Abstract
The tight junctions of bile duct epithelium form a barrier between the toxic bile and liver parenchyma. Disruption of tight junctions appears to play a crucial role in the pathogenesis of various liver diseases. In this study, we investigated the disruptive effect of hydrogen peroxide and the protective effect of epidermal growth factor (EGF) on the tight junctions and adherens junctions in the bile duct epithelium. Oxidative stress in NRC-1 and Mz-ChA-1 cell monolayers was induced by administration of hydrogen peroxide. Barrier function was evaluated by measuring electrical resistance and inulin permeability. Integrity of tight junctions, adherens junctions and the actin cytoskeleton was determined by imunofluorescence microscopy. Role of signaling molecules was determined by evaluating the effect of specific inhibitors. Hydrogen peroxide caused a rapid disruption of tight junctions and adherens junctions leading to barrier dysfunction without altering the cell viability. Hydrogen peroxiderapidly increased the levels of p-MLC (myosin light chain) and c-Src(pY418). ML-7 and PP2 (MLCK and Src kinase inhibitors) attenuated hydrogen peroxide-induced barrier dysfunction, tight junction disruption and reorganization of actin cytoskeleton. Pretreatment of cell monolayers with EGF ameliorated hydrogen peroxide-induced tight junction disruption and barrier dysfunction. The protective ffect of EGF was abrogated by ET-18-OCH3 and the Ro-32-0432 (PLCγ and PKC inhibitors). Hydrogen peroxide increased tyrosine-phosphorylation of ZO-1, claudin-3, E-cadherin and β-catenin, and pretreatment of cells with EGF attenuated tyrosine-phosphorylation of these proteins. These results demonstrate that hydrogen peroxide disrupts tight junctions, adherens junctions and the actin cytoskeleton by an MLCK and Src kinase-dependent mechanism in the bile duct epithelium. EGF prevents hydrogen peroxide-induced tight junction disruption by a PLCγ and PKC-dependent mechanism.
Keywords: Adherens junctions, cholangiocyte, EGF, oxidative stress, protein kinase, tight junction
The luminal surface of intra and extra-hepatic bile ducts is lined by a monolayer of cholangiocytes (epithelial cells). This cholangiocyte monolayer forms a barrier to the diffusion of the various injurious agents from the bile duct lumen into the hepatic parenchyma (1). The tight junctions, a multi-protein complex, along the apical end of the cell provide the barrier function of different epithelia. Disruption of bile duct epithelial integrity is implicated in the pathogenesis of liver diseases that affect biliary tree, such as primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC) (2–5). Disruption of tight junction integrity would allow regurgitation of bile acids or other noxious agents into the hepatic parenchyma from the bile duct lumen (6). As of now, most of the knowledge we have on the structure and regulation of tight junctions has been adapted from what is known about the tight junctions of intestinal and renal epithelia. Very little is known about the structure and regulation of tight junctions in the bile duct epithelium.
The tight junctions are composed of many proteins, including transmembrane proteins such as occludin, claudins and junctional adhesion molecules, and intracellular scaffold proteins such as zona occludens (ZO-1, ZO-2, ZO-3). The scaffold proteins and other associated tight junction proteins interact with the perijunctional actomyosin ring. The integrity of actomyosin ring and MLCK (myosin light chain kinase) activity play a crucial role in the regulation of tight junction integrity. Additionally, numerous signaling proteins such as c-Src, phosphatidylinositol 3-Kinase, ERK, PKCζ, PKCη, PP1 and PP2A directly interact with tight junction proteins, indicating their potential role in the regulation of tight junction integrity (7). The pharmacologic and molecular evidence support fact that tight junctions and paracellular permeability are indeed regulated by signaling molecules such as intracellular calcium, cyclic AMP, GTPase switch protein, protein kinases and protein phosphatases in the intestinal and renal epithelia (7, 8). However, the role of such signaling mechanisms in the regulation of bile duct epithelium is poorly understood. Adherens junctions are prominent junctional complexes in the intestinal and other epithelia. E-cadherin and catenins are the predominant proteins that are involved in the assembly of adherens junctions. But, there is no much information available regarding the adherens junctions in bile duct epithelium. One study, reported nearly two decades ago, described the presence of an adherens junction-like structure in the bile duct epithelium (9).
A significant body of evidence indicates that oxidative stress plays an important role in the pathogenesis of liver diseases. Compromised antioxidant defense mechanism and elevated oxidative stress was detected in the liver of patients with PSC and PBC (10–12). Serum oxidative stress level was shown to be elevated in patients with cholecystic bile duct injury (13). Hepatic oxidative stress was also detected in experimental cholecystic injury (14–16). Superoxide dismutase gene therapy attenuated the experimental cholestasis-induced liver fibrosis (17). The mechanism of oxidative stress-induced liver injury is poorly understood. Very little information is available regarding the oxidative stress effect on bile duct epithelial function. One study demonstrated that nitric oxide-mediated inhibition of DNA repair potentiates DNA damage in cholangiocytes (18). However, there is no information available regarding the effect of hydrogen peroxide or oxidative stress on the bile duct epithelial functions.
In the present study, we investigated the effect of hydrogen peroxide-induced oxidative stress on tight junction and adherens junction integrity and the mechanism associated with it in cholangiocyte monolayers. We further evaluated the EGF (epidermal growth factor)-mediated amelioration of tight junction disruption. EGF is a well-established gastrointestinal mucosal protective factor. However, its effect on bile duct function is unknown. NRC-1 cell monolayers were mainly used for this study. The effect of hydrogen peroxide and EGF on barrier function was confirmed in Mz-ChA-1 cell monolayers.
MATERIALS AND METHODS
Chemicals
Cell culture supplies were obtained from Invitrogen (San Jose, CA), and Transwell inserts and other cell culture plastic wares were purchased from Costar (Cambridge, MA). Rat-tail collagen (Type I), ML-7, PP2, genistein and FITC-inulin were obtained from Sigma chemical company (St Louis MO). Ro-32-0432 and ET-18-OCH3 were purchased from EMD Biochemicals (Gibbstown, NJ). Other fine chemicals and lab supplies were purchased from Fisher Scientific (Tustin, CA) or Sigma chemical company.
Antibodies
Mouse monoclonal anti-occludin antibody and rabbit polyclonal anti-occludin, anti-ZO-1, anti-claudin-3 and anti-β-catenin antibodies were purchased from Zymed laboratories (South San Francisco, CA). Biotin-conjugated anti-phospho-tyrosine (p-Tyr), anti-E-cadherin, and anti-actin antibodies were purchased from BD Transduction laboratories (Lexington, KY). Mouse monoclonal anti-Src, anti-p-MLC and anti-c-Src(pY418) antibodies were purchased from Upstate Biotechnology Inc. (Lake Placid, NY). AlexaFlour-488-conjugated anti-mouse IgG was obtained from Molecular Probes (Eugene, OR). Cy3-conjugated anti-rabbit IgG was purchased from Sigma Immunochemicals (St. Louis, MO).
Cell culture
NRC-1 cells (normal rat cholangiocytes) were cultured in DMEM-F12 supplemented with 10% fetal bovine serum, vitamin mix, chemically defined lipid mix, insulin-transferrin-selenium mix (ITS), non essential amino acids and antibiotics (penicillin and streptomycin) as described before (5). These cells were originally derived from intra hepatic cholangiocytes. Cells were cultured on plates or Transwell inserts (12 mm or 24 mm diameter) coated with rat-tail type I collagen. All experiments were performed on 5–6 days post seeding in confluent monolayers. Mz-ChA-1 cells were grown in CMRL Medium-1066 containing 10% fetal bovine serum, L-glutamine and antibiotics (penicillin and streptomycin). Cells were passaged at 90% confluence and seeded on to Transwell inserts (12 mm). Experiments were conducted on 4–5 days post seeding.
