Abstract
Background
We examined whether any differences in brain volumes at entry into alcohol dependence treatment differentiate subsequent Abstainers from Relapsers.
Methods
Individuals in alcohol dependence treatment (N=75) underwent magnetic resonance imaging approximately 6 ± 4 days after their last alcoholic drink, and 40 age-matched non-smoking light drinkers were studied as controls. At follow-up 7.8 ± 2.6 months later, 23 alcoholics (31%) had abstained from drinking and 52 (69%) had relapsed. Deformation morphometry compared Relapsers, Abstainers, and light drinkers.
Results
Compared to light drinkers, future Abstainers had smaller brain tissue volumes in the left amygdala, hippocampal head, and entorhinal cortex, and bilaterally in the thalamus and adjacent subcortical white matter (WM), and had larger volume in the left lateral orbitofrontal region. Compared to light drinkers, future Relapsers had smaller brain tissue volumes in the right middle temporal, occipital, and superior frontal WM. Compared to future Abstainers, future Relapsers had smaller tissue volumes primarily in bilateral orbitofrontal cortex and surrounding WM. Results were virtually unaffected after controlling for common comorbidities.
Conclusion
At entry into alcohol dependence treatment, the brain structure of future Relapsers differs from that of future Abstainers. Future Relapsers have smaller brain volumes in regions of the mesocorticolimbic reward system that are critically involved in impulse control, emotional regulation, craving, and evaluation and anticipation of stimulus salience and hedonics. Structural abnormalities of this circuitry may confer greater risk for resumption of hazardous drinking after treatment and may contribute to the definition of a neurobiological relapse risk profile in alcohol dependence.
Keywords: abstinence, deformation morphometry, relapse, longitudinal
Introduction
Magnetic resonance imaging (MRI) has been extensively used to study morphological changes associated with alcohol use disorders (for reviews see e.g., (1–3)) and the recovery of brain tissue volume that accompanies sobriety. We have employed deformation based morphometry (DBM) (4–7) to visualize and quantify shape differences between brains on a voxel-by-voxel basis without a priori definitions of regions of interest (ROIs): in treatment-naïve individuals with an alcohol use disorder (AUD) versus light-drinking controls, we found dose-related, focal cortical gray matter (GM) and diffuse CSF volume differences (less GM and more CSF), with no significant white matter (WM) differences (8). We also used DBM to study the effects of chronic alcohol consumption on brain morphology in participants seeking treatment for AUD after about one week of abstinence and to examine longitudinal morphological changes during a 6–9 month interval following treatment entry (9): at one week of abstinence, alcohol dependent participants showed significant tissue loss in the frontal and temporal lobes. Over 6–9 months of abstinence, tissue volumes increased throughout the brain, particularly in the parietal and frontal lobes, with slower recovery of volume in individuals who drank alcohol within 15 days of their follow-up scan. These results suggest that resumption of hazardous levels of alcohol consumption impedes or eliminates brain tissue volume increases that occur in early abstinence.
It is estimated that at least 60% of individuals who seek treatment for an AUD will resume hazardous levels of alcohol consumption (10, 11), typically within 6 months following treatment (12–14). However, a significant portion of those with AUD do not return to a chronically relapsing/remitting course after treatment (15–18), suggesting variable individual vulnerability to relapse. Psychological, psychiatric and sociodemographic correlates of relapse have been investigated in previous work (17, 19–28). However, the neurobiological underpinnings associated with relapse and sustained abstinence after treatment for AUD are not well understood.
In an earlier report (29), we examined brain lobar measures of GM, WM, N-acetylaspartate (NAA), and choline-containing metabolites (Cho), and found that select lobar NAA and Cho levels predicted resumption of drinking. Although the relationships between relapse and lobar volumes of GM or WM failed to reach significance after correction for multiple comparisons, the results suggested volumetric abnormalities and clearly implicated metabolic abnormalities as neurobiological factors underlying relapse.
The mesocorticolimbic system is involved in the generation of “reward,” and modifications of these pathways may be involved in the development and maintenance of substance use disorders (30, 31). Major components of this brain reward system (BRS) include the dorsolateral prefrontal cortex, orbitofrontal cortex, insula, anterior cingulate cortex, hippocampus, amygdala, thalamus, nucleus accumbens, ventral tegmental area and other nuclei in the basal forebrain and ventral pallidum. In more recent work (32), we identified morphological correlates of relapse by comparing neocortical thickness, surface area, and volume in the cortical regions of the BRS among non-smoking light drinking controls (LD), individuals who remained abstinent (Abstainers), and those who relapsed after treatment (Relapsers). At one week of abstinence, Relapsers and Abstainers both demonstrated markedly smaller neocortical thickness and volume in multiple regions of the BRS compared to LD. However, Relapsers compared to Abstainers had significantly thinner cortices and smaller volumes in the lateral orbitofrontal region and in corresponding summary measures of select components of the BRS. While these results reveal clear volumetric differences between treatment-seeking Abstainers and Relapsers at one week of abstinence, the analyses specifically focused on neocortical components of the BRS; therefore, it is uncertain if these groups also showed volume differences outside of this collection of GM regions.
