Abstract
Amide proton transfer (APT) imaging has shown promise as an indicator of tissue pH and as a marker for brain tumors. Sources of error in APT measurements include direct water saturation, and magnetization transfer (MT) from membranes and macromolecules. These are typically suppressed by post-processing asymmetry analysis. However, this approach is strongly dependent on B0 homogeneity and can introduce additional errors due to intrinsic MT asymmetry, aliphatic proton features opposite the amide peak, and radiation damping-induced asymmetry. Although several methods exist to correct for B0 inhomogeneity, they tremendously increase scan times and do not address errors induced by asymmetry of the z-spectrum. In this paper, a novel saturation scheme - saturation with frequency alternating RF irradiation (SAFARI) - is proposed in combination with a new magnetization transfer ratio (MTR) parameter designed to generate APT images insensitive to direct water saturation and MT, even in the presence of B0 inhomogeneity. The feasibility of the SAFARI technique is demonstrated in phantoms and in the human brain. Experimental results show that SAFARI successfully removes direct water saturation and MT contamination from APT images. It is insensitive to B0 offsets up to 180Hz without using additional B0 correction, thereby dramatically reducing scanning time.
Keywords: APT – Amide proton transfer imaging, CEST – chemical exchange saturation transfer, SAFARI – saturation with alternating frequency RF irradiation, MT – magnetization transfer
Introduction
Chemical Exchange Saturation Transfer (CEST) imaging (1–3) is a method to detect molecules with exchangeable protons by saturating the exchangeable resonance and then observing the effects of the saturation transferred to water by exchange. This technique is of interest both for detecting particular molecules or types of molecules and also as a measure of pH, since the exchange rate of protons is often pH sensitive (4, 5). Among the stronger in-vivo contributions to CEST contrast are hydroxyl protons at approximately 1ppm above water, that have been used to assess glycogen (6) and glycosaminoglycans (7) in tissues where these molecules dominate, rapidly exchanging amine protons of amino acids and other molecules between 2–3 ppm above water, and the more slowly exchanging amides on the backbone of proteins, at approximately 3.5 ppm. Because amide protons have a larger chemical shift and their slow exchange permits saturation at power levels more compatible with human applications, amide proton transfer (APT) imaging (8, 9) is particularly attractive for pH measurement with endogenous CEST contrast.
At pH values occurring in-vivo, the chemical exchange of amide protons is base-catalyzed (10), therefore APT contrast decreases with decreasing intracellular pH. This has been exploited for the study of acute cerebral ischemia (8, 11–16). It has been shown that the APT contrast correlates with pH (8, 13) and may help distinguish benign oligemia from the ischemic penumbra (14). As a result, APT imaging could become an important marker to predict progression to infarction in stroke.
In addition to being sensitive to pH, APT contrast is also related to the protein content inside cells, although it is not yet clear which specific proteins in which compartments contribute to the signal. Nevertheless, this property of APT imaging has been exploited for the study of tumors (17–22), multiple sclerosis (23, 24) and to design a CEST reporter gene to map a specific gene expression pattern (25). In cancer imaging, APT imaging appears to correlate with tumor grade (21) and may distinguish active tumor regions from edema and normal-appearing white matter (18), as well as from necrotic tumor cores (20, 26). APT imaging has also shown potential to differentiate between tumor recurrence and radiation necrosis (22), which otherwise appear identical on radiological images.
Although, APT imaging has emerged as a new source of MRI contrast with several promising clinical applications, including stroke and cancer imaging, it has so far been limited to research use due to the difficulty of measuring the APT effect in-vivo without errors. The RF irradiation needed to generate APT contrast also induces direct water saturation and magnetization transfer (MT) from semisolid protons in membranes and macromolecules. These effects both decrease the signal of the APT-weighted image, confounding the APT measurement. The standard method to correct for direct water saturation and MT is MT ratio asymmetry (MTRasym) analysis, where the APT image is subtracted from a control image acquired with RF irradiation at the frequency opposite the amide protons with respect to the water line. This method, however, is dependent on the water frequency being centered and leads to severe artifacts when B0 is not homogeneous, as occurs in-vivo.
Errors induced by B0 inhomogeneity can be corrected to generate APT maps free of B0 artifacts. This is typically accomplished by acquiring a z-spectrum and recentering the water line before performing the asymmetry subtraction. Because the z-spectrum involves acquiring many images with a range of RF irradiation frequencies (20–40 offsets), this method increases scan time beyond what is suitable for clinical application. Several alternative B0 correction methods have been developed to reduce scan time. These include variations on the z-spectrum method (21, 26, 27), multiple offset acquisitions with additional B0 mapping (28, 29), and fitting of an analytical model (30). Yet, even with B0 correction asymmetry analysis introduces additional errors in the APT measurement due to asymmetries in the z-spectrum.
Several sources of asymmetry of the z-spectrum – other than amide protons – contribute to the MTRasym measurement, introducing errors in the quantification of the amide proton signal. First, aliphatic protons that have resonances in the range from −1.3ppm to −4.8ppm (8, 9) can decrease the signal of the control image by saturation transfer. At low RF power, this tends to cancel out the amide proton signal in the MTRasym calculation (31). Second, probe detuning can cause radiation damping-induced asymmetry of the z-spectrum (32) introducing additional errors in MTRasym. Third, the center of symmetry of the MT line is shifted with respect to the water line (33–35) and, therefore, does not completely cancel in the asymmetry subtraction. Again, this will cancel out the amide proton signal leading to MTRasym values near zero or negative. Recently, an approximate analytical model (36), and sophisticated alternative acquisition methods (37, 38) have been proposed to separate the amide proton signal from MT.
In this paper, we propose a novel APT acquisition method using saturation with frequency alternating RF irradiation (SAFARI) along with a new magnetization transfer ratio= (MTRSAFARI) to remove direct water saturation and MT from APT images without additional B0 correction. This technique was first tested in phantoms including a control relaxation phantom without chemical exchange in order to establish that SAFARI does not introduce contrast based on T2 rather than CEST, and a bovine serum albumin protein phantom to test the sensitivity of SAFARI to amide proton exchange and aliphatic proton contamination. The phantoms were also imaged with increasing RF frequency offsets to test the range at which MTRSAFARI remains insensitive to B0 shifts while still saturating amide protons. The performance of APT-SAFARI in the presence of CEST and MT was then evaluated in a set of experiments performed in humans. High Signal-to-Noise Ratio (SNR) APT images of the brain were acquired to compare MTRasym and MTRSAFARI maps without B0 correction. Lower SNR APT images were also acquired at 51 frequency offsets, akin to a z-spectrum, in order to compare both the B0 and MT sensitivity of MTRasym with B0 correction and MTRSAFARI without B0 correction. Finally, the sensitivity of MTRSAFARI to chemical exchange was studied by varying the average saturation power, similar to the “quantification of exchange as a function of saturation power” (QUESP) technique (39).