Hydrogen peroxide treatment
Cell monolayers were preincubated in DMEM for one hour with varying concentrations of hydrogen peroxide (100–500 μM) at both the apical and basal chambers and placed in 37°C incubator. At varying times after hydrogen peroxide the paracellular permeability was evaluated by measuring transepithelial electrical resistance (TER) and unidirectional flux of FITC-inulin. Inhibitors such as PP2 (3 μM) and ML-7 (10 μM) were administered 60 min prior to hydrogen peroxide administration. EGF (30 nM) was added 10 min prior to hydrogen peroxide. ET-18-OCH3 (15 μM) or Ro-32-0432 (1 μM) was administered 50 min before EGF administration. Control monolayers received the inhibitor in the absence of EGF and hydrogen peroxide.
Cell viability assay
Cell viability was assessed by measuring lactate dehydrogenase (LDH) activity in the incubation medium three hours after incubation with or without 500 μM hydrogen peroxide. LDH activity measured using a kit form Sigma Chemical Company (St Louis, MO). The metabolic activity of cells was evaluated by using WST-1 cell viability assay kit from Clontech (Mountain View, CA) according to vendor’s instructions. This assay involves cleavage of stale tetrazolium salt of WST-1 to a soluble formazan, which is measured by colorimetric method. The conversion of WST-1 occurs at cell surface by metabolically active cells, largely dependent on glycolytic production of NADH. Therefore, color produced is directly proportional to the number of metabolically active cells. A loss of cell viability results in reduced rate of WST-1 conversion and low color development.
Measurement of transepithelial electrical resistance (TER)
TER was measured as described before (5) using a Millicell-ERS electrical resistance system (Millipore, Bedford, MA). The TER recorded in empty Transwell inserts (usually 50–80 Ohms•cm2) was subtracted from all values.
Unidirectional Flux of Inulin
Inulin permeability was measured by incubating cell monolayers in the presence of 0.5 μg/ml FITC-inulin in the apical chamber. At varying times, 100 μl aliquot of basal medium was withdrawn and fluorescence was measured in a micro plate fluorescence reader (FLx-800, Bio TEK Instruments, Winooski, VT). Flux of FITC-inulin into the basal well, was calculated as the percentage of total fluorescence administered into the apical well per hour per cm2 surface area.
Immunofluorescence Microscopy
Under various experimental conditions, cell monolayers were fixed in ice-cold acetone:methanol (1:1, v/v) for 5 min. The fixed cells were rehydrated in PBS (Dulbecco’s saline containing 1.2 mM CaCl2 and 1 mM MgCl2) and permeabilized with 0.2% Triton-X100 in PBS. Cell monolayers were blocked with 4% nonfat milk in TBST (20 mM Tris, pH 8.0, containing 150 mM NaCl and 0.5% Tween 20). Cells were then stained with a mixture of mouse monoclonal anti-occludin and rabbit polyclonal anti-ZO-1 antibodies or mouse monoclonal anti-E-cadherin and rabbit polyclonal anti-β-catenin antibodies. A mixture of AlexaFlour-488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG antibodies was used as secondary antibodies. Actin cytoskeleton was stained by incubation of paraformaldehyde-fixed cells with AlexaFluor 488-conjugated phalloidin. Cells were mounted and images collected using a Zeiss LSM 5 PASCAL laser scanning confocal microscope and the LSM 5 PASCAL software (Release 3.2) as a series of images from 1.0 μm XY sections. Images were stacked by using the Image J software and processed by Adobe Photoshop (Adobe Systems, San Jose, CA).
Analysis of tyrosine phosphorylated proteins
Proteins were extracted under denatured conditions using lysis buffer D (50 mM Tris buffer, pH 8.0, containing 0.3% SDS, 2 mM vanadate, 10 mM sodium fluoride and protease inhibitors as described above) and heated at 100°C for 10 min. Biotin-conjugated anti-p-Tyr was used to immunoprecipitate p-Tyr. Immunocomplexes were precipitated with streptavidin-agarose and immunoblotted for different proteins as described below.
Immunoblot analysis
Proteins were separated by SDS-PAGE and transferred to PVDF membranes. Blots were probed for occludin, ZO-1, c-Src, p-MLC, c-Src(pY418), E-cadherin, β-catenin and claudin-3. HRP-conjugated anti-mouse IgG or anti-rabbit IgG antibodies were used as secondary antibodies. The blots were developed using the enhanced chemiluminescence method (Amersham, Arlington Heights, IL).
Statistics
Comparison between two groups was made by the Student’s t tests for grouped data. The significance in all tests was derived at 95% or greater confidence level.
RESULTS
Hydrogen peroxide induces barrier dysfunction in cholangiocyte monolayers
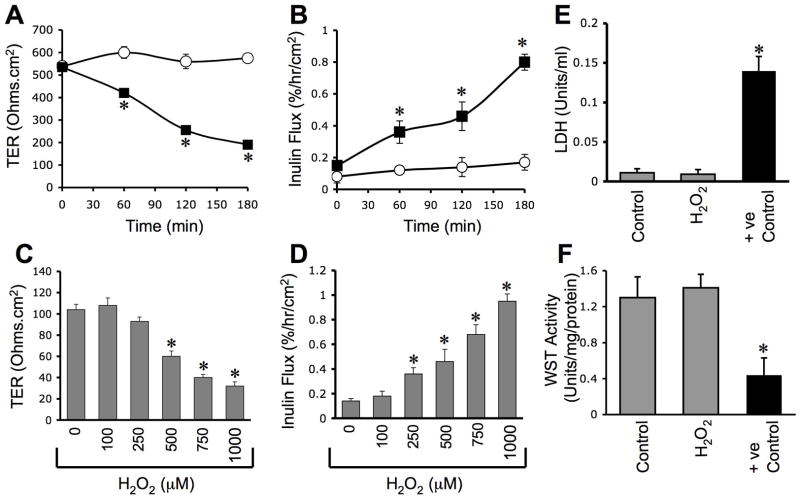
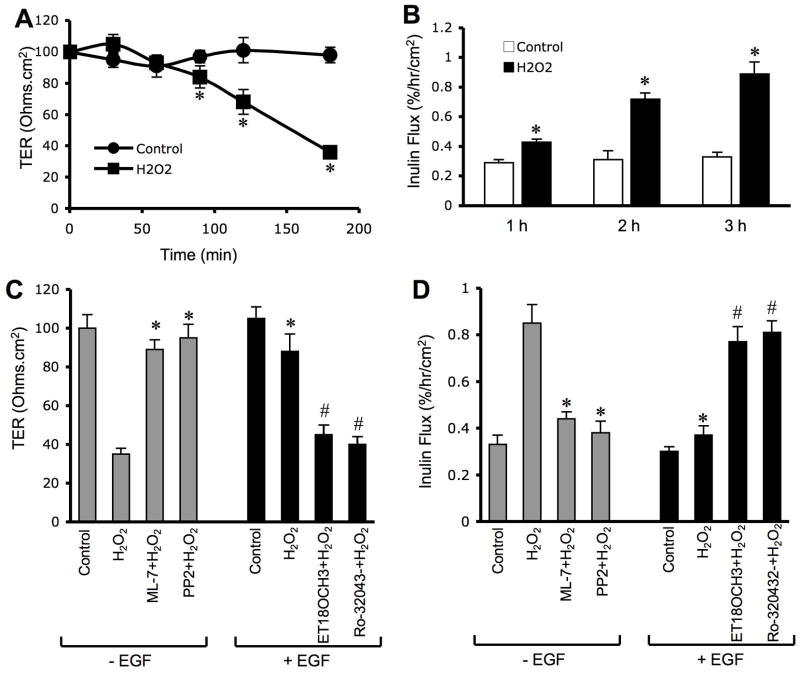
To determine the influence of oxidative stress on the barrier function of bile duct epithelium, NRC-1 cell monolayers were exposed to hydrogen peroxide and the barrier function was evaluated by measuring TER and inulin permeability. Hydrogen peroxide reduced TER and increased inulin permeability in a time (Fig. 1A & 1B) and dose (Fig. 1C & 1D)-dependent manner, indicating that hydrogen peroxide increases the paracellular permeability. To determine the effect of hydrogen peroxide on cell viability we evaluated its effect on LDH release and cell metabolic capacity as assessed by WST-1 assay. Under our experimental conditions, hydrogen peroxide did not increase LDH release (Fig. 1E) or decrease WST-1 activity (Fig. 1F).
