Abstract
Objective
To determine whether EEG spectral analysis could be used to demonstrate awareness in patients with severe brain injury.
Methods
We recorded EEG from healthy controls and three patients with severe brain injury, ranging from minimally conscious state (MCS) to locked-in-state (LIS), while they were asked to imagine motor and spatial navigation tasks. We assessed EEG spectral differences from 4 to 24 Hz with univariate comparisons (individual frequencies) and multivariate comparisons (patterns across the frequency range).
Results
In controls, EEG spectral power differed at multiple frequency bands and channels during performance of both tasks compared to a resting baseline. As patterns of signal change were inconsistent between controls, we defined a positive response in patient subjects as consistent spectral changes across task performances. One patient in MCS and one in LIS showed evidence of motor imagery task performance, though with patterns of spectral change different from the controls.
Conclusion
EEG power spectral analysis demonstrates evidence for performance of mental imagery tasks in healthy controls and patients with severe brain injury.
Significance
EEG power spectral analysis can be used as a flexible bedside tool to demonstrate awareness in brain-injured patients who are otherwise unable to communicate.
Keywords: Consciousness, EEG spectral analysis, motor imagery, traumatic brain injury, locked-in-state, minimally conscious state
1. INTRODUCTION
Recent studies using functional MRI (fMRI) and event-related potentials (ERP) demonstrate that some severely brain-injured patients retain a range of cognitive capacities despite minimal or no behavioral evidence of awareness (Kotchoubey et al., 2005; Owen et al., 2006; Perrin et al., 2006; Schnakers et al., 2008; Monti et al., 2010; Bardin et al., 2011). Importantly, Monti et al. (2010) used fMRI detection of motor and spatial navigation imagery to establish communication with a patient who had no overt behavioral ability to communicate.
These results, while compelling, raise an important ethical obligation to seek out patients who may retain significant cognitive abilities not evidenced by behavioral testing as in principle such patients may have a desire and capacity to participate in their own decision-making (Fins and Schiff, 2010). Currently available methods are limited in the types of patients they can assess and in the paradigms available for determination of awareness. For example, fMRI cannot be used in patients who are unable to be transported to the scanner, have implanted ferromagnetic material or make frequent head movements. The need to bring patients to the scanner also makes repeated assessments difficult, and can overlook evidence of awareness in patients whose arousal levels fluctuate through the day (Bardin et al., 2011). ERPs, meanwhile, require exact and consistent timing of subject performance. This limits the range of applicable behavioral paradigms and risks false negative results in patients with delayed or variable response times.
An alternative is a quantitative approach to EEG using power spectral analysis. Unlike fMRI, EEG can be recorded at the bedside, allowing for multiple testing sessions across different states of arousal. EEG measurements can be carried out in patients with ferromagnetic implants, and the EEG signal can be parsed with a precise temporal resolution, allowing for removal of transient movement artifacts. Unlike ERP-based analysis methods, power spectral analysis of EEG allows for detection of responses that are delayed or not tightly synchronized to a stimulus. Finally, EEG power spectral analysis has already been used as a communication tool in patients with stroke and motor neuron disease (Bai et al., 2008) and therefore can in principle serve both as a diagnostic method and basis for development of a communication device.
With this motivation, we investigated whether spatially- and spectrally-localized changes in EEG power spectra can identify behaviorally covert responses to commands in healthy subjects and patients with severe brain injury.
2. METHODS
2.1 Subjects
Five healthy control (HC) volunteers with no history of neurological disease (three males; mean age 34 years, range 25 to 52 years) participated in the study. The three patient subjects (PSs) chosen for this study were drawn from a convenience sample enrolled in a multi-modal imaging and behavioral study of the natural history of recovery from severe brain injury. Clinical profiles of the PSs are in Table 1, Figure 1 and Supplementary Appendix A. The five HCs and three PSs in this study demonstrated the capacity to generate mental imagery on the same tasks used here, via independent fMRI studies (Bardin et al., 2011). Studies described herein were approved by the Weill Cornell Medical College Institutional Review Board. HCs gave their written consent. Consent was obtained for PSs from their legally authorized representatives.
Table 1.
Summary of Patient Subjects’ clinical status and experiments performed
| Patient Subject | Age | Time since injury (months) | Mechanism of injury | Diagnosis | Timings of Studies | |
|---|---|---|---|---|---|---|
| 1 | 25 | 25 | trauma | LIS | 2 runs in 2 hours | |
| 2 | Visit 1 | 19 | 6 | trauma | MCS | 3 Runs in 30 minutes |
| Visit 2 | 19 | 10 | Emerged from MCS | Day1: Runs 1–4 over 2 hours Day2: Runs 5&6 over 1 hour |
||
| 3 | Visit 1 | 24 | 31 | stroke | MCS | 4 Runs over 2 hours |
| Visit2 | 25 | 43 | MCS | Day 1: Run 1 in afternoon. Day 2: Run 2 in morning, Run 3 in afternoon |
Abbreviations: CNS – Central Nervous System; LIS – Locked-In-State; MCS – Minimally Conscious State
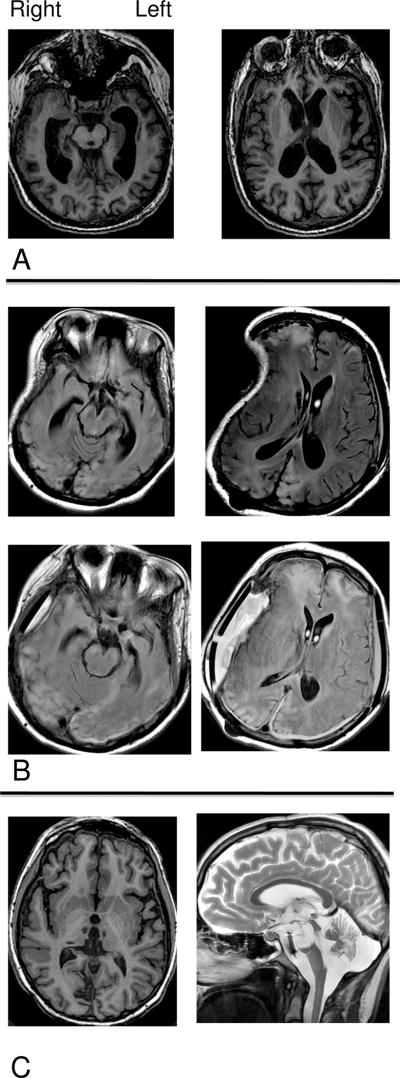
Figure 1. Brain MRIs showing major features of structural damage in the three patient subjects (PSs).
A. PS 1: T1-weighted MRI shows diffuse atrophy. B. PS 2: T2 FLAIR MRI shows focal injuries to frontal and occipital lobes and distortion from craniectomy on right (visit 1, top), and right occipital and bifrontal injuries, and fluid collection under cranioplasty site on right (visit 2, bottom). C. PS 3: T1-weighted axial image shows bithalamic and right medial temporo-occipital lobe strokes with minimal cerebral atrophy; T2-weighted sagittal image shows loss of majority of midline pons and midbrain.
2.2 Experimental paradigm
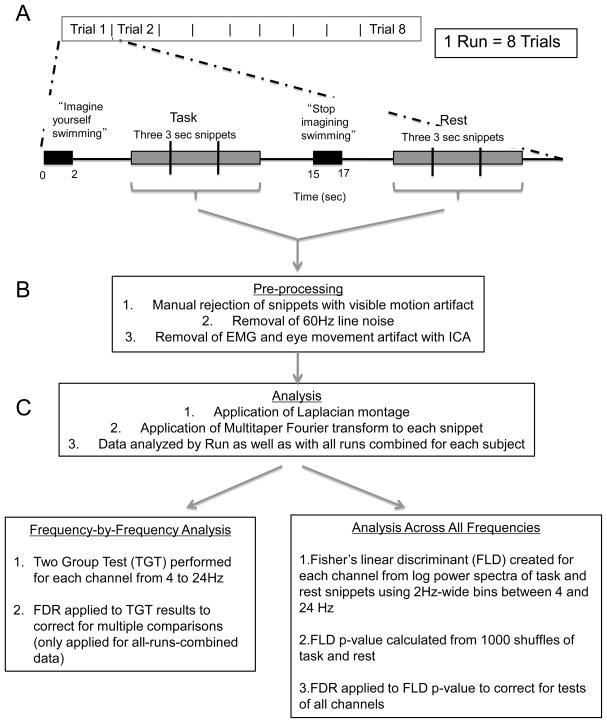
HCs and PSs were asked to perform multiple trials of one or two imagery tasks while the EEG was recorded (Figure 2). Each trial consisted of a pair of pre-recorded commands in a male voice, delivered 15 seconds apart to the subjects via noise-cancelling headphones (JVC, Wayne, NJ). Commands lasted approximately 2 seconds, providing subjects with 13 seconds to perform the mental imagery task. A sequence of 8 contiguous trials of the same start – stop command pair constituted a run; so a run lasted approximately 4 minutes.
Figure 2. Overview of task paradigm and signal processing.
A. Timeline of audio presentation and response period during EEG recording. Illustrated commands are those used in the motor imagery task. Only EEG from the period “Three 3 sec snippets” is used for further analysis. B. Data preprocessing steps. C. Data analysis steps.
