Abstract
Objective(s)
We previously reported that elevated anti-inflammatory cervical cytokines in early pregnancy were associated with spontaneous preterm birth. Our objective was to explore the relation between serum folate vitamers and the lower genital tract inflammatory milieu.
Study Design
Pregnant women (n=417) at <16 wk gestation had serum samples analyzed for folate species (5-methyltetrahydrofolate (5MeTHF), 5-formyltetrahydrofolate (5FoTHF)) and cervical fluid assayed for cytokine concentrations. Patterns in pro-inflammatory (PRO) cytokines (interleukin (IL)-1β, IL-6, IL-8, IL-10, monocyte chemotactic protein-1) and anti-inflammatory (ANTI) cytokines (IL-4, IL-10, IL-13) were identified with factor analysis.
Results
After confounder adjustment, maternal serum 5MeTHF concentrations had a strong, negative association with elevated ANTI scores, while serum 5FoTHF concentrations were positively associated with elevated ANTI scores (both p<0.05). Maternal folate was not associated with PRO scores.
Conclusion
Maternal serum folate vitamers are associated with cervical cytokine concentrations, suggesting a possible mechanistic link between folate and preterm birth risk.
Keywords: folate, cytokines, inflammation, preterm birth
INTRODUCTION
A relation between folate deficiency and prematurity was originally suggested in 1944 by Callender, who reported an increased incidence of prematurity in women with megaloblastic anemia in pregnancy.1 Since that time, a number of studies have explored whether maternal folate status is linked with preterm birth.2–11 More recent investigations of dietary, supplemental, or biomarker folate in relation to preterm birth have produced conflicting results that were likely due to varying ranges and timing of folate assessment, assessment methods of gestational age, and population characteristics. The 3 primary folate species that comprise total folate in serum are 5-methyltetrahydrofolate (5MeTHF), 5-formyltetrahydrofolate (5FoTHF), and folic acid. These different folate species contribute to the range of biological effects of folate.
We recently demonstrated that the relative concentrations of folate species in maternal circulation in early pregnancy might be more critical than total folate in determining the risk of preterm birth.12 We found a significant interaction between serum 5MeTHF and 5FoTHF concentrations and risk of preterm birth (P = 0.01). When serum 5MeTHF concentrations are low, there is a positive linear relation between 5FoTHF and risk of preterm birth. When 5MeTHF concentrations are high, there is a strong negative relation between 5FoTHF and preterm birth.
In 1970, Baumslag wrote “The mechanism whereby folic acid reduces the incidence of prematurity is unknown.”11 In the last 40 years, few explorations of mechanisms underlying this association have been undertaken. In the present manuscript, we present our study exploring the relation between serum folate and the lower genital tract inflammatory milieu, as a possible link between folate and spontaneous preterm birth.
The inflammatory milieu of the cervix has been hypothesized to play a role in contributing to the risk of preterm birth. Both pro- and anti-inflammatory components of the cervical immune system may contribute to the risk of subsequent intrauterine infection and preterm birth. In the current work, we hypothesized that the lower genital tract inflammatory milieu in early pregnancy is a biologic link between folate and preterm birth risk.
MATERIALS AND METHODS
The Study of Nutrition and Pregnancy (SNAP) is an ongoing prospective cohort study of pregnant women seeking care at Magee-Womens Hospital resident antepartum clinics in Pittsburgh, PA. The antepartum clinics serve a predominantly publically insured, low-income population that is approximately 55% black and 44% white. Eligible women were <16 weeks gestation and non-Hispanic white or non-Hispanic black based on self-report, and had singleton pregnancies with no known preexisting conditions, vaginal bleeding, fetal anomalies, or current or planned cervical cerclage. All women provided informed, written consent. The study was approved by the University of Pittsburgh Institutional Review Board.
At enrollment, which occurred at a mean of 9.5 weeks gestation, women underwent a standard pelvic examination and provided a nonfasting blood sample that was banked for later analysis. Women also completed an interviewer-administered questionnaire to collect data on sociodemographic characteristics; medical, reproductive, and sexual history; and maternal behaviors.
