Abstract
This review highlights some of the many contributions of the late Dr. Jerry J. Buccafusco to the neurobiology of nicotinic acetylcholine receptors (nAChRs) and cognition over a 25 year period. The article is written by two of Dr. Buccafusco's professional colleagues, one from academia and one from the pharmaceutical industry. While Dr. Buccafusco's expertise in the cholinergic field was extensive, his insights into the practical relevance of his work (with a long-term goal of formulating new drug development strategies) were unique, and a great asset to both the basic science community and pharmaceutical companies. In 1988, Dr. Buccafusco's laboratory was the first to report the cognitive enhancing action of low doses of nicotine in non-human primates. Since that time he studied a large number of novel pro-cognitive agents from several pharmacological classes in rodents as well as monkeys. Based on years of observing paradoxical effects of nicotinic ligands in vitro and in vivo, Dr. Buccafusco made the provocative argument that it might be possible to develop new chemical entities (with pro-cognitive actions) that have the ability to desensitize nAChRs without producing an antecedent agonist action. Some of his more recent work focused on development of single molecular entities that act on multiple CNS targets (including nAChRs) to enhance cognition, provide neuroprotection, and/or provide additional therapeutic actions (e.g., antipsychotic effects). Dr. Buccafusco's influence will live on in the work of the numerous graduate students, postdoctoral fellows, and junior faculty that he mentored over the years who now serve in prestigious positions throughout the world.
Introduction
The purpose of this review is to highlight just a few of the many contributions of Dr. Jerry J. Buccafusco (see photograph, Fig 1) to the neurobiology of nicotinic acetylcholine receptors (nAChRs) and cognition. Dr. Buccafusco's contributions to this specific focus area as well as the more general subject of novel drug discovery and development for disorders of cognition spanned more than 25 years. As will be evident in the following paragraphs, Dr. Buccafusco had a unique capability of thinking and functioning in truly “translational” context, a great asset to both the basic science community and the pharmaceutical industry. This review is written by two of Dr. Buccafusco's professional colleagues, Dr. Alvin V. Terry Jr., and Dr. Michael W. Decker, the former, an academic colleague and the later a colleague from the pharmaceutical industry. Over the course of a 20 year professional relationship Dr. Buccafusco served as a mentor, colleague, collaborator, and close personal friend to Dr. Terry. Likewise, Dr. Buccafusco was a close collaborator and friend of Dr. Decker for over 15 years.
Fig 1.
Jerry J. Buccafusco, Ph.D. (August 20, 1949 – March 6, 2010), Regents Professor of Pharmacology and Toxicology and Director, Alzheimer's Research Center, Medical College of Georgia. Dr. Buccafusco's contributions to the field of cholinergic neurobiology and novel drug discovery for disorders of cognition spanned more than 25 years.
A Brief Biography-Jerry J. Buccafusco (“Dr. B.,” as he was known to many) was born on August 20th, 1949 in Jersey City, New Jersey to Dominick and Rose Buccafusco. He was educated in Jersey City public schools, and his scientific career began with a chemistry set in his father's basement. He attended St. Peter's College, graduating with a bachelor's degree in Chemistry in 1971. He later received a Master's degree in Inorganic Chemistry from Canisius College in 1973 and a Ph.D. in Pharmacology from the University of Medicine and Dentistry of New Jersey in 1978 (research advisor, Henry E. Brezenoff). During graduate school, he married Regina Neilan, a long-time friend, because, according to her, his car broke down and he needed transportation. Dr. Buccafusco subsequently received postdoctoral training at the Roche Institute of Molecular Biology, Department of Physiological Chemistry and Pharmacology from 1977-1979 under the guidance of the department head, Dr. Sydney Spector. Later in 1979, Jerry and Regina moved to Georgia when he was offered a position as Assistant Professor in the Department of Pharmacology and Toxicology at the Medical College of Georgia (MCG), Augusta, GA. Over the next thirty years, Dr. Buccafusco was appointed Director of the Neuropharmacology Laboratory at the Veteran's Affairs Medical Center in Augusta, and he was the founder and director of both the Alzheimer's Research Center and the Animal Behavior Center at MCG. He was also the inspiration and the original President and CEO of a successful contract research organization (CRO), Prime Behavior Testing Laboratories (PBTLI, Inc.) which specializes in the preclinical evaluation of novel therapeutic agents for learning and memory-related disorders.
Dr. Buccafuso's research, which comprises more than 200 peer-reviewed articles in scientific journals, four edited books, and 5 US patents, has made significant contributions to a variety of fields, including hypertension, drug abuse, Gulf War Illness, Alzheimer's Disease and schizophrenia research. In addition, he served as an associate editor of the Journal of Pharmacology and Experimental Therapeutics, was a member of the scientific advisory board of the Alzheimer's Drug Discovery Foundation & the Institute for the Study of Aging, and he served as the chair of a National Institutes of Health study section, “Drug Discovery for the Nervous System”, ZRG1 MDCN-C (91) from 2006-2010. Over the course of his career, Dr. Buccafusco received numerous accolades including Regents’ Professor of the University of Georgia System, the Distinguished Alumnus Award from the University of Medicine and Dentistry of New Jersey, Research Career Scientist Award, Department of Veterans Affairs, and the Society for Pharmacology and Experimental Therapeutics – ASPET Award for Experimental Therapeutics. Dr. Buccafusco's research was continually supported by federally-sponsored grants, private foundations, as well as the pharmaceutical industry for more than 25 years.
Cholinergic Neurobiology: The Early Years
Dr. Buccafusco's graduate training in pharmacology, his postdoctoral work, and the early years of his academic career focused on the role of cholinergic neurons in the central nervous system's regulation of the cardiovascular system. This seminal work contributed to our understanding of how cholinergic neurons in the brain (particularity the brain stem) influence blood pressure as well as how several antihypertensive agents (e.g., clonidine, methyldopa) act on central cholinergic neurons either directly or indirectly to decrease blood pressure [1] and [2]. Later his focus shifted to studying the means to prevent or reverse the acute toxicity associated with the most toxic forms of cholinesterase inhibitors – the chemical nerve agents such as soman and sarin. His laboratory was first to demonstrate the protective potential of clonidine and related centrally-acting α2-adrenergic receptor agonists in soman poisoning in rodents [3]. Clonidine was shown not only to enhance survivability, but to prevent the long-term behavioral impairments in survivors of soman exposure. Around this same time Dr. Buccafusco also began working in the field of drug abuse research, the beginnings of his long time participation in the Veteran's Administration Merit system. These studies focused on the role of central cholinergic neurons in the development of physical dependence on opiates, the expression of withdrawal symptoms, and the return to drug-seeking behavior [4].