For this study, we undertook a voxel-wise DBM analysis of tissues from the entire brain, in order to identify both GM and WM regions associated with relapse, without a priori definition of ROIs. We examined whether regional brain volumes at entry into outpatient treatment for AUD differentiate individuals who demonstrated sustained abstinence from those who relapsed in the 12 months following initiation of treatment. Morphology patterns in 1-week-abstinent alcohol dependent individuals were measured and visualized in comparison to LD. Based on our earlier results examining brain morphology at treatment entry and literature that implicate neurobiological abnormalities in the BRS as critical to the maintenance of abstinence, we tested the following a priori hypotheses: 1) Compared to LD, both Abstainers and Relapsers at treatment entry have smaller volumes of the frontal cortex, insula, hippocampus, and amygdala; 2) compared to LD, Relapsers show volume differences of greater magnitude than Abstainers in the frontal cortex, insula, hippocampus, and amygdala; and 3) compared to Abstainers, Relapsers have smaller volumes in the dorsolateral prefrontal and orbitofrontal neocortices.
Methods and Materials
Participants
Seventy-five outpatient participants (including 6 females) were recruited from the VA Medical Center Substance Abuse Day Hospital and the Kaiser Permanente Chemical Dependence Recovery Program in San Francisco. Primary inclusion criteria for the alcohol dependent participants were fluency in English, DSM-IV diagnosis of alcohol dependence or alcohol abuse at the time of enrollment, consumption of greater than 150 standard alcohol-containing drinks (i.e., 13.6 grams of ethanol per drink) per month for at least 8 years prior to enrollment for men, or consumption of greater than 80 drinks per month for at least 6 years prior to enrollment for women. Light-drinking controls (LD; n = 40; 4 females) were recruited from the local community. Participants were between 28 and 69 years of age. See Table 1 for group demographic data. All participants provided written informed consent prior to study according to the Declaration of Helsinki and the study was approved by the University of California San Francisco and the San Francisco VA Medical Center. Participants were urine-tested for illicit substances immediately before all assessments (i.e., cannabinoids, opiates, phencyclidine, cocaine, and amphetamines) and did not test positive for these substances at any assessment. Exclusion criteria are described in previous publications (33, 34).
Table 1.
Participant Demographics
| LD (Light Drinkers) | Abstainers | Relapsers | Group Differences (p-value) | |
|---|---|---|---|---|
| N | 40 | 23 | 52 | |
| Age | 47 ± 8 | 50 ± 12 | 50 ± 8 | 0.361 |
| %smokers | NA | 48 | 60 | 0.801 |
| % female | 10 | 13 | 6 | 0.553 |
| % prev. alcoholism treatment | NA | 65 | 46 | 0.142 |
| # of treatment attempts | NA | 2 ± 2 (range 0–6) | 2 ± 1 (range 1–5) | 0.200 |
| 1 yr avg #drinks/mo | 14 ± 15 | 338 ± 164 | 396 ± 168 | <0.001* |
| Lifetime avg #drinks/mo | 14 ± 12 | 199 ± 122 | 246 ± 151 | <0.001* |
| Months of heavy drinking | NA | 230 ± 109 | 272 ± 114 | 0.145 |
| Fagerstrom total** | NA | 6 ± 1 | 5 ± 2 | 0.317 |
| Smoking duration (yrs) | NA | 20 ± 6 | 20 ± 11 | 0.975 |
| Cigarettes per day | NA | 23 ± 13 | 23 ± 11 | 1.000 |
| %Substance use comorbidities | NA | 17 | 21 | 1.000 |
| %psychiatric comorbidities | NA | 26 | 50 | 0.080 |
| %medical comorbidities | NA | 57 | 52 | 0.804 |
| Days abstinent at MRI study | NA | 6 ± 4 | 6 ± 3 | 0.477 |
LD < Abstainers, LD < Relapsers, p < 0.05, Abstainers vs. Relapsers, no significant difference NA (not applicable)
Fagerstrom total is a measure of nicotine dependence, on a scale of 0 (none) to 10. In population studies, about 30% of smokers score 4–5 on the scale, indicating more than average dependence and withdrawal symptoms. About 15% of smokers score 6–7 on the scale, indicating strong dependence and withdrawal symptoms.
Clinical Measures
For the alcohol dependent cohort, clinical and MR procedures were conducted 6 ± 4 days after last alcoholic drink, and included assessments of lifetime alcohol and nicotine use; detailed descriptions can be found in (33, 34). At the time of the assessment, all alcohol dependent participants were actively involved in stabilization/early recovery outpatient treatment, which typically lasted 14–28 days.
