exposing sensory neuronal cultures to APE1siRNA caused an 84% reduction in native APE1 expression as indicated by Western blotting (Figures 8C and 8D), whereas viral infection with the 266-177-APE1 construct results in expression of this mutant (Figures 8C and 8D). In these cultures, we treated cells with 20 μM APX3330 for 3 days prior to the first IR exposure and throughout the 3 days of radiation treatment (10 Gy/day). In contrast to the neuroprotective effects of APX3330 in sensory neurons with native APE1 (see Figure 7), the compound was not effective in cultures overexpressing the APE1 mutant 226-177. As observed in previous experiments, exposing cultures to 10 GY of radiation per day for three days resulted in 75 ± 1 % viability in control cells, 56 ± 4 % in cells with APE1 knock-down and 53 ± 3 % in cells with APE1 knock-down and overexpression of 226-177-APE1. Viability after APX3330 was 51 ± 3 % (Figure 8A). In a similar manner, IR reduced the evoked release of iCGRP from 10.7 ± 0.5 % of total peptide content to 7.5 ± 0.4 % of total content (Figure 8B). With APE1 knock-down and 266-177 overexpression release was 5.6 ± 0.2 % of total content in the absence of APX3330 and 5.9 ± 0.5 % with APX3330 treatment. As a positive control for these experiments, we examined whether APX3330 was neurorprotective to neuronal cultures exposed to SCsiRNA. In neuronal cultures treated with SCsiRNA, 20 μM APX3330 did not result in a significant change in cell viability (as measured by trypan blue exclusion) or in the capsaicin-evoked release of ICGRP (Table 1). Exposing cultures treated with SCsiRNA to 10 Gy of radiation for 3 consecutive days resulted in a significant loss in cell viability and a decrease in evoked release of iCGRP as was observed in other experiments. In these cells, the radiation-induced neurotoxicity was blocked by treating cultures with 20 μM APX3330 (Table 1) in a manner analogous to cultures not exposed to siRNA (see Figure 7). Taken together, these results show that in the presence of APE1 that has only redox activity APX3330 is not neuroprotective, whereas in cells with intact APE1, the drug attenuates radiation-induced toxicity. These data support the notion that the APE1 repair function is a necessary component in the neuroprotective actions of APX3330.
Figure 8. APX3330 does not attenuate IR-induced cell death and decreases in iCGRP release from sensory neurons expressing the redox competent APE1 mutant.
A: Columns represent the mean ± SEM of % cells surviving 24 h after no exposure to radiation (open columns) or after exposure to 3 doses of 10 Gy of IR (shaded columns). Cultures were treated with siRNA, lentivirus, and/or APX3330 as indicated. B: Columns show the mean ± SEM of the release of iCGRP evoked by 30 nM capsaicin as the % of total iCGRP content over a 10-min period. Open columns are cultures not exposed to IR; filled columns are cells exposed to 10 Gy/day for 3 days. Cultures were treated with siRNA, lentivirus, and/or APX3330 as indicated. C: A representative Western blot showing APE1 HA-tagged 266-177-APE1 and actin from cultures exposed to either SCsiRNA or APE1siRNA or virus as indicated. D: The ordinate represents the density of APE1 bands from Western blots normalized to actin. Each column represents the mean ± SEM from 3 separate harvests of cells used in the experiments in the top figures and exposed to either SCsiRNA, APE1siRNA and/or virus as indicated. An asterisk indicates a statistically significant reduction in APE1 expression compared to values obtained on cultures exposed to SCsiRNA.
Table 1.