The Pro-Cognitive effects of Nicotine in Non-human primates
It the mid 1980s Dr. Buccafusco began to collaborate with a fellow faculty member at the Medical College of Georgia (Department of Physiology), Dr. William J. Jackson, an experimental psychologist who specialized in non-human primate behavior, especially cognition. The synergy produced by the combined efforts of a cholinergic neuropharmacologist and a primate behavioral scientist lead to a number of important contributions that would be recognized by the academic community as well as the pharmaceutical industry. In 1988, Drs Buccafusco and Jackson (with graduate student, Karey Elrod) were the first to report that low doses of nicotine could enhance performance of a working/short-term memory task, the Delayed Match to Sample (DMTS) task in non-human primates [5]. Later they showed that this effect could be produced in both young and aged monkeys [6], an observation that had obvious ramifications for the potential treatment of age-related disorders of cognition (e.g., Alzheimer's Disease). Another observation that especially intrigued Dr. Buccafusco throughout his career was that when the doses of nicotine were optimized for individual monkeys (i.e., for effects on DMTS performance), the pro-cognitive effects lasted until the following day's testing session (i.e., 24 hours later) without any additional nicotine being administered. Given the known pharmacokinetics of nicotine (half life approximately 2-3 hours), this observation suggested that either a metabolite of nicotine (e.g., cotinine) might be contributing to the pro-cognitive effects of nicotine or that some plasticity-related process was being activated. These observations are also discussed further in the section below entitled “Long Lasting Cognitive Improvement with Nicotinic Receptor Agonists”.
Pro-Cognitive Effects of Other Nicotinic Ligands
The pro-cognitive effects of nicotine in non-human primates described above stimulated a number of collaborations between Dr. Buccafusco and the pharmaceutical industry that extended throughout the rest of his career. Interest in the role of the cholinergic system in cognitive performance has a long history but was not a primary focus of the pharmaceutical industry until a series of observations revealed that Alzheimer's disease is characterized by cholinergic deficits [7]. Early efforts to develop cognition enhancers focused mainly on cholinesterase inhibitors and muscarinic agonists, with the former resulting in the approval of tacrine, the first medication approved for symptomatic treatment in AD. Continued work in this area culminated in additional cholinesterase inhibitors with improved safety profiles, including the current market leader, donepezil, but efforts to develop muscarinic agonists have not been successful.
In these early days, there was little attention on nAChRs for the treatment of AD. It was known, of course, that nicotine could improve performance in humans, but since subjects in many of these studies were smokers, the interpretation that nicotine acted by reversing withdrawal effects on cognitive performance provided a reasonable alternative explanation of the results. Dr. Buccafusco's demonstration that nicotine improved cognitive performance in nonhuman primates and similar reports in rodents provided strong support for the notion that nicotine itself had cognition-enhancing properties [8]. Moreover, during this period, cloning of neuronal nAChR subunits and the ensuing efforts to understand the different roles of nAChR subtypes [9] led to an increase in interest in the therapeutic potential of nAChR ligands that continues to this day.
Abbott first entered into collaboration with Dr. Buccafusco in the early 1990s through the efforts of Dr. Steve Arneric, who, along with Dr. Mike Williams, had just established the nAChR drug discovery program at Abbott, and the collaboration continued for nearly 20 years until Dr. Buccafusco's untimely death. During this time, Dr. Buccafusco assessed the effects of multiple nicotinic compounds on delayed-matching-to-sample (DMTS) performance in nonhuman primates, including the first Abbott compound to advance to the clinic, ABT-418 [10] and [11]. The efficacy Dr. Buccafusco observed in both mature and aged nonhuman primates provided critical support in that it demonstrated that the pro-cognitive effects previously established in rodents translated to primates. Moreover, a small study subsequently conducted by Dr. Paul Newhouse and colleagues in AD patients, confirmed the prediction that acute administration of ABT-418 could improve performance on cognitive measures in AD [12]. However, these encouraging results did not translate into efficacy in a subsequent large phase 2, multi-center trial in AD.
Later, Abbott and Dr. Buccafusco's laboratory collaborated on the development of a modification of the DMTS procedure to assess the effects of nAChR agonists on attention by introducing distractor stimuli [13]. Dr. Mark Prendergast and Dr. Buccafusco then demonstrated robust effects of nicotine and ABT-418 in this new procedure [14]. Notably, there was evidence of efficacy for these same compounds in small clinical trials in adults with ADHD. Both Drs. Keith Conners and Ed Levin and collaborators had demonstrated positive effects of nicotine in small ADHD trials [15] [16] and [17], and Dr. Tim Willens had demonstrated efficacy with ABT-418 in a cross-over trial in adults with ADHD [18]. However, the advancement of either compound was limited by tolerability issues and poor pharmacokinetics. Abbott did have ABT-089, an α4β2-selective partial agonist with improved preclinical tolerability, safety, and PK characteristics at the time [19] and [20], but it was uncertain if the pilot clinical findings in ADHD with nicotine and ABT-418 could be generalized to ABT-089 because nicotine and ABT-418 are full agonists and both are less subtype selective than ABT-089. It was therefore of critical importance to determine if a subtype-selective partial agonist with very low intrinsic agonist activity like ABT-089 could demonstrate efficacy in a relevant animal model. The subsequent demonstration that ABT-089 had efficacy as good as or better than that demonstrated by nicotine and ABT-418 provided the impetus to move ABT-089 forward in a new indication, ADHD [14]. Results from initial trials with ABT-089 in ADHD were encouraging. An efficacy signal in an initial small cross-over trial in adults [21] was confirmed in a larger, multi-center trial [22]. However, these effects were not replicated in subsequent parallel group studies in children with ADHD [23].