Quantification of folate vitamers
Non-fasting serum samples were allowed to coagulate at room temperature for 30 minutes. Subsequently, tubes were kept on ice and covered with foil to protect from light. Within 2 hours of the blood draw, tubes were centrifuged, and serum was aliquotted into amber vials. Vials were stored at −80°F until they were transported on dry ice in 2009 to the local lab of Dr. Raman Venkataramanan. No samples were thawed before assay for folate vitamers. The 3 primary folate vitamers (5MeTHF, 5FoTHF, and folic acid) were quantified with high-performance liquid chromatography-tandem mass spectrometry based on a published method 13, the details of which were described previously.12 The mean percent deviations were less than 15% for each concentration level of all calibration curves including the lower limit of quantification, 1.8 ng/ml. The intra-day and inter-day coefficient of variation was less than 10%. The method was also accurate with bias of within the 15% acceptable limits at all levels tested.
Serum total folate concentrations were calculated by summing the concentrations of 5MeTHF, 5FoTHF, and free folic acid. Folate vitamers were categorized into tertiles for analysis.
Lower genital tract inflammation
During a speculum examination at enrollment, a Dacron swab was placed in the cervix and left there for 10 seconds to achieve saturation. The swab was then placed in a plastic tube containing 400 μl of PBS (final dilution of 1:5), immediately transported to the laboratory, and stored at −20°C. The buffer did not contain any other additives, including protease inhibitors. For analysis, the specimens were thawed at room temperature. The swab and the remaining diluent were centrifuged in a spin-X centrifuge filter unit (Costar, Cambridge, MA) at 12,000 rpm for 20 minutes. Cytokine concentrations of interleukin (IL)-1β, IL-6, IL-8, IL-4, IL-10, IL-13 and monocyte chemotactic protein (MCP)-1 were measured using the Luminex LabMAP® multiplex system and a BeadLyte bead kit (Upstate, Lake Placid, NY). Each assay was run with an intra- and inter-assay variation of <10%. For all analytes, the lower limit of sensitivity of the assay was 3.5 pg/ml. The percent of women with cytokine concentrations below the detection limit were 12% (IL-1β), 7% (IL-4), 2% (IL-6), 0% (IL-8), for 14% (IL-10), 18% (IL-13), and 0.2% (MCP-1). Women with IL-4, IL-10, and IL-13 below the detection limit (n=5) or IL-1β and IL-6 below the detection limit (n=2), were not included in the analysis because we felt that inability to detect concentration of multiple cytokines reflected an unusable sample. For the remaining observations with a value below the detection limit, we imputed the limit of detection divided by the square-root of 2.
Cervical cytokine factor analysis
A factor analysis was performed on cytokines using a principal factor method 14, 15, as described in detail previously (Simhan et al, Paediatr Perinat Epidemiol 2010, in press). Factor analysis was used to discover the underlying structure of cytokines in the lower genital tract immunologic milieu. Briefly, the number of factors extracted was based on the eigenvalues, examination of the scree plot, and the interpretability of the solution. Two factors were identified: Factor 1, which highly loaded on pro-inflammatory/immunomodulatory cytokines (IL-1β, IL-6, IL-8, MCP-1, and IL-10), and Factor 2, which highly loaded on anti-inflammatory cytokines (IL-4, IL-10, and IL-13). Each subject has a score for Factor 1 and 2, which are continuous variables. Factor scores were categorized into thirds based on tertiles of the distribution and dichotomized as elevated (highest tertile) or not elevated (lower two tertiles).
Covariates
Women self-reported their race/ethnicity as non-Hispanic white or non-Hispanic black. Data on maternal age, smoking status in the 3 months before pregnancy, maternal education, marital status, parity, household yearly income, and employment status were ascertained from the baseline interviewer-administered questionnaire. Prepregnancy body mass index (BMI, weight (kg)/height (m)2) was based on maternal self-report of pregravid weight and measured height at enrollment. Gestational age was based on best obstetric estimate (a reliable, self-reported estimate of last menstrual period or an ultrasound early in pregnancy).
Women were also classified as having a sexually transmitted infection (STI, Trichomonas vaginalis, Chlamydia trachomatis or Neisseria gonorrhoeae) based on specimens obtained from the enrollment pelvic exam 16. Also at the enrollment visit, vaginal swabs collected for culture and identification of vaginal flora. BV was diagnosed by vaginal pH > 4.7 and a score of 7 through 10 from a Gram-stained vaginal smear interpreted using the method of Nugent et al 17. Intermediate flora was defined as having a Nugent score of 4 through 6 and normal flora was defined as having a Nugent score of 0 through 3.