Theory
The key to optimizing APT contrast is achieving full amide proton saturation while minimizing the effects of direct water saturation and MT. We propose a novel scheme to produce amide proton transfer contrast based on saturation with frequency alternating RF irradiation (SAFARI). The APT-SAFARI technique relies on the non-linearity of the saturation process: once the amide proton line has been fully saturated, increasing the RF irradiation power should not affect the saturation significantly. Therefore, if the applied RF power is sufficient to produce complete amide proton saturation, the APT effect becomes independent of power. The broad macromolecular line and the water line, however, are not fully saturated. As a result, the effect of direct water saturation and MT vary mostly linearly with power. The APT-SAFARI acquisition and post-processing method exploits this different power dependence of the amide proton line compared to the water line to cancel out the effects of MT and direct water saturation, even in the presence of B0 inhomogeneity and MT asymmetry, as described below.
The SAFARI image is acquired with RF irradiation applied with equal power at both the control (−3.5ppm) and label (+3.5ppm) frequencies. This is achieved within a pulsed-RF irradiation module by alternating every other saturation pulse between the two frequencies. If the total RF power applied at 3.5ppm is sufficient, the amide proton line will be fully saturated. Therefore, the signal in the SAFARI image can be modeled as a combination of the chemical exchange saturation transfer (CEST), as well as signal loss due to direct water saturation (W) and magnetization transfer (MT) from both off-resonance frequencies:
| [1] |
where S0 is the unsaturated reference signal intensity, δB0 is the B0 inhomogeneity, δMT is the center of MT asymmetry, ωs=3.5ppm is the amide proton frequency and P represents the RF irradiation power. In the presence of B0 inhomogeneity, such as susceptibility effects in-vivo, the water line is shifted and direct water saturation is no longer symmetric around 0ppm, taken into account by the δB0 variable. The CEST effect, however, is independent of δB0 as long as the RF saturation bandwidth is broad enough to maintain full amide proton saturation despite the shift of the line. The center of MT symmetry is known to be intrinsically shifted with respect to the water line, and is represented here by δMT. The SAFARI image, therefore, contains the individual asymmetric effects of direct water saturation and MT at both the positive and negative saturation frequencies.
In addition, we acquire the standard CEST image with RF irradiation of the amide proton line only. This is achieved by applying all of the RF pulses of the saturation module at the label frequency only. As a result, the total RF power deposited on the amide proton line is doubled compared to the SAFARI image. Consequently, the MT and direct water saturation effects double, while the APT effect does not change:
| [2] |
Similarly, the signal in the control image with all RF pulses applied at the control frequency only can be modeled as:
| [3] |
The signal amplitudes resulting from the three different saturation preparations of the APT-SAFARI scan are illustrated in Figure 1.
Figure 1.
Schematic illustration of the APT-SAFARI experiment. The spectral line represents the free water signal. W, MT and CEST are the signal loss attributed to direct water saturation, conventional magnetization transfer and amide proton transfer, respectively. The plus and minus subscripts indicate the effects induced by RF saturation applied at the amide proton frequency (ωs=+3.5ppm) and the opposite frequency (−ωs), respectively. For clarity, W is depicted to be symmetric around the water line, while MT is not. Because of the MT asymmetry, the standard two-offset CEST difference MTRasym = [Ssat(− ωs)− Ssat (+ωs)]/S0 is not equal to the CEST effect. The SAFARI image with half the saturation power applied simultaneously at the control and label frequencies contains the contributions of W and MT at both frequencies, as well as signal decrease due to APT. Therefore, the difference MTRSAFARI = [Ssat(− ωs) + Ssat (+ωs) − 2Ssat(SAFARI)]/S0 isolates the CEST effect without contamination from B0 inhomogeneity and asymmetric MT.
The common approach for separating CEST contrast from other effects is asymmetry analysis, where the reference image is subtracted from the CEST image. The contrast generated by standard asymmetry analysis is given by ([3]–[2]):
| [4] |
MTRasym fails to isolate the CEST contrast because direct water saturation and MT are not symmetric around 0ppm. Instead of the standard asymmetry analysis, we employ a three-way subtraction where the SAFARI image is doubled and subtracted from the sum of the positive and negative frequency images ([2]+[3]−2[1]):
| [5] |
As a result, both MT and direct water saturation cancel out exactly, even in the presence of B0 inhomogeneity and MT asymmetry.
Methods
APT Pulse sequence design
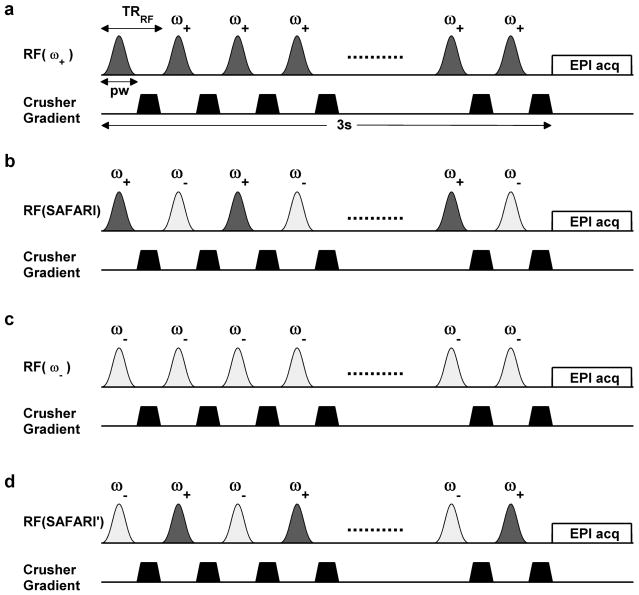
The APT-SAFARI imaging pulse sequence is shown in Figure 2. Four images are acquired for each APT-SAFARI scan: one label image Ssat(ω+) with all saturation pulses applied at a single frequency ω+ (Fig. 2a), one SAFARI image Ssat(SAFARI) with saturation pulses alternating between ω+ and ω− (Fig. 2b), one control image Ssat(ω−) with all saturation pulses applied at a single frequency ω− (Fig. 2c), and another SAFARI image Ssat(SAFARI′) with saturation pulses alternating between ω− and ω+ (Fig. 2d). Note that the first and third images constitute a standard CEST experiment. It was necessary to acquire two SAFARI images with reversed ordering of the pulses to ensure that identical RF powers were deposited on the positive and negative frequencies.