Dr. Buccafusco's collaboration with Abbott continued as Abbott shifted focus to the α7 nAChR more recently. Dr. Buccafusco compared the effects of α7-targeted compounds with those targeting subtypes with higher affinity for nicotine and discovered that the profiles produced were distinct. For example, ABT-594, a full agonist with little affinity for α7 nAChRs has robust effects at shorter delays in DMTS and is active in the distractor DMTS, whereas the effects of the α7 agonist A-582941 in DMTS are restricted to the long delays [24]. This more pronounced effect on the long delay is also observed with other α7 compounds, including ABT-107 [25]. Thus, α7 ligands appear to have more pronounced effects on memory, with other nAChR ligands having more robust effects on attention. This finding suggests that alternative therapeutic indications should be considered for these different groups of nAChR agonists and that combining these agents could produce additional benefit.
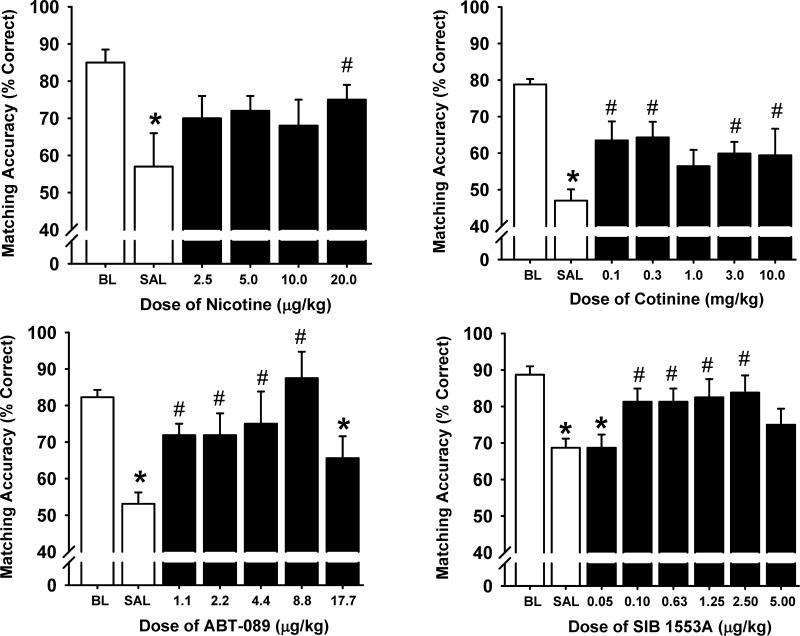
Abbott was an early industry collaborator of Dr. Buccafusco's, but was by no means alone in this endeavor. Dr. Buccafusco maintained productive collaborations with a variety of pharmaceutical companies. His activities in drug discovery also extended well beyond nAChRs, but given the focus of this review on nAChRs, his work with SIBIA, a pioneer of nAChR drug discovery is of particular note. Through this collaboration, Dr. Buccafusco provided preclinical validation of SIB-1553A, an nAChR agonist that is unusual in that it shows a preference for β4-containing nAChRs, demonstrating that SIB-1553A improved the performance of nonhuman primates in DMTS in both the presence and absence of a distractor stimulus [26] and [27]. Figure 3 illustrates positive results obtained with the Abbott compound ABT-089, the SIBIA compound SIB-1553A as well as nicotine and its major metabolite cotinine.
Fig 3.
Effects of nicotinic receptor ligands on performance of the Delayed Match to Sample task with distractors (DMTS-D) in monkeys. Short delay performance during interference sessions is illustrated. Data are represented as mean percentage correct ± S.E.M. BL= saline baseline for standard DMTS performance; SAL = saline baseline performance associated with 3-s distractor trials. *= significantly different (p<0.05) compared to vehicle baseline levels of DMTS accuracy. # = significantly different (p<0.05) compared to the saline distractor trial-related level of accuracy. N= 5-8
Despite the preclinical success of these collaborations and some evidence of the translation of signals of efficacy into early stage clinical trials, several nAChR ligands have failed in more advanced trials. There are still no nAChR ligands yet approved for any indication other than smoking cessation (varenicline and, of course, nicotine). Never the less, the work that Dr. Buccafusco conducted in collaboration with industry has been critical in advancing the most appropriate compounds for testing hypotheses regarding the potential of nAChR ligands. The failure of a compound to treat a disease in a clinical trial is difficult to interpret without evidence that the pharmacology of the compound observed in rodents can be translated to nonhuman primates and ultimately in some fashion to humans. In other words, if we are not confident that the compound engages the molecular target in primates, we do not know if the lack of effect in a clinical trial is the result of limited therapeutic utility of the target or failure of the specific compound to engage the target appropriately in a higher species. Dr. Buccafusco's collaboration with industry did not eliminate this concern, given potential differences between effects in nonhuman primates and humans, but clearly efficacy in nonhuman primates provides greater assurance than efficacy restricted to rodents.
Cotinine: Nicotine metabolite with Pro-Cognitive and Antipsychotic-Like Properties
As noted above, the protracted (pro-cognitive) effects of nicotine after a single dose suggested the possibility that a long-lived metabolite of nicotine and/or the induction of some prolonged pharmacodynamic or plasticity-related process (activated by nicotine or one or more of its metabolites) might be responsible. Cotinine is a primary metabolite of nicotine with a long pharmacological half-life (range of approximately 16-20 hr depending on the body fluid analyzed) relative to nicotine (range of approximately 30 min to 3 hr) [28] [29] [30] [31] and [32]. After nicotine consumption the duration of cotinine's presence in blood and brain greatly exceeds that of nicotine. However, most likely due to its comparatively low potency in receptor binding and functional assays (conducted many years ago for the most part) relatively little attention had been given to the behavioral effects of cotinine (especially in the context of memory and cognition). Drs. Buccafusco and Terry collaborated on several projects to systematically evaluate cotinine in behavioral assays in rodents and non-human primates for pro-cognitive effects as well as for potential antipsychotic effects. In monkeys, cotinine elicited dose-dependent improvements of a DMTS task (see Fig 2) as well as an attention-related version of the task (DMTS-D) where randomly-presented (task-relevant) distractors were presented [33]. Cotinine also attenuated deficits in DMTS produced by the glutamate NMDA receptor antagonist ketamine [34]. In rats cotinine was evaluated for its ability to improve prepulse inhibition (PPI) of the acoustic startle response, a property that may predict the efficacy of compounds as antipsychotic agents as well as cognitive enhancers. In this study, PPI was disrupted in Wistar rats in three pharmacologic models: dopamine receptor agonism by apomorphine, NMDA receptor antagonism by MK801, or muscarinic acetylcholine receptor antagonism by scopolamine. Cotinine (depending on dose) improved PPI deficits in all three PPI disruption models [33].