Figure 1. hydrogen peroxide disrupts barrier function in NRC-1 cell monolayers.
NRC-1cell monolayers were incubated with (μ) or without (○) varying concentrations of hydrogenperoxide (C & D) for varying times (A & B). TER (A & C) and inulin permeability (B & D)were measured. The incubation medium was assayed for LDH activity (E) and the cells weresubjected to WST-1 viability test (F). Values are mean ± sem (n = 6). Asterisks indicate thevalues that are significantly (p<0.05) different from corresponding control values.
Hydrogen peroxide disrupts tight junctions and adherens junctions
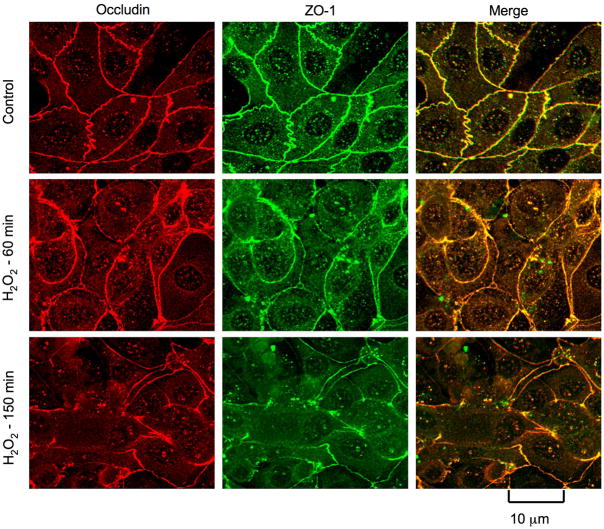
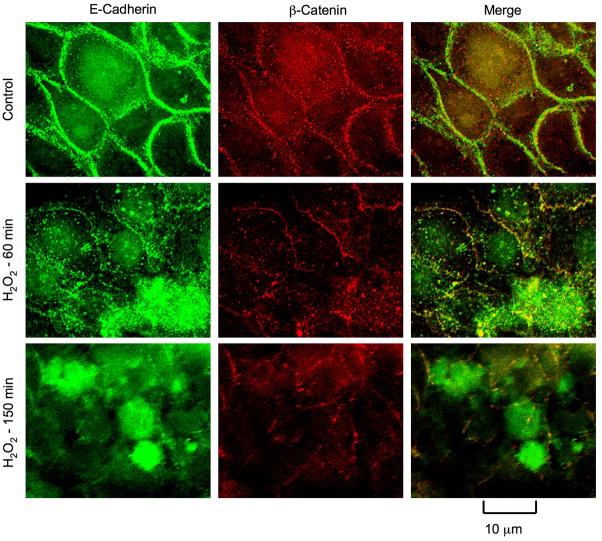
Above data suggest that hydrogen peroxide increases paracellular permeability in NRC-1 cell monolayers without causing any loss of cell viability. Paracellular permeability in epithelial monolayers is restricted due to the presence of intact tight junctions. Although adherens junctions do not provide a physical barrier, they are known to indirectly regulate the integrity of tight junctions. Therefore, we examined the effect of hydrogen peroxide on the integrity of tight junctions and adherens junctions by immunofluorescence analysis of sub cellular localization of occludin, ZO-1, E-cadherin and β-catenin. Occludin and ZO-1 were co-localized at the intercellular junctions in the control cell monolayers, indicating the presence of intact tight junctions. hydrogen peroxide treatment induced a redistribution of occludin and ZO-1 from the intercellular junctions into the intracellular compartments in a time-dependent manner (Fig. 2). E-cadherin and β-catenin were co-localized at the intercellular junctions in control cell monolayers, and hydrogen peroxide induced a redistribution of both E-cadherin and β-catenin from the intercellular junctions in a time-dependent manner (Fig. 3).
Figure 2. hydrogen peroxidedisrupts tight junctions.
NRC-1 cell monolayers were incubatedwith or without hydrogen peroxide (500 μM) for varying times. Cell monolayers were fixed andstained for occludin and ZO-1 by immunofluorescence method. Fluorescence images collected using a confocal microscope.
Figure 3. hydrogen peroxide disrupts adherens junctions.
NRC-1 cell monolayers were incubated with or without hydrogen peroxide (500 μM) for varying times. Cell monolayers were fixed and stained for E-cadherin and β-catenin by immunofluorescence method. Fluorescence images collected using a confocal microscope.
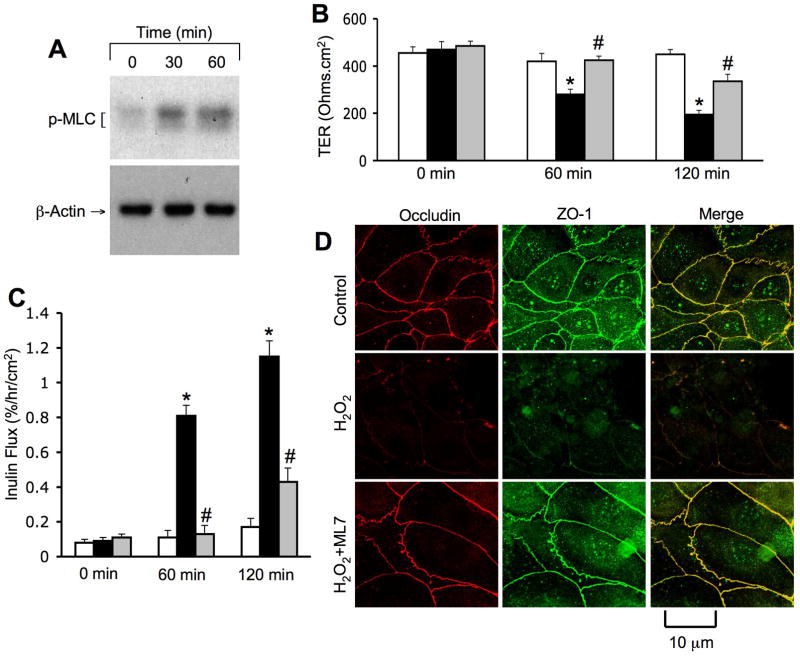
MLCK activity mediates hydrogen peroxide-induced tight junction disruption and barrier dysfunction
MLCK is well known to play a role in tight junction regulation in the intestinal (19) and lung (20) epithelial monolayers. Studies were therefore designed to determine the effect of hydrogen peroxide on MLCK activation and the effect of ML-7, an MLCK-selective inhibitor, on tight junction disruption. Pretreatment of cell monolayers with ML-7 significantly attenuated hydrogen peroxide-induced decrease in TER (Fig. 4B) and increase in inulin permeability (Fig. 4C). ML-7 also attenuated hydrogen peroxide-induced redistribution of occludin and ZO-1 from the intercellular junctions into the intracellular compartments (Fig. 4D). ML-7 by itself did not influence TER, inulin flux or junctional distribution of occludin and ZO-1.
Figure 4. MLCK activity mediates hydrogen peroxide-induced tight junction disruption.
NRC-1 cell monolayers were incubated with (gray) or without ML-7 (white and black bars) followed by incubation with (black and gray bars) or without (white bars) hydrogen peroxidefor 120 min. Protein extracts were immunoblotted for p-MLC and β-actin (A). TER (B) and inulin flux (C) were measured. Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from corresponding control values. Symbol, #, indicates the value that is significantly (p<0.05) different from cells treated with hydrogen peroxide in the absence of ML-7. Cell monolayers were fixed and stained for occludin and ZO-1 (D). Fluorescence images collected using a confocal microscope.