Figure 2.
Pulse sequence for APT-SAFARI imaging with a pulsed off-resonance saturation module followed by a single-slice EPI readout. A 3 second train of Blackman inversion pulses (pw=9ms, TRRF=15ms, flip angle = 180°, average RF power = 0.78μT) was used. Each scan consists of acquiring four images: a) Standard CEST image with saturation at the labile proton frequency (ω+ = 3.5ppm for amide protons). b) SAFARI image with saturation alternating between the label and control frequencies. c) Standard CEST control image with saturation at the control frequency (ω− = −3.5ppm for amide protons). d) SAFARI image with saturation alternating between the control and label frequencies.
For all four images, the off-resonance pulsed-RF irradiation consisted of a 3 second train of Blackman shaped inversion pulses with pulse width (pw) = 9 ms repeated every TRRF = 15 ms while incrementing the change in phase shift linearly by 117° for spoiling of transverse magnetization (40). The corresponding continuous wave (CW) RF power is given by (41), where B1(t) is the Blackman waveform given by the MATLAB (Mathworks, Natick MA) “blackman” function. These pulse parameters were optimized to saturate the amide protons at 3.5ppm even in the presence of B0 shifts, while within the constraint of not producing strong direct water saturation (42). Crusher gradients were turned on between every RF pulse to destroy remaining transverse magnetization. The saturation module was followed by a single-shot echo-planar acquisition described below.
Phantom preparation
Control phantoms were prepared with eight different concentrations of MnCl2 in water. Each solution was divided amongst four 15mm-diameter centrifuge tubes. The tubes were arrayed in a single 4 by 8 Styrofoam holder for imaging. T1 and T2 relaxation times have been measured previously (43). T1 values range from 2000ms to 400ms and T2 values range from 280 ms to 40ms. A protein phantom was prepared by dissolving 5g of bovine serum albumin (BSA, Sigma-Aldrich) in 50ml phosphate saline buffer at pH=5.5. The BSA solution was transferred to a 29.1mm-diameter tube for imaging.
Imaging
All studies were performed on a 3T GE SIGNA EXCITE MR system using the body coil for transmit. A standard eight-channel receive-only head array was used for signal reception for both phantom and human imaging. A single shot spin-echo echo-planar imaging (EPI) readout was used for all control phantom and human studies with TR = 4s, TE between 63 and 64 ms, FOV = 24cm, matrix size = 96×96, and slice thickness = 12mm. The BSA phantom was imaged with the same parameters, except for a FOV = 15cm and TE = 17.8 ms.
The control phantoms were imaged with the long axis of the tubes along the direction of the magnetic field. A modified z-spectrum was acquired to test the robustness of MTRSAFARI against B0 inhomogeneity. Shifts in the water line due to B0 offsets were mimicked by introducing an offset f to the off-resonance irradiation frequency. Instead of acquiring APT scans with off-resonance irradiation at the amide proton frequency ωs/2π= 450Hz, the applied frequencies were ω+ = 2πf + ωs and ω− = 2πf − ωs, where the frequency offset f was swept from −900 Hz to +900Hz in steps of 30 Hz. Therefore, the frequency shift f simulates an offset of the water line due to a B0 shift of −f. The modified z-spectrum acquisition resulted in a total of 61 APT scans (244 images). Note that for the single frequency images the data set constitutes a standard z-spectrum acquired out of order. For the SAFARI images, however, it differs from a z-spectrum because the offset f leads to RF irradiation at frequencies with increasing asymmetry around the water line, rather than symmetric frequencies with increasing offsets. Total scan time was 15 minutes for each phantom.
The BSA phantom was imaged with the long axis of the tube along the direction of the magnetic field. The amide proton resonance of BSA is located at 350Hz (see Figure 4b), therefore, the modified z-spectrum was acquired with applied frequencies ω+ = 2π(f +350Hz) and ω− = 2π(f − 350Hz), where the frequency offset f was swept from −350 Hz to +350Hz in steps of 14 Hz. The modified z-spectrum acquisition resulted in a total of 51 APT scans (204 images) in just under 14 minutes. Because the BSA amide resonance is 100Hz closer to the water resonance than the amide proton resonance in the brain, the experiment was also repeated with a modified RF irradiation module with pw = 12 ms repeated every TRRF = 20 ms to provide a more selective saturation bandwidth and reduce direct water saturation..
Figure 4.
APT imaging of the BSA phantom. Data plotted for saturation with RF parameters pw = 12ms and TRRF = 20ms. a) Mean MTRSAFARI in the BSA phantom as a function of frequency offset f. Error bars represent the standard deviation across the phantom. MTRSAFARI has annearly constant amplitude for frequency offsets of at least ±100Hz. b) The z-spectrum of the BSA phantom shows the amide proton peak at 350Hz and no aliphatic proton features. The vertical line is located at 350Hz. Error bars have been omitted for clarity.
Human imaging was performed following a protocol approved by the institutional review board. Four healthy volunteers (2 men, 2 women; age range 23–40 years, mean age 30 years) were scanned after giving written informed consent. For each volunteer, a single slice was located at the level of the ventricles. One unsaturated S0 image was acquired for reference with 12 averages. One high SNR APT-SAFARI scan with 12 time-interleaved repetitions of the sequence (48 images total) was acquired with off-resonance irradiation at the amide proton frequency. Scan time was 3 minutes. In order to evaluate the robustness of MTRSAFARI against B0 inhomogeneity, 3 high SNR APT-SAFARI scans were acquired with frequency offsets f = 100, 150, and 200Hz, corresponding to RF irradiation at the frequency pairs +550Hz/−350Hz, +600Hz/−300Hz, and +650Hz/−250Hz, respectively. Scan time for the high SNR scans with frequency offsets was 3 minutes each for a total of 9 minutes. In addition, a lower SNR (no scan repetitions) modified z-spectrum was acquired similar to the phantom protocol. 51 APT-SAFARI scans (204 images) were acquired with the frequency offset f swept from −450Hz to +450Hz in steps of 18Hz, thereby simulating B0 shifts of the water line. Total scan time was just under 14 minutes.