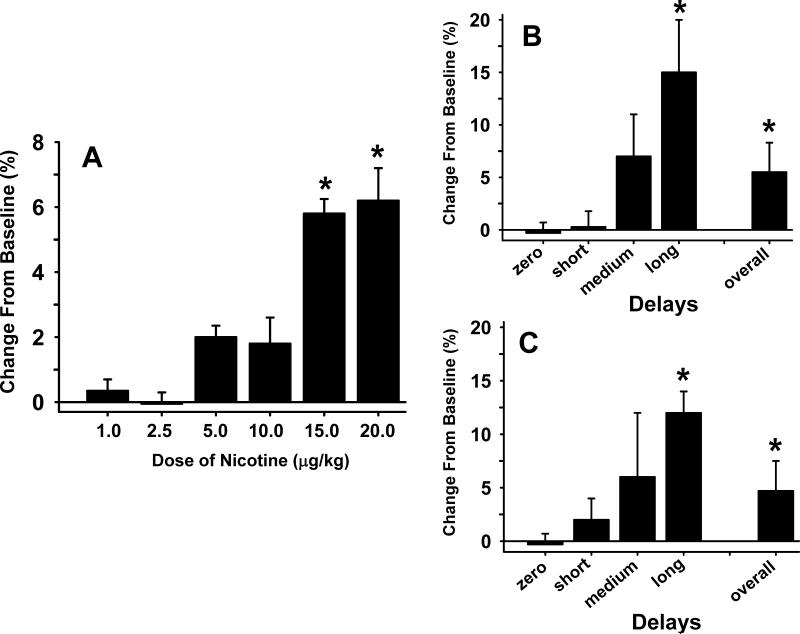
Fig 2.
Effects of Nicotine on Delayed Match to Sample (DMTS) performance in monkeys. Fig 1A illustrates the dose-effect relationship (2-3 replicates per dose) in the DMTS task (averaged across delay) in 5 adult pigtail monkeys, 10 min after the intramuscular administration of nicotine. Each bar represents the mean percentage change from vehicle baseline ± SEM over 96 trials per session. Fig 2B illustrates the effects of the individualized (and replicated) optimum dose of nicotine on each delay in the DMTS. Fig 2C illustrates the effect of the optimum dose of nicotine DMTS accuracy when the test was administered 24 h after dosing. *= significantly different (p<0.05 compared) to vehicle baseline levels of DMTS accuracy.
Collectively, the results described above indicate that cotinine improves information processing, attention, and memory-related task function in animal models. The data support the role of cotinine as a prototypical representative of a novel class of therapeutic agents for disorders of cognition such as AD. The positive effects observed in the PPI studies and the DMTS-D experiments suggest that cotinine might be also useful in neuropsychiatric conditions not necessarily associated with advanced aged such as schizophrenia. Given the reported safety profile of cotinine compared to nicotine in humans especially on blood pressure, heart rate and the electrocardiogram [35], the cotinine structure might serve as a platform for novel drug development. Dr. Terry's laboratory currently has studies underway to test this hypothesis.
Nicotinic Receptor Antagonism and Desensitization: Paradoxical Effects on Cognition
Nicotinic drug discovery platforms designed to improve cognition have to date focused primarily on enhancing activity at nAChRs in an effort to promote the release of neurotransmitters in memory-related brain regions. A variety of nAChR agonists, partial agonists and allosteric modulators have been developed for this purpose and some may have significant potential as therapeutic agents for the treatment of AD and other neuropsychiatric disorders [36]. However, in a recent review, Dr. Buccafusco (based on a number of paradoxical observations in his laboratories and those of others) made the argument that such an approach may be too simplistic [37]. This argument is supported by observations that, in number of settings, nAChR agonists and antagonists can have very similar physiologic effects. Moreover, a large discrepancy often exists between the high concentrations of nicotinic ligands required to activate nicotinic responses in vitro and the lower doses used to induce various behavioral responses in vivo. Nicotine can both activate and desensitize its receptors over a relatively short time course leading to the question of whether (in fact) nAChR desensitization when compared to receptor activation, plays an equal if not more important role in the overall behavioral effects. In recently published experiments, the effectiveness of four compounds (nicotine, cotinine, and two novel analogs of choline, JWB1-84-1 and JAY2-22-33, see [38] as pro-cognitive agents in the monkey DMTS task was linearly related to their effectiveness in producing desensitization of a nAChR agonist response in rats [37]. Only nicotine evoked a significant agonist-like action in these studies indicating that it is possible to develop new chemical entities (e.g., choline analogs, cotinine analogs) that have the ability to desensitize nAChRs without a significant antecedent agonist action (i.e., silent desensitizers). Since the side effects of nicotine (e.g., cardiovascular, gastrointestinal) are often associated with its agonist effects, such an approach could offer the advantage of better tolerability.
Long Lasting Cognitive Improvement with Nicotinic Receptor Agonists
As discussed above in the paragraphs devoted to the pro-cognitive effects of nicotine, one hypothesis for its sustained effects is that a long-lived metabolite (e.g. cotinine) might be responsible. Another hypothesis is that some pharmacodynamic or plasticity-related process activated by nicotine or one of its metabolites might be responsible. The validity of the later argument is supported by the fact that a variety of additional nicotinic agonists and partial agonists (e.g., isoarecolone, ABT-089, GTS-21,) also appear to produce the prolonged cognitive effect. As reviewed by Dr. Buccafuso and colleagues [39], the mechanism of this sustained pro-cognitive effect may be related to the nACHR agonist-initiated cascade of cellular and molecular signals that begin with the influx of calcium and other cations. Elevations of intracellular calcium concentrations have been linked to a variety of events that influence information processing and cognition such as enhanced neurotransmitter release, activation of specific kinases (e.g., calmodulin-dependent kinase, MAPK), long-term potentiation (LTP), and CREB phosphorylation.