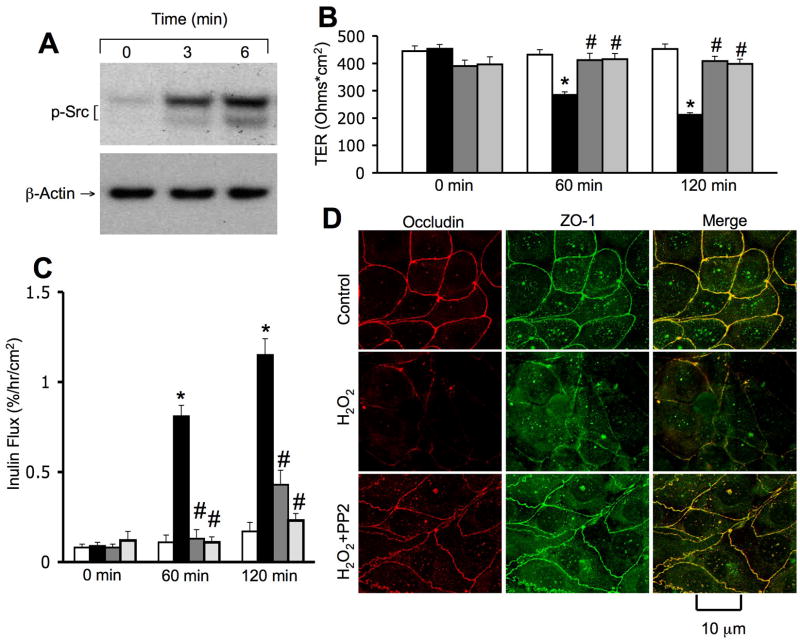
Hydrogen peroxide-induced disruption of tight junctions is mediated by Src kinase activity
Our previous studies demonstrated that tyrosine kinases, especially c-Src, play an important role in tight junction regulation in the intestinal epithelium. We therefore, evaluated the role of tyrosine kinase activity in hydrogen peroxide-induced tight junction disruption in NRC-1 cell monolayers. Hydrogen peroxide rapidly increased the level of c-Src(pY418) (Fig. 5A), indicating an activation of c-Src in NRC-1 cell monolayers. Pretreatment of cell monolayers with genistein (a broad range tyrosine kinase inhibitor) or PP2 (a Src kinase inhibitor) significantly attenuated hydrogen peroxide-induced decrease in TER (Fig. 5B) and increase in inulin permeability (Fig. 5C). PP2 also attenuated hydrogen peroxide-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 5D). Genistein or PP2 under the concentrations used, did not influence TER, inulin permeability or junctional distribution of occludin and ZO-1.
Figure 5. Src kinase activity mediates hydrogen peroxide-induced tight junction disruption.
NRC-1 cell monolayers were incubated without (white and black bars) or with genistein (dark gray bars) or PP2 (light gray bars) followed by incubation with (black bars) or without (white bars) hydrogen peroxide for 120 min. Protein extracts were immunoblotted for c-Src(pY418) and β-actin (A). TER (B) and inulin flux (C) were measured. Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from corresponding control values. Symbol, #, indicates the values that are significantly (p<0.05) different from values for cells treated with hydrogen peroxide in the absence of PP2. Cell monolayers were fixed and stained for occludin and ZO-1 (D). Fluorescence images collected using a confocal microscope.
hydrogen peroxide-induced activation of MLCK and c-Src are independent of each other
To investigate the interdependency of MLCK activation and c-Src activation we determined the effect of ML-7 and PP2 on MLCK and c-Src activation. Pretreatment of cell monolayers with ML-7 attenuated hydrogen peroxide-induced increase in p-MLC levels (Fig. 6), but p-MLC levels were unaffected by PP2. Similarly, hydrogen peroxide-induced increase in the levels of c-Src(pY418) was attenuated by PP2, but not by ML-7 (Fig. 6). These results indicate independent activation of MLCK and c-Src by hydrogen peroxide.
Figure 6. Hydrogen peroxide-induced activation of MLCK and c-Src are independent of each other.
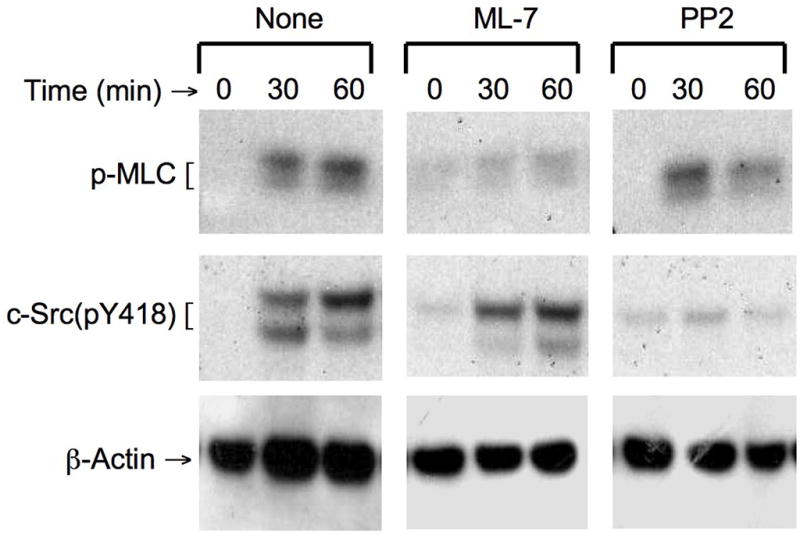
NRC-1 cell monolayers were incubated with or without ML-7 or PP2 for 30 min prior to incubation with hydrogen peroxide (500 μM) for varying times. Protein extracts were immunoblotted for p-MLC, c-Src(pY418) and βactin.
Hydrogen peroxide induces reorganization of actin cytoskeleton by an MLCK and Src kinase-dependent mechanism
The tight junction protein complex is known to intimately associate with the actomyosin ring, and disruption of actin cytoskeleton leads to disruption of tight junctions (21–23). In the control cell monolayers, actin is organized distinctly at the apical, middle and basal parts (Fig. 7). At the apical part, actin stains appear as uniformly distributed punctuates. In the middle part of the cell, the F-actin filaments appear to be organized as a ring at the perijunctional region. Network of weak stress fibers were seen at the basal part of the cell. Treatment with hydrogen peroxide resulted in a dramatic disorganization of F-actin arrangements at all three levels of the cell (Fig. 7). Pretreatment of cell monolayers with ML-7 or PP2 preserved the normal organization of the actin cytoskeleton in hydrogen peroxide-treated cells.
Figure 7. Hydrogen peroxide induces reorganization of actin cytoskeleton by an MLCK and Src kinase-dependent mechanism.