The sensitivity of APT-SAFARI imaging to chemical exchange was characterized by studying the effect of saturation power on the image intensity, similarly to the QUESP technique (39). Power was modulated by changing the delay time between saturation pulses while keeping the total saturation time constant. Due to RF duty cycle limitations on the scanner, the minimum TRRF allowed for a 9ms pulse was 15ms. Therefore, TRRF could only be increased, thereby decreasing power. Seven APT-SAFARI scans were acquired at the amide proton frequency (f = 0Hz) with TRRF = 20, 30, 45, 60, 80, 100 and 150 ms corresponding to a CW RF power BCW= 0.58, 0.39, 0.26, 0.19, 0.15, 0.12, and 0.08μT, respectively. As TRRF increases from 15ms to 150ms, the number of saturation pulses applied in the 3 second saturation module decreases from 200 to 20, thereby reducing the average saturation power. Each scan was repeated 6 times to increase SNR, resulting in a total acquisition time of 11 minutes.
Image analysis
Image analysis was performed in MATLAB 7.6 (Mathworks, Natick MA). All images were normalized voxel-by-voxel by the unsaturated reference image S0. For the high SNR APT-SAFARI scans, quantitative maps of MTRasym and MTRSAFARI were calculated voxel-by-voxel according to: [6]
| [6] |
| [7] |
where ω− =2πf−ωs and ω+=2πf+ωs. MTRasym and MTRSAFARI were each averaged across the entire slice excluding the skull and ventricles. Results are presented as the mean MTR for each volunteer with standard deviations across the slice. In addition, the error in the APT measurement due to the offset f was calculated voxel-by-voxel by error = [MTR(f) − MTR(f = 0)]/MTR(f = 0), and averaged across all volunteers.
For the lower SNR modified z-spectrum scans, B0 correction of the single frequency images was performed following a previously published method (8) in order to remove unintentional B0 inhomogeneity shifts across the FOV before calculating MTRasym. The single frequency images Ssat(ω−) and Ssat(ω+) were reordered to generate a standard z-spectrum. The z-spectrum was fit voxel-by-voxel to a 30th order polynomial. The resulting polynomial was evaluated with a frequency resolution of 1Hz. The frequency with the minimum fit intensity was assumed to be the water peak. A map of the water frequency deviation from 0 was generated. To correct for B0 inhomogeneity across the FOV, the z-spectrum was interpolated by a piecewise cubic spline to a resolution of 1Hz and shifted by the water frequency deviation such that the water resonance occurred at 0Hz for all voxels. After B0 correction, the z-spectrum was resampled back to its original resolution (30Hz steps for control phantom data, 14Hz steps for BSA phantom data, 18Hz steps for human data). The resulting corrected images were used to calculate MTRasym according to Eq. [6]. MTRSAFARI was calculated from the original data without B0 correction according to Eq. [7]. The control phantom MTR maps were then averaged for each of the eight solutions across the four tubes with identical relaxivities. The human MTR maps were averaged across the entire brain slice, excluding the skull and ventricles. Results are presented as the mean MTR across all volunteers with standard errors.
For the APT-SAFARI scans as a function of saturation power, the MTR parameters were calculated voxel-by-voxel according to Eqs. [6] and [7] with f = 0Hz. MTRasym and MTRSAFARI were each averaged across the entire slice excluding the skull and ventricles. Results are presented as the mean MTR across all volunteers with standard errors.
Results
The control phantom study confirms that MTRSAFARI does not generate erroneous contrast in the absence of CEST agent. Figure 3 illustrates the effect of frequency shifts on APT imaging in the control phantom without chemical exchange. For clarity, only three of the eight different MnCl2 solutions are shown, including the longest and shortest T2 solutions. It can be seen that when the off-resonance saturation is applied at the amide proton frequency (f = 0Hz), both MTRSAFARI and MTRasym are zero regardless of T2, as expected. Moreover, MTRSAFARI remains below 0.5% for frequency offsets of at least ± 120Hz for all relaxation times. Increasing T2 increases the stability of MTRSAFARI, with the longest T2 solution remaining at MTRSAFARI <0.5% for up to ±180 Hz. Therefore, T2 does not affect the amplitude of MTRSAFARI as long as water saturation remains small. Instead, it modulates the frequency offset at which direct water saturation becomes significant and can no longer be removed by MTRSAFARI. MTRasym, in contrast, deviates from zero as the frequency offset increases. |MTRasym| exceeds 1% before the smallest offset of ±30Hz. It reaches a value greater than 3%, which is on the order of the expected in-vivo APT effect, at a frequency offset of only ±60 Hz, except for the longest T2 solution, which remains below 3% up to ±90Hz. In addition, the rate of change of MTRasym increases with decreasing T2, which induces additional errors in the APT measurement.
Figure 3.
Modified z-spectra in the control phantom (no CEST agent) demonstrating the effect of RF saturation frequency shifts – similarly to a B0 offset – on the APT measurements. Results are shown for three MnCl2 solutions. a) MTRSAFARI is consistent with zero and independent of frequency offset and relaxivity up to ~ ±180Hz. b) MTRasym varies quickly with frequency offset and relaxivity parameters.
A BSA protein phantom was imaged to test the sensitivity of SAFARI to amide and aliphatic proton features and to study the robustness of SAFARI against frequency offsets in the presence of chemical exchange. The standard z-spectrum shown in Figure 4b reveals the amide proton peak at 350Hz or 2.75ppm, consistent with previous measurements (31). Even the more selective saturation preparation (pw=12ms, TRRF=20ms, data shown in Figure 4) has a fairly broad saturation bandwidth, as can be seen by the width of the water and amide peaks. The water peak has a full width at half maximum of 320Hz. Therefore, when the RF preparation is applied at the amide proton frequency, these should remain mostly fully saturated even if B0 offsets shift the line by up to ±160Hz. Note, also, that neither of the two RF preparations used produced noticeable saturation transfer from aliphatic protons and no peak was detected in the aliphatic region of the spectrum. Prior work (31) with BSA has shown a more pronounced aliphatic peak when much lower power is applied. Our data cannot exclude some level of aliphatic contribution to the SAFARI signal, however, the major contribution to the MTRSAFARI measurement is expected to be from amide protons alone. The MTRSAFARI spectrum is shown in Figure 4a. On resonance with the amide protons (f = 0 Hz), the baseline SAFARI signal is 2.0% ± 0.3%. In the presence of frequency offsets f, MTRSAFARI remains within 0.5% of baseline in the range of offsets from −140Hz to 100Hz. Therefore, the SAFARI strategy can successfully saturate amide protons even when B0 offsets shift the line and remains insensitive to B0 shifts of at least 100Hz. These phantom results are expected to provide a lower bound for the performance of SAFARI in-vivo. Since the resonance of in-vivo amide protons is centered around 450Hz instead of 350Hz, the robustness of SAFARI to B0 shifts should increase, as will be seen below.