The primary follow-up for the alcohol dependent participants occurred at 7.8 ± 2.6 months after treatment-entry studies as detailed in (32). Participants were designated as Abstainers if they met all the following criteria: a) self-report of no alcohol consumption between the initial assessment and follow-up; b) no report of alcohol consumption between entry and follow-up recorded in medical charts; and c) available laboratory indicators of alcohol consumption (e.g., gamma glutamyltransferase; GGT) at follow-up within normal limits. Participants were designated as Relapsers if they met any or all of the following criteria: a) self-report of alcohol consumption at any time between the initial assessment and follow up as assessed by telephone or in-person interview; b) use of alcohol indicated in medical records; and c) report of alcohol use by a relative or close friend of the participant obtained via telephone or in-person interview. The average duration of abstinence among Relapsers was 121 ± 78 days (range 2–337 days).
Magnetic Resonance Imaging Acquisition and Analyses
A volumetric magnetization-prepared rapid gradient echo (MPRAGE) was acquired with TR/TE/TI = 9.7/4/300 ms, 15° flip angle, 1×1 mm2 in-plane resolution, and 1.5-mm-thick coronal partitions oriented perpendicular to the main long axes of bilateral hippocampi as seen on sagittal scout MRI. See Gazdzinski and colleagues (35) for detailed information on MR acquisition methods at 1.5T.
A B-Spline Free Form deformation algorithm driven by normalized mutual information (36) was used to register individual scans to a 36-year old male reference atlas (37). The Jacobian determinant of this transformation, giving the pattern of fractional volume contraction or expansion at each voxel required to force the individual anatomy to conform to the reference, was used to characterize the shape difference for deformation morphometry. Smoothing was applied using an intensity consistent filtering approach (38), yielding tissue volume maps with resolution (1×1×1.5) mm3.
Statistical measures were applied to locate points where voxel level differences in the maps were associated with group (LD, Abstainers, or Relapsers). Using all participants, we performed multiple regression analyses with the continuous independent variables of age and intracranial volume, and the categorical variables denoting alcohol status. Deformation maps were dependent variables. Including only the alcohol dependent participants, we performed multiple regression to determine differences between Abstainers and Relapsers in deformation at treatment entry, again using age and intracranial volume as covariates. Nonstationary random field theory was used to correct the statistical clusters above p=0.01 for multiple comparisons (39).
RESULTS
Demographic, Alcohol, and Cigarette Consumption Variables
Of the 75 alcohol dependent participants, 23 (31%) were Abstainers and 52 (69%) were Relapsers. All treatment-seeking participants met DSM-IV criteria for alcohol dependence (with physiological dependence) at study enrollment. For the Relapsers for whom we had quantity and frequency alcohol consumption data, 95% met Project MATCH criteria for an alcohol relapse (men: ≥ 3 consecutive days of consumption of ≥6 drinks per day; women: ≥ 3 consecutive days of consumption of ≥ 4 drinks per day). Abstainers and Relapsers were not different on age, education, and the frequency of previous treatments for AUD (see Table 1). Abstainers and Relapsers were also equivalent on number of months of heavy drinking, number of drinks per month over one year and over lifetime. Smoking frequency was equivalent among Relapsers and Abstainers and groups also did not differ on cigarette consumption variables (see Table 1). Relapsers and Abstainers did not differ significantly on the proportion of subjects with psychiatric (primarily major depression, or substance-induced mood disorder with depressive features) or medical comorbidities (primarily hypertension and hepatitis C) or other substance abuse disorders (methamphetamine, cannabis, opioid, or cocaine). Thus, substance use or comorbidity data at entry into treatment were not related to group membership (Abstainer or Relapser).
Alcohol Dependent Cohort vs. Light Drinking Controls
The alcohol dependent cohort had consistently smaller tissue volume than LD in a large cluster located mostly in the right temporal lobe (including insula), extending into the frontal lobe (p=0.001, 8.5% tissue loss compared to LD). The alcohol dependent sample showed no regions of significantly larger tissue volumes than LD. This result extends and confirms previous work on a smaller cohort that was a subset of the current cohort (9).