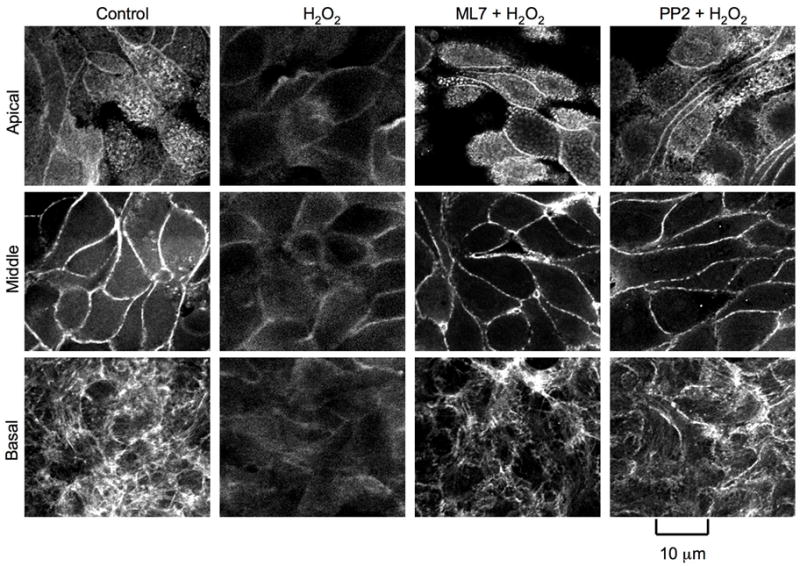
NRC-1 cell monolayers were pretreated with or without ML-7 or PP2 for 30 min prior to incubation with hydrogen peroxide (500 μM) for 90 min. F- actin in paraformaldehyde-fixed cell monolayers was stained with AlexaFlour 488-phalloidin. Fluorescence images from 1 μm optical sections collected using a confocal microscope. Images from 1 μm section of apical end (Apical), mid cell (Middle) and basal end (Basal) are presented.
EGF ameliorates hydrogen peroxide-induced tight junction disruption in NRC-1 cell monolayers
EGF, a gastrointestinal mucosal protective factor, has been extensively investigated for its mucosal protective role in the gastrointestinal mucosa (24). But, there is no information available on the potential protective role of EGF in the bile ducts. We investigated the effect of EGF on hydrogen peroxide-induced tight junction disruption in NRC-1 cell monolayers. Pretreatment of cell monolayers with EGF for 10 minutes prior to hydrogen peroxide administration significantly attenuated hydrogen peroxide-induced decrease in TER (Fig. 8A) and increase in inulin permeability (Fig. 8B). EGF also attenuated hydrogen peroxide-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 8C). EGF by itself did not cause significant change in TER, inulin flux or junctional distribution of occludin and ZO-1. The effect of EGF on hydrogen peroxide-induced tight junctions was significantly attenuated by pretreatment of cell monolayers with 0.3 μM AG1478, a selective inhibitor of EGF receptor tyrosine kinase (data not shown).
Figure 8. EGF attenuates hydrogen peroxide-induced tight junction disruption.
A & B: NRC-1 cell monolayers were incubated with or without EGF (30 nM) for 10 min followed by administration of hydrogen peroxide (500 μM) for 30 min. TER (A) and inulin flux (B) were measured. Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from corresponding control values. Symbol, #, indicates the value that is significantly (p<0.05) different from values for cells treated with hydrogen peroxide in the absence of EGF. C: Cell monolayers were fixed and stained for occludin and ZO-1. Fluorescence images were collected using a confocal microscope.
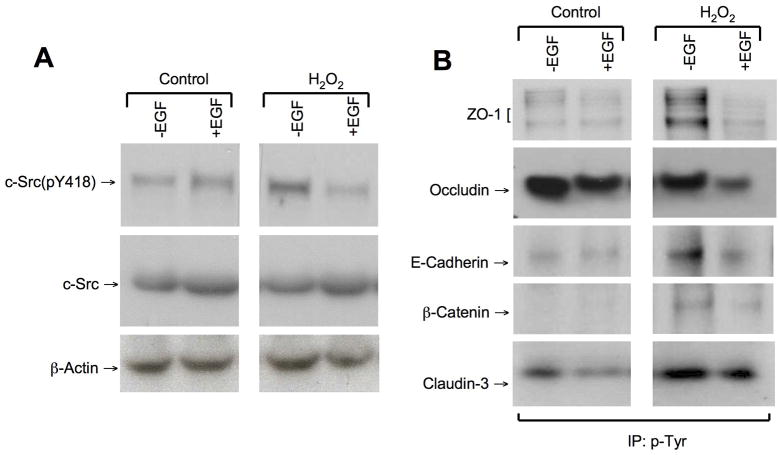
EGF attenuates hydrogen peroxide-induced c-Src activation and tyrosine phosphorylation of tight junction and adherens junction proteins
Previous studies showed that the integrity of tight junctions and adherens junctions is regulated by Src-mediated tyrosine-phosphorylation of tight junction and adherens junction proteins (25, 26). The present study shows that EGF pretreatment attenuates hydrogen peroxide-induced increase in the level of c-Src(pY418) in NRC-1 cell monolayers (Fig. 9A). Hydrogen peroxide treatment increased the levels of tyrosine-phosphorylated ZO-1, E-cadherin, β-catenin and claudin-3 (Fig. 9B). EGF pretreatment reduced Tyr-phosphorylation of these proteins in hydrogen peroxide-treated cells. Tyr-phosphorylated occludin levels were high in both untreated and hydrogen peroxide-treated cell monolayers (Fig. 9B). EGF treatment reduced the levels of tyrosine-phosphorylated occludin in both untreated and hydrogen peroxide-treated cell monolayers.
Figure 9. EGF attenuates hydrogen peroxide-induced Tyr-phosphorylation of tight junction and adherens junction proteins.
A: NRC-1 cell monolayers were incubated with or without EGF (30 nM) for 10 min followed by administration of hydrogen peroxide (500 μM) for 30 min. Protein extracts were immunoblotted for c-Src(pY418) and c-Src. B: Cell monolayers pretreated with or without EGF were incubated with or without hydrogen peroxide (500 μM) for 90 min. Phospho-tyrosine from the denatured protein extracts was immunoprecipitated and immunoblotted for tight junction and adherens junction proteins.
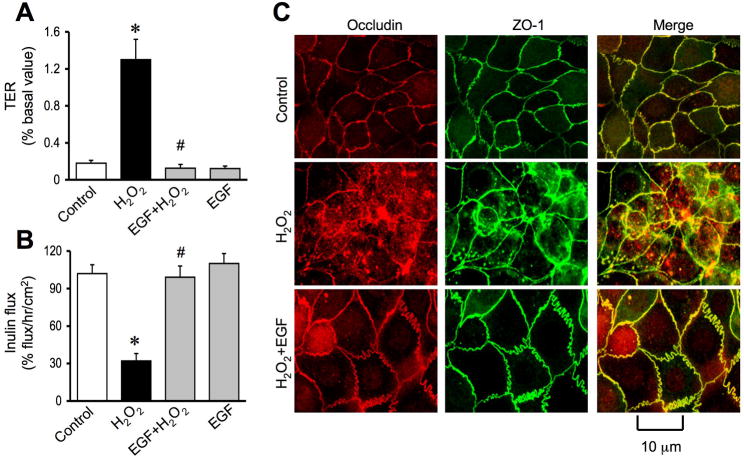
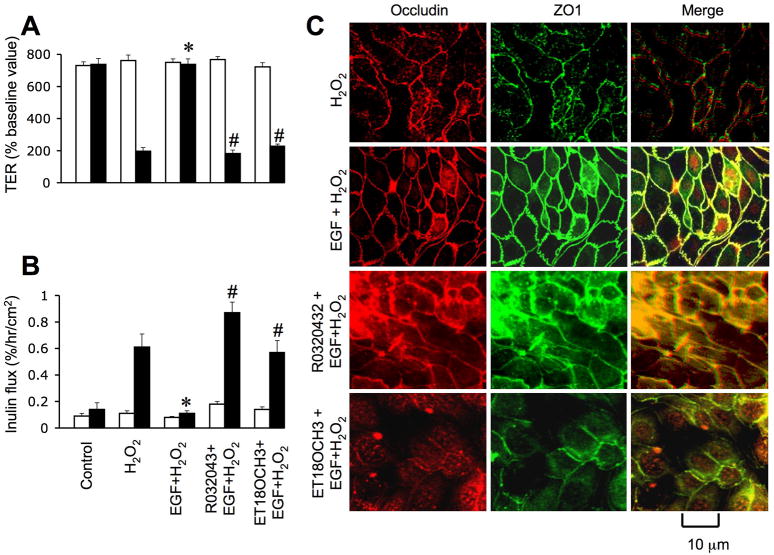
PLCγ and PKC activities are involved in EGF-mediated protection of tight junctions
One of the major intracellular signaling pathways activated by EGF is PLCγ-mediated PKC activation as shown in Caco-2 cell monolayers (27). In the present study, we investigated the role of PLCγ and PKC in EGF-mediated tight junction protection in NRC-1 cell monolayers. Pretreatment of cell monolayers with ET-18-OCH3, a PLCγ-selective inhibitor significantly attenuated EGF-mediated prevention of hydrogen peroxide-induced decrease in TER (Fig. 10A) and increase in inulin permeability (Fig. 10B). ET-18-OCH3 also prevented EGF-mediated attenuation of hydrogen peroxide-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 10C). Similarly, Ro-32-0432, a PKC-selective inhibitor, prevented EGF-mediated attenuation of hydrogen peroxide-induced decrease in TER, increase in inulin permeability and redistribution of occludin and ZO-1 from the intercellular junctions. ET-18-OCH3 or Ro-32-0432 by themselves produced no significant influence on TER, inulin flux or junctional distribution of occludin and ZO-1 in control and hydrogen peroxide-treated cell monolayers.