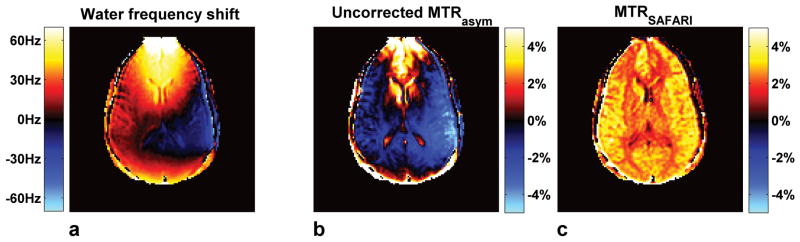
Figure 5 compares a representative high SNR in-vivo MTRSAFARI map at f = 0Hz with the corresponding uncorrected MTRasym map and the water frequency map derived from the lower SNR z-spectrum. The water frequency map (Fig. 5a) shows large positive B0 shifts at the front of the brain, negative B0 shifts on the left side of the brain and small positive or zero shifts elsewhere. As shown in Figure 3, positive B0 shifts (f < 0) lead to increased MTRasym values, while negative B0 shifts (f > 0) lead to decreased MTRasym values. However, the MTRasym map (Fig. 5b) displays mostly negative values throughout the brain (except at the front of the brain), even in regions with zero or small positive B0 shifts where MTRasym should be increased, indicating that the contrast is dominated by intrinsic MT asymmetry rather than APT or small B0 shifts. In comparison, the MTRSAFARI map (Fig. 5c) values are positive suggesting that the proposed APT-SAFARI method can successfully separate the amide proton transfer signal from the MT signal. MTRSAFARI also performs much better in the presence of B0 inhomogeneity than MTRasym. Comparing Figures 5a and 5b shows that MTRasym closely follows the B0 inhomogeneity distribution. Elevated MTRasym values are found at the front of the brain and decreased MTRasym values on the left side of the brain, corresponding to susceptibility artifacts near air-tissue interfaces seen on the water frequency map. In contrast, the MTRSAFARI map is homogeneous throughout the brain except for a small gray-white matter contrast. There are no apparent erroneous MTRSAFARI values induced by off-resonance effects indicating that MTRSAFARI is insensitive to the range of B0 inhomogeneity found in the brain.
Figure 5.
APT imaging results in a representative human volunteer. a) B0 map calculated from the low SNR z-spectrum images by the B0 correction algorithm. b) MTRasym map and c) MTRSAFARI map calculated from the high SNR APT images without B0 correction. The uncorrected MTRasym map shows large spatial intensity variation over the brain, which corresponds to the water frequency shift map. The uncorrected MTRSAFARI map shows a remarkable improvement in homogeneity over the brain.
The effect of B0 inhomogeneity was further investigated by acquiring APT-SAFARI scans with asymmetric saturation around the water line. Figure 6 shows quantitative MTR maps for RF frequency offsets f = 0, 100, 150 and 200 Hz. In addition, mean values of MTRasym and MTRSAFARI for each volunteer are shown in Table 1. In the presence of a 100Hz offset simulating a B0 shift of −100Hz across the entire field of view, MTRasym decreases on average by 300% compared to baseline and completely fails at detecting the APT effect. Instead it is dominated by asymmetric direct water saturation yielding large negative MTRasym values. At offsets above 100Hz, the errors in MTRasym increase even more reaching over 700% at f = 200Hz. In contrast, MTRSAFARI performs much better and mean values remain within one standard deviation of the f = 0Hz MTRSAFARI map for offsets f = 100Hz and f = 150Hz. An average change of ~155% is seen in the map at f = 200Hz, roughly half the error seen in the MTRasym map at f = 100Hz.
Figure 6.
Comparison of MTRasym and MTRSAFARI maps calculated from high SNR APT images as a function of RF frequency offset. The frequency offset f mimics shifts of the water line due to B0 shifts of −f. a) When displayed with the same contrast, MTRasym deviates from baseline becoming more and more negative as f increases, while MTRSAFARI remains invariant. b) The same MTRSAFARI images from (a) displayed with more contrast to better show the quality of the MTRSAFARI images and the deviation with frequency offset.
Table 1.
Average values of high SNR MTR maps across the healthy human brain (n=4) without B0 correction
| MTRasym [%] | MTRSAFARI [%] | |||||||
|---|---|---|---|---|---|---|---|---|
| f = 0Hz | f = 100Hz | f = 150Hz | f = 200Hz | f = 0Hz | f = 100Hz | f = 150Hz | f = 200Hz | |
| 1 | −0.3 ± 2.3 | −8.6±2.9 | −14.4±3.7 | −19.9±5.6 | 2.6±0.9 | 3.1±1.0 | 4.4±1.2 | 6.3±2.2 |
| 2 | 0.7 ± 2.1 | −7.6±2.7 | −12.6±3.7 | −18.2±5.2 | 2.0±0.8 | 2.5±0.8 | 3.4±1.1 | 5.2±2.0 |
| 3 | −0.5 ±1.5 | −7.9±2.5 | −15.0±4.1 | −19.5±5.8 | 2.3±0.9 | 3.0±0.9 | 4.3±1.5 | 5.8±2.2 |
| 4 | −0.04 ±1.9 | −9.1±2.6 | −14.8±3.3 | −21.3±4.6 | 2.3±0.7 | 3.1±0.9 | 4.3±1.0 | 6.5±1.8 |
| mean ± SD | 0.1± 0.5 | −8.3±0.7 | −14.3±1.1 | −20.0±1.4 | 2.3±0.3 | 2.9±0.3 | 4.1±0.5 | 6.1±0.8 |
| mean error ± SD [%] | 0 | −290±150 | −510±270 | −720±360 | 0 | 27±4 | 78±8 | 155±15 |
The same trends seen in the high SNR images (Figures 5 and 6) are observed on the in-vivo modified z-spectrum illustrated in Figure 7. At f = 0Hz, MTRSAFARI = 2.0% ± 0.3% is in good agreement with the high SNR measurements in Table 1. MTRasym = −3.8% ± 0.2%, however, is quite different from the values derived from the high SNR maps without B0 correction, because the large positive MTRasym values due to B0 inhomogeneity above the sinuses have now been removed. Again, we see that MTRSAFARI is positive and MTRasym is negative. In addition, while MTRasym (Fig. 7b) varies quickly with the RF frequency offset, MTRSAFARI (Fig. 7a) gives a much flatter response up to f ~ ±200Hz. The error in MTRSAFARI remains under 50% of baseline for a frequency range f = −216Hz to f = 162 Hz. In contrast, the error in MTRasym is less than 50% only in the range f = ±18 Hz. At offsets above ~ ±200Hz, both parameters increase steeply and peak at f = ± 450 Hz when the saturation is directly on resonance with the water line (saturation at +900/0Hz and 0/−900Hz).