Abstainers vs. Light Drinking Controls
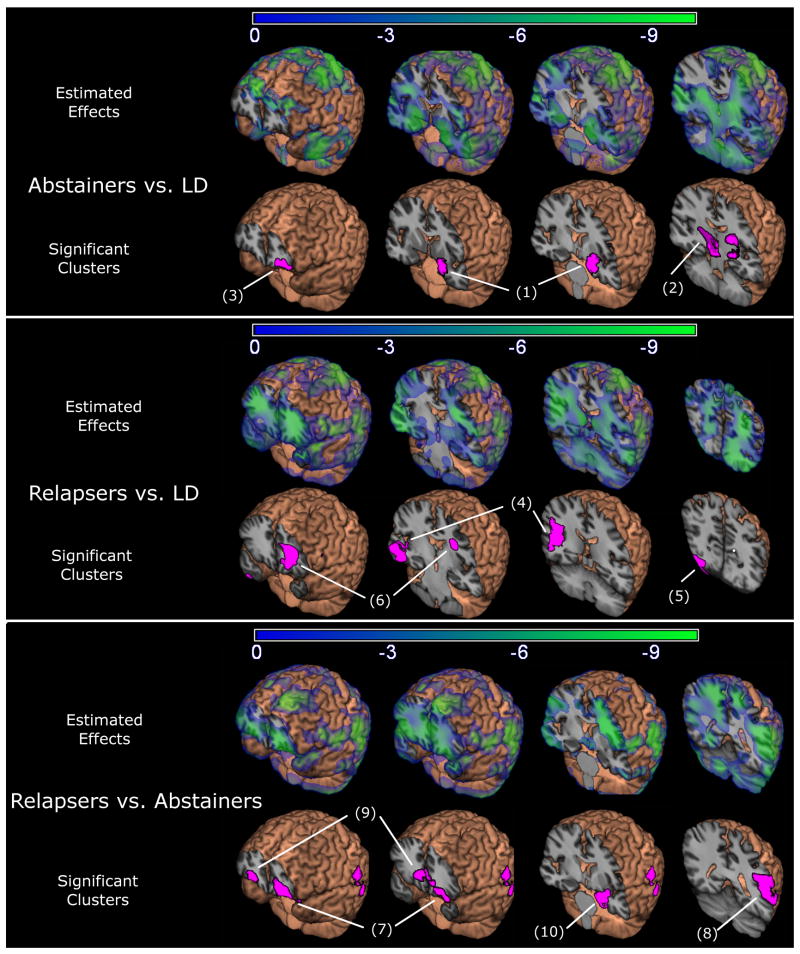
Panel A of Figure 1 shows maps of beta coefficients (i.e., estimated effects) showing regions of volume differences in Abstainers compared to LD, as well as clusters where tissue volume in Abstainers and LD were significantly different after correction for multiple comparisons. As shown by the blue/green shading in the figure, Abstainers showed smaller tissue volumes in the subcortex, medial temporal lobes, insula, and frontal lobes. After correction for multiple comparisons, volume differences were significant in two regions: A region including the left hippocampus, entorhinal cortex and amygdala, extending into the surrounding subcortical WM and left thalamus (identified as region 1 in Table and Figure, p<0.001, 9.5% smaller volume compared to LD), and a region including the right thalamus and surrounding WM (region 2, p=0.016, 11.4% smaller). The only region in which Abstainers had more tissue than LD was lateral orbitofrontal in the left hemisphere (p=0.035, 10.1% larger volume, region 3). Figure S1 in the supplement shows the average Jacobian within these clusters plotted by group.
Figure 1.
Estimated effects (beta coefficients) from the regression models and regions of significant relative volume differences are overlaid on the average spatially normalized brain. Voxels shaded blue/green show smaller volume in Abstainers relative to light drinkers (LD, Panel A), Relapsers relative to LD (Panel B), and Relapsers relative to Abstainers (Panel C). Voxels shaded pink show regions (clusters) where volume differences were significant (p<0.05, corrected).
Relapsers vs. Light Drinking Controls
Panel B of Figure 1 shows maps of beta coefficients showing regional differences between Relapsers and LD, as well as clusters where tissue volume in Relapsers was significantly smaller after correction for multiple comparisons. As shown by the blue/green shading in the figure, Relapsers showed smaller volume in the temporal lobes, extending through the subcortical WM and into the parietal and occipital WM. Significantly smaller volumes were observed after multiple comparison correction in left superior corona radiata (region 6, p=0.043, 10.3% lower volume), a region encompassing the right posterior superior temporal gyrus, posterior insula, supramarginal gyrus and underlying WM (region 4, p<0.001, 9.1%), and the right lateral occipital lobe GM and underlying WM (region 5, p=0.031, 12.6%). When comparing the maps of estimated group effects in Figure 1, it becomes apparent that Relapsers had an entirely different spatial pattern of volume loss than Abstainers. Figure S1 in the supplement shows the average Jacobian within these clusters plotted by group.
Relapsers vs. Abstainers
Panel C of Figure 1 shows estimated effects where tissue volume in Relapsers was significantly smaller compared to Abstainers, as well as clusters of volume differences. As shown by the blue/green shading in the estimated effects panel, Relapsers showed widespread smaller volumes in frontal GM and WM regions, extending into the posterior temporal/parietal regions. After correction for multiple comparisons, Relapsers showed significantly smaller volumes in the lateral orbitofrontal cortex and underlying WM of both hemispheres (left: region 7, p<0.001, 10.8% smaller volume; right: region 9, p=0.033, 8.7%), in left posterior middle and superior temporal gyri and the supramarginal gyrus and underlying WM (region 8, p=0.014, 14.4%). There was a trend in Relapsers for greater tissue volumes in the left amygdala and hippocampus (region 10, p=0.115, 9.1%). Figure S1 in the supplement shows the average Jacobian within these clusters plotted by group.