Figure 10. PLCγand PKC activities mediate EGF-mediated protection of tight junction from hydrogen peroxide.
A & B: NRC-1 cell monolayers were incubated with or without ET- 18-OCH3 (15 μM) or Ro-32-0432 (1μM) for 60 min followed by incubation with or without hydrogen peroxide for 120 min. TER (A) and inulin flux (B) were measured before (white bars) and after (Black bars) hydrogen peroxide treatment. Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from corresponding values for hydrogen peroxide group. Symbol, #, indicates the values that are significantly (p<0.05) different from values for cells treated with hydrogen peroxide and EGF in the absence of ET-18- OCH3 or Ro-32-0432. C: Cell monolayers were fixed and stained for occludin and ZO-1. Fluorescence images collected using a confocal microscope.
EGF prevents hydrogen peroxide-induced barrier dysfunction in Mz-ChA-1 cell monolayers
Mz-ChA-1 cell is a human bile duct epithelial cell line that forms a polarized cholangiocyte monolayer when grown in culture. The barrier function of this cholangiocyte monolayer is weak compared to that of NRC-1 cell monolayers. However, treatment of Mz-ChA-1 cell monolayers with hydrogen peroxide significantly decreased TER (Fig. 11A) and increased inulin permeability (Fig. 11B). This effect of hydrogen peroxide was attenuated by pretreatment of cell monolayers with ML-7 or PP2. The hydrogen peroxide-induced effect on TER and inulin flux was attenuated by the pretreatment of cells with EGF. This effect of EGF was prevented by ET-18-OCH3 or Ro-32-0432 (Fig. 11).
Figure 11. Hydrogen peroxide disrupts barrier function and EGF prevents this effect in Mz-ChA-1 cell monolayers.
A & B: Mz-ChA-1 cell monolayers were incubated with (ν) or without (○) 200 μM hydrogen peroxide for varying times. TER (A) and inulin permeability (B) were measured at varying times. Values are mean± sem (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from corresponding control values. C & D: Cell monolayers were pretreated with different inhibitors either 60 min prior to hydrogen peroxide (ML-7 and PP2) or 50 min prior to EGF administration (ET-18-OCH3 and R0-32-0432). EGF was administered 10 min prior to hydrogen peroxide. TER (C) and inulin permeability (D) were measured at two hours after hydrogen peroxide. Values are mean ±sem (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from corresponding values for hydrogen peroxide group. The symbol # indicates the values that are significantly (p<0.05) different from corresponding values for hydrogen peroxide+EGF group.
DISCUSSION
Although evidence indicates that oxidative stress is associated with the bile duct-associated pathology of liver (10, 28, 29) the mechanism of oxidative stress-induced bile duct injury is unclear. In the present study, using a primary cell line of rat bile duct epithelium and a human cholangiocarcinoma cell line, we show that oxidative stress induced by hydrogen peroxide results in compromised epithelial barrier function. The barrier function of bile duct epithelium is vital to the liver health as it prevents the diffusion of toxic bile components into liver parenchyma, thus protecting the hepatocytes and other cells from bile acid-induced injury. Disruption of barrier function and bile acid-induced hepatocyte injury plays a crucial role in the pathogenesis of liver disease. Furthermore, the factors that prevent oxidative stress-induced barrier function of bile duct epithelium is crucial to our understanding of the regulation of bile duct epithelial barrier function. The present study shows that EGF, a gastrointestinal mucosal protective factor, prevents hydrogen peroxide-induced tight junction disruption and barrier dysfunction.
Decrease in TER and increase in inulin permeability without altering cell viability show that hydrogen peroxide causes increase in paracellular permeability in the bile duct epithelium. Redistribution of tight junction proteins, occludin and ZO-1, from the intercellular junctions into the intracellular compartments indicated that hydrogen peroxide-induced barrier dysfunction was caused by the disruption of tight junctions. Occludin and ZO-1 are the most widely characterized tight junction proteins. Organization of these proteins involves an interaction of ZO-1 with the intracellular, C-terminal domain of occludin (30). Redistribution of these proteins from the intercellular junctions into the intracellular compartment in hydrogen peroxide-treated cholangiocyte monolayer is a clear indication of disrupted tight junctions. The hydrogen peroxide concentration used in this study is similar to those (400–1000 micromolar) used in previous experiments in other laboratories (31, 32). Accurate measurement of hydrogen peroxide concentration in vivo is difficult due to high reactivity of this molecule and spatial differences in hydrogen peroxide at sub cellular levels. Evidence indicates that very high level of hydrogen peroxide is detected in neutrophil-nonphagocytosable surface contact points, which is not accessible to catalase (33).
Co-localization of E-cadherin and β-catenin at the intercellular junctions in NRC-1 cell monolayers indicated that cholangiocytes do form adherens junctions. Very little is known about the adherens junctions in bile duct epithelium. Distribution of E-cadherin and β-catenin at the junctions of NRC1 cell monolayers appeared diffuse unlike much more discrete organization of these proteins in other epithelial cells (34). This is consistent with the flat and wide morphologic appearance of cholangiocytes. Incubation with hydrogen peroxide induced a redistribution of both E-cadherin and β-catenin from the intercellular junctions indicating that hydrogen peroxide also disrupts adherens junctions. Previous studies have shown that disruption of adherens junctions leads to disruption of tight junctions (35). Therefore, it is likely that adherens junction disruption plays a role in facilitating tight junction disruption in bile duct epithelium.
Activation of MLCK is known to cause disruption of tight junctions and increased paracellular permeability in the intestinal and lung epithelia (20, 36). MLCK activation in bile duct epithelium and its influence on tight junction permeability is unknown. A rapid increase in the levels of p-MLC indicates that hydrogen peroxide activates MLCK. Attenuation of hydrogen peroxide-induced tight junction disruption and barrier dysfunction by ML-7 indicated that MLCK is involved in hydrogen peroxide-induced tight junction disruption in NRC-1 cell monolayers. This is the first report of MLCK activation in a bile duct epithelium and its influence on the integrity of tight junctions and barrier function.
Our previous studies showed that c-Src plays a crucial role in regulation of tight junction integrity in the intestinal epithelium (37). The present study shows that Src kinase activity is also involved in hydrogen peroxide-induced tight junction disruption in NRC-1 cell monolayers. Rapid increase in the level of c-Src(pY418) indicated that hydrogen peroxide activates c-Src in NRC-1 cells, and attenuation of tight junction disruption and barrier dysfunction by PP2 demonstrates that Src kinase activity is involved in the mechanism of hydrogen peroxide-induced tight junction disruption and barrier dysfunction. The present study also indicates that hydrogen peroxide-induced activations of MLCK and c-Src are independent of each other. Hydrogen peroxide-induced increase in p-MLC was attenuated by ML-7, but unaffected by PP2. On the other hand, hydrogen peroxide-induced increase in c-Src(pY418) was attenuated by PP2, but not by ML-7. Therefore, hydrogen peroxide activates multiple signaling pathways with multiple targets that in concert may affect the integrity of tight junctions.