Figure 7.
APT modified z-spectra averaged across the entire brain slice in four healthy volunteers. Error bars represent the standard error of the mean over the 4 volunteers. a) MTRSAFARI as a function of induced frequency offset shows the APT measurement remains fairly stable up to f~ ±200Hz. At |f| > 250Hz, MTRSAFARI increases steeply as direct water saturation becomes significant. b) MTRasym – calculated after B0 correction - as a function of induced frequency offset varies quickly with f because it is dominated by direct water saturation. The negative value of MTRasym at f = 0Hz is due to asymmetry in the conventional MT effects.
The power dependence of MTRasym and MTRSAFARI is shown in Figure 8. At the highest power (TRRF=15ms, BCW=0.78μT), MTRasym = −0.3% ± 0.3% is negative, as seen previously. Again, the value of MTRasym calculated here without B0 correction does not match the value calculated from the modified z-spectrum data with B0 correction, but is in good agreement with the uncorrected high SNR measurements in Table 1. This again, indicates that without B0 correction, MTRasym fails at quantifying the APT effect. As TRRF increases beyond the chemical exchange time, the APT contrast should decrease due to inefficient amide proton saturation. This was observed experimentally by increasing TRRF up to 150ms. MTRasym initially decreases, then reaches a minimum at −3.4% when TRRF=80ms (BCW=0.15μT), and starts increasing. Because MTRasym is the sum of the MT effect (negative) and the APT effect (positive), as TRRF increases and the APT effect decreases, MTRasym becomes more negative. The increase in MTRasym when TRRF exceeds 80ms could be explained by the fact that the MT asymmetry is a function of saturation power (35). As the power decreases, the MT asymmetry lessens, and therefore MTRasym becomes less negative. MTRSAFARI has an initial value of +2.5% ± 0.1 % and appears to decrease exponentially towards zero as the power decreases. Note that if amide protons are fully saturated, MTRSAFARI should in principle remain constant as TRRF increases, until the amide proton saturation begins to decrease. Simulations (44) suggest that a minimum CW RF saturation power of BCW≈0.5μT is required to fully saturate amide protons. Therefore, the two shortest TRRF used here should produce full saturation and have similar MTRSAFARI measurements. Although, MTRSAFARI (TRRF = 15ms) and MTRSAFARI (TRRF = 20ms) are within one standard deviation of each other no clear plateau was reached at low TRRF, indicating that higher RF power may be needed to fully saturate amide protons. However, the saturation level achieved at TRRF = 15ms was sufficient for the APT-SAFARI strategy to be successful as illustrated in the other results. As TRRF becomes larger and amide proton saturation decreases, the assumption that the CEST effect is independent of power no longer holds. Therefore, the CEST effect will tend to cancel out in the MTRSAFARI calculation similar to direct water saturation and MT.
Figure 8.
APT results in four healthy volunteers as a function of the saturation module RF pulse repetition time. Error bars represent the standard error of the mean over the 4 volunteers. The data point at TRRF =15ms was derived from the high SNR images in Fig 4 recalculated with only 6 averages to match the SNR of the images acquired as a function of TRRF. a) MTRasym – without B0 correction – is initially negative due to MT asymmetry. As TRRF increases, MTRasym initially becomes more negative and then reaches a minimum around a TRRF of 80 ms. This reflects amide proton exchange and MT asymmetry effects. b) MTRSAFARI is initially positive and decreases approximately exponentially towards 0 as TRRF increases.
Discussion
We have introduced a novel acquisition technique – saturation with frequency alternating RF irradiation or SAFARI - combined with a new MTR subtraction to measure the amide proton transfer effect in-vivo. The SAFARI technique was designed to be insensitive to B0 inhomogeneity and MT asymmetry. We have shown by comparing uncorrected MTRasym and MTRSAFARI phantom and in-vivo results, that MTRSAFARI can reliably measure the APT effect even in the presence of B0 shifts and MT. Both phantom and human studies indicate that MTRSAFARI can measure the APT signal accurately for frequency offsets up to about ±180Hz at 3T. This frequency range is well above the range of susceptibility offsets that were observed in the brain (see Figure 5a), removing the need for additional B0 correction of APT images. MTRasym, in contrast, relies on symmetric subtraction around the water line to cancel direct water saturation. This approach is intrinsically dependent on the B0 homogeneity as was seen in both modified z-spectra data and quantitative brain maps. Unless specialized B0 correction is used, standard MTRasym images closely reflect the field inhomogeneity rather than the APT effect, as was observed in Figure 5. In addition, even after B0 correction, MTRasym maps are negative even though the APT effect should yield positive MTRasym values. This is caused by intrinsic asymmetry in the z-spectrum, with the negative offset frequencies having lower intensities than the positive frequencies. This asymmetry is a well-known effect of conventional MT from semisolid protons (33–35) and has been observed in other APT experiments (9, 13, 17, 18, 20, 41). Not only does MTRSAFARI largely remove the need for B0 correction, it also appears to eliminate MT contamination from the APT maps. Because the APT-SAFARI scan only acquires four images, instead of dozens needed to produce a full z-spectrum for B0 correction, scan times can be dramatically reduced. Therefore, the SAFARI approach could help make APT, and more generally CEST, a clinically viable research tool.