In further analyses, smoking and medical comorbidities were shown not to be significantly associated with regional morphology. Presence of a psychiatric comorbidity on the other hand was associated with significantly greater tissue volumes in the regions of the cerebellum and WM underlying the right insula, and the presence of comorbid substance abuse was associated with significantly greater tissue volume in several regions of left anterior temporal, right parietal, and right temporal WM. Although these regions are mostly non-overlapping with those differentiating Relapsers and Abstainers, we controlled for psychiatric and substance abuse comorbidities, but observed little change in the pattern of volume differences: Smaller bilateral orbitofrontal regions were still observed in Relapsers (left: p<0.001, 10.5% smaller volume; right: p=0.01, 9.4%), volume differences in the left temporal/parietal region also remained significant (p=0.006, 14.6%, 10.1 cc), and the trend for larger left amygdala/hippocampus in Relapsers reached significance (p=0.043, 9.2%, 3.9 cc).
Associations of Morphology with Pre-Treatment Alcohol and Cigarette Use in Alcohol Dependent Participants
For each alcohol dependent participant, the Jacobian determinant was averaged within each significant cluster from the comparisons of Abstainers vs. LD, Relapsers vs. LD, and Relapsers vs. Abstainers. Associations of these morphology measures with pre-treatment alcohol and cigarette consumption measures were examined with linear regression covaried for age and intracranial volume. None of the group-discriminating brain regions were significantly associated to pre-treatment alcohol or cigarette consumption variables at alpha=0.05, unadjusted for the multiplicity of tests. The lack of associations between smoking variables and structural brain abnormalities is surprising in light of our previous work showing smaller cortical tissue volumes in smoking vs. non-smoking non-treatment seeking heavy drinkers using volumetric lobar ROI approaches (40).
Discussion
DBM of structural MRI obtained within one week of abstinence from alcohol identified distinct regional brain volume abnormalities in those treatment-seeking alcoholics who demonstrated sustained abstinence versus those who relapsed to hazardous levels of alcohol consumption within one year after treatment. The pattern and magnitude of abnormalities was not influenced by comorbid substance abuse, or psychiatric conditions, or to pre-treatment alcohol or cigarette consumption. Our previous analyses (87% participant overlap with this study) focused on identifying brain abnormalities in cortical regions of the BRS (32). Since DBM allows visualization of morphological differences throughout the brain without prior classification of tissue into WM or GM or anatomical ROIs, we employed DBM to determine if Abstainers and Relapsers showed volume differences in non-BRS regions or in subcortical nuclei of the BRS (e.g. the ventral tegmental area, ventral pallidum, nucleus accumbens). The DBM indicated that most volume differences within treatment seeking alcoholics occur in neocortical and WM regions of the BRS, and that examination of other brain regions yields little and sacrifices power. Higher-field and higher resolution imaging and/or larger sample sizes may be necessary to detect potential changes in smaller subcortical nuclei.
Our observation of brain structural differences between Abstainers and Relapsers at entry into AUD treatment complements our previous knowledge of brain structural, biochemical and neurocognitive functional abnormalities in this cohort gleaned from magnetic resonance spectroscopy, perfusion, and structural studies of lobar brain regions (29) and components of the BRS (32–34). Early work (42% participant overlap with this study) with participants at one month of abstinence found that future Relapsers, compared to Abstainers, had less frontal and temporal GM NAA (a measure of neuronal viability), frontal WM NAA, and frontal GM Cho (a measure of cell membrane turnover) (29). Relapsers were also deficient on measures of processing speed and had more unipolar mood disorders. At the same time in treatment, i.e., at one month of abstinence, Relapsers and Abstainers did not differ on measures of lobar GM or WM volume. A follow-up spectroscopy study (70% participant overlap) explored differences between Relapsers and Abstainers in the BRS at about one week of abstinence (34). Relapsers had less NAA in the dorsolateral prefrontal cortex, anterior cingulate, insula, cerebellar vermis, and less NAA, Cho, and Cr in the superior corona radiata. A perfusion study (71% participant overlap) showed reduced GM perfusion in the frontal and temporal lobes in Relapsers compared to Abstainers after about one week of abstinence, an effect that persisted after about one month of abstinence (33). In recent work closely related to this study (87% participant overlap), Relapsers had smaller volumes of right middle frontal gyrus, right and left orbitofrontal cortex, and total volume of the BRS as defined by 16 cortical ROIs (32). In summary, our previous work consistently shows Relapsers demonstrate significantly greater neurobiological abnormalities that Abstainers in multiple components of the BRS.