In the motor imagery task, subjects alternately heard the commands “imagine yourself swimming,” and “stop imagining swimming”. Prior to each run we instructed subjects to imagine themselves swimming from the time they heard the command, until they heard the command to stop. In the spatial navigation task, subjects alternately heard the commands “imagine walking through the rooms of your house” and “stop imagining walking through your house”. Prior to each run we instructed subjects that after they were given the command, to then imagine entering their house and to move through the rooms, looking around each room. For PSs, we requested that they move through a home selected by a family member as one they would be mostly likely to recall. PSs were only tested when their eyes were open to ensure they were awake. For this reason, HCs were required to keep their eyes open during the experimental runs (blinks were allowed). Subjects performed 2 to 4 runs for each task based on time constraints and subject fatigue.
Experimental runs were identical for all subjects, although not all subjects performed both tasks. PS 1 and PS 2 performed the motor task only. HC 5 performed both tasks, but the motor task data was discarded because the subject’s eyes were inadvertently closed in one of the two runs. Experiments with HCs were performed within one three-hour EEG recording session, while experiments with PSs were variably spaced over one to two days of an inpatient visit (see Table 1).
2.3 Data acquisition
The EEG was recorded using 29 (HC 1, PS 1, PS 2, PS 3 visit 1) or 37 electrodes (Nihon Kohden (Japan) silver-collodion disc electrodes, 1.5 mm) placed via an enhanced 10–20 system (Jasper, 1958), using the Natus XLTEK (San Carlos, CA) EEG system (typical inter-electrode spacing of 3 to 4 cm). Signals were amplified and digitized at either 200 Hz (29 electrode studies) or 256 Hz (37 electrode studies) using an anti-aliasing high pass filter with a corner frequency at 0.4 × the digitization rate. To ensure accurate timing of the EEG activity with respect to the voice commands, an auxiliary channel of the EEG system recorded the audio signal. Synchronous video recordings of the subjects were obtained via the Natus XLTEK system so that periods of large amplitude movement could be removed from the analysis and to ensure that subjects had their eyes open throughout the experiment.
2.4 Data analysis
EEG from 9 of the approximately 13 second response period was used for analysis. The 9-second period began 1 second after the end of the audio command to ensure that the analysis did not include a non-specific alerting response to hearing the command. We terminated the analysis period 3 seconds before the start of the subsequent command based on feedback from the HCs who reported difficulties maintaining task performance for the entire 13-second response period. The 9 seconds of data were cut into three-second “snippets”, resulting in a total of 48 snippets per run (Figure 2). Snippets with artifact due to subject movements (as determined by review of the video) were removed. Analysis of the EEG was performed one run at a time and consisted of three further steps: (1) removal of artifacts from line noise, muscle (EMG), eye movement, and eye blink, (2) calculation of power spectra, and (3) determination of the statistical significance of differences between conditions. These steps are described in Figure 2 and in detail below, and were carried out in Matlab (The Mathworks, Natick, MA) using in-house software except where noted.
2.5 Artifact Removal
To remove 60 Hz line noise, we applied a frequency-domain regression based on Thomson’s F-test, (Thomson, 1982; Percival and Walden, 1993) implemented by the code rmlinesc from the Chronux toolbox (Mitra and Bokil, 2007, http://chronux.org). To remove muscle, eye movement and eye blink artifact, we used independent components analysis (ICA), as implemented by EEGLAB’s runica tool (Delorme and Makeig, 2004, http://sccn.ucsd.edu/eeglab/). To improve ICA performance and eliminate low-frequency artifacts due to movement, sweat, and other sources, we applied a 1 Hz high pass filter prior to the ICA, as implemented by EEGLAB’s infinite impulse response (IIR) filter plug-in. We used the methods described in McMenamin et al. to identify and remove “myogenic” ICA components, as well as those representing blinks and large amplitude eye movements (McMenamin et al., 2010). Components containing an apparent mixture of “myogenic” and “neurogenic” activity (as defined by McMenamin et al.) were not removed to avoid removing signal of interest. EMG artifact from microsaccades likely had minimal effect on these data, as there was no requirement for visual fixation, the Laplacian montage filtered these signals out of most channels, and spectral changes were only analyzed to 24 Hz while the power from this artifact type occurs from 20 to 80 Hz (Schwartzman and Kranczioch, 2011). ICA artifact rejection, across all subjects, led to removal of 20 to 94% of the variance (see Supplementary Table S1). The fraction of variance removed generally correlated with amount of artifact in the recordings as assessed by visual inspection of the EEG.
2.6 Laplacian Montage
After artifact removal, EEG signals were converted to the Hjorth Laplacian montage (HLM) as a step to improve source localization (Hjorth, 1975). To make this conversion, we calculated the difference between the voltages at each individual electrode placement and the weighted average (at the same time point) of the voltages at the surrounding electrodes (four nearest neighbors for electrodes on the interior of the array; three for electrodes on the edge). The weight attributed to each electrode was the reciprocal of the distance to the central electrode, measured as arc length on an assumed spherical head (Hjorth, 1980; Thickbroom et al., 1984).
2.7 Power Spectral Density
We calculated power spectral density for each HLM channel separately for each three-second snippet, using Thomson’s multi-taper method (Thomson, 1982; Percival and Walden, 1993; Mitra and Pesaran, 1999), as implemented by the code mtspectrumc in the Chronux toolbox (Mitra and Bokil, 2007, http://chronux.org). We used 5 tapers, resulting in a frequency resolution of 2 Hz and estimates spaced 1/3 Hz apart. Further analysis was restricted to the frequency range 4 to 24 Hz, to avoid overlap with frequencies typically contaminated with artifact (eye movement below 4Hz and muscle above 24Hz) (Shackman et al., 2010). Power spectral changes in the analyzed range are known to correlate with actual and imagined motor and spatial imagery task performance in healthy subjects (Pfurtscheller and Lopes da Silva, 1999; Hung et al., 2005; Li et al., 2009) and, when localized, overlap with cortical areas activated in fMRI (Yuan et al., 2010). Example spectra averaged across a run can be seen in Figure 3A.
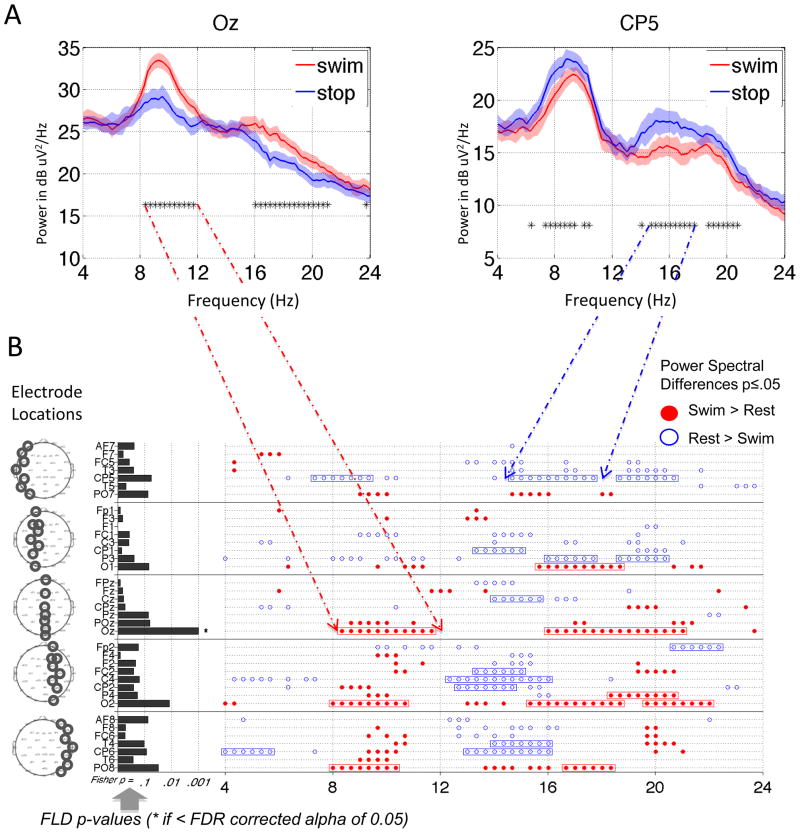
Figure 3. Power spectral analysis of EEG in HC 1 during one run of the motor imagery task.
A. Power spectra (with 95% jackknife error bars) for two Laplacian channels from Run 1 of HC 1 performing motor imagery versus rest. Frequencies with differences (TGT p ≤ 0.05 by jackknife) are noted by *s in the lower part of the graph. B. Summary for Run 1 across all channels: circles represent frequencies with differences between task and rest (TGT p ≤ 0.05 by jackknife): red-filled means more power in the task condition; blue-open means more power in the rest condition. Rectangles are drawn around contiguous groups of frequencies that span a range of greater than 2 Hz (Section 2.8). Horizontal bars on the left represent p-values of the FLD: * to right of bar of Oz implies this FLD p-value is significant after FDR (0.05) correction. Head maps on the left show locations of the channels.
2.8 Statistical Analyses
We used two complementary methods to determine the significance of differences in the frequency content of the EEG signal between the task and rest conditions: a univariate (frequency-by-frequency) approach and a multivariate approach. For each subject, both analyses were applied on a channel-by-channel basis to each run individually and to all runs combined.