Effect of APX3330 on ionizing radiation-induced cell death and decrease in iCGRP release in sensory neurons treated with scramble siRNA
| Treatment | n | Percent Survival | CAP Evoked Release of iCGRP (% of Total Content) |
|---|---|---|---|
| SCsiRNA (100nM) | 9 | 94 ± 2 | 10.2 ± 0.31 |
| SCsiRNA (100nM) + APX3330 (20 μM) | 9 | 97 ± 2 | 10.2 ± 0.27 |
| SCsiRNA (100nM) + 10Gy/dose × 3 | 9 | 76 ± 4* | 7.9 ± 0.28* |
| SCsiRNA (100nM) + 10Gy/dose × 3 + APX3330 (20 μM) | 9 | 93 ± 6+ | 9.6 ± 0.25+ |
Data are presented as mean ± S.E.M. An asterisk indicated a significant different (p< .05) compared to SCsiRNA without IR, whereas a + indicates a significant difference between cells in the absence or presence of APX3330 treatment
4. Discussion
The data presented here establish that APE1 is neuroprotective for IR-induced damage. Using cultures of adult hippocampal and sensory neurons, we confirm previous work showing that exposing cells to increasing doses of IR increases cell death, which correlates with DNA damage [13, 14, 16, 36]. We also demonstrate for the first time that radiation reduces capsaicin-evoked CGRP release from sensory neurons, suggesting reduced sensory neuron function. Reducing APE1 expression in hippocampal and sensory neuronal cultures using siRNA augments IR-induced cell death, double strand breaks (indicated by increased histone 2AX phosphorylation) [32], and inhibition of transmitter release. In contrast, overexpression of APE1 in cells with depleted or “normal” APE1 levels (those treated with SCsiRNA) blocks IR’s adverse effects on all three endpoints.
We initially used a CMV promoter to overexpress APE1, and those experiments demonstrated that overexpression confers neuroprotection in hippocampal and sensory neuronal cultures. The question remains whether the protective effects of APE1 are occurring in neurons or in non-neuronal cells present in primary cultures. This issue is significant because IR could directly damage neurons or cause secondary effects to support cells [2, 7, 37]. For example, IR increases cytokine production in glial cells, which influences neuronal function. To answer this question, we developed lentiviral constructs that contained the neuronal-specific promoter for α CaM kinase II and overexpressed APE1 and modified APE1 mutant proteins [33]. Use of this promoter resulted in selective APE1 expression in cultured neurons and the level of APE1 expression in cells infected with the virus was similar to that observed in non-treated cells. Of importance, overexpression of APE1 in neurons attenuated radiation-induced neurotoxicity, strongly supporting the notion that IR damage occurs directly to the neurons.
APE1’s multifunctionality has long been appreciated. It is involved in repairing oxidative damage to DNA, as can occur from IR [38]. APE1 also is a redox co-activator of many transcription factors [18–20]. The repair and redox activities of APE1 are located in two functionally distinct regions within the protein [18, 19, 39]. By selectively overexpressing one of these functions in mutated APE1 molecules, we address the question of whether APE1’s repair, redox or combined functions are critical for neuroprotection. Cys65 mutations remove the redox function of APE1 but do not affect the repair function [19, 39–41], whereas mutation of a variety of amino acids (e.g., Arg 177 and Asn 226) eliminates the repair function but leaves the redox function intact [42]. Overexpressing the redox-deficient Cys65 mutation of APE1 in sensory neurons reverses IR’s toxic effects in a manner analogous to that seen with WT-APE1. However, overexpressing the repair-deficient mutation is not neuroprotective. These results demonstrate that only APE1’s DNA repair function is essential for post-mitotic neuronal cell survival and function after exposure to oxidative damage [17, 29].
Of interest, an APE1 redox-specific inhibitor, APX3330, also protected neuronal cells from IR-induced cell death and the deleterious effects of iCGRP release inhibition. We previously had determined that APE1 can exist in an unfolded form [41]. We also demonstrated that 1) APX3330 binds to partially unfolded APE1, 2) the partially or locally unfolded form is the redox-active form [41] existing in equilibrium with the fully folded state, and 3) APX3330 can perturb this equilibrium, trapping a partially unfolded state of APE1 [41]. Thus, in using APX3330 to block APE1’s redox function, APE1’s repair function is fully expressed, leading to the protective effects shown in Figure 6. The results with APX3330 parallel those using the C65A APE1 mutant, in that blocking APE1’s redox function still allows for neuronal protection. This “trapped” APE1 could also have a reduced affinity for other proteins with which APE1 interacts—allowing its repair function to dominate [19, 43, 44]. This possibility is further supported by the fact that APX3330 is not neuroprotective in neurons expressing the redox competent APE1 mutant, but only when native APE1 is expressed.