Although actin cytoskeleton seems to play an important role in the regulation of cholangiocyte functions (38, 39), very little is known about the organization of actin cytoskeleton in bile duct epithelium. The present study shows that actin cytoskeleton in NRC-1 cell monolayers is organized into apical microvillar bundles, middle cortical network and basal network of stress fibers. Interestingly, hydrogen peroxide treatment resulted in a dramatic loss of actin organizations at all three levels. Attenuation of hydrogen peroxide-induced actin reorganization by ML-7 and PP2 indicated the roles of both MLCK and Src kinase activities in disruption of actin cytoskeleton. It is not clear how these activities are involved in the disruption of actin cytoskeleton. Modulation of actomyosin ring structure and tyrosine-phosphorylation of actin binding proteins are likely mechanisms.
It is well established that EGF protects the gastrointestinal mucosa from a variety of insults (24). EGF was shown to play a role in liver regeneration and hepatocellular carcinoma as a potent mitogen (40-42). EGF effect in bile duct epithelium however is unknown. The present study, for the first time, demonstrates that EGF plays a protective role in normal cholangiocytes. EGF attenuated hydrogen peroxide-induced disruption of tight junctions and barrier dysfunction. EGF is predominantly produced in salivary glands and kidney and is released into the gastrointestinal lumen and renal tubules. A significant body of evidence indicates that EGF is also released into circulation and liver is the principal organ that clears the plasma EGF including its biliary secretion EGF (43). Therefore, EGF receptor activation in the bile duct epithelium is likely to influence the bile duct function under physiologic and pathophysiologic conditions.
The present study shows that one of the mechanisms by which EGF protects tight junctions in NRC-1 cell monolayers involves suppression of hydrogen peroxide-induced c-Src activation. Our previous studies showed that c-Src-induced Tyr-phosphorylation of occludin on specific tyrosine residues results in loss of its interaction with ZO-1 and weakening of tight junction integrity (25). Similarly, c-Src-induced Tyr-phosphorylation of β-catenin result in loss of adherens junction integrity (26). The potential role of Src kinase activity in hydrogen peroxide-induced tight junction disruption raised the question whether H2O2 increased tyrosine-phosphorylation of tight junction and adherens junction proteins in NRC-1 cell monolayers. Results indicate that hydrogen peroxide treatment increased the levels of tyrosine-phosphorylated E-cadherin and β-catenin, suggesting a potential loss of interaction between E-cadherin and β-catenin in hydrogen peroxide-treated cell monolayers.
Occludin was found to be highly tyrosine-phosphorylated in both untreated and hydrogen peroxide-treated cell monolayers. But, Tyr-phosphorylation of ZO-1 and claudin-3 was increased by hydrogen peroxide. Although ZO-1 was previously shown to undergo tyrosine-phosphorylation during the tight junction disruption in Caco-2 cell monolayers, the function of ZO-1 phosphorylation in the mechanism of tight junction disruption is unclear. EGF treatment reduced the levels of tyrosine-phosphorylated E-cadherin, β-catenin, ZO-1 and claudin-3, which is likely one of the mechanisms associated with EGF-mediated protection of tight junctions from hydrogen peroxide in NRC1 cell monolayers. Although high levels of tyrosine-phosphorylated occludin were equally high in both untreated and hydrogen peroxide-treated cell monolayers, EGF treatment caused a reduction of occludin phosphorylation in both untreated and hydrogen peroxide-treated cell monolayers. In general, tyrosine-phosphorylation of tight junction and adherens junction proteins is high in hydrogen peroxide-treated cell monolayers, while EGF attenuates such protein tyrosine-phosphorylation.
The mechanism of EGF-mediated protection of epithelial integrity is known to involve activation of several intracellular signaling pathways. One such mechanism is activation of PLCγand PLCγ-mediated activation of PKC. Our recent study showed that EGF rapidly activates PLCγand PKC in Caco-2 cell monolayers (27). Attenuation of EGF-mediated protection of tight junctions from hydrogen peroxide by ET-18-OCH3 and Ro-32-0432 indicated that PLCγand PKC activities are involved in EGF-mediated protection of tight junctions in NRC-1 cell monolayers.
We further examined the effect of hydrogen peroxide and EGF on barrier function in Mz-ChA-1 cell monolayers. Mz-ChA-1 cell is a human cholangiocarcinoma cell line that grows in culture to form a differentiated monolayer of cholangiocytes. Although this cell monolayer is relatively leaky compared to NRC-1 cell monolayers, hydrogen peroxide treatment significantly disrupted the barrier function (decrease in TER and increase in inulin permeability). MLCK and Src kinase inhibitors attenuated this effect of hydrogen peroxide. EGF prevented hydrogen peroxide-induced barrier dysfunction, which was attenuated by PLCγand PKC inhibitors. These results demonstrate that the phenomenon of hydrogen peroxide and EGF effects on barrier function is likely to exist in all types of bile duct epithelia, and not confined to a single cell line.
In conclusion, oxidative stress induced by hydrogen peroxide disrupts tight junctions and adherens junctions, and induces barrier dysfunction in bile duct epithelium by an MLCK and Src kinase-dependent mechanism. Furthermore, EGF ameliorates hydrogen peroxide-induced tight junction disruption in bile duct epithelium by a PLCγand PKC-dependent mechanism.
Acknowledgments
Source of support:
This study was supported mainly by a grant from Mussette and Allen Morgan Foundation for Primary Sclerosing Cholangitis and partially by National Institute of Health grants R01-DK55532 and R01-AA12307.