For the univariate approach, we used a z-statistic, the Two Group Test (TGT) (Bokil et al., 2007), as implemented by the Chronux toolbox routine, two_group_test_spectrum (http://chronux.org), with a cutoff of p≤0.05 by jackknife method. Because spectral estimates within 2 Hz of each other are correlated by the taper functions, a difference identified by the TGT was only considered significant if it was present for all frequencies contiguously over a range greater than 2 Hz. This implies significance over at least two neighboring but non-overlapping windows of the multi-taper estimate and is indicated in figures with a rectangle drawn around the results (Figure 3B). Spectral differences over ranges narrower than 2 Hz represent only a trend to significance. To compensate for multiple comparisons (60 frequencies per channel in 29 or 37 channels), the False Discovery Rate (FDR) (Benjamini and Hochberg, 1995; Benjamini and Yekutieli, 2001) was applied to the TGT p-values determined from analyses of all runs combined.
To look for spectral differences that might only be apparent if combinations of frequencies are considered, we employed a multivariate approach, Fisher’s linear discriminant (FLD) (Fisher, 1936). This approach has been used successfully for classification of EEG responses to motor imagery (Hung et al., 2005; Bai et al., 2007). To limit dimensionality, we binned the log spectra from 4 to 24 Hz into 2 Hz windows, reducing the spectrum to 10 values. The FLD was then defined as the linear combination of these quantities that maximized the ratio of the power variance between the conditions to the power variance within the conditions. To determine the significance of the FLD, we used a shuffle method: we recomputed the FLD from 1000 shuffles of the two conditions, and determined the p-value as the fraction of shuffled datasets that yielded an equal or larger value to the actual FLD. To take into account the possibility that neighboring snippets had similar spectra because of a slowly changing underlying brain state (rather than the task) (Menzer et al., 2010), the shuffled datasets kept the snippets from the nine-second-response period after each command together during all shuffles. To control for multiple comparisons (since the FLD was applied separately to each channel), the FLD p-value was only considered significant for a channel if it was less than an FDR-corrected rate of 0.05. This is shown as an asterisk on the summary figures (e.g. channel Oz in Figure 3B). For each subject, this analysis was applied to each run individually, and to all runs combined.
2.9 Overall outcome measures
As our imagery tasks had no observable behavioral measures of performance, we used the responses of the HCs to develop outcome measures to apply to the PSs, with the explicit assumption that the HCs performed the task. Based on HC results (Section 3.1), we chose to use the TGT for the primary outcome measure. Furthermore, we chose within-subject between-run consistency of spectral changes as our primary outcome measure in PSs, rather than a specific “template” of changes, for two reasons. First, although the data showed consistent and statistically significant task-related changes within each HC, it showed notable variations in the pattern of these changes between HCs, both in scalp location and frequency. Second, this strategy allows for the possibility that PSs’ brain injuries resulted in spectral changes different from HCs. We codified the definition of a positive response in PSs into two overall outcome measures: an inclusive measure (outcome measure 1) that could detect responses that were only present in a subset of runs; and a strict measure (outcome measure 2) that requires consistency across all runs.
Outcome measure 1 – A positive finding required that one run had a channel with a significant TGT result (contiguous over greater than a 2 Hz range as described above), and a second run showed at least a trend towards a significant TGT result in the same channel and within the same frequency range. Considered in isolation, outcome measure 1 was subject to false positive results as there was no correction for multiple comparisons.
Outcome measure 2 - A positive finding required that when all runs were combined, at least one of the individual spectral differences identified by the TGT remained significant after FDR correction (0.05) for all frequencies and channels tested.
Overall results were designated “positive” if outcome measures 1 and 2 were met, “indeterminate” when only outcome measure 1 was met, and negative otherwise. The reason to retain the “indeterminate” designation was that PSs may indeed have had fluctuating levels of awareness and performance. Thus, if EEG power changes were seen on some runs but not others, we simply could not determine whether the changes were merely a statistical artifact from the multiple comparisons, or in fact reflected these fluctuations.
3. RESULTS
3.1 Healthy Controls
We begin by stepping through the detailed analysis of two HCs performing the motor imagery task. We then summarize the findings across all HCs on both the motor and the navigation imagery tasks. In all comparisons shown below, we describe increases or decreases in power of the EEG spectrum as task relative to a rest (stop imagining) condition.
Motor Imagery Task (imagination of swimming)
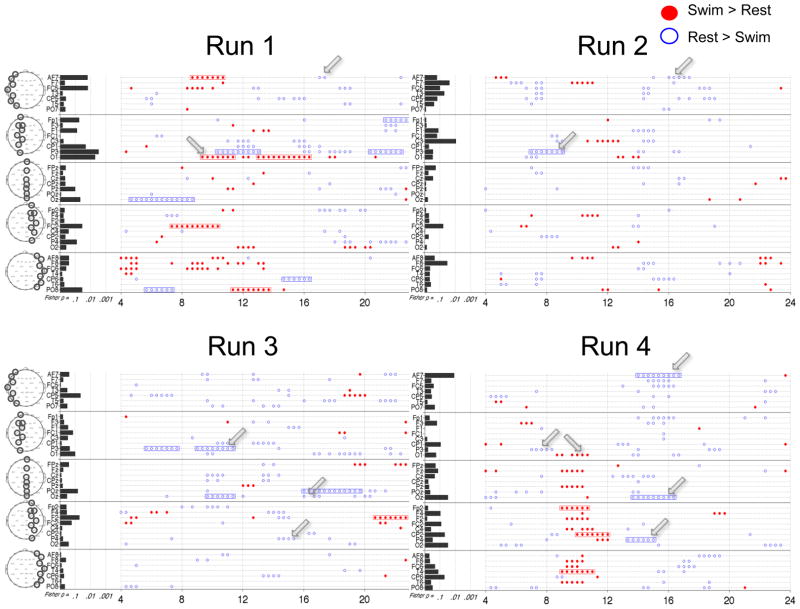
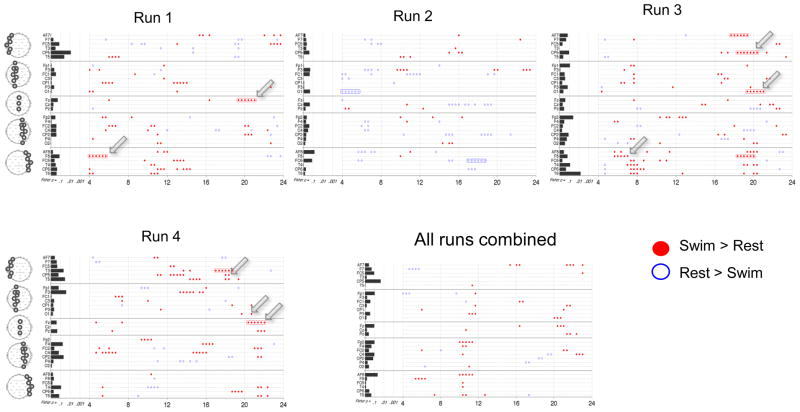
Results from HC 1 on the motor imagery task are shown in Figure 3. We begin with the univariate (frequency-by-frequency) analysis on a single run. Figure 3A shows spectra from two Laplacian channels (Oz and CP5) from the first run, averaged across all 8 trials for each condition. During mental imagery there was a significant increase in power from 8 to 12 Hz and from 16 to 21 Hz at Oz, and a significant decrease at partially overlapping bands of frequencies at CP5, as indicated by the significant TGT results. Figure 3B summarizes the imagery versus resting state comparisons at all frequencies and channels. For this subject, significant changes (greater than 2 Hz in width, see Section 2.8) were as follows: decreased power from 4 to 6 Hz in CP6, 7 to 10 Hz in CP5 (see blue arrows), and 13 to 20 Hz in multiple central and parietal channels; increased power was seen from 8 to 12 Hz and 16 to 22 Hz in multiple posterior channels (red arrows point to Oz). The results of the multivariate analysis, via the FLD, are summarized on the left of Fig. 3B. For this run, only channel Oz was significant via the FLD after FDR correction. It is also notable that the FLD p-values generally correlated with the number of significant univariate differences in each channel.
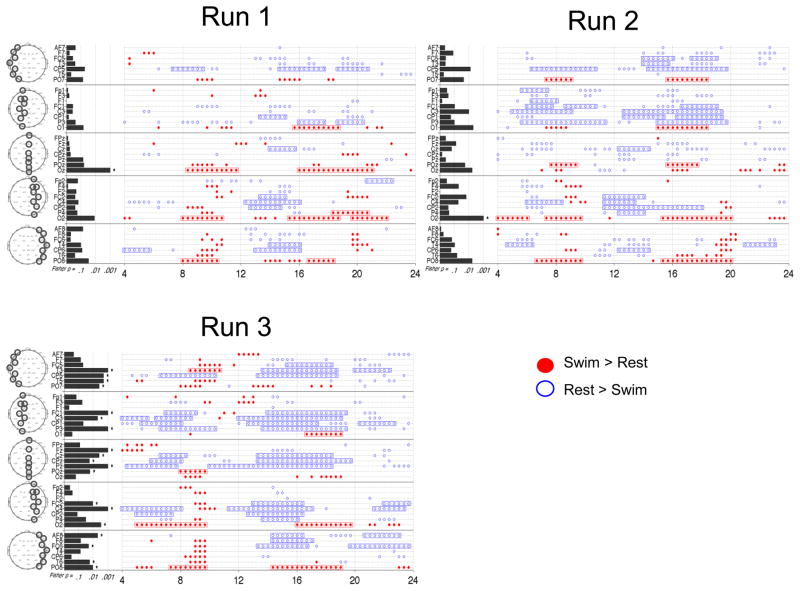
Figure 4 summarizes this analysis for the 3 runs of HC 1. There was a general consistency between the runs in regards to channel location and direction of change in the frequencies identified as significant by the TGT. Superimposed on this overall pattern there were variations in the overall number and local patterns of significant frequencies between runs. For example, Runs 2 and 3 had more frequencies with significant differences than Run 1 and a stronger pattern of significant power decreases between 5 and 10 Hz in central channels. In addition, Run 3 had more channels with significant FLD p-values. Of interest the results from several channels in Run 3 were deemed significant by FLD but not by the TGT (e.g. PO7), however, this was not typical of other datasets.