In this DBM study, observed regions of brain tissue loss were similar to those detected by regional measurements of cortical thickness or surface area in a cohort composed of 87% of the participants of this study (32). This provides complementary evidence for regional tissue loss as a functionally significant substrate of relapse propensity. With both methods, Abstainers had abnormal measurements in left amygdala/hippocampus, and Relapsers had abnormal measures in the regions of the insula and superior frontal gyrus. Similarly, with both methods, the greatest morphological differences between Abstainers and Relapsers were observed in the orbitofrontal cortex, where Relapsers showed reduced thickness, volume, and surface area. Neurobiological abnormalities in the OFC have been linked to emotional and behavior disturbances that may confer risk for the relapse/remit cycle commonly observed in all substance use disorders (30, 31). Specifically, the OFC is involved in emotion-related learning and regulation of internal affective and drive states (41, 42). One of the hallmark manifestations of OFC injury/dysfunction is impulsivity/disinhibition, which is critically linked to risk for relapse.
The results of this and the previous study (32) are in contrast to our study that did not find lobar volume differences (i.e., total frontal GM or WM) between Relapsers and Abstainers studied after about one month of abstinence (29). It is possible that volume differences originally present at one week of abstinence largely dissipate after one month, or that regionally more specific volume changes as observed by voxel-wise analysis methods or BRS ROIs are more strongly associated with relapse than the large volumes of lobar regions.
DBM identified a few additional regions differentiating Abstainers and Relapsers from LD and each other: smaller volumes in the amygdala/hippocampus/entorhinal cortex, thalamus, and subcortical WM within Abstainers, smaller occipital volume and frontal WM within Relapsers, and smaller volume in a left fronto-temporo-parietal region of Relapsers versus Abstainers. The latter volume difference is worth further exploration. A recent analysis of fMRI hemispheric asymmetries revealed that the left temporoparietal junction is engaged during the processing of immoral stimuli, such as drinking and driving (43). To the degree that anatomy underlies the effect observed in fMRI, it is possible that smaller brain volumes in the left temporoparietal junction in Relapsers may affect their judgment of “wrong” and “not wrong.” DBM also identified smaller amygdala in Abstainers. It has recently been shown that in fMRI of abstinent alcoholics, amygdala activation during emotional face processing is inversely proportional to lateral prefrontal activity, with the interpretation that increased activation of prefrontal regions compensates for reduced amygdala function. In this study, we observed preserved frontal volume and smaller amygdala volume in Abstainers, consistent with the fMRI study. In contrast, Relapsers had preserved amygdala volume and smaller frontal WM volumes. Future work could explore whether this difference underlies the inability of a Relapser to modify their behavior in an emotionally or socially challenging situation.
In comparisons with LD, we predicted a similar pattern of volume differences in Abstainers and Relapsers, with Relapsers showing either greater magnitude of differences or a spatially more expanded pattern, or both. Instead, we observed distinct and mostly non-overlapping areas of differences of similar estimated effect sizes and slightly larger spatial extents (cluster sizes) in Relapsers. Interestingly, the difference in the orbitofrontal region appeared to be driven by greater-than-normal volume within the Abstainers, not volume loss within the Relapsers. This underlines the importance of the orbitofrontal region in an individual’s ability to remain abstinent after treatment and suggests that orbitofrontal volume may be a useful predictor of vulnerability to relapse. Considering the lack of associations between these volume abnormalities and measures of drinking (or smoking) severity, these data may suggest a premorbid, perhaps genetic component in the relationship between orbitofrontal cortex volume, impulsivity, alcohol dependence and/or relapse.
Furthermore, as the volumes of the brain regions that discriminated the alcohol dependent groups were not related to pre-treatment alcohol or cigarette consumption measures, this again suggests that the morphological patterns that differentiate Relapsers from Abstainers are influenced by comorbid or premorbid (heritable) factors that were not assessed in this study, such as diet, exercise, or genetics. We could not adequately examine whether patterns of volume differences within Relapsers at treatment entry were associated with post-treatment drinking because we had quantity and frequency information on only 24 of 52 Relapsers, but this is an avenue for future research.
Limitations of this study include the reliance on self-report and/or medical records for the determination of drinking status at follow-up for some participants (for further details see (32)), our inability to examine sex effects due to the small number of female participants in our cohort of US veterans, and the modest number of participants in the Abstainer group. Psychiatric, medical, smoking, and substance abuse comorbidities are part of the clinical syndrome of alcohol dependence and were common in the Relapsers and Abstainers, but absent in LDs. In comparisons to LD, initial attempts to covary for these comorbidities revealed a complicated picture of independent, mediating and interacting effects beyond the scope of this study. As such, the patterns of volume differences in comparison to our non-smoking LD are influenced by these comorbidites within our Relapsers and Abstainers. In comparing Relapsers to Abstainers, however, covarying for these comorbidities did not affect the pattern of volume differences, perhaps because these comorbidities equally affected both groups. Notwithstanding these limitations, our study shows that future Relapsers are not significantly different from future Abstainers on past alcohol and substance use variables, proportion of common comorbidities, or number of previous treatment attempts. Not unexpectedly, therefore, they do not simply manifest greater morphological abnormalities at entry into alcohol dependence treatment than future Abstainers. Instead, Abstainers and Relapsers show distinct patterns of volume differences compared to LD. This suggests that the clinical syndrome of alcohol dependence is associated with different morphological abnormalities in our Relapsers and Abstainers apparent at entry into AUD treatment. Knowledge of such morphological brain differences may contribute to the development of neurobiological risk profiles that assist in the early identification of those with greater relapse vulnerability after AUD treatment.