In this work, we have applied SAFARI imaging to acquire CEST images of amide protons on the backbone of endogenous proteins. In principle, the SAFARI technique is not limited to amide proton exchange. SAFARI could also be used to acquire CEST images of other exchangeable protons as long as the two main assumptions are met. Recall that for SAFARI to be successful, saturation of the water line must be linear with power, i.e. direct water saturation must be small, and the exchangeable protons must be almost fully saturated. These assumptions impose limits on the design of the RF saturation module. The pulse shape, width and repetition rate must be optimized to maximize saturation of exchangeable protons while minimizing direct water saturation. For the case of amide proton exchange, we found that a 9ms Blackman-shape pulse with TRRF = 15ms produced a saturation bandwidth broad enough to saturate the amide protons at 3.5ppm even in the presence of B0 shifts, while within the constraint that the bandwidth not be so broad as to produce strong direct water saturation. Given these parameters, direct water saturation remains insignificant for frequency offsets up to f = 180 Hz, as we’ve seen from the z-spectra data. At this frequency offset, the off-resonance saturation is applied at a frequency ν = ωs/2π−180Hz = 270Hz from the water line. Therefore, the saturation module used here can be applied for SAFARI imaging of exchangeable protons with chemical shifts greater than 270Hz (2.1ppm at 3 Tesla). For protons with smaller chemical shifts, different pulse parameters must be employed to provide a more selective saturation.
The second assumption also imposes a limit on the maximal chemical exchange rate of the protons of interest. If the exchange time is so fast that saturation of the amide protons is not achieved then the SAFARI approach will not be successful. This places requirements on the amplitude and repetition rate of the RF pulse train. For the pulse parameters used here, simulations (42) suggest that SAFARI will only detect exchangeable protons with exchange rates smaller than 200Hz. For faster exchanging protons, higher RF power and potentially simultaneous dual frequency irradiation with pulsed or continuous wave RF would be possible options to pursue.
The experiments described were optimized for the frequency and expected exchange rates of amide protons, but the possibility of other contributions to the SAFARI signal in-vivo cannot be excluded. Nearby amine protons (2) would likely be affected by the saturation pulses we employed, but their typically very high exchange rates would likely ensure saturation was not achieved and therefore the contribution to SAFARI in our experiments should be small. Lines on the other side of water may also contribute to SAFARI. In particular, a number of lines which exchange with water through spin exchange rather than chemical exchange have been reported, for example see (4, 7–9, 45). A positive contribution of these lines to our SAFARI images, and a negative contribution to asymmetry analysis should be expected.
Conclusion
We have developed a new method for measuring amide proton transfer contrast without errors caused by direct water saturation and magnetization transfer. This feasibility study demonstrates that MTRSAFARI is much more robust in the presence of B0 inhomogeneity than MTRasym and minimizes the need for specialized B0 correction. In addition, the confounding MT asymmetry is removed from MTRSAFARI maps, allowing for accurate quantification of the CEST effect. Further evaluation is needed to assess whether APT-SAFARI imaging will permit improved characterization of brain pathology in clinical applications.
References
- 1.Guivel-Scharen V, Sinnwell T, Wolff SD, Balaban RS. Detection of proton chemical exchange between metabolites and water in biological tissues. J Magn Reson. 1998;133(1):36–45. doi: 10.1006/jmre.1998.1440. [DOI] [PubMed] [Google Scholar]
- 2.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, van Zijl PCM. Chemical exchange saturation transfer imaging and spectroscopy. Progr NMR Spectr. 2006;48:109–36. [Google Scholar]
- 4.Mori S, Eleff SM, Pilatus U, Mori N, van Zijl PC. Proton NMR spectroscopy of solvent-saturable resonances: A new approach to study pH effects in situ. Magn Reson Med. 1998;40(1):36–42. doi: 10.1002/mrm.1910400105. [DOI] [PubMed] [Google Scholar]
- 5.Ward KM, Balaban RS. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST) Magn Reson Med. 2000;44(5):799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.van Zijl PC, Jones CK, Ren J, Malloy CR, Sherry AD. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST) Proc Natl Acad Sci U S A. 2007;104(11):4359–64. doi: 10.1073/pnas.0700281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci U S A. 2008;105(7):2266–70. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085–90. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 9.van Zijl PC, Zhou J, Mori N, Payen JF, Wilson D, Mori S. Mechanism of magnetization transfer during on-resonance water saturation. A new approach to detect mobile proteins, peptides, and lipids. Magn Reson Med. 2003;49(3):440–9. doi: 10.1002/mrm.10398. [DOI] [PubMed] [Google Scholar]
- 10.Englander SW, Downer NW, Teitelbaum H. Hydrogen exchange. Annu Rev Biochem. 1972;41:903–24. doi: 10.1146/annurev.bi.41.070172.004351. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Wilson DA, Sun PZ, Klaus JA, Van Zijl PC. Quantitative description of proton exchange processes between water and endogenous and exogenous agents for WEX, CEST, and APT experiments. Magn Reson Med. 2004;51(5):945–52. doi: 10.1002/mrm.20048. [DOI] [PubMed] [Google Scholar]
- 12.Sun PZ, Zhou J, Huang J, van Zijl P. Simplified quantitative description of amide proton transfer (APT) imaging during acute ischemia. Magn Reson Med. 2007;57(2):405–10. doi: 10.1002/mrm.21151. [DOI] [PubMed] [Google Scholar]
- 13.Jokivarsi KT, Grohn HI, Grohn OH, Kauppinen RA. Proton transfer ratio, lactate, and intracellular pH in acute cerebral ischemia. Magn Reson Med. 2007;57(4):647–53. doi: 10.1002/mrm.21181. [DOI] [PubMed] [Google Scholar]
- 14.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27(6):1129–36. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 15.Sun PZ, Murata Y, Lu J, Wang X, Lo EH, Sorensen AG. Relaxation-compensated fast multislice amide proton transfer (APT) imaging of acute ischemic stroke. Magn Reson Med. 2008;59(5):1175–82. doi: 10.1002/mrm.21591. [DOI] [PubMed] [Google Scholar]