Another nACHR agonist-related phenomenon (investigated by Dr. Buccafusco and his colleagues) that might contribute to their sustained effects on cognition involves positive effects on neurotrophins and/or their receptors. This hypothesis was initially based on in vitro experiments in PC-12 cells [40] where exposure to nicotine enhanced the expression of the receptors for the prototypical neurotrophin, nerve growth factor (NGF). This neurotrophin is well documented to support adult basal forebrain cholinergic neurons (i.e., neurons well documented to influence memory and cognition, see Bartus, 2000 for review [41]). A number of subsequent in vivo reports indicated nicotine-related increases in NGF mRNA in the hippocampus [42], NGF protein in the hippocampus [43], and NGF-like immunoreactivity in the fronto-parietal cortex [44]. Nicotine-related increases in the high affinity NGF receptor TrKA and its activated form phospho-TrkA in the hippocampus have also been reported [45].
Multiple CNS Drug Targets
Some of Dr. Buccafusco's recent collaborative work was also directed at the development of single molecular entities that act on multiple CNS targets (including nAChRs). The goal was to develop novel agents that (in addition to enhancing cognition) could provide neuroprotection, alter the disposition/metabolism of amyloid, and/or have additional (desirable) properties such as antipsychotic activity. In illnesses such as AD, the complex pathophysiology and diverse symptoms provide a significant challenge from a therapeutic target standpoint. While there are compounds available to treat the cognitive deficits associated with AD (e.g., donepezil, galantamine, memantine), their effects are modest at best and there is little evidence that they have significant (and sustained) disease-modifying actions. The so-called non-cognitive behavioral symptoms (e.g., agitation, hallucinations, etc) of AD also present a particularly challenging problem since the available remedies (i.e., the antipsychotics) have been associated with severe side effects and increased mortality in AD patients [46] and [47]. Thus, a compound exhibiting pro-cognitive and antipsychotic properties might be especially valuable in AD as well as conditions like schizophrenia which is characterized by abnormal behavioral symptoms as well as cognitive deficits. A single compound with activity at several therapeutic targets would also address the limitation of combining two or more drugs with potentially different degrees of bioavailability, pharmacokinetics, and metabolism [48]. An additional theoretical advantage to a single drug with multiple therapeutic actions would be the simplification of dosing regimen (an advantage that could be especially important from a compliance perspective in individuals with memory-related disorders). Over the last several years Dr Buccafusco and his collaborators have reported the positive effects of several prototypical agents with two or more therapeutic actions. For example MHP-133 (a pyridostigmine analog with mild acetylcholinesterase inhibitor properties and modest activity at several subtypes of cholinergic, serotonergic, and imidazoline receptors) was found to have neuroprotective properties in cell culture and the ability to improve cognition in rats and monkeys [49]. JWB1-84-1, a piperazine-based choline analog was found to have neuroprotective activity in cell culture [50] [51] and caenorhabditis elegans [51] and to improve memory-related task performance in AD-Tg mice and aged macaques. In collaboration with Dr. Terry, JWS-USC-75IX (3-[[[2-[[(5-dimethylaminomethyl)-2-furanyl]methyl]thio]ethyl]amino]-4-nitropyridazine), a ranitidine analog with antagonist activity at M2 acetylcholine receptors and acetylcholinesterase, was found to exhibit pro-cognitive and antipsychotic-like activity in animal models. Specifically, in rats, JWS-USC-75IX improved performance of a spontaneous novel object recognition task and a five choice serial reaction time task, and it improved prepulse inhibition of the acoustic startle response. In monkeys, JWS-USC-75IX improved the performance of a DMTS task as well as an attention-related version of the task where randomly-presented (task-relevant) distractors were presented [52].
Other Contributions
Dr. Buccafusco's most recent work also spanned a number of non-nicotinic areas that are beyond the scope of this review. However, it is noteworthy to briefly discuss one particular area of focus that might eventually lead to better diagnostic tools for Alzheimer's disease (AD) as well as its treatment. In collaboration with Dr. Steven Miller at the University of Georgia, recent experiments were begun to clarify the role of specific immune responses in AD and IgGs directed against amyloid-beta (Aβ) and the neuronal membrane receptor for advanced glycation end products (RAGE). Aβ proteins form the so-called “amyloid plaques” in AD, while RAGE binds advanced glycation end products (AGE) that are detected in normal aging processes in tissues. Experiments conducted to date support the premise that both Aβ and RAGE increase significantly with age and that they both play an active role in AD-related pathological events and cognitive decline [53] [54] and [55]. This line of research is currently being pursued by Scott Webster (former graduate student in Dr. Buccafusco's laboratory) who is further evaluating these proteins as potential biomarkers of AD. He is also evaluating a protein complex vaccine that targets both RAGE and Aβ1-42 as a new therapeutic strategy for AD.
Conclusion
As is evident in the preceding paragraphs, Dr. Buccafusco's numerous publications and accolades are testament to his scientific achievements across a number of important fields in neuroscience and in particular, the neurobiology of nAChRs and cognition. He was also an excellent teacher at both the professional and graduate level, he served on numerous institutional and national committees and he was a great colleague and collaborator to other scientists around the world. His scholarly activities, goodwill, and leadership will truly be missed. His influence, however, will live on in the work of the numerous graduate students, postdoctoral fellows and junior faculty that he mentored (see Table 1) who now serve in prestigious positions throughout the world.
Table 1.