Figure 4. Power spectral analysis of EEG in HC 1 for all runs of the motor imagery task.
Plotting conventions as in Figure 3B.
Figure 5 shows the summary figures of the 4 runs of motor imagery for HC 2. Compared to HC 1, this subject’s runs had markedly fewer frequencies with significant changes via the TGT, and no channels with significant changes via the FLD. Nonetheless, bands of significantly different spectra were observed across multiple runs in the same channel and frequency range. Increased power was seen during imagery from 9 to 11 Hz in O1 (significant in Run 1, trend in Run 4). Decreased power was seen from: 6 to 9 Hz in P3 (significant in Runs 2 and 3, trend in Run 4); 14 to 17 Hz in AF7 (significant in Run 4, trend in Runs 1 and 2); 13 to 15 Hz in P4 (significant in Run 4, trend in Run 3); and 14 to 17 Hz in Oz (significant in Run 4, trend in Run 3). For clarity, arrows in Figure 5 indicate the findings. Of note, compared to HC 1, this subject had significantly more muscle artifact in all runs, with an average of 72% of the variance removed by ICA versus 32% of the variance removed for HC 1 (Supplementary Table S1).
Figure 5. Power spectral analysis of EEG in HC 2 for all runs of the motor imagery task.
Plotting conventions as in Figure 3B. Arrows refer to findings discussed in Section 3.1.
Results from HCs 3 and 4 (Supplementary Figures S1 and S2) were intermediate to HCs 1 and 2, both in terms of number of significant changes identified by TGT and FLD on each run, as well as consistency between runs. In both subjects, the univariate analysis via the TGT identified significant changes that were consistent across all runs, and additional changes that were consistent across subsets of runs. Results of the multivariate analysis via the FLD generally agreed with the TGT and only rarely identified significant changes at a channel at which no change was determined by the TGT. Supporting the finding in HC 2 of artifact impeding visualization of signal change, HC 4, across runs, demonstrated an inverse correlation between number of significant changes and percentage of variance removed (variance removed was: 61% Run 1, 48% Run 2, and 19% Run 3). This was also observed to a lesser degree in other subjects (variance removed reported in Supplementary Table S1).
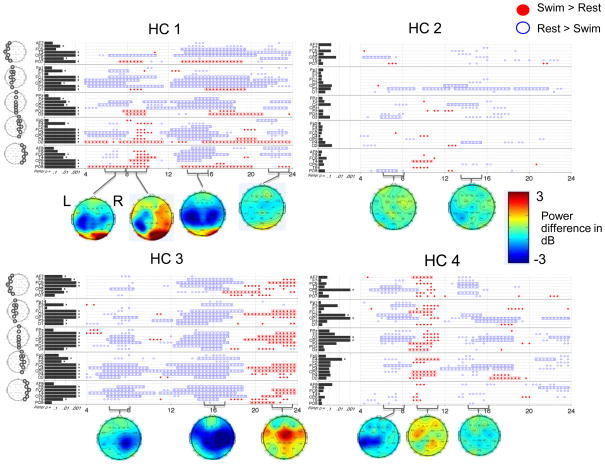
To identify the overall patterns of EEG change and compare across subjects we carried out the above analyses for each HC after averaging all runs of the motor imagery task for each subject (Figure 6). There was a clear pattern of signal change for each HC along with evidence of both commonalities and individual variations across HCs. In all HCs, we observed a decrease in power in the 6 to 9 Hz and 13 to 18 Hz ranges, predominantly in the central and parietal EEG channels. Findings common to subsets of HCs were: increased power from 9 to 11 Hz in HC 1 (posterior channels), HC 2 (temporal channels), and HC 4 (diffusely); increased power from 16 to 20 Hz in HCs 1 and 4 in posterior channels; and decreased power from 21 to 24 Hz in HCs 1 and 4 (whereas HC 3 demonstrated increased power in this range). To assess outcome measure 2, we applied FDR correction for multiple comparisons to all the TGT p-values across all channels, and found significant results in all subjects. Of the results displayed, the percentage that remained significant after FDR correction were: HC 1 – 77%, HC 2 – 3%, HC 3 – 74%, HC 4 – 36%. The all-runs-averaged data from HCs 1, 3 and 4 also demonstrated channels with significant FLD.
Figure 6. Power spectral analysis of EEG for each HC, for all runs of the motor imagery task combined within each subject.
Plotting conventions for the upper section of each panel as in Figure 3B; lower sections indicate differences in log power (task minus baseline) at selected frequencies. Headmaps rendered via EEGLAB’s “topoplot” command (http://sccn.ucsd.edu/eeglab).
Navigation task (Imagination of walking around one’s house)
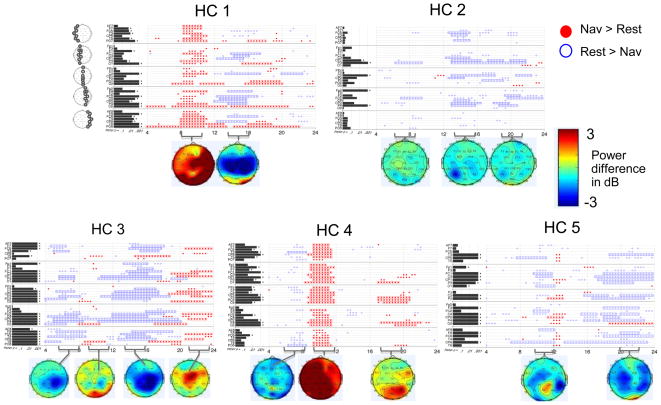
For the navigation imagery task, we also found that HCs showed significant task-related modulation of the EEG, with some findings at consistent channels and frequency bands across runs. Individual subject results are presented in Supplementary Figures S3–S7. In contrast to the motor imagery task, when data from each HC were averaged over all of their runs (Figure 7), there was no change that was consistent across all HCs, although some changes were consistent across subsets of HCs. These include: decreased power during navigation imagery from 6 to 9 Hz in central and parietal channels (HCs 2, 3, 4 and 5); increased power from 9 to 11 Hz diffusely (HCs 1 and 4) and posteriorly in HC 3 (also seen weakly around 12 Hz only in HC 5); decreased power from 12 to 17 Hz centrally (HCs 1, 2, and 3); increased power 16 to 20 Hz in posterior channels (HCs 1, 3 and 4); and decreased power from 18 to 24 Hz centrally (HCs 2 and 5 and one channel in HC 1; HC 3 shows increased power in this range). For outcome measure 2, the percentages of significant TGT results that remained significant after FDR correction for each subject were: HC 1 – 69%, HC 2 – 21%, HC 3 – 75%, HC 4 – 58%, HC 5 – 62%. All subjects had channels with significant changes via FLD, but, as was seen for motor imagery, the FLD rarely identified changes that were not also identified by the TGT.
Figure 7. Power spectral analysis of EEG for each HC, for all runs of the navigation imagery task combined within each subject.
Plotting conventions as in Figure 6.
Summary of Findings in HCs
For HCs, EEG spectral analysis showed task-dependent changes on both the motor and navigation imagery tasks on all runs and met the criteria for outcome measure 1 (consistent differences by the TGT across runs) and outcome measure 2 (significant differences by the TGT after FDR correction). Observed signal patterns and strengths varied both within HCs across runs, and between subjects using all-runs-combined results. Variation between HCs was especially notable in navigation imagery, where no feature was present in all 5 HCs. When comparing the response between the motor and navigation tasks, there were clear similarities in pattern within subjects, though differences were seen as well (Figures 6 and 7).
3.2 Patient Subjects
We now turn to the data from the three PSs, all of whom showed fMRI evidence of command following in work conducted in our laboratory (Bardin et al., 2011). As detailed below, two of the PSs demonstrated evidence of task-related modulation of the EEG, though with patterns of change different from HCs. The remainder of the PS studies were indeterminate due to variability between runs.