- 16.Jokivarsi KT, Hiltunen Y, Tuunanen PI, Kauppinen RA, Grohn OH. Correlating tissue outcome with quantitative multiparametric MRI of acute cerebral ischemia in rats. J Cereb Blood Flow Metab. 2010;30(2):415–27. doi: 10.1038/jcbfm.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50(6):1120–6. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]
- 18.Jones CK, Schlosser MJ, van Zijl PC, Pomper MG, Golay X, Zhou J. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006;56(3):585–92. doi: 10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 19.Grande S, Luciani AM, Rosi A, Guidoni L, Viti V. Identification of amide protons of glutathione in MR spectra of tumour cells. NMR Biomed. 2008;21(10):1057–65. doi: 10.1002/nbm.1280. [DOI] [PubMed] [Google Scholar]
- 20.Salhotra A, Lal B, Laterra J, Sun PZ, van Zijl PC, Zhou J. Amide proton transfer imaging of 9L gliosarcoma and human glioblastoma xenografts. NMR Biomed. 2008;21(5):489–97. doi: 10.1002/nbm.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Blakeley JO, Hua J, Kim M, Laterra J, Pomper MG, van Zijl PC. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med. 2008;60(4):842–9. doi: 10.1002/mrm.21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Yan K, Wang S, Fu D, Ford E, Laterra J, van Zijl Peter CM. Differentiation between glioma and radiation necrosis using molecular imaging of endogenous proteins and peptides. Proc Intl Soc Mag Reson Med. 2010;18:340. doi: 10.1038/nm.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Radaideh A, Mougin OE, Lim S, Tench C, Constantinescu C, Gowland PA. Magnetization transfer (MT) and endogenous chemical exchange saturation transfer (CEST) effects in patients with clinically isolated syndrome. Proc Intl Soc Mag Reson Med. 2010;18:4310. [Google Scholar]
- 24.Dula AN, Dortch RD, Landman BA, Pawate S, Lavin PJ, Welch EB, Gore JC, Smith SA. Application of CEST imaging to study amide proton transfer (APT) in healthy controls and multiple sclerosis pathology at 7 tesla. Proc Intl Soc Mag Reson Med. 2010;18:4311. [Google Scholar]
- 25.Gilad AA, McMahon MT, Walczak P, Winnard PT, Jr, Raman V, van Laarhoven HW, Skoglund CM, Bulte JW, van Zijl PC. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol. 2007;25(2):217–9. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 26.Wen Z, Hu S, Huang F, Wang X, Guo L, Quan X, Wang S, Zhou J. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage. 2010;51(2):616–22. doi: 10.1016/j.neuroimage.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61(6):1441–50. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keupp J, Eggers H. CEST-dixon MRI for sensitive and accurate measurement of amide proton transfer in humans at 3T. Proc Intl Soc Mag Reson Med. 2010;18:338. [Google Scholar]
- 29.Wei W, Jia G, Sammet S, Wassenaar P, Zhou J, Knopp MV. Improving amide proton transfer imaging with dual echo B0 mapping for field inhomogeneity correction at 3T. Proc Intl Soc Mag Reson Med. 2010;18:2986. [Google Scholar]
- 30.Sun PZ, Farrar CT, Sorensen AG. Correction for artifacts induced by B(0) and B(1) field inhomogeneities in pH-sensitive chemical exchange saturation transfer (CEST) imaging. Magn Reson Med. 2007;58(6):1207–15. doi: 10.1002/mrm.21398. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard PL, Närväinen J, Kauppinen RA, Morris GA. Unexpected magnetization transfer to aliphatic resonances in z-spectroscopy in model systems and in vivo. Proc Intl Soc Mag Reson Med. 2007;15:3464. [Google Scholar]
- 32.Williamson DC, Narvainen J, Hubbard PL, Kauppinen RA, Morris GA. Effects of radiation damping on Z-spectra. J Magn Reson. 2006;183(2):203–12. doi: 10.1016/j.jmr.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Stein AD, Roberts DA, McGowan J, Reddy R, Leigh JS. Asymmetric cancellation of magnetization transfer effects. 1994:880. [Google Scholar]
- 34.Pekar J, Jezzard P, Roberts DA, Leigh JS, Jr, Frank JA, McLaughlin AC. Perfusion imaging with compensation for asymmetric magnetization transfer effects. Magn Reson Med. 1996;35(1):70–9. doi: 10.1002/mrm.1910350110. [DOI] [PubMed] [Google Scholar]
- 35.Hua J, Jones CK, Blakeley J, Smith SA, van Zijl PC, Zhou J. Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain. Magn Reson Med. 2007;58(4):786–93. doi: 10.1002/mrm.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaiss MW, Schmitt B, Stieltjes B, Bachert P. Enhancement of MT and CEST contrast via heuristic fitting of Z-spectra. Proc Intl Soc Mag Reson Med. 2010;18:5136. [Google Scholar]
- 37.Friedman JI, McMahon MT, Stivers JT, Van Zijl PC. Indirect detection of labile solute proton spectra via the water signal using frequency-labeled exchange (FLEX) transfer. J Am Chem Soc. 2010;132(6):1813–5. doi: 10.1021/ja909001q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song X, Gilad AA, Liu G, van Zijl Peter CM, Bulte JWM, McMahon MT. Improved CEST detection in frequency domain using the length and offset VARiation of saturation (LOVARS-CEST) acquisition scheme. Proc Intl Soc Mag Reson Med. 2010;18:5139. [Google Scholar]
- 39.McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JW, van Zijl PC. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): Ph calibration for poly-L-lysine and a starburst dendrimer. Magn Reson Med. 2006;55(4):836–47. doi: 10.1002/mrm.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zur Y, Wood ML, Neuringer LJ. Spoiling of transverse magnetization in steady-state sequences. Magn Reson Med. 1991;21(2):251–63. doi: 10.1002/mrm.1910210210. [DOI] [PubMed] [Google Scholar]
- 41.Sun PZ, Benner T, Kumar A, Sorensen AG. Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn Reson Med. 2008;60(4):834–41. doi: 10.1002/mrm.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheidegger R, Vinogradov E, Alsop DC. Optimization of RF saturation to minimize B0 inhomogeneity effects in pulsed amide proton transfer imaging. Proc Intl Soc Mag Reson Med. 2010;18:2987. [Google Scholar]
- 43.de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: Preliminary results. Radiology. 2004;230(3):652–9. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 44.Sun PZ, van Zijl PC, Zhou J. Optimization of the irradiation power in chemical exchange dependent saturation transfer experiments. J Magn Reson. 2005;175(2):193–200. doi: 10.1016/j.jmr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Avni R, Mangoubi O, Bhattacharyya R, Degani H, Frydman L. Magnetization transfer magic-angle-spinning z-spectroscopy of excised tissues. J Magn Reson. 2009;199(1):1–9. doi: 10.1016/j.jmr.2009.03.007. [DOI] [PubMed] [Google Scholar]