Statistical analysis
Pearson chi-squared tests were used to test for differences in the distribution of maternal characteristics tertiles of folate vitamers. We used Spearman rank correlation coefficients to test the association between folate vitamer concentrations and Factor 1 and Factor 2 scores. To assess the independent association between maternal folate status and elevated Factor 1 and 2 scores, we used multivariable Poisson regression to estimate prevalence ratios (PR; using the “log” link) 18. PR was selected instead of an odds ratio because this was a cross-sectional analysis and elevated factor scores were common outcomes 19. Potential confounders were race/ethnicity, smoking status before pregnancy, maternal age, marital status, education, income, employment status, pregravid BMI, gestational age at enrollment, presence of STI, and vaginal flora. We removed confounders if their inclusion did not satisfy our a priori change-inestimate criterion (>8% change in a PR), yet none of these factors met our definition of confounding. We maintained maternal race/ethnicity and STI in the model out of convention. We tested for interaction between the 5MeTHF and 5FoTHF using a likelihood ratio test (α=0.10).
RESULTS
From 2003 to 2007, 548 eligible women enrolled in the study (75% response rate). Of these, we excluded 31 women who did not have cervical fluid available for cytokine assay, 100 women without an adequate volume of banked serum to perform the folate assays. A total of 417 women were included in the final analysis. There were no meaningful differences in maternal race, age, parity, smoking, education or the prevalence of preterm birth between women included and excluded from the analysis (data not shown). Most women in the cohort were 20 to 29 years, multiparous, high-school educated, unemployed, and low-income (Table 1). Non-Hispanic black women made up 56% of the cohort. More than half of women smoked. Bacterial vaginosis was common (45%), and 11% of women had an STI.
Table 1.
Maternal characteristics for the total population and by tertile (T) of 5-methyltetrahydrofolate (5MeTHF) and 5-formyltetrahydrofolate (5FoTHF) concentrations at <16 weeks gestation
| Total population | Maternal serum 5MeTHF
|
Maternal serum 5FoTHF
|
|||||
|---|---|---|---|---|---|---|---|
| n (%) | T1 (low) | T2 | T3 (high) | T1 (low) | T2 | T3 (high) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Maternal race | |||||||
| Non-Hispanic white | 183 (44) | 46 (32) | 53 (40) | 84 (60)1 | 48 (34) | 59 (42) | 76 (56)2 |
| Non-Hispanic black | 234 (56) | 99 (68) | 80 (60) | 55 (40) | 92 (66) | 82 (58) | 60 (44) |
| Maternal age | |||||||
| <20 y | 53 (13) | 12 (8) | 19 (14) | 22 (16) | 18 (13) | 17 (12) | 18 (13) |
| 20 to 29 y | 305 (73) | 114 (79) | 94 (71) | 97 (70) | 98 (70) | 106 (75) | 101 (74) |
| ≥30 y | 59 (14) | 19 (13) | 20 (15) | 20 (14) | 24 (17) | 18 (13) | 17 (13) |
| Parity | |||||||
| 0 | 69 (17) | 16 (11) | 18 (14) | 35 (25)2 | 16 (11) | 21 (15) | 32 (24)3 |
| 1 | 183 (44) | 55 (39) | 64 (48) | 64 (46) | 59 (43) | 60 (43) | 64 (47) |