Patient Subject 1
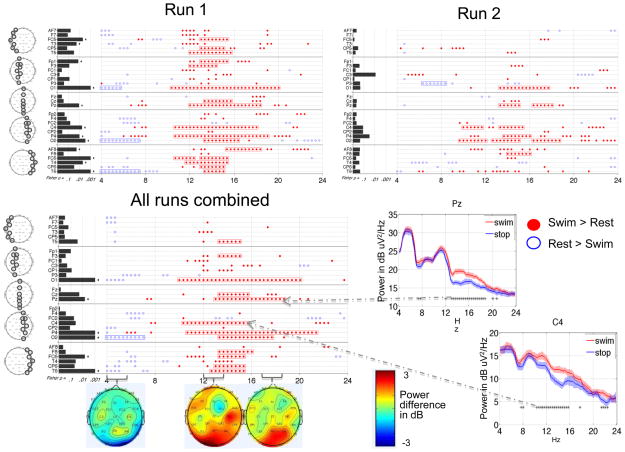
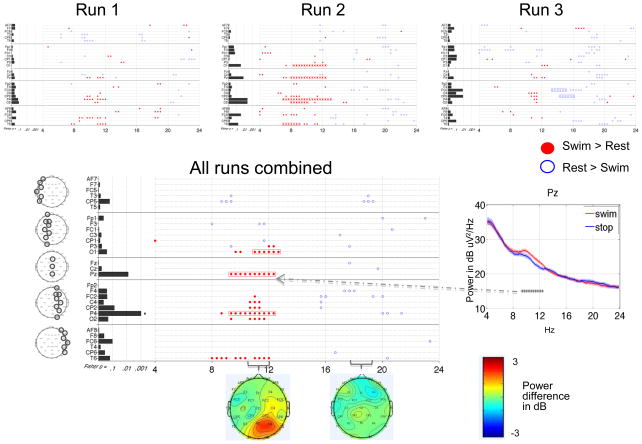
PS 1 completed two runs of the motor imagery task during one visit (Figure 8). The results were positive as there were consistent changes between runs (positive outcome measure 1) and with runs combined, 53% of the TGT p-values remained significant after FDR correction (positive outcome measure 2). The primary finding of the runs-combined analysis was significantly increased power over a broad range of frequencies (11 to 20 Hz) across multiple channels, with maximal signal strength concentrated posteriorly. Of note, this finding was not seen in any of the HCs, though HC 1 did demonstrate increased power in the 13 to 20 Hz range in some posterior channels (Figure 6). The other finding from the runs-combined analysis was a decrease in power over posterior channels between 4 and 8 Hz, also seen in HCs 2, 3 and 4 (Figure 6). In Run 2 compared to Run 1, spectral changes were more spatially restricted to the right central and posterior channels. The fewer significant differences in Run 2 may have been due to removal of more data due to patient motion (18 snippets removed from Run 2 versus 3 snippets from Run 1 - Supplementary Table S1).
Figure 8. Power spectral analysis of PS 1 for the motor imagery task.
each run analyzed separately (upper panels, plotted as in Figure 3B) and both runs combined (plotted as in Figure 6). Right lower panel shows example power spectra (Laplacian channels Pz and C4).
Patient Subject 2
PS 2 completed three runs of the motor imagery task on the first visit, and six runs of the motor imagery task on the second visit. On visit 1 (Figure 9), this subject met the outcome measure 1 criterion: increased power from 8 to 12 Hz in posterior channels in all runs (significant only in Run 2); and decreased power from 15 to 17 Hz (significant in Run 3, trend in Run 2). Outcome measure 2 was also positive: 14% of the differences in the runs-combined analysis were significant after FDR correction. Note that HCs 1 and 4 also showed decreases in EEG power in the same frequency band and scalp region (Figure 6). On visit 2 (Supplementary Figure S8), significant differences were seen in common to subsets of runs (positive outcome measure 1), though none were significant in all-runs-combined (negative outcome measure 2). As such, we designated this subject’s response on visit 2 to be indeterminate. Of note, this patient’s recordings had the greatest amount of variance removed by ICA (78% or more in all runs - see Supplementary Table S1). This reflects the removal of frequent eye blinks (typically 1 to 3 per second) and a moderate amount of muscle artifact.
Figure 9. Power spectral analysis of PS 2 for the motor imagery task on visit 1.
each run analyzed separately (upper panels, plotted as in Figure 3B) and both runs combined (plotted as in Figure 6). Right lower panel shows example power spectra (Laplacian channel Pz).
Patient Subject 3
PS 3 completed motor and navigation imagery during two visits. The findings on both tasks at both visits were indeterminate. There were isolated significant changes on subsets of runs (positive outcome measure 1), but none remained significant after FDR correction with all runs combined (negative outcome measure 2). Because consistent results were seen in subsets of runs, we speculate that PS 3 was in fact able to perform the task, but that this ability fluctuated over time, potentially due to subclinical seizure activity. Findings from visit 1 motor imagery are detailed below; while those of visit 1 navigation imagery, and visit 2, motor and navigation imagery, are in Supplementary Figures S9–S11.
In visit 1 motor imagery (Figure 10), all runs had bands of frequencies with significant differences, and Runs 1, 3 and 4 had more than one channel with a significant FLD. Common findings between runs included: increased power at F8 from 4 to 6 Hz (significant in Run 1, trend in Run 3); increased power at Fz from 19 to 22 Hz (significant in Runs 1 and 4); and increased power at CP5 and O1 at 20 Hz (significant in Run 3, trend in Run 4). For clarity, arrows in Figure 10 indicate the findings. When runs were combined, none of the spectral differences were significant after FDR correction (negative outcome measure 2). None of the HCs had similar increases in power from 4 to 6 Hz in right frontal channels, though HC 3 had an increase in power in frontal channels between 20 and 24 Hz (Figure 6).
Figure 10. Power spectral analysis of PS 3 for the motor imagery task on visit 1.
each run analyzed separately (plotted as in Figure 3B) and both runs combined (plotted as in Figure 6). Arrows refer to findings discussed in Section 3.2.
4. DISCUSSION
In this study we have shown that EEG power spectral analysis can be used to denote performance of a mental imagery task in healthy controls. In addition, we used outcome measures developed from the HC studies to demonstrate evidence of motor imagery task performance in patients with severe brain injury. In the HCs, imagination of either swimming or walking around their house changed EEG spectral power at multiple channels and frequency bands as indicated by the TGT. Based on the HC results, we developed and applied outcome criteria to the PS results that made no a priori assumptions about patterns of signal change. Rather, these criteria were based on rigorous statistical criteria including consistency across runs and correction for multiple comparisons. Using this approach we observed a range of findings in our PSs. PS 1, who remained in LIS from traumatic brain injury (TBI), demonstrated a widespread significant change in EEG power spectra on the motor imagery task. Notably, the pattern of signal change differed markedly from the HCs. PS 2 who, at visit 1 was in MCS from TBI, also demonstrated a positive finding on the motor imagery task, though with signal change in a relatively restricted number of EEG channels compared to most HCs and PS 1. On PS 2’s second visit, despite an improved behavioral exam (emergence from MCS), we measured an indeterminate response. PS 3, who remained in MCS following strokes of the brainstem and thalami, showed an indeterminate response at both time points of assessment using both motor and spatial imagery tasks.
These findings imply that the quantitative EEG methodology developed here can be used to determine awareness in patients with apparent disorders of consciousness who cannot demonstrate command following through movement. At present, the clinical evaluations and rating scales used to diagnose level of consciousness require movement to demonstrate awareness (Giacino et al., 2004), but motor output may be limited by peripheral or central nervous system injury. These motor deficits, along with fluctuating arousal levels, have been shown to lead to a high misdiagnosis rate: patients who actually are MCS may be mistaken for VS (Childs et al., 1993; Andrews et al., 1996; Schnakers et al., 2009), and patients who are in the fully conscious LIS may be mistaken for MCS (Smart et al., 2008, Personal experience.). The distinction between VS and MCS (or potentially higher levels of function) is essential, as available data support a better prognosis for those in MCS (Giacino and Kalmar, 1997; Lammi et al., 2005; Estraneo et al., 2010).
4.1 Comparison to Other Methods
The methods developed here have a complementary role to fMRI approaches (Owen et al., 2006; Monti et al., 2010; Bardin et al., 2011) for assessing awareness and cognitive function in patients who lack a behavioral means of communication. EEG allows for assessment of patients who cannot be transported, who have ferromagnetic implants, or have intermittent head movements that preclude fMRI interpretation. Bedside testing also allows for repeated examinations, and at short notice. This increases the likelihood of capturing responses associated with spontaneous fluctuations in arousal, and facilitates assessing response to a treatment. EEG also allows for determination of the patient’s level of arousal through clinical exam (the patient can be directly interacted with) as well as through clinical review of the EEG at the time of the study. FMRI, however, offers a much higher spatial resolution, potentially allowing for a higher degree of differentiation of response between tasks.
Power spectral analysis of the EEG, as performed here, also has advantages over standard event-related potential analysis (ERPs). Standard ERP analysis involves averaging the time-domain EEG signal to extract cerebral activity that is time-locked to a particular event. For example, the P3, a type of ERP, is a positive deflection that occurs when an infrequent stimulus is introduced in the setting of a regularly repeating background stimulus. While the presence of the P3 has been shown to correlate with behavioral evidence of consciousness, (Kotchoubey et al., 2005; Schnakers et al., 2008) and, in small studies, correlate with recovery (Wijnen et al., 2007; Qin et al., 2008), there are some caveats concerning this approach. ERPs require responses that are time-locked to a stimulus, leading to false negatives in patients with delayed responses and precluding paradigms with prolonged response periods. With our methodology, strict time locking is not required, since we are measuring event-related changes in the power spectrum, i.e., event-related synchronization and desynchronization (Pfurtscheller and Lopes da Silva, 1999). This approach treats the signal within the analysis window as statistically stationary and compares it to a reference state; thus the analysis is sensitive to relative changes in signal power at a given frequency arising at any time across the period sampled. Since this analysis is carried out over several seconds, it is much more likely to identify a substantially delayed response than an ERP technique. A delayed response, though implying pathology, still provides evidence of cognitive function and the potential for communication. Additionally, since strict time locking is not required, event-related spectral changes can be used in a wider variety of behavioral paradigms.