We assessed three endpoints to determine whether reducing APE1 expression increases IR-induced neurotoxicity. Our cell viability studies show that dose-dependent cell death is greater in neuronal cultures with reduced APE1 expression 24 h after IR treatment. Overexpressing APE1 significantly reduced cell death in neuronal cultures. This confirms previous work demonstrating that in situ or in vitro exposure to radiation triggers neuronal apoptosis [13, 15] and supports the idea that the cell death is secondary to DNA damage, which is our second endpoint. We also show significant DNA damage within 1 h of radiation treatment, evidenced by substantially increased H2A.X phosphorylation. Findings for DNA damage parallel cell viability: both increase significantly in cultures with reduced APE1 expression and both were decreased significantly by APE1 overexpression.
We measured the resting and capsaicin-evoked release of the neuropeptide CGRP as our third endpoint. Increasing the number of exposures of sensory neurons to 10 Gy of radiation reduces the capsaicin-evoked release of CGRP without altering its resting release. Interestingly, the total CGRP content in the cultures is not reduced until 5 doses of 10 Gy are delivered, suggesting that the cumulative amount causes loss of CGRP-containing neurons or decreases peptide expression. The reduction in radiation-induced release is more pronounced in cultures with reduced APE1 expression and is reversed by APE1 overexpression.
A major neurological side effect of IR is loss of cognitive function; [7] this correlates with decreased hippocampal neurogenesis [4, 5]. Although numerous interventions have been proposed to minimize the cognitive side effects of IR, to date none have proven effective. Our results suggest that radiation can affect adult hippocampal neurons, and that augmenting APE1’s repair function largely reverses the toxic effects. Additionally, because APX3330’s redox inhibition of APE1 is neuroprotective for sensory neurons after IR exposure, further studies are warranted to determine if this compound can minimize IR’s effect of cognitive dysfunction. This is an intriguing novel finding because previous studies demonstrated APX3330’s cytostatic effect on cancer cells [25, 26, 45, 46].
Although peripheral neuropathy is not considered a major side effect of IR, its effects on small-diameter sensory neurons could have important clinical ramifications. Numerous studies suggest that small-diameter sensory neurons contribute to neurogenic inflammation [47, 48] and wound healing [49, 50]. Not surprisingly, studies show that toxic effects of radiation on the GI tract alter the neuropeptides SP and CGRP, which small-diameter sensory neurons synthesize and release [10, 11]. Indeed, ablation of capsaicin-sensitive small-diameter sensory neurons and CGRP receptor antagonists increases the severity of IR-induced damage to the gut [12]. Because IR decreases CGRP release in isolated sensory neurons, this effect could contribute to IR-induced tissue damage.
5. Conclusion
In conclusion, we demonstrate here that APE1’s repair function is required for protection of hippocampal and DRG neuronal cultures, while APE1’s redox activity does not appear to be involved in neuronal function or survival. Because APX3330 also demonstrates neuronal protection from IR, these studies form the foundation for additional studies in vivo to ascertain APX3330’s protective effect and the role of APE1’s repair component in reversing neurocognitive impairment and peripheral neuropathy following IR treatment.
Acknowledgments
Financial support for this work was provided by the National Institutes of Health NS048565 and NS069915 to MRV, National Cancer Institute CA121168, CA114571, and CA121168S1 to MRK, and through the NCRR Research Facilities Improvement Program Gant C06 RR015481-01to MRV. Financial Support was also provided by the Riley Children’s Foundation to MRK.