Supplementary Material
Table 2.
Regions with significant volume differences in group comparisons.
| Contrast | Location of region | Cluster Volume (cc) | Corrected p-value | Effect* (%) |
|---|---|---|---|---|
| Relapsers+Abstainers vs. LD | Right temporal (including insula), frontal lobe | 11.7 | 0.001 | −8.5 |
| Abstainers vs. LD | (1) Left amygdala, head of hippocampus, entorhinal cortex, thalamus, adjacent WM | 11.7 | <0.001 | −9.5 |
| (2) Right thalamus, right colliculi, adjacent WM | 4.9 | 0.016 | −11.4 | |
| (3) Left orbitofrontal | 2.1 | 0.035 | +10.1 | |
| Relapsers vs. LD | (4) Right middle temporal (including insula) | 15.7 | <0.001 | −9.1 |
| (5) Right occipital | 6.0 | 0.031 | −12.6 | |
| (6) Left superior corona radiata | 12.5 | 0.043 | −10.3 | |
| Relapsers vs. Abstainers | (7) Left lateral orbitofrontal | 8.1 | <0.001 | −10.8 |
| (8) Left fronto-temporo-parietal | 9.9 | 0.014 | −14.4 | |
| (9) Right lateral orbitofrontal | 3.8 | 0.033 | −8.0 | |
| (10) Left amygdala, head of hippocampus, entorhinal cortex | 3.6 | 0.115 | +9.1 |
negative numbers indicate % tissue loss in alcohol groups vs. LD or in Relapsers vs. Abstainers Numbers in parenthesis identify locations in Figure 1.
Acknowledgments
This material is the result of work supported by National Institutes of Health [R01AA10788, K08DA24136, R03EB008136, P01AA11493, P41RR023953] and with resources and the use of facilities at the San Francisco Veterans Administration Medical Center, San Francisco CA. We thank Mary Rebecca Young, Bill Clift, Jeanne Eichenbaum and Drs. Peter Banys and Ellen Herbst of the Veterans Administration Substance Abuse Day Hospital and Dr. David Pating, Karen Moise and their colleagues at the Kaiser Permanente Chemical Dependency Recovery Program in San Francisco for their valuable assistance in recruiting participants. We also wish to extend our gratitude to the study participants, who made this research possible.
Footnotes
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Structural Brain Alterations Associated with Alcoholism. Alcohol Health & Research World. 1995;19:266–272. [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan EV. NIAAA Research Monograph No. 34: Human brain vulnerability to alcoholism: Evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s neuroscience and behavioral research portfolio. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2000. pp. 473–508. [Google Scholar]
- 3.Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- 4.Gaser C, Volz HP, Kiebel S, Riehemann S, Sauer H. Detecting structural changes in whole brain based on nonlinear deformations-application to schizophrenia research. Neuroimage. 1999;10:107–113. doi: 10.1006/nimg.1999.0458. [DOI] [PubMed] [Google Scholar]
- 5.Studholme C, Cardenas V, Schuff N, Rosen H, Miller B, Weiner M. Detecting Spatially Consistent Structural Differences in Alzheimer’s and Fronto Temporal Dementia Using Deformation Morphometry. Proceedings of Medical Image Computing and Computer Assisted Interventions; Utrecht. 2001. pp. 41–48. [Google Scholar]
- 6.Machado AM, Gee JC, Campos MF. Atlas warping for brain morphometry; proceedings of Medical Imaging; SPIE Press; 1998. pp. 642–651. [Google Scholar]
- 7.Davatzikos C, Vaillant M, Resnick SM, Prince JL, Letovsky S, Bryan RN. A computerized approach for morphological analysis of the corpus callosum. J Comput Assist Tomogr. 1996;20:88–97. doi: 10.1097/00004728-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krampe H, Stawicki S, Hoehe MR, Ehrenreich H. Outpatient Long-term Intensive Therapy for Alcoholics (OLITA): a successful biopsychosocial approach to the treatment of alcoholism. Dialogues Clin Neurosci. 2007;9:399–412. [PMC free article] [PubMed] [Google Scholar]
- 11.McKay JR, Franklin TR, Patapis N, Lynch KG. Conceptual, methodological, and analytical issues in the study of relapse. Clin Psychol Rev. 2006;26:109–127. doi: 10.1016/j.cpr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Maisto SA, Zywiak WH, Connors GJ. Course of functioning 1 year following admission for treatment of alcohol use disorders. Addict Behav. 2006;31:69–79. doi: 10.1016/j.addbeh.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Maisto SA, Clifford PR, Stout RL, Davis CM. Moderate drinking in the first year after treatment as a predictor of three-year outcomes. J Stud Alcohol Drugs. 2007;68:419–427. doi: 10.15288/jsad.2007.68.419. [DOI] [PubMed] [Google Scholar]
- 14.Udo T, Clifford PR, Davis CM, Maisto SA. Alcohol use post AUD treatment initiation as a predictor of later functioning. Am J Drug Alcohol Abuse. 2009;35:128–132. doi: 10.1080/00952990802707059. [DOI] [PubMed] [Google Scholar]