| ≥2 | 161 (39) | 70 (50) | 51 (38) | 40 (29) | 63 (46) | 58 (42) | 40 (29) |
| Smoking status 3 months before pregnancy | |||||||
| Nonsmoker | 186 (46) | 51 (36) | 59 (45) | 76 (56)2 | 65 (48) | 59 (44) | 62 (46) |
| Smoker | 220 (54) | 90 (64) | 71 (55) | 59 (44) | 70 (52) | 76 (56) | 74 (54) |
| Maternal education | |||||||
| Less than high school | 99 (24) | 38 (26) | 36 (27) | 25 (18) | 31 (22) | 41 (29) | 27 (20)2 |
| High school or equivalent | 279 (67) | 98 (68) | 87 (65) | 94 (68) | 103 (74) | 90 (64) | 86 (63) |
| Greater than high school | 39 (9) | 9 (6) | 10 (8) | 20 (14) | 6 (4) | 10 (7) | 23 (17) |
| Marital status | |||||||
| Unmarried | 351 (84) | 128 (88) | 117 (88) | 106 (76)2 | 123 (88) | 118 (84) | 110 (81) |
| Married | 66 (16) | 17 (12) | 16 (12) | 33 (24) | 17 (12) | 23 (16) | 26 (19) |
| Employment status | |||||||
| Unemployed | 217 (52) | 76 (52) | 69 (52) | 72 (52) | 81 (58) | 65 (46) | 71 (52) |
| Employed part-time | 92 (22) | 27 (19) | 36 (27) | 29 (21) | 29 (21) | 34 (24) | 29 (21) |
| Employed full-time | 108 (26) | 42 (29) | 28 (21) | 38 (27) | 30 (21) | 42 (30) | 36 (27) |
| Family’s yearly income | |||||||
| <$10,000 | 173 (42) | 61 (43) | 64 (49) | 48 (35) | 60 (43) | 55 (40) | 58 (43) |
| $10,000 to <$25,000 | 149 (36) | 57 (40) | 42 (32) | 50 (37) | 51 (37) | 55 (40) | 43 (32) |
| ≥$25,000 | 88 (21) | 25 (17) | 24 (18) | 39 (28) | 27 (20) | 28 (20) | 33 (25) |
| Prepregnancy BMI | |||||||
| <25.0 kg/m2 | 178 (43) | 56 (39) | 51 (38) | 71 (51)3 | 54 (39) | 62 (44) | 62 (46) |
| 25.0 to 29.9 kg/m2 | 101 (24) | 30 (21) | 40 (30) | 31 (22) | 34 (24) | 38 (27) | 29 (21) |
| 30.0 to 34.9 kg/m2 | 70 (17) | 25 (17) | 25 (19) | 20 (14) | 22 (16) | 19 (14) | 29 (21) |
| ≥35.0 kg/m2 | 67 (16) | 33 (23) | 17 (13) | 17 (12) | 30 (21) | 21 (15) | 16 (12) |
| Presence of an STI | |||||||
| Yes | 372 (89) | 121 (83) | 122 (92) | 129 (93)3 | 125 (89) | 125 (89) | 122 (90) |
| No | 45 (11) | 24 (17) | 11 (8) | 10 (7) | 15 (11) | 16 (11) | 14 (10) |
| Vaginal flora | |||||||
| Bacterial vaginosis | 188 (45) | 53 (37) | 55 (42) | 80 (58)1 | 54 (39) | 63 (45) | 71 (52) |
| Intermediate flora | 53 (13) | 14 (10) | 18 (14) | 21 (15) | 19 (14) | 20 (14) | 14 (10) |
| Normal vaginal flora | 173 (42) | 77 (53) | 58 (44) | 38 (27) | 65 (47) | 57 (41) | 51 (38) |
P<0.001,
P<0.01, and
P<0.05 based on Pearson chi-squared tests.
Missing data is as follows: n=1 missing prepregnancy BMI; n=3 missing STI and vaginal flora; n=4 missing parity; n=7 missing income; n=11 missing smoking
In this cohort, 5MeTHF and 5FoTHF concentrations made up 65% [IQR, 54%–77%] and 33% [20%–44%] of total folate concentrations, respectively. Only 8% of women had detectable concentrations of free folic acid. Thus, analyses were limited to the study of 5MeTHF and 5FoTHF. Women who were white, nulliparous, non-smokers, married, lean, and without an STI or bacterial vaginosis were significantly more likely to have serum 5MeTHF in the upper tertile at <16 weeks. Women in the upper tertile of serum 5FoTHF were more often white, nulliparous, and well-educated compared to women with lower concentrations of 5FoTHF.