Abbreviations
- APE1
human apurinic/apyrimidinic (AP) endonuclease
- BER
base excision repair
- CGRP
calcitonin gene-related peptide
- CMV
cytomegalovirus
- CNS
central nervous system
- DRG
dorsal root ganglion
- EGFP
enhanced green fluorescent protein
- HA
hemagglutinin antibodies
- iCGRP
immunoreactive calcitonin gene-related peptide
- IR
ionizing radiation
- IRES
internal ribosome entry site
- Ref-1
redox effector factor 1
- ROS
reactive oxygen species
- SCsiRNA
scramble siRNA
- SP
(neuropeptide) substance P
- WT-APE1
wild type APE1
Footnotes
Conflict of Interest Statement
Mark Kelley declares that he is employed as a consultant at ApeX Therapeutics which has licensed IP from his work. No other authors have any potential conflicts of interest to declare.
References
- 1.Trnovec T, Kallay Z, Bezek S. Effects of ionizing radiation on the blood brain barrier permeability to pharmacologically active substances. Int J Radiat Oncol Biol Phys. 1990;19:1581–1587. doi: 10.1016/0360-3016(90)90376-u. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez MC, Benitez A, Ortloff L, Green LM. Alterations in glutamate uptake in NT2-derived neurons and astrocytes after exposure to gamma radiation. Radiat Res. 2009;171:41–52. doi: 10.1667/RR1361.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tikka T, Usenius T, Tenhunen M, Keinanen R, Koistinaho J. Tetracycline derivatives and ceftriaxone, a cephalosporin antibiotic, protect neurons against apoptosis induced by ionizing radiation. J Neurochem. 2001;78:1409–1414. doi: 10.1046/j.1471-4159.2001.00543.x. [DOI] [PubMed] [Google Scholar]
- 4.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 5.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich J, Monje M, Wefel J, Meyers C. Clinical Patterns and Biological Correlates of Cognitive Dysfunction Associated with Cancer Therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 7.Wong CS, Van der Kogel AJ. Mechanisms Of Radiation Injury To The Central Nervous System: Implications For Neuroprotection. Molecular interventions. 2004;4:273–284. doi: 10.1124/mi.4.5.7. [DOI] [PubMed] [Google Scholar]
- 8.Hochberg FH, Slotnick B. Neuropsychologic impairment in astrocytoma survivors. Neurology. 1980;30:172–177. doi: 10.1212/wnl.30.2.172. [DOI] [PubMed] [Google Scholar]
- 9.Crowe R, Vale J, Trott KR, Soediono P, Robson T, Burnstock G. Radiation-induced changes in neuropeptides in the rat urinary bladder. J Urol. 1996;156:2062–2066. [PubMed] [Google Scholar]
- 10.Hockerfelt U, Franzen L, Kjorell U, Forsgren S. Parallel increase in substance P and VIP in rat duodenum in response to irradiation. Peptides. 2000;21:271–281. doi: 10.1016/s0196-9781(99)00200-4. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Hauer-Jensen M. Neuroimmune interactions: potential target for mitigating or treating intestinal radiation injury. Br J Radiol, 80 Spec No. 2007;1:S41–48. doi: 10.1259/bjr/33057885. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Zheng H, Kulkarni A, Ou X, Hauer-Jensen M. Regulation of early and delayed radiation responses in rat small intestine by capsaicin-sensitive nerves. Int J Radiat Oncol Biol Phys. 2006;64:1528–1536. doi: 10.1016/j.ijrobp.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Scott M, Shepherd HS, Charlotte H, Nadja C, Vilhelm AB. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–2. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh MM, Hegde V, Kelley MR, Deutsch WA. Activation of APE/Ref-1 redox activity is mediated by reactive oxygen species and PKC phosphorylation. Nucleic Acids Res. 2001;29:3116–3122. doi: 10.1093/nar/29.14.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belzile JP, Choudhury SA, Cournoyer D, Chow TYK. Targeting DNA Repair Proteins: A Promising Avenue for Cancer Gene Therapy. Current Gene Therapy. 2006;6:111–123. doi: 10.2174/156652306775515538. [DOI] [PubMed] [Google Scholar]