Jerry J. Buccafusco, Ph.D., Professional Trainees
| Graduate Students | ||
|---|---|---|
| Name | Degree Received | Year |
| Dennis C. Marshall | Ph.D. | 1985 |
| Nevine F. Makari | M.S. | 1988 |
| Karey Elrod | Ph.D. | 1989 |
| Xiaohong Yang | Ph.D. | 1993 |
| Frances F. Martin | M.S. | 1993 |
| Jian Wei | Ph.D. | 1994 |
| David Feldman | M.D., Ph.D. | 1995 |
| Mahanandeeshwar Gattu | Ph.D. | 1996 |
| Lu Zhang | Ph.D. | 1997 |
| J. Bradly Summers | M.S. | 1997 |
| Ramamohana Jonnala | Ph.D. | 2002 |
| Xinyu (Daniel) Li | M.D., Ph.D. | 2007 |
| Ajay Sood | Ph.D. | 2007 |
| Postdoctoral Fellows and Junior Faculty | ||
| Name | Institution of Origin | Years |
| Dr. Mariangela Serra, | University of Cagliari, Italy | 1984-1985 |
| Giuseppe R. Trimarchi, M.D | Institute of Pharmacology, Messina, Italy | 1985-1987 |
| Anthony G. Molloy | Royal College of Surgeons, Dublin, Ireland | 1986 |
| Venera Magri', M.D | Institute of Pharmacology, Messina, Italy | 1986-1988 |
| Hiroaki Takahashi, D.V.M., Ph.D | The Institute of Environmental Toxicology, Mitsukaido, Japan, | 1988-1990 |
| Alvin V. Terry, Jr., Ph.D | University of South Carolina, Columbia, SC | 1991-1993 |
| Leland N. Holland, Ph.D | Medical College of Georgia, Augusta, GA | 1991-1993 |
| Carol A. Lapp, Ph.D | Medical College of Georgia, Augusta, GA | 1992-1994 |
| Mark A. Prendergast, Ph.D | University of Nebraska, Omaha, NE | 1994-1997 |
| Jerry A. Davis, II, M.D | Medical College of Georgia, Augusta, GA | 1996 |
| Ganapathy K. Bhat, Ph.D | University of Mysore, India | 1998 |
| J. Derek Stone, Ph.D. | Medical College of Georgia, Augusta, GA | 1998-2000 |
| Shyamala Mruthinti, Ph.D | VA Medical Center, Augusta, GA | 1999-2006 |
| Bao-Ling Adam, Ph.D. | Medical College of Georgia, Augusta, GA | 2009-2010 |
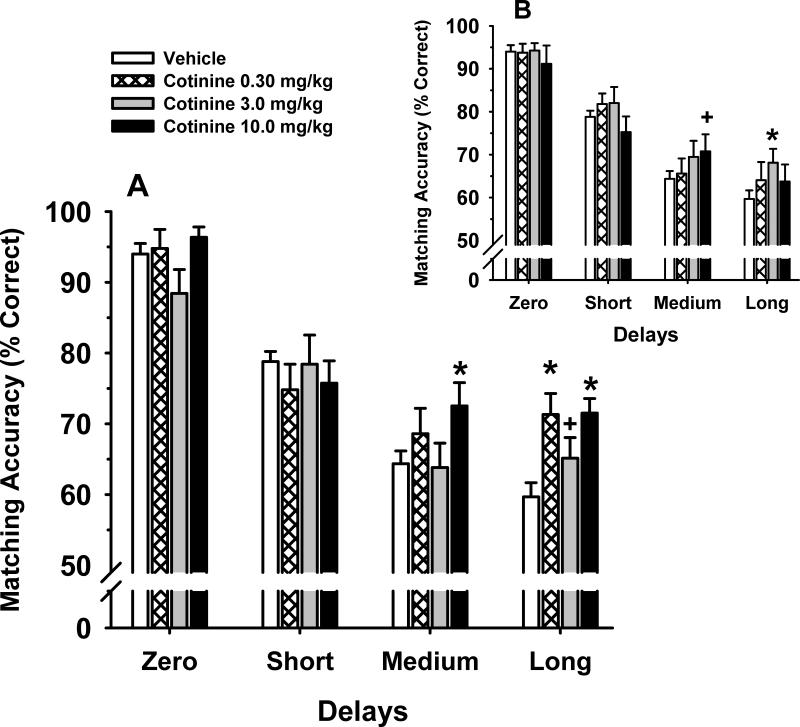
Fig 4.
Effects of cotinine on Delayed Match to Sample (DMTS) performance in monkeys. Fig 2A illustrates the dose-effect relationship for each delay in the DMTS task in 8 adult rhesus macaques, 15 min after the intramuscular administration of cotinine. Each bar represents the mean (% correct) ± SEM over 96 trials per session. The baseline (Vehicle) was determined from the average of all vehicle sessions run throughout the study. Inset (Fig 2B): the effect of cotinine on standard DMTS accuracy when the test was administered 24 h after dosing. *= significantly different (p<0.05 compared) to vehicle baseline levels of DMTS accuracy. + = p<0.07.
Acknowledgments
The authors would like to thank Ms. Ashley Davis for her administrative assistance in preparing this article. This corresponding author's laboratories are supported by the National Institute of Aging (AG032140 and AG029617), the National Institute of Environmental Health Sciences (ES012241), and the National Institute on Drug Abuse (DA029127). The corresponding author has also provided consultation or performed research (relevant to this article) in the last three years either contractually or in collaboration with a several pharmaceutical companies including Abbott Laboratories, Helicon, Merck, Neuralstem, Neurosearch, Roche Palo Alto, and Servier.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buccafusco JJ, Spector S. Role of central cholinergic neurons in experimental hypertension. J. Cardiovascular Pharmacol. 1980;2:347–55. doi: 10.1097/00005344-198007000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Buccafusco JJ, Brezenoff HE. Brain neurotransmitters and the development and maintenance of experimental hypertension. Prog. Drug. Res. 1986;30:127–50. doi: 10.1007/978-3-0348-9311-4_4. [DOI] [PubMed] [Google Scholar]
- 3.Buccafusco JJ, Aronstam RS. Clonidine protection from the toxicity of soman, an organophosphate acetylcholinesterase inhibitor, in the mouse. J. Pharmacol. Exp. Ther. 1986;239:43–7. [PubMed] [Google Scholar]
- 4.Marshall DC, Buccafusco JJ. Spinal cholinergic neurons and the expression of morphine withdrawal symptoms in the rat. J. Neurosci. 1987;7:621–8. doi: 10.1523/JNEUROSCI.07-03-00621.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elrod K, Buccafusco JJ, Jackson WJ. Nicotine enhances delayed matching-to-sample performance by primates. Life Sci. 1988;43:277–87. doi: 10.1016/0024-3205(88)90318-9. [DOI] [PubMed] [Google Scholar]
- 6.Buccafusco JJ, Jackson WJ. Beneficial effects of nicotine administered prior to a delayed matching-to-sample task in young and aged monkeys. Neurobiol. Aging. 1991;12:233–8. doi: 10.1016/0197-4580(91)90102-p. [DOI] [PubMed] [Google Scholar]
- 7.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982 Jul 30;217(4558):408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 8.Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998 Aug;138(3-4):217–30. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 9.Lindstrom J. Neuronal nicotinic acetylcholine receptors. In: Narahashi T, editor. Ion channels. Vol. 4. Plenum; New York: 1996. pp. 377–450. [DOI] [PubMed] [Google Scholar]
- 10.Buccafusco JJ, Jackson WJ, Terry AV, Marsh KC, Decker MW, Arneric SP. Improvement in performance of a delayed matching-to-sample task by monkeys following ABT-418: A novel cholinergic channel activator for memory enhancement. Psychopharmacology. 1995;120(3):256–66. doi: 10.1007/BF02311172. [DOI] [PubMed] [Google Scholar]
- 11.Prendergast MA, Terry AV, Jackson WJ, Marsh KC, Decker MW, Arneric S,P, et al. Improvement in accuracy of delayed recall in aged and non-aged, mature monkeys after intramuscular or transdermal administration of the CNS nicotinic receptor agonist ABT-418. Psychopharmacology. 1997;130(3):276–84. doi: 10.1007/s002130050240. [DOI] [PubMed] [Google Scholar]