Abbreviations
- AG1478
4-(-3-chloroanilino)-6,7-dimethoxyquinozoline
- EGF
epidermal growth factor
- ET-18-OCH3
1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphorylcholine
- HRP
horse radish peroxidase
- LDH
lactate dehydrogenase
- ML-7
(5-iodonaphthalene-1-sulfonyl) homopiperazine
- MLCK
myosin light chain kinase
- NRC-1
normal rat cholangiocyte-1 cell line
- PBC
primary biliary cirrhosis
- PBS
phosphate buffered saline
- PKC
protein kinase C
- PLC
phospholipase C
- p-MLC
phospho-myosin light chain
- PMSF
phenylmethyl sulfonyl fluoride
- PP2
4-amino-5[chlorophyll]-7-[t-butyl]pyrazolo[3-4-d]pyrimidine
- PSC
primary sclerosing cholangitis
- Ro-32-0432
2-(8-[(dimethylamino)methyl]-6,7,8,9-tetrahydropyridol[1,2-a]indol-3-yl}-3-(1-methylinfol-3-yl)maleimide
- SDS
sodium dodecyl sulfate
- TER
transepithelial electrical resistance
- ZO-1
zona occludens-1
References
- 1.Luedde T, Heinrichsdorff J, de Lorenzi R, De Vos R, Roskams T, Pasparakis M. IKK1 and IKK2 cooperate to maintain bile duct integrity in the liver. Proc Natl Acad Sci U S A. 2008;105(28):9733–9738. doi: 10.1073/pnas.0800198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127(5):1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Hanada S, Harada M, Koga H, Kawaguchi T, Taniguchi E, Kumashiro R, et al. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int. 2003;23(1):3–11. doi: 10.1034/j.1600-0676.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 4.Nagtzaam IF, van Geel M, Driessen A, Steijlen PM, van Steensel MA. Bile duct paucity is part of the neonatal ichthyosis-sclerosing cholangitis phenotype. Br J Dermatol. 163(1):205–207. doi: 10.1111/j.1365-2133.2010.09794.x. [DOI] [PubMed] [Google Scholar]
- 5.Sheth P, Delos Santos N, Seth A, LaRusso NF, Rao RK. Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G308–318. doi: 10.1152/ajpgi.00582.2006. [DOI] [PubMed] [Google Scholar]
- 6.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127(1):261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci. 2008;13:7210–7226. doi: 10.2741/3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joly P, Gilbert D, Thomine E, Delpech A, Verdier S, Lauret P, et al. Immunofluorescence and immunoelectron microscopy analyses of a human monoclonal anti-epithelial cell surface antibody that recognizes a 185-kD polypeptide: a component of the paraneoplastic pemphigus antigen complex? J Invest Dermatol. 1993;101(3):339–345. doi: 10.1111/1523-1747.ep12365500. [DOI] [PubMed] [Google Scholar]
- 10.Cecere A, Tancredi L, Gattoni A. Primary sclerosing cholangitis. Panminerva Med. 2002;44(4):313–323. [PubMed] [Google Scholar]
- 11.Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Identification of novel molecules and pathogenic pathways in primary biliary cirrhosis: cDNA array analysis of intrahepatic differential gene expression. Gut. 2001;49(4):565–576. doi: 10.1136/gut.49.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salem TA, El-Refaei MF, Badra GA. Study of antioxidant enzymes level and phagocytic activity in chronic liver disease patients. Egypt J Immunol. 2003;10(1):37–45. [PubMed] [Google Scholar]
- 13.Miranda-Diaz AG, Hermosillo-Sandoval JM, Ortiz GG, Lizardi-Garcia D, Cardona-Munoz EG, Pacheco-Moises F. Serum oxidative stress is increased in patients with post cholecystectomy bile duct injury. Rev Esp Enferm Dig. 102(6):352–356. doi: 10.4321/s1130-01082010000600002. [DOI] [PubMed] [Google Scholar]
- 14.Lissidini G, Piccinni G, Portincasa P, Grattagliano I, Gurrado A, Testini M. Surgically-induced bile duct injury is followed by early hepatic oxidative stress. A preliminary experimental study in rats. Hepatogastroenterology. 2009;56(91–92):602–605. [PubMed] [Google Scholar]
- 15.Tiao MM, Lin TK, Wang PW, Chen JB, Liou CW. The role of mitochondria in cholestatic liver injury. Chang Gung Med J. 2009;32(4):346–353. [PubMed] [Google Scholar]
- 16.Portincasa P, Grattagliano I, Testini M, Caruso ML, Wang DQ, Moschetta A, et al. Parallel intestinal and liver injury during early cholestasis in the rat: modulation by bile salts and antioxidants. Free Radic Biol Med. 2007;42(9):1381–1391. doi: 10.1016/j.freeradbiomed.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Zhong Z, Froh M, Wheeler MD, Smutney O, Lehmann TG, Thurman RG. Viral gene delivery of superoxide dismutase attenuates experimental cholestasis-induced liver fibrosis in the rat. Gene Ther. 2002;9(3):183–191. doi: 10.1038/sj.gt.3301638. [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 2001;120(1):190–199. doi: 10.1053/gast.2001.20875. [DOI] [PubMed] [Google Scholar]
- 19.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115(10):2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dull RO, Dinavahi R, Schwartz L, Humphries DE, Berry D, Sasisekharan R, et al. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2003;285(5):L986–995. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- 21.Madara JL, Stafford J, Barenberg D, Carlson S. Functional coupling of tight junctions and microfilaments in T84 monolayers. Am J Physiol. 1988;254(3 Pt 1):G416–423. doi: 10.1152/ajpgi.1988.254.3.G416. [DOI] [PubMed] [Google Scholar]
- 22.Meza I, Ibarra G, Sabanero M, Martinez-Palomo A, Cereijido M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J Cell Biol. 1980;87(3 Pt 1):746–754. doi: 10.1083/jcb.87.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson BR, Begg DA. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J Cell Sci. 1994;107 ( Pt 3):367–375. doi: 10.1242/jcs.107.3.367. [DOI] [PubMed] [Google Scholar]
- 24.Rao RK. Biologically active peptides in the gastrointestinal lumen. Life Sci. 1991;48(18):1685–1704. doi: 10.1016/0024-3205(91)90205-p. [DOI] [PubMed] [Google Scholar]
- 25.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, et al. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284(3):1559–1569. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, et al. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J. 2007;402(2):291–300. doi: 10.1042/BJ20060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki T, Seth A, Rao R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J Biol Chem. 2008;283(6):3574–3583. doi: 10.1074/jbc.M709141200. [DOI] [PubMed] [Google Scholar]
- 28.Assimakopoulos SF, Grintzalis K, Thomopoulos KC, Papapostolou I, Georgiou CD, Gogos C, et al. Plasma superoxide radical in jaundiced patients and role of xanthine oxidase. Am J Med Sci. 2008;336(3):230–236. doi: 10.1097/MAJ.0b013e3181601158. [DOI] [PubMed] [Google Scholar]
- 29.Harada K, Nakanuma Y. Molecular mechanisms of cholangiopathy in primary biliary cirrhosis. Med Mol Morphol. 2006;39(2):55–61. doi: 10.1007/s00795-006-0321-z. [DOI] [PubMed] [Google Scholar]
- 30.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127(6 Pt 1):1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabaretnam T, Harris MJ, Kockx M, Witting PK, Le Couteur DG, Kritharides L. Effects of hydrogen peroxide and apolipoprotein E isoforms on apolipoprotein E trafficking in HepG2 cells. Clin Exp Pharmacol Physiol. 2009;36(12):e96–102. doi: 10.1111/j.1440-1681.2009.05306.x. [DOI] [PubMed] [Google Scholar]
- 32.Sato H, Takeo T, Liu Q, Nakano K, Osanai T, Suga S, et al. Hydrogen peroxide mobilizes Ca2+ through two distinct mechanisms in rat hepatocytes. Acta Pharmacol Sin. 2009;30(1):78–89. doi: 10.1038/aps.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vissers MC, Day WA, Winterbourn CC. Neutrophils adherent to a nonphagocytosable surface (glomerular basement membrane) produce oxidants only at the site of attachment. Blood. 1985;66(1):161–166. [PubMed] [Google Scholar]
- 34.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368(Pt 2):471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem. 2007;282(15):11487–11498. doi: 10.1074/jbc.M610597200. [DOI] [PubMed] [Google Scholar]
- 36.Clayburgh DR, Rosen S, Witkowski ED, Wang F, Blair S, Dudek S, et al. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J Biol Chem. 2004;279(53):55506–55513. doi: 10.1074/jbc.M408822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278(14):11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 38.Doctor RB, Dahl R, Fouassier L, Kilic G, Fitz JG. Cholangiocytes exhibit dynamic, actin-dependent apical membrane turnover. Am J Physiol Cell Physiol. 2002;282(5):C1042–1052. doi: 10.1152/ajpcell.00367.2001. [DOI] [PubMed] [Google Scholar]
- 39.Doctor RB, Fouassier L. Emerging roles of the actin cytoskeleton in cholangiocyte function and disease. Semin Liver Dis. 2002;22(3):263–276. doi: 10.1055/s-2002-34504. [DOI] [PubMed] [Google Scholar]
- 40.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 41.Furuse J. Growth factors as therapeutic targets in HCC. Crit Rev Oncol Hematol. 2008;67(1):8–15. doi: 10.1016/j.critrevonc.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Michalopoulos GK. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990;4(2):176–187. [PubMed] [Google Scholar]
- 43.Marti U, Burwen SJ, Jones AL. Biological effects of epidermal growth factor, with emphasis on the gastrointestinal tract and liver: an update. Hepatology. 1989;9(1):126–138. doi: 10.1002/hep.1840090122. [DOI] [PubMed] [Google Scholar]