- 16.Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don’t divide what’s to repair? Mutation Research. 2007;614:24–36. doi: 10.1016/j.mrfmmm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Guo C, Fishel ML, Wang ZY, Vasko MR, Kelley MR. Role of APE1 in differentiated neuroblastoma SH-SY5Y cells in response to oxidative stress: Use of APE1 small molecule inhibitors to delineate APE1 functions. DNA Repair. 2009;8:1273–1282. doi: 10.1016/j.dnarep.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo M, He H, Kelley MR, Georgiadis M. Redox Regulation of DNA Repair: Implications for Human Health and Cancer Therapeutic Development. Antioxid Redox Signal. 2010;12:1247–1269. doi: 10.1089/ars.2009.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The Many Functions of APE1/Ref-1: Not Only a DNA Repair Enzyme. Antioxid Redox Signal. 2009;11:601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutation Research. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 21.Paap B, Wilson DM, III, Sutherland BM. Human abasic endonuclease action on multilesion abasic clusters: implications for radiation-induced biological damage. Nucl Acids Res. 2008;36:2717–2727. doi: 10.1093/nar/gkn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson DM, III, McNeill DR. Base excision repair and the central nervous system. Neuroscience. 2007;145:1187–1200. doi: 10.1016/j.neuroscience.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Jean-Pierre P, Winters P, Ahles T, Antoni M, Armstrong D, Penedo F, Lipshultz SE, Miller TL, Morrow GR, Fiscella K. Prevalence of memory problems that limit daily functioning in adult cancer patients: A national representative sample of the U.S. population. Journal of Clinical Oncology. 2010;28:15s. [Google Scholar]
- 24.Kelley MR, Luo M, Reed A, Su D, Delaplane S, Borch RF, Nyland RL, II, Gross ML, Georgiadis M. Functional analysis of new and novel analogs of E3330 that block the redox signaling activity of the multifunctional AP endonuclease/redox signaling enzyme APE1/Ref-1. Antioxid Redox Signal. 2011;14:1387–1401. doi: 10.1089/ars.2010.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley MR, Luo M, Reed A, Su D, Delaplane S, Borch RF, Nyland RL, II, Gross ML, Georgiadis M. Functional analysis of new and novel analogs of E3330 that block the redox signaling activity of the multifunctional AP endonuclease/redox signaling enzyme APE1/Ref-1. Antioxid Redox Signal. 2011;14:1387–1401. doi: 10.1089/ars.2010.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyland RL, Luo M, Kelley MR, Borch RF. Design and Synthesis of Novel Quinone Inhibitors Targeted to the Redox Function of Apurinic/Apyrimidinic Endonuclease 1/Redox Enhancing Factor-1 (Ape1/Ref-1) Journal of Medicinal Chemistry. 2010;53:1200–1210. doi: 10.1021/jm9014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 28.Vasko MR, Guo C, Kelley MR. The multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes survival of neurons after oxidative stress. DNA Repair (Amst) 2005;4:367–379. doi: 10.1016/j.dnarep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y, Guo C, Vasko MR, Kelley MR. Implications of Apurinic/Apyrimidinic Endonuclease in Reactive Oxygen Signaling Response after Cisplatin Treatment of Dorsal Root Ganglion Neurons. Cancer Res. 2008;68:6425–6434. doi: 10.1158/0008-5472.CAN-08-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JJ, Barber LA, Dymshitz J, Vasko MR. Peptidase inhibitors improve recovery of substance P and calcitonin gene-related peptide release from rat spinal cord slices. Peptides. 1996;17:31–37. doi: 10.1016/0196-9781(95)02091-8. [DOI] [PubMed] [Google Scholar]
- 31.Tong JX, Vogelbaum MA, Drzymala RE, Rich KM. Radiation-induced apoptosis in dorsal root ganglion neurons. J Neurocytol. 1997;26:771–777. doi: 10.1023/a:1018566431912. [DOI] [PubMed] [Google Scholar]