- 12.Potter A, Corwin J, Lang J, Piasecki M, Lenox R, Newhouse PA. Acute effects of the selective cholinergic channel activator (nicotinic agonist) ABT-418 in Alzheimer's disease. Psychopharmacology (Berl) 1999;142(4):334–42. doi: 10.1007/s002130050897. [DOI] [PubMed] [Google Scholar]
- 13.Prendergast MA, Jackson WJ, Terry AV, Kille NJ, Arneric SP, Decker M,W, et al. Age-related differences in distractibility and response to methylphenidate in monkeys. Cerebral Cortex. 1998;8(2):164–72. doi: 10.1093/cercor/8.2.164. [DOI] [PubMed] [Google Scholar]
- 14.Prendergast MA, Jackson WJ, Terry AV, Decker MW, Arneric SP, Buccafusco JJ. Central nicotinic receptor agonists ABT-418, ABT-089, and (-)-nicotine reduce distractibility in adult monkeys. Psychopharmacology. 1998;136(1):50–8. doi: 10.1007/s002130050538. [DOI] [PubMed] [Google Scholar]
- 15.Levin E, Conners C, Sparrow E, Hinton S, Erhardt D, Meck W, et al. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology. 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- 16.Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD). Psychopharmacology Bulletin. 1996;32(1):67–73. [PubMed] [Google Scholar]
- 17.Conners CK, Levin ED, March J, Sparrow E, Erhardt D. Neurocognitive and behavioral effects of nicotine in adult attention deficit hyperactivity disorder (ADHD). Psychopharmacology Bulletin. 1995;31(3):559. [PubMed] [Google Scholar]
- 18.Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, et al. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156(12):1931–7. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- 19.Decker MW, Bannon AW, Curzon P, Gunther KL, Brioni JD, Holladay MW, et al. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine dihydrochloride]: II. A novel cholinergic channel modulator with effects on cognitive performance in rats and monkeys. J Pharmacol Exp Ther. 1997;283:247–58. [PubMed] [Google Scholar]
- 20.Arneric SP, Campbell JE, Carroll S, Daanen JF, Holladay MW, Johnson P, et al. ABT-089 (3-(2(S)-pyrrolidinylmethoxy)-2-methyl-pyridine): An orally effective cholinergic channel modulator with potential once-a-day dosing and cardiovascular safety. Drug Devel Res. 1997;41(1):31–43. [Google Scholar]
- 21.Wilens T, Verlinden MH, Adler LA, Wozniak PA, West SA. ABT-089, A Neuronal Nicotinic Receptor Partial Agonist, for the Treatment of Attention-Deficit/Hyperactivity Disorder in Adults: Results of a Pilot Study. Biological Psychiatry. 2006;59(11):1065–70. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Abi-Saab W, Wilens TE, Apostol G. Efficacy and safety of ABT-089 in adults with attention deficit/hyperactivity disorder.. 161st Annual American Psychiatric Association Meeting; Washington, D.C.. May 3-8; 2008. pp. Poster #NR6–043. al. e. [Google Scholar]
- 23.Wilens TE, Gault LM, Childress A, Kratochvil CJ, Bensman L, Hall CM, et al. Safety and Efficacy of ABT-089 in Pediatric Attention Deficit/Hyperactivity Disorder: Results from Two Randomized Placebo-Controlled Clinical Trials. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:73–84. doi: 10.1016/j.jaac.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buccafusco JJ, Terry AV, Jr, Decker MW, Gopalakrishnan M. Profile of nicotinic acetylcholine receptor agonists ABT-594 and A-582941, with differential subtype selectivity, on delayed matching accuracy by young monkeys. Biochemical Pharmacology. 2007;74(8):1202–11. doi: 10.1016/j.bcp.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Bitner RS, Bunnelle WH, Decker MW, Drescher KU, Kohlhaas KL, Markosyan S, et al. In Vivo Pharmacological Characterization of a Novel Selective alpha 7 Neuronal Nicotinic Acetylcholine Receptor Agonist ABT-107: Preclinical Considerations in Alzheimer's Disease. J. Pharmacol. Exp. Ther. 2010;334(3):875–86. doi: 10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- 26.Bontempi B, Whelan KT, Risbrough VB, Rao TS, Buccafusco JJ, Lloyd GK, et al. SIB-1553A, (+/-)-4-{(2-(1-methyl-2-pyrrolidinyl)ethyl)thio}phenol hydrochloride, a subtype-selective ligand for nicotinic acetylcholine receptors with putative cognitive-enhancing properties: Effects on working and reference memory performances in aged rodents and nonhuman primates. J. Pharmacol. Exp. Ther. 2001;299(1):297–06. [PubMed] [Google Scholar]
- 27.Terry AV, Risbrough VB, Buccafusco JJ, Menzaghi F. Effects of (+/-)-4-{(2-(1-methyl-2-pyrrolidinyl)ethyl)thio}phenol hydrochloride (SIB-1553A), a selective ligand for nicotinic acetylcholine receptors, in tests of visual attention and distractibility in rats and monkeys. J. Pharmacol. Exp. Ther. 2002;301(1):284–92. doi: 10.1124/jpet.301.1.284. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL, Jacob P, 3rd, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982 May;221(2):368–72. [PubMed] [Google Scholar]
- 29.Feyerabend C, Ings RM, Russel MA. Nicotine pharmacokinetics and its application to intake from smoking. Br J Clin Pharmacol. 1985 Feb;19(2):239–47. doi: 10.1111/j.1365-2125.1985.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988 Jun;78(6):696–8. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benowitz NL, Jacob P. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–93. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 32.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 33.Terry AV, Jr, Hernandez CM, Hohnadel EJ, Bouchard KP, Buccafusco JJ. Cotinine. A Neuroactive Metabolite of Nicotine: Potential for Treating Disorders of Impaired Cognition. CNS Drug Reviews. 2005;11:229–52. doi: 10.1111/j.1527-3458.2005.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buccafusco JJ, Terry AV., Jr A reversible model of the cognitive impairment associated with schizophrenia in monkeys: potential therapeutic effects of two nicotinic acetylcholine receptor agonists. Biochemical Pharmacology. 2009;78:852–62. doi: 10.1016/j.bcp.2009.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatsukami DK, Grillo M, Pentel PR, Oncken C, Bliss R. Safety of cotinine in humans: physiologic, subjective, and cognitive effects. Pharmacol Biochem Behav. 1997 Aug;57(4):643–50. doi: 10.1016/s0091-3057(97)80001-9. [DOI] [PubMed] [Google Scholar]