4.2 Observed signal characteristics
HC results led to important observations regarding interpretation of PS data in this and future studies. First, significant variability was seen when comparing findings across HCs on the same task. On the motor imagery task, while HC subjects had findings in common (power decreases from 6 to 9 Hz and 13 to 15 Hz in central channels), there was also a significant amount of variation between them (Figure 6). In the navigation imagery task, there were no findings in common to all HCs (Figure 7). We speculate that this variability is due to variation in task performance, though cannot confirm this, as the task has no behavioral output. It is also possible that the variability between subjects is due to neuroanatomical differences that affect the source geometry of the EEG signal.
Due to this variability in HC results, along with the fact that those with severe brain injury have differences in neuroanatomy and connectivity due to injury and the recovery process, we designed our PS outcome measure without an a priori signal pattern. Of note, this is different than the approach of some fMRI analyses (Owen et al., 2006; Monti et al., 2010), where a standardized region of interest (ROI) was used to define a positive outcome measure. We allowed for flexibility in the outcome signal by determining significance at individual frequencies instead of pre-defined frequency bands, and by making the primary outcome measure be consistency of signal change across runs. The most notable finding allowed by this approach is the positive result in PS 1 (Figure 8), despite a marked difference in frequency location and direction of the signal change compared with HCs (Figure 6). It is not possible to determine whether the reason for the difference in this patient’s spectral pattern reflects variation in the way the task was performed, or an injury-induced reorganization in cerebral networks supporting the behavior. This patient’s consistent bedside command following and communication using head movements is a strong reason to believe that he was able to perform the mental imagery task, though clearly the mental imagery task samples an independent set of cerebral networks.
A second finding from the HC results was that the TGT was more sensitive to determining changes in the spectra than the FLD. This means that no combination of frequency band changes better differentiated the conditions than the individual changes alone. As such, the data indicate that for the determination of patient awareness via EEG spectral changes, the univariate approach (the TGT) with correction for multiple comparisons suffices. Though in principle a multivariate approach may be more sensitive, a search for patterns across frequencies entails many more degrees of freedom, and there appears to be no net benefit of that approach in this application.
A third observation from the HC data was that subjects demonstrated changes in their EEG spectra to two different tasks, motor and spatial imagery. This is important, as patients may be unable to perform one category of task (Monti et al., 2010) due to causes such as focal injury to a cortical area involved in the task.
One potential limitation to interpretation of PS data is the possibility of changes in the EEG spectra due to non-volitional cortical activation from the auditory command presentation. Our analysis methods greatly reduce this possibility through ignoring data from the first second after command presentation and requiring consistency across runs as the primary outcome measure. Furthermore, any non-specific alerting response to sound wouldn’t differentiate the two types of commands, and there is no indication that unconscious patients have sustained (over 1 second) cortical activation that can differentiate between commands (Owen et al., 2007).
4.2 Interpretation of Indeterminate Results
PS 2 at visit 2, and PS 3 had indeterminate results, as the significant spectral differences between task and rest on individual runs, were no longer significant when averaged and corrected for multiple comparisons. We chose to retain an explicit “indeterminate” designation, to recognize the potential for intermittent task performance and limitations of EEG power spectral analysis. In fact, the clinical and imaging evidence in these two patient subjects indicates that it might be misleading to consider these results as “negative”. Both PS 2 and PS 3 showed behavioral evidence of following simple commands (Supplementary Appendix A), and, notably, PS 2 had an improved behavioral exam at visit 2 (emergence from MCS). Furthermore, both of these subjects showed evidence of performance of the same mental imagery task via an independent fMRI study (Bardin et al., 2011), during visits where their EEG response was deemed indeterminate.
One potential cause of indeterminate results is variability in task performance across runs. HCs, who also demonstrated variation in patterns of EEG spectral change across runs, may have performed the tasks differently as they started naïve to the tasks. Variable task performance may also be due to differences in overall level of arousal. This may have occurred in HCs as the EEG testing session lasted over 3 hours. As for the PSs, it is well known that patients with severe brain injury have fluctuating arousal throughout a day (Giacino et al., 2002; Hart et al., 2006; Schiff et al., 2007). In fact, behavioral testing of both PS 2 and PS 3 showed variations in responsiveness to spoken commands over different testing periods. In PS 3 this may have been due to intermittent subclinical seizure activity. A previous study, using fMRI, also demonstrated variability in signal strength and location across imaging sessions of one patient (Bardin et al., 2011). Future work may benefit from repeated testing of patients over many days to look for repeated patterns of signal change, suggestive of intermittent task performance.
There are other more general limitations to EEG analysis that may result in indeterminate results. One patient-specific factor is cortical atrophy, which may result in a weaker EEG signal. Another issue for both patients and controls is muscle electrical activity (EMG) contamination of the EEG signal. EMG has been shown to appear in the EEG power spectrum well into the frequency range involved in motor imagery (McMenamin et al., 2010) and therefore can confound interpretation of spectral power changes in this range. ICA is successful in removing this artifact, though is time consuming and requires a trained investigator to select components to remove. In the setting of a significant amount of EMG, such as HC 2 in this study, ICA artifact removal may also remove some of the underlying EEG, limiting the ability to identify changes in EEG spectral power. Thus, the use of EEG power spectral analysis as a clinical diagnostic tool, or potentially a communication device, will be facilitated by further development of algorithms to remove muscle electrical artifact quickly and reliably.
4.3 Conclusions and Future Directions
Overall, based on our positive results in 5 healthy subjects and 2 patients with severe brain injury, we find that EEG power spectral analysis of response to imagery commands is a promising tool for identifying awareness in patients who have no clear behavioral output. Determination of patient awareness requires a flexible approach that takes into account variability in the frequency bands and scalp locations at which EEG spectral changes may be present. EEG power spectral analysis has advantages of being inexpensive, portable, and flexible in terms of task paradigms and detection of delayed responses. Before this can be used as a clinically diagnostic tool, a larger group of patients will need to be assessed at multiple time points and results correlated with behavioral and other imaging measures. Additionally, a larger pool of healthy control subject data is required to determine if there is a normal range of patterns, and if these can serve as a marker of recovery in brain injured patients. If future work finds patients who are able to use imagery tasks to modulate EEG spectra consistently, this tool could potentially be used as a bedside “yes/no” brain computer interface, complementing this application of fMRI (Monti et al., 2010; Bardin et al., 2011).
Supplementary Material
Supplementary Table S1: Summary for all subjects of: tasks and number of runs performed; count of snippets analyzed (others removed due to artifact); ICA components rejected (out of a total of 29 or 37 corresponding to number of EEG channels); and percentage of variance removed by rejecting ICA components (a marker of amount of artifact in the data).
Supplementary Figure S1: Power spectral analysis of EEG in HC 3 for all runs of the motor imagery task. Plotting conventions as in Figure 3B.
Findings – all runs demonstrated significant power decreases between 14 and 18 Hz and increases between 20 and 24 Hz in central channels. Runs 2, 3 and 4 also showed significantly decreased power from 6 to 10 Hz in central channels. Other findings described in Section 3.1.
Supplementary Figure S2: Power spectral analysis of EEG in HC 4 for all runs of the motor imagery task. Plotting conventions as in Figure 3B.
Findings - all runs demonstrated significant power decreases from 8 to 9 Hz in CP5, and power increases from 9 to 11 Hz in central channels (trend in Run 1, significant in Runs 2 and 3). Subsets of runs showed: decreased power from 13 to 16 Hz in central channels (trend in Run 1, significant in Run 3); increased power from 17 to 19 Hz in posterior channels (trend in Run 1 and significant in Run 2). Runs 1 and 2 had fewer significant spectral frequencies than Run 3, which correlated with Run 3 being the only one with significant FLD p-values. Other findings described in Section 3.1.
Supplementary Figure S3: Power spectral analysis of EEG in HC 1 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - all runs showed significantly increased power from 8 to 11 Hz and 15 to 20 Hz over central and parietal channels, and significantly decreased power from 13 to 16 Hz over central channels. Each run had at least one channel with significant FLD p-values that corresponded to channels with many significant spectral differences. Other findings described in Section 3.1.
Supplementary Figure S4: Power spectral analysis of EEG in HC 2 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - HC 2 showed fewer significant frequencies than other HCs, though consistent patterns remained. All runs showed significantly decreased power from 7 to 9 Hz and 13 to 15 Hz in frontal and parietal channels. Findings in common to subsets of runs were: increased power from 10 to 12 Hz in parietal and occipital channels (significant in Runs 1 and 3, trend in Run 2); and significantly decreased power from 19 to 21 Hz in frontal and central channels (Runs 2, 3 and 4). Only Run 3 had channels with significant FLD. As in the motor imagery task, this subject had marked muscle artifact, as indicated by ICA removing an average of 85% of the variance. Other findings described in Section 3.1.
Supplementary Figure S5: Power spectral analysis of EEG in HC 3 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - all runs showed significantly decreased power from 14 to 17 Hz in central channels and increased power from 21 to 23 Hz in central channels. Findings in common to subsets of runs were: decreased power from 5 to 8 Hz in central and parietal channels (trend in Run 1, significant in Runs 2 and 3); increased power from 8 to 12 and 16 to 20 Hz in posterior channels (significant in Runs 1, 3 and 4) but interestingly not in Run 2 which otherwise had the most significant differences. Multiple channels in Runs 1, 2 and 4 had significant FLD p-values, correlating with many significantly different spectral values. Variation between runs is not likely to be due to variation in amount of artifact since ICA removed only 21 to 30% of the variance. Other findings described in Section 3.1.