- 32.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. The Journal of Biological Chemistry. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 33.Mima K, Deguchi S, Yamauchi T. Characterization of 5′ flanking region of alpha isoform of rat Ca2+/calmodulin-dependent protein kinase II gene and neuronal cell type specific promoter activity. Neurosci Lett. 2001;307:117–121. doi: 10.1016/s0304-3940(01)01941-3. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein ME, House SB, Gainer H. NF-L and peripherin immunoreactivities define distinct classes of rat sensory ganglion cells. J Neurosci Res. 1991;30:92–104. doi: 10.1002/jnr.490300111. [DOI] [PubMed] [Google Scholar]
- 35.Hiramoto M, Shimizu N, Sugimoto K, Tang J, Kawakami Y, Ito M, Aizawa S, Tanaka H, Makino I, Handa H. Nuclear targeted suppression of NF-kappa B activity by the novel quinone derivative E3330. J Immunol. 1998;160:810–819. [PubMed] [Google Scholar]
- 36.Gobbel GT, Chan PH. Neuronal death is an active, caspase-dependent process after moderate but not severe DNA damage. J Neurochem. 2001;76:520–531. doi: 10.1046/j.1471-4159.2001.00070.x. [DOI] [PubMed] [Google Scholar]
- 37.Gilmore SA, Sims TJ. Glial-glial and glial-neuronal interfaces in radiation-induced, glia-depleted spinal cord. J Anat. 1997;190( Pt 1):5–21. doi: 10.1046/j.1469-7580.1997.19010005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelley MR, Fishel ML. DNA repair proteins as molecular targets for cancer therapeutics. Anticancer Agents Med Chem. 2008;8:417–425. doi: 10.2174/187152008784220294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Georgiadis M, Luo M, Gaur R, Delaplane S, Li X, Kelley M. Evolution of the redox function in mammalian Apurinic/apyrimidinic Mutation Research. 2008;643:54–63. doi: 10.1016/j.mrfmmm.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bapat A, Glass LS, Luo M, Fishel ML, Long EC, Georgiadis MM, Kelley MR. Novel small molecule inhibitor of Ape1 endonuclease blocks proliferation and reduces viability of glioblastoma cells. J Pharmacol Exp Ther. 2010;334:988–998. doi: 10.1124/jpet.110.169128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su D, Delaplane S, Luo M, Rempel D, Vu B, Kelley MR, Gross ML, Georgiadis M. Interactions of APE1with a redox inhibitor: Evidence for an alternate conformation of the enzyme. Biochemistry. 2011;50:82–92. doi: 10.1021/bi101248s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNeill DR, Wilson DM. 3rd, A Dominant-Negative Form of the Major Human Abasic Endonuclease Enhances Cellular Sensitivity to Laboratory and Clinical DNA-Damaging Agents. Mol Cancer Res. 2007;5:61–70. doi: 10.1158/1541-7786.MCR-06-0329. [DOI] [PubMed] [Google Scholar]
- 43.Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7:367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 44.Vascotto C, Cesaratto L, Zeef LA, Deganuto M, D’Ambrosio C, Scaloni A, Romanello M, Damante G, Taglialatela G, Delneri D, Kelley MR, Mitra S, Quadrifoglio F, Tell G. Genome-wide analysis and proteomic studies reveal APE1/Ref-1 multifunctional role in mammalian cells. Proteomics. 2009;9:1058–1074. doi: 10.1002/pmic.200800638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang A, Gao H, Kelley MR, Qiao X. Inhibition of APE1/Ref-1 Redox Activity with APX3330 Blocks Retinal Angiogenesis in vitro and in vivo. Vision Res. 2011;51:93–100. doi: 10.1016/j.visres.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou GM, Luo MH, Reed A, Kelley MR, Yoder MC. Ape1 regulates hematopoietic differentiation of embryonic stem cells through its redox functional domain. Blood. 2007;109:1917–1922. doi: 10.1182/blood-2006-08-044172. [DOI] [PubMed] [Google Scholar]
- 47.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 48.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. The Journal of Pharmacology and Experimental Therapeutics. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 49.Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology. 1997;37:133–152. doi: 10.1016/s0162-3109(97)00055-6. [DOI] [PubMed] [Google Scholar]
- 50.Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res. 2002;307:281–291. doi: 10.1007/s00441-001-0477-8. [DOI] [PubMed] [Google Scholar]