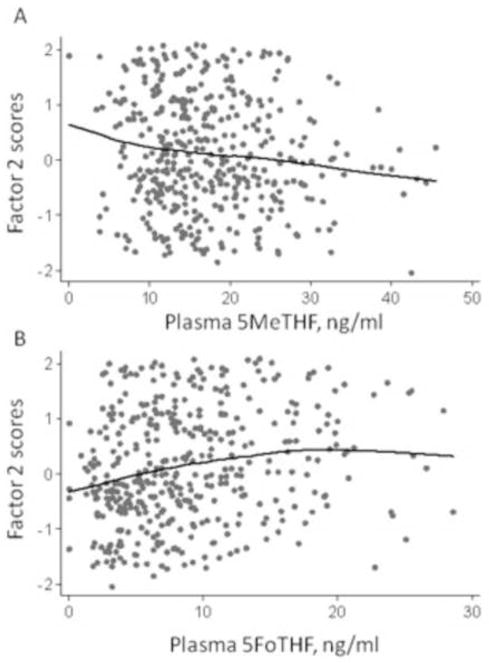
Figure 1 shows that factor 2 (anti-inflammatory cytokine) scores were negatively associated with serum 5MeTHF concentrations (r=−0.11, p<0.05; Panel A) and positively associated with serum 5FoTHF (r=0.19, p<0.001; Panel B). Factor 1 (pro-inflammatory/immunomodulatory) scores were not associated with 5MeTHF (r=−0.01, p=0.85) or 5FoTHF (r=−0.03, p=0.43). Neither factor score was associated with total folate (data not shown).
Figure 1.
Factor 2 (anti-inflammatory cytokine) scores against maternal serum 5-methyl-tetrahydrofolate (5MeTHF, Panel A) and serum 5-formyl-tetrahydrofolate (5FoTHF, Panel B) at <16 weeks gestation (n=417). Lines were fit using locally weighted regression. Spearman correlation coefficient = −0.11 (p<0.05) for 5MeTHF and 0.19 (p<0.001) for 5FoTHF.
Factor 1 scores were not associated with serum total folate, 5MeTHF, or 5FoTHF concentrations in unadjusted or adjusted analyses (Table 2). In contrast, maternal folate status at <16 weeks gestation was associated with Factor 2 scores. Each 1-SD increase in 5MeTHF concentration was associated with a 30% reduction in the prevalence of elevated Factor 2 scores (prevalence ratio 0.7 (95% CI: 0.6, 0.9)) after adjustment for race/ethnicity and STIs, whereas a 1-SD increase in 5FoTHF was associated with a 20% increased prevalence of elevated Factor 2 scores (1.2 (1.1, 1.4)). When the folate vitamers were categorized, women in the middle and upper tertiles of serum 5FoTHF concentrations were 50% and 60% more likely to have elevated Factor 2 scores compared with those in the lowest tertile, independent of confounders. Mothers in the upper tertile of 5MeTHF concentrations had a 30% reduction in the prevalence of elevated Factor 2 scores, but this did not reach statistical significance. Serum total folate was not associated with Factor 2 scores.
Table 2.
Association between maternal concentrations of total folate and folate vitamers and the prevalence of elevated pro- and anti-inflammatory cervical cytokine factor scores (n=417)
| Elevated1 factor 1 scores (Pro-inflammatory/immunomodulatory cytokine)
|
Elevated1 factor 2 scores (Anti-inflammatory cytokine)
|
||||||
|---|---|---|---|---|---|---|---|
| No | Yes | Adjusted2 PR (95% CI) | No | Yes | Adjusted2 PR (95% CI) | ||
| Serum total folate | 1-SD (12.7-ng/ml) increase | 1.0 (0.9, 1.2) | 0.9 (0.7, 1.1) | ||||
| T1 (low) | 93 (33) | 50 (36) | 1.0 (ref) | 95 (34) | 48 (35) | 1.0 (ref) | |
| T2 | 99 (36) | 39 (28) | 0.8 (0.5, 1.2) | 87 (31) | 51 (37) | 1.1 (0.7, 1.6) | |
| T3 (high) | 86 (31) | 50 (36) | 1.0 (0.7, 1.5) | 96 (35) | 40 (29) | 0.9 (0.6, 1.3) | |
| Serum 5MeTHF | 1-SD (7.7-ng/ml) increase | 1.0 (0.9, 1.2) | 0.7 (0.6, 0.9) | ||||
| T1 (low) | 94 (34) | 51 (37) | 1.0 (ref) | 90 (32) | 55 (40) | 1.0 (ref) | |
| T2 | 95 (34) | 38 (27) | 0.8 (0.5, 1.3) | 88 (32) | 45 (32) | 0.8 (0.6, 1.3) | |
| T3 (high) | 89 (32) | 50 (36) | 1.0 (0.7, 1.6) | 100 (36) | 39 (28) | 0.7 (0.5, 1.1) | |
| Serum 5FoTHF | 1-SD (6.1-ng/ml) increase | 1.0 (0.8, 1.1) | 1.2 (1.1, 1.4) | ||||
| T1 (low) | 93 (33) | 47 (34) | 1.0 (ref) | 106 (38) | 34 (25) | 1.0 (ref) | |
| T2 | 89 (32) | 52 (37) | 1.1 (0.7, 1.6) | 88 (32) | 53 (38) | 1.5 (1.1, 2.4) | |
| T3 (high) | 96 (35) | 40 (29) | 0.9 (0.6, 1.3) | 84 (30) | 52 (37) | 1.6 (1.1, 2.5) | |
5MeTHF, 5-methyl-tetrahydrofolate; 5FoTHF, 5-formyl-tetrahydrofolate; CI, confidence interval; PR, prevalence ratio
Elevated scores are those in the upper tertile of factor scores.