Supplementary Figure S6: Power spectral analysis of EEG in HC 4 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - all runs showed a significant pattern of increased power from 9 to 11 Hz in most channels and from 17 to 21 Hz in central and posterior channels. Findings in subsets of runs were: significantly decreased power from 6 to 9 Hz in frontal channels (Runs 2 and 3); and from 12 to 14 Hz in frontal channels (Runs 2 and 3). All runs had significant FLD p-values, which correlated with number of significant spectral differences. Other findings described in section 3.1.
Supplementary Figure S7: Power spectral analysis of EEG in HC 5 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - both runs demonstrated significantly decreased power from 8 to 12 Hz in frontal channels, 14 to 20 Hz in posterior channels, and 20 to 24 Hz in predominantly left central channels. A trend to an increase in power was seen at 12 Hz in both runs in many channels. FLD p-values were significant in multiple channels, typically those with more significant TGT p-values. Other findings described in section 3.1.
Supplementary Figure S8: Power spectral analysis of EEG in PS 2 for all runs of the motor imagery task on visit 2. Plotting conventions as in Figure 3B.
Findings – all runs except Run 2 had frequency bands with changes in power identified as significant by TGT, and Runs 1 and 3 each had one channel that showed a significant change by FLD. Findings in common to subsets of runs (positive outcome measure 1) were: increased power from 12 to 18 Hz in left frontal channels (significant in Run 1, trend in Runs 2, 4 and 5); and increased power from 7 to 9 Hz and 13 to 15 Hz in P3 (significant in Run 3, trend in Runs 2, 5 and 6). Neither of these sets of findings was seen in the HCs (Figure 6). Outcome measure 2 was negative, so the overall response was indeterminate.
Supplementary Figure S9: Power spectral analysis of EEG in PS 3 for all runs of the navigation imagery task on visit 1. Plotting conventions as in Figure 3B.
Findings - all runs had at least one band of significantly different frequencies and at least one channel with a significant FLD. Findings in common to subsets of runs (positive outcome measure 1) were: increased power from 17 to 19 Hz in T5 and C4 (significant in Run 2, trend in Run 4); decreased power from 10 to 12 Hz in FC1 (significant in Run 1, trend in Run 4). Outcome measure 2 was negative so the overall response was indeterminate. Other findings described in Section 3.2
Supplementary Figure S10: Power spectral analysis of EEG in PS 3 for all runs of the motor imagery task on visit 2. Plotting conventions as in Figure 3B.
Findings - all runs had at least one frequency band with significant differences and Runs 2 and 3 had channels with significant FLD. Findings in common to subsets of runs (outcome measure 1) were: increased power from 4 to 6 Hz in left anterior frontal channels (significant in Run 3, trend in Run 1); and decreased power from 14 to 16 Hz in P4 (significant in Run 3, trend in Run 1). Of note, Runs 1 and 3 were performed in the evening on two different days, while Run 2 was performed in the morning, which may correlate with changes in the subject’s state. Outcome measure 2 is negative so the overall response is indeterminate. Significant findings from this visit are not consistent with those in visit 1 (Figure. 6). Other findings described in Section 3.2
Supplementary Figure S11: Power spectral analysis of EEG in PS 3 for all runs of the navigation imagery task on visit 2. Plotting conventions as in Figure 3B.
Findings - all runs had at least one frequency band with significant differences and at least one channel with significant FLD. Findings in common to subsets of runs (outcome measure 1) were: increased power in T5 from 21 to 23 Hz (significant in Run 2, trend in Run 1) and decreased power in FC6 from 17 to 19 Hz (significant in Run 3, trend in Run 1). Outcome measure 2 was negative so the overall response was indeterminate. The change at T5 was not seen in any of the HCs but the change at FC6 is seen in HC1 and 5 (Figure 7). Other findings described in Section 3.2.
Acknowledgments
We thank Hemant Bokil for assistance with the use of the Chronux toolbox and for many helpful conversations on digital signal processing. We thank Jennifer Hersh for assistance with the data collection. This work was supported by NIH-NICHD 51912, the James S McDonnell Foundation, and Weill-Cornell CTSC UL 1RR02499. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication
Abbreviations
- FDR
False Discovery Rate
- FLD
Fisher Linear Discriminant
- HC
Healthy Control
- HLM
Hjorth Laplacian montage
- PS
Patient Subject
- TGT
Chronux Two Group Test
- TBI
Traumatic Brain Injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews K, Murphy L, Munday R, Littlewood C. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. BMJ. 1996;313:13–16. doi: 10.1136/bmj.313.7048.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai O, Lin P, Vorbach S, Floeter MK, Hattori N, Hallett M. A high performance sensorimotor beta rhythm-based brain–computer interface associated with human natural motor behavior. J Neural Eng. 2008;5:24–35. doi: 10.1088/1741-2560/5/1/003. [DOI] [PubMed] [Google Scholar]
- Bai O, Lin P, Vorbach S, Li J, Furlani S, Hallett M. Exploration of computational methods for classification of movement intention during human voluntary movement from single trial EEG. Clin Neurophysiol. 2007;118:2637–2655. doi: 10.1016/j.clinph.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin JC, Fins JJ, Katz DI, Hersh J, Heier LA, Tabelow K, et al. Dissociations between behavioral and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain. 2011;134:769–782. doi: 10.1093/brain/awr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- Benjamini Y, Yekutieli D. The Control of the False Discovery Rate in Multiple Testing under Dependency. Ann Statist. 2001;29:1165–1188. [Google Scholar]
- Bokil H, Purpura K, Schoffelen J-M, Thomson D, Mitra P. Comparing spectra and coherences for groups of unequal size. J Neurosci Methods. 2007;159:337–345. doi: 10.1016/j.jneumeth.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology. 1993;43:1465–1467. doi: 10.1212/wnl.43.8.1465. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Estraneo A, Moretta P, Loreto V, Lanzillo B, Santoro L, Trojano L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology. 2010;75:239–245. doi: 10.1212/WNL.0b013e3181e8e8cc. [DOI] [PubMed] [Google Scholar]
- Fins JJ, Schiff ND. In the blink of the mind’s eye. Hastings Cent Rep. 2010;40:21–23. doi: 10.1353/hcr.0.0257. [DOI] [PubMed] [Google Scholar]
- Fisher RA. The Use of Multiple Measurements in Taxonomic Problems. Ann Eugen. 1936;7:179–188. [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K. The Vegetative and Minimally Conscious States: A Comparison of Clinical Features and Functional Outcome. J Head Trauma Rehabil. 1997;12:36–51. [Google Scholar]
- Hart T, Whyte J, Millis S, Bode R, Malec J, Richardson RN, Hammond F. Dimensions of Disordered Attention in Traumatic Brain Injury: Further Validation of the Moss Attention Rating Scale. Arch Phys Med Rehabil. 2006;87:647–655. doi: 10.1016/j.apmr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Hjorth B. An on-line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr Clin Neurophysiol. 1975;39:526–530. doi: 10.1016/0013-4694(75)90056-5. [DOI] [PubMed] [Google Scholar]
- Hjorth B. Source Derivation Simplifies Topographical EEG Interpretation. Ame J EEG Technol. 1980;20:121–132. [Google Scholar]
- Hung C-I, Lee P-L, Wu Y-T, Chen L-F, Yeh T-C, Hsieh J-C. Recognition of Motor Imagery Electroencephalography Using Independent Component Analysis and Machine Classifiers. Ann Biomed Eng. 2005;33:1053–1070. doi: 10.1007/s10439-005-5772-1. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The Ten Twenty Electrode System of the International Federation. Electroenceph Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Kotchoubey B, Lang S, Mezger G, Schmalohr D, Schneck M, Semmler A, et al. Information processing in severe disorders of consciousness: Vegetative state and minimally conscious state. Clin Neurophysiol. 2005;116:2441–2453. doi: 10.1016/j.clinph.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Lammi MH, Smith VH, Tate RL, Taylor CM. The minimally conscious state and recovery potential: A follow-up study 2 to 5 years after traumatic brain injury. Arch Phys Med Rehab. 2005;86:746–754. doi: 10.1016/j.apmr.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Umeno K, Hori E, Takakura H, Urakawa S, Ono T, et al. Global synchronization in the theta band during mental imagery of navigation in humans. Neurosci Res. 2009;65:44–52. doi: 10.1016/j.neures.2009.05.004. [DOI] [PubMed] [Google Scholar]
- McMenamin BW, Shackman AJ, Maxwell JS, Bachhuber DRW, Koppenhaver AM, Greischar LL, et al. Validation of ICA-based myogenic artifact correction for scalp and source-localized EEG. NeuroImage. 2010;49:2416–2432. doi: 10.1016/j.neuroimage.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzer DL, Bokil H, Ryou JW, Schiff ND, Purpura KP, Mitra PP. Characterization of trial-to-trial fluctuations in local field potentials recorded in cerebral cortex of awake behaving macaque. J Neurosci Methods. 2010;186:250–261. doi: 10.1016/j.jneumeth.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PP, Pesaran B. Analysis of Dynamic Brain Imaging Data. Biophys J. 1999;76:691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. 1. Oxford University Press; USA: 2007. [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful Modulation of Brain Activity in Disorders of Consciousness. N Engl J Med. 2010;362:579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Jolles D, et al. Response to Comments on “Detecting Awareness in the Vegetative State. Science. 2007;315:1221. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting Awareness in the Vegetative State. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Percival DB, Walden AT. Spectral analysis for physical applications: multitaper and conventional univariate techniques. Cambridge University Press; 1993. [Google Scholar]
- Perrin F, Schnakers C, Schabus M, Degueldre C, Goldman S, Bredart S, et al. Brain Response to One’s Own Name in Vegetative State, Minimally Conscious State, and Locked-in Syndrome. Arch Neurol. 2006;63:562–569. doi: 10.1001/archneur.63.4.562. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Qin P, Di H, Yan X, Yu S, Yu D, Laureys S, et al. Mismatch negativity to the patient’s own name in chronic disorders of consciousness. Neurosci Lett. 2008;448:24–28. doi: 10.1016/j.neulet.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Perrin F, Schabus M, Majerus S, Ledoux D, Damas P, et al. Voluntary brain processing in disorders of consciousness. Neurology. 2008;71:1614–1620. doi: 10.1212/01.wnl.0000334754.15330.69. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, Moonen G, Laureys S. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman DJ, Kranczioch C. In the blink of an eye: the contribution of microsaccadic activity to the induced gamma band response. Int J Psychophysiol. 2011;79:73–82. doi: 10.1016/j.ijpsycho.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. NeuroImage. 2010;51:1319–1333. doi: 10.1016/j.neuroimage.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart CM, Giacino JT, Cullen T, Moreno DR, Hirsch J, Schiff ND, et al. A case of locked-in syndrome complicated by central deafness. Nat Clin Pract Neurol. 2008;4:448–453. doi: 10.1038/ncpneuro0823. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Mastaglia FL, Carroll WM, Davies HD. Source derivation: Application to topographic mapping of visual evoked potentials. Electroencephalogr Clin Neurophysiol. 1984;59:279–285. doi: 10.1016/0168-5597(84)90045-5. [DOI] [PubMed] [Google Scholar]
- Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70:1055–1096. [Google Scholar]
- Wijnen VJM, van Boxtel GJM, Eilander HJ, de Gelder B. Mismatch negativity predicts recovery from the vegetative state. Clin Neurophysiol. 2007;118:597–605. doi: 10.1016/j.clinph.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Yuan H, Liu T, Szarkowski R, Rios C, Ashe J, He B. Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: An EEG and fMRI study of motor imagery and movements. NeuroImage. 2010;49:2596–2606. doi: 10.1016/j.neuroimage.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Summary for all subjects of: tasks and number of runs performed; count of snippets analyzed (others removed due to artifact); ICA components rejected (out of a total of 29 or 37 corresponding to number of EEG channels); and percentage of variance removed by rejecting ICA components (a marker of amount of artifact in the data).