- 36.Terry AV, Callahan PM, Hall B, Webster SJ. Alzheimer's Disease and Age-Related Memory Decline (Preclinical) in the Special Issue on Cognitive Enhancers and Neuropsychiatric Disorders. Pharmacology, Biochemistry and Behavior. 2011 doi: 10.1016/j.pbb.2011.02.002. in press. doi:10.1016/j.pbb.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buccafusco JJ, Beach JW, Terry AV. Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. Invited Minireview J. Pharmacol. Exp. Ther. 2009;328:364–70. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sood A, Beach JW, Webster SJ, Terry AV, Jr., Buccafusco JJ. The Effects of JWB 1-84-1 on Memory-Related Task Performance by Amyloid Aβ Transgenic Mice and Aged Rhesus Monkeys. Neuropharmacology. 2007;53:588–600. doi: 10.1016/j.neuropharm.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Buccasfusco JJ, Letchworth SR, Bencherif M, Lippiello P. Long-lasting cognitive improvement with nicotinic receptor agonists: mechanisms of pharmacokinetic-pharmacodynamic discordance. Trends Pharmacol Sci. 2005 Jul;26(7):352–60. doi: 10.1016/j.tips.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Terry AV, Clarke MS. Nicotine stimulation of nerve growth factor receptor expression. Life Sci. 55:PL-91–8. doi: 10.1016/0024-3205(94)00653-9. [DOI] [PubMed] [Google Scholar]
- 41.Bartus R. On Neurodegenerative Disease, Models, and Treatment Strategies: Lessons Learned and Lessons Forgotten a Generation Following the Cholinergic Hypothesis. Exp Neurol. 2000 Jun;163(2):495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 42.French SJ, Humby T, Horner CH, Sofroniew MV, Rattray M. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Mol Brain Res. 1999;67:124–36. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 43.Jonnala RR, Terry AV, Buccasfusco JJ. Nicotine increases the expression of high affinity nerve growth factor receptors in both in vitro and in vivo. Life Sci. 2002;70:1543–54. doi: 10.1016/s0024-3205(01)01529-6. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Rodriquez R, Roledano A, Alvarez M, Turegano L, Colman O, Roses P, Gomez de Segura I, De Miguel E. Chronic nicotine administration increases NGF-like immunoreactivity in frontoparietal cerebral cortex. J Neurosci Res. 2003;73:708–16. doi: 10.1002/jnr.10688. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez CM, Terry AV., Jr Repeated nicotine exposure in rats: Effects on memory function, cholinergic markers and nerve growth factor. Neuroscience. 2005;130:997–1012. doi: 10.1016/j.neuroscience.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 47.Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, et al. DART-AD investigators. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009 Feb;8(2):151–7. doi: 10.1016/S1474-4422(08)70295-3. [DOI] [PubMed] [Google Scholar]
- 48.Youdim MBH, Buccafusco JJ. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends in Pharmacological Sci. 2005;26:27–35. doi: 10.1016/j.tips.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Buccafusco JJ, Powers JC, Hernandez MA, Prendergast MA, Terry AV, Jonnala RR. MHP-133, a Drug with Multiple CNS Targets: Potential for Neuroprotection and Enhanced Cognition. Neurochemical Research. 2007;32:1224–37. doi: 10.1007/s11064-007-9294-0. [DOI] [PubMed] [Google Scholar]
- 50.Buccafusco JJ, Beach JW, Terry AV, Jr, Doad GS, Sood A, Arias E, Misawa H, Masai M, Fujii T, Kawashima K. Novel analogs of choline as potential neuroprotective agents. Journal of Alzheimer's Disease. 2004;6(6 Suppl):S85–92. doi: 10.3233/jad-2004-6s612. [DOI] [PubMed] [Google Scholar]
- 51.Keowkase R, Aboukhatwa M, Adam BL, Beach JW, Terry AV, Jr, Buccafussco JJ, Luo Y. Neuroprotective effects and mechanism of cognitive-enhancing choline analogs JWB 1-84-1 and JAY 2-22-33 in neuronal culture and Caenorhabditis elegans. Mol Neurodegener. 2010;5:59. doi: 10.1186/1750-1326-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terry AV, Buccafusco JJ, Herman EJ, Callahan PM, Beck WD, Warner S, et al. Prototypical ranitidine analog, JWS-USC-75-IX, improves information processing and cognitive function in animal models. J. Pharmacol. Exp. Ther. 2011;336:751–66. doi: 10.1124/jpet.110.175422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mruthinti S, Buccafusco JJ, Hill WD, Waller JL, Jackson TW, Zamrini EY, Schade RF. Autoimmunity in Alzheimer's disease: increased levels of circulating IgGs binding Abeta and RAGE peptides. Neurobiol Aging. 2004 Sep;25(8):1023–32. doi: 10.1016/j.neurobiolaging.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Wilson JS, Mruthinti S, Buccafusco JJ, Schade RF, Mitchell MB, Harrell DU, Gulati NK, Miller LS. Anti-RAGE and Abeta immunoglobulin levels are related to dementia level and cognitive performance. J Gerontol A Biol Sci Med Sci. 2009 Feb;64(2):264–71. doi: 10.1093/gerona/gln002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell MB, Buccafusco JJ, Schade RF, Webster SJ, Mruthinti S, Harrell DU, Gulati NK, Miller LS. RAGE and Abeta immunoglobulins: relation to Alzheimer's disease-related cognitive function. J Int Neuropsychol Soc. 2010 Jul;16(4):672–8. doi: 10.1017/S1355617710000469. [DOI] [PubMed] [Google Scholar]