Total folate models were adjusted for maternal race and presence of sexually transmitted infections. Serum 5MeTHF models were additionally were adjusted for 5FoTHF concentrations, and serum 5FoTHF were additionally adjusted for 5MeTHF concentrations.
COMMENT
In this biracial cohort of low-income pregnant women at <16 weeks gestation, we have shown that maternal serum folate vitamers are associated with cervical cytokine concentrations. Serum 5MeTHF concentrations were negatively associated with the prevalence of elevated anti-inflammatory cytokine factor scores, while serum 5FoTHF concentrations were positively associated with prevalence of high anti-inflammatory factor scores. These findings were independent of measured confounders. The direction of these associations is consistent with our previous findings regarding the association of folate vitamers and preterm birth risk. We have previously demonstrated that women with low 5MeTHF and high 5FoTHF had the highest risk of preterm birth. Interestingly, in this paper we identify this same subgroup of women as having a unique environment in their lower genital tract. Specifically women with low 5MeTHF and high 5FoTHF have the highest Factor 2 or anti-inflammatory scores.
The notion that folate status, in part, acts via lower genital tract inflammatory milieu to modify the risk of preterm birth is novel. There are few data regarding maternal nutritional status and inflammation in reproductive tissues. In a population of HIV-infected Tanzanian women, Fawzi et al found no relation between multivitamin supplement use or Vitamin A supplement use and IL-1β concentrations as determined by cervicovaginal lavage.20 With respect to folate and upper genital tract inflammation, in a porcine pregnancy model, Guay et al found that endometrial granulocyte/macrophage colony stimulating factor expression was reduced among folic acid supplemented animals.21
The mechanisms linking folate status and lower genital tract inflammation are unknown. Folate metabolism is integral to purine synthesis and to DNA methylation; gene expression can be altered through either of these pathways. Future work could explore the relation between folate status and the methylation profile of the genes responsible for regulating lower genital tract inflammation.
Our sample size and study design limited our ability to study the relation between folate status and infection/inflammation in the upper genital tract. Additionally, while our assessment of the vitamers that comprise total folate is innovative, a fuller view of the contribution of the complexity of folate metabolism to preterm birth could be gained by measuring additional elements of the pathway such as homocysteine, vitamin B-12, methionine, SAM, and S-adenosylhomocysteine.
Our study’s prospective design, rigorous characterization of the lower genital tract inflammatory milieu, and quantification of 3 folate metabolites with the gold-standard method of LC-MS/MS are major strengths. Our findings prompt the speculation that the preterm births most closely associated with folate status and altered lower genital tract inflammatory milieu would be those subtypes of preterm birth caused by ascending microbial invasion of the upper genital tract. In ongoing and future studies, we hope to accrue sufficient sample size with accurate phenotype data to permit this exploration. In particular, cohorts with placental histopathologic examination, microbial assays, and adequate numbers of very early spontaneous preterm births would help further this line of investigation.