Supplementary Figure S1: Power spectral analysis of EEG in HC 3 for all runs of the motor imagery task. Plotting conventions as in Figure 3B.
Findings – all runs demonstrated significant power decreases between 14 and 18 Hz and increases between 20 and 24 Hz in central channels. Runs 2, 3 and 4 also showed significantly decreased power from 6 to 10 Hz in central channels. Other findings described in Section 3.1.
Supplementary Figure S2: Power spectral analysis of EEG in HC 4 for all runs of the motor imagery task. Plotting conventions as in Figure 3B.
Findings - all runs demonstrated significant power decreases from 8 to 9 Hz in CP5, and power increases from 9 to 11 Hz in central channels (trend in Run 1, significant in Runs 2 and 3). Subsets of runs showed: decreased power from 13 to 16 Hz in central channels (trend in Run 1, significant in Run 3); increased power from 17 to 19 Hz in posterior channels (trend in Run 1 and significant in Run 2). Runs 1 and 2 had fewer significant spectral frequencies than Run 3, which correlated with Run 3 being the only one with significant FLD p-values. Other findings described in Section 3.1.
Supplementary Figure S3: Power spectral analysis of EEG in HC 1 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - all runs showed significantly increased power from 8 to 11 Hz and 15 to 20 Hz over central and parietal channels, and significantly decreased power from 13 to 16 Hz over central channels. Each run had at least one channel with significant FLD p-values that corresponded to channels with many significant spectral differences. Other findings described in Section 3.1.
Supplementary Figure S4: Power spectral analysis of EEG in HC 2 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - HC 2 showed fewer significant frequencies than other HCs, though consistent patterns remained. All runs showed significantly decreased power from 7 to 9 Hz and 13 to 15 Hz in frontal and parietal channels. Findings in common to subsets of runs were: increased power from 10 to 12 Hz in parietal and occipital channels (significant in Runs 1 and 3, trend in Run 2); and significantly decreased power from 19 to 21 Hz in frontal and central channels (Runs 2, 3 and 4). Only Run 3 had channels with significant FLD. As in the motor imagery task, this subject had marked muscle artifact, as indicated by ICA removing an average of 85% of the variance. Other findings described in Section 3.1.
Supplementary Figure S5: Power spectral analysis of EEG in HC 3 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - all runs showed significantly decreased power from 14 to 17 Hz in central channels and increased power from 21 to 23 Hz in central channels. Findings in common to subsets of runs were: decreased power from 5 to 8 Hz in central and parietal channels (trend in Run 1, significant in Runs 2 and 3); increased power from 8 to 12 and 16 to 20 Hz in posterior channels (significant in Runs 1, 3 and 4) but interestingly not in Run 2 which otherwise had the most significant differences. Multiple channels in Runs 1, 2 and 4 had significant FLD p-values, correlating with many significantly different spectral values. Variation between runs is not likely to be due to variation in amount of artifact since ICA removed only 21 to 30% of the variance. Other findings described in Section 3.1.
Supplementary Figure S6: Power spectral analysis of EEG in HC 4 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - all runs showed a significant pattern of increased power from 9 to 11 Hz in most channels and from 17 to 21 Hz in central and posterior channels. Findings in subsets of runs were: significantly decreased power from 6 to 9 Hz in frontal channels (Runs 2 and 3); and from 12 to 14 Hz in frontal channels (Runs 2 and 3). All runs had significant FLD p-values, which correlated with number of significant spectral differences. Other findings described in section 3.1.
Supplementary Figure S7: Power spectral analysis of EEG in HC 5 for all runs of the navigation imagery task. Plotting conventions as in Figure 3B.
Findings - both runs demonstrated significantly decreased power from 8 to 12 Hz in frontal channels, 14 to 20 Hz in posterior channels, and 20 to 24 Hz in predominantly left central channels. A trend to an increase in power was seen at 12 Hz in both runs in many channels. FLD p-values were significant in multiple channels, typically those with more significant TGT p-values. Other findings described in section 3.1.
Supplementary Figure S8: Power spectral analysis of EEG in PS 2 for all runs of the motor imagery task on visit 2. Plotting conventions as in Figure 3B.
Findings – all runs except Run 2 had frequency bands with changes in power identified as significant by TGT, and Runs 1 and 3 each had one channel that showed a significant change by FLD. Findings in common to subsets of runs (positive outcome measure 1) were: increased power from 12 to 18 Hz in left frontal channels (significant in Run 1, trend in Runs 2, 4 and 5); and increased power from 7 to 9 Hz and 13 to 15 Hz in P3 (significant in Run 3, trend in Runs 2, 5 and 6). Neither of these sets of findings was seen in the HCs (Figure 6). Outcome measure 2 was negative, so the overall response was indeterminate.
Supplementary Figure S9: Power spectral analysis of EEG in PS 3 for all runs of the navigation imagery task on visit 1. Plotting conventions as in Figure 3B.
Findings - all runs had at least one band of significantly different frequencies and at least one channel with a significant FLD. Findings in common to subsets of runs (positive outcome measure 1) were: increased power from 17 to 19 Hz in T5 and C4 (significant in Run 2, trend in Run 4); decreased power from 10 to 12 Hz in FC1 (significant in Run 1, trend in Run 4). Outcome measure 2 was negative so the overall response was indeterminate. Other findings described in Section 3.2
Supplementary Figure S10: Power spectral analysis of EEG in PS 3 for all runs of the motor imagery task on visit 2. Plotting conventions as in Figure 3B.
Findings - all runs had at least one frequency band with significant differences and Runs 2 and 3 had channels with significant FLD. Findings in common to subsets of runs (outcome measure 1) were: increased power from 4 to 6 Hz in left anterior frontal channels (significant in Run 3, trend in Run 1); and decreased power from 14 to 16 Hz in P4 (significant in Run 3, trend in Run 1). Of note, Runs 1 and 3 were performed in the evening on two different days, while Run 2 was performed in the morning, which may correlate with changes in the subject’s state. Outcome measure 2 is negative so the overall response is indeterminate. Significant findings from this visit are not consistent with those in visit 1 (Figure. 6). Other findings described in Section 3.2
Supplementary Figure S11: Power spectral analysis of EEG in PS 3 for all runs of the navigation imagery task on visit 2. Plotting conventions as in Figure 3B.
Findings - all runs had at least one frequency band with significant differences and at least one channel with significant FLD. Findings in common to subsets of runs (outcome measure 1) were: increased power in T5 from 21 to 23 Hz (significant in Run 2, trend in Run 1) and decreased power in FC6 from 17 to 19 Hz (significant in Run 3, trend in Run 1). Outcome measure 2 was negative so the overall response was indeterminate. The change at T5 was not seen in any of the HCs but the change at FC6 is seen in HC1 and 5 (Figure 7). Other findings described in Section 3.2.