- 15.Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- 16.Moos RH, Moos BS, Timko C. Gender, treatment and self-help in remission from alcohol use disorders. Clin Med Res. 2006;4:163–174. doi: 10.3121/cmr.4.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delucchi KL, Weisner C. Transitioning into and out of problem drinking across seven years. J Stud Alcohol Drugs. 2010;71:210–218. doi: 10.15288/jsad.2010.71.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritvo JI, Park C. The psychiatric management of patients with alcohol dependence. Curr Treat Options Neurol. 2007;9:381–392. [PubMed] [Google Scholar]
- 20.Zywiak WH, Stout RL, Trefry WB, Glasser I, Connors GJ, Maisto SA, et al. Alcohol relapse repetition, gender, and predictive validity. J Subst Abuse Treat. 2006;30:349–353. doi: 10.1016/j.jsat.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- 22.Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: predictors of outcome 6 months after treatment. Eur Addict Res. 2005;11:132–137. doi: 10.1159/000085548. [DOI] [PubMed] [Google Scholar]
- 23.Bradizza CM, Stasiewicz PR, Paas ND. Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: a review. Clin Psychol Rev. 2006;26:162–178. doi: 10.1016/j.cpr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKay JR. Studies of factors in relapse to alcohol, drug and nicotine use: a critical review of methodologies and findings. J Stud Alcohol. 1999;60:566–576. doi: 10.15288/jsa.1999.60.566. [DOI] [PubMed] [Google Scholar]
- 26.Kodl MM, Fu SS, Willenbring ML, Gravely A, Nelson DB, Joseph AM. The impact of depressive symptoms on alcohol and cigarette consumption following treatment for alcohol and nicotine dependence. Alcohol Clin Exp Res. 2008;32:92–99. doi: 10.1111/j.1530-0277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 27.Glenn SW, Parsons OA. Prediction of resumption of drinking in posttreatment alcoholics. Int J Addict. 1991;26:237–254. doi: 10.3109/10826089109053186. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18:589–597. doi: 10.1037/0894-4105.18.3.589. [DOI] [PubMed] [Google Scholar]
- 29.Durazzo TC, Gazdzinski S, Yeh PH, Meyerhoff DJ. Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol Alcohol. 2008;43:683–691. doi: 10.1093/alcalc/agn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 31.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 32.Durazzo TC, Tosun D, Buckley ST, Gazdzinski S, Mon A, Fryer SL, et al. Cortical thickness, surface area and volume of the brain reward system in alcohol dependence: Relationships to relapse and extended abstinence. Alcohol: Clinical and Experimental Research. doi: 10.1111/j.1530-0277.2011.01452.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durazzo T, Gazdzinski S, Mon A, Meyerhoff D. Cortical perfusion in alcohol-dependent individuals during short-term abstinence: relationships to resumption of hazardous drinking after treatment. Alcohol. 2010;44:201–210. doi: 10.1016/j.alcohol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J Stud Alcohol Drugs. 2010;71:278–289. doi: 10.15288/jsad.2010.71.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- 36.Studholme C, Cardenas V, Weiner M. Multi-Scale Image and Multi-Scale Deformation of Brain Anatomy for Building Average Brain Atlases. SPIE Medical Imaging Conference; 2001. pp. 557–568. [Google Scholar]
- 37.Studholme C, Cardenas V, Blumenfeld R, Schuff N, Rosen HJ, Miller B, et al. Deformation Tensor Morphometry of Semantic Dementia with Quantitative Validation. NeuroImage. 2004;21:1387–1398. doi: 10.1016/j.neuroimage.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Studholme C, Cardenas V, Maudsley A, Weiner M. An intensity consistent filtering approach to the analysis of deformation tensor derived maps of brain shape. Neuroimage. 2003;19:1638–1649. doi: 10.1016/s1053-8119(03)00183-6. [DOI] [PubMed] [Google Scholar]
- 39.Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- 40.Durazzo TC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Non-treatment-seeking heavy drinkers: effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend. 2007;87:76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: Systematic review of behavioral decision-making and neuroimaging studies. Br J Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- 42.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 43.Cope LM, Schaich Borg J, Harenski CL, Sinnott-Armstrong W, Lieberman D, Nyalakanti PK, et al. Hemispheric Asymmetries during Processing of Immoral Stimuli. Front Evol Neurosci. 2:110. doi: 10.3389/fnevo.2010.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.