Acknowledgments
Supported by National Institutes of Health grants R01 HD041663 and R01 HD052732 (HNS) and K01 MH074092 and R01 HD056999 (LMB). This publication was also made possible by Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Callender STE. A critical review of pernicious anaemia of pregnancy. Quarterly Journal of Medicine. 1944;13:75–105. [Google Scholar]
- 2.Bukowski R, Malone FD, Porter FT, Nyberg DA, Comstock CH, Hankins GD, et al. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med. 2009;6:e1000061. doi: 10.1371/journal.pmed.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmermans S, Jaddoe VW, Hofman A, Steegers-Theunissen RP, Steegers EA. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: the Generation R Study. Br J Nutr. 2009;102:777–785. doi: 10.1017/S0007114509288994. [DOI] [PubMed] [Google Scholar]
- 4.Zeng L, Dibley MJ, Cheng Y, Dang S, Chang S, Kong L, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337:a2001. doi: 10.1136/bmj.a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christian P, West KP, Khatry SK, Leclerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78:1194–1202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 6.Siega-Riz AM, Savitz DA, Zeisel SH, Thorp JM, Herring A. Second trimester folate status and preterm birth. Am J Obstet Gynecol. 2004;191:1851–1857. doi: 10.1016/j.ajog.2004.07.076. [DOI] [PubMed] [Google Scholar]
- 7.Rolschau J, Kristoffersen K, Ulrich M, Grinsted P, Schaumburg E, Foged N. The influence of folic acid supplement on the outcome of pregnancies in the county of Funen in Denmark. Part I. Eur J Obstet Gynecol Reprod Biol. 1999;87:105–110. discussion 103–104. [PubMed] [Google Scholar]
- 8.Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr. 2000;71:1295S–1303S. doi: 10.1093/ajcn/71.5.1295s. [DOI] [PubMed] [Google Scholar]
- 9.Smits LJ, Essed GG. Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion. Lancet. 2001;358:2074–2077. doi: 10.1016/S0140-6736(01)07105-7. [DOI] [PubMed] [Google Scholar]
- 10.Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Dietary and serum folate: their influence on the outcome of pregnancy. Am J Clin Nutr. 1996;63:520–525. doi: 10.1093/ajcn/63.4.520. [DOI] [PubMed] [Google Scholar]
- 11.Baumslag N, Edelstein T, Metz J. Reduction of incidence of prematurity by folic acid supplementation in pregnancy. Br Med J. 1970;1:16–17. doi: 10.1136/bmj.1.5687.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodnar LM, Himes KP, Venkataramanan R, Chen JY, Evans RW, Meyer JL, et al. Maternal serum folate species in early pregnancy and risk of preterm birth. Am J Clin Nutr. 2010;92:864–871. doi: 10.3945/ajcn.2010.29675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeiffer CM, Fazili Z, McCoy L, Zhang M, Gunter EW. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin Chem. 2004;50:423–432. doi: 10.1373/clinchem.2003.026955. [DOI] [PubMed] [Google Scholar]
- 14.Comrey AL, Lee HB. A first course in factor analysis. 2. Hillsdale, N.J: Erlbaum Associates; 1992. [Google Scholar]
- 15.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5. Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- 16.Simhan HN, Bodnar LM, Krohn MA. Paternal race and bacterial vaginosis during the first trimester of pregnancy. Am J Obstet Gynecol. 2008;198:196, e191–194. doi: 10.1016/j.ajog.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 19.Thompson ML, Myers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med. 1998;55:272–277. doi: 10.1136/oem.55.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fawzi W, Msamanga G, Antelman G, Xu C, Hertzmark E, Spiegelman D, et al. Effect of prenatal vitamin supplementation on lower-genital levels of HIV type 1 and interleukin type 1 beta at 36 weeks of gestation. Clin Infect Dis. 2004;38:716–722. doi: 10.1086/381673. [DOI] [PubMed] [Google Scholar]
- 21.Guay F, Matte JJ, Girard CL, Palin MF, Giguere A, Laforest JP. Effect of folic acid plus glycine supplement on uterine prostaglandin and endometrial granulocyte-macrophage colony-stimulating factor expression during early pregnancy in pigs. Theriogenology. 2004;61:485–498. doi: 10.1016/s0093-691x(03)00213-9. [DOI] [PubMed] [Google Scholar]