Abstract
Gammaretroviral vectors are an efficient means to effect gene therapy. However, genotoxicity from insertion at non-random sites can confer a competitive advantage to transduced cells resulting in clonal proliferation or neoplasia. Six pig-tailed macaques (Macaca nemestrina) underwent total body irradiation and reconstitution with autologous stem cells genetically modified by a gammaretroviral vector overexpressing HOXB4. Two animals were euthanized due to irradiation/transplantation-associated toxicity, whereas the other four had successful reconstitution.9,10 Of the four macaques with successful reconstitution, one has no long-term follow-up information, one was euthanized due to infection with simian varicella virus infection 18 months post-TBI,4 and the two others are described herein as case Nos. 1 and 2. After being stable for 3 years case No. 1 developed pancytopenia and petechiation, and after 2 years of stability case No. 2 developed anemia and thrombocytopenia. Despite therapy, the animals deteriorated and were euthanized. Gross findings included emaciation, and case No. 1 also had hemorrhage, peritonitis, and cholecystitis. Histologically, bone marrow was hypercellular with predominately blast cells of all hematopoietic lineages though with myeloid predominance, and with maturation arrest and blast cell dysplasia (myelodysplasia). Myelodysplasia likely was from a combination of insertional mutagenesis by the retroviral vector and overexpression of HOXB4. Consequences of myelodysplasia included the blood dyscrasias, and in case No. 1 hemorrhage, bacterial cholecystitis, hepatitis, and peritonitis.
Keywords: retroviral vector, gene therapy, HOXB4, Macaca nemestrina, insertional mutagenesis, myelodysplasia
Gene therapy holds tremendous promise for therapy of a wide variety of diseases, and retroviral vectors are one of the most efficient means to effect such treatments.1–3,6 Gammaretroviral vectors in particular have demonstrated efficacy to cure genetic disease such as Severe Combined Immunodeficiency (SCID) and Chronic Granulomatous Disease.1–3,6 However, despite early successes, a significant percentage of patients treated with retroviral vector gene therapy and cured of SCID subsequently developed leukemia, due to retroviral-induced insertional mutagenesis.1–3 Further investigations into possible genotoxicity of retroviral vectors has suggested that insertion of the retroviral vector at specific, non-random sites may confer a competitive advantage to transduced cells that can eventually result in clonal proliferation or neoplasia.1–3, 5,7,10
Two (case Nos. 1 and 2) juvenile, male pig-tailed macaques (Macaca nemestrina), as part of a cohort of six pig-tailed macaques, were total body irradiated (TBI) with 800 cGy and then transplanted with autologous stem cells that had been genetically modified by a gammaretroviral vector expressing homeobox protein B-4 (HOXB4), as previously described.9,10 Case No. 1 was animal K03290 and case No. 2 was animal K00339 in Zhang XB, et al, Differential effects of HOXB4 on nonhuman primate short- and long-term repopulating cells, PLoS Med 2006;3(5):0687–0698, and in Zhang XB, et al, High incidence of leukemia in large animals after stem cell gene therapy with a HOXB4-expressing retroviral vector, J Clin Invest 2008;118(4):1502–1510.9,10 HOXB4 is a homeobox gene that encodes a DNA binding protein whose overexpression can expand hematopoietic stem cells.7,9,10 As such, HOXB4 is a candidate for therapeutic hematopoietic stem cell expansion. Overexpression of HOXB4 was documented in case Nos. 1 and 2 indicating successful insertion.9 Of the six macaques in the above-described cohort, two were euthanized due to irradiation/transplantation-associated toxicity,9,10 whereas the other four had successful reconstitution. Of the four macaques with successful reconstitution, one has no long-term follow-up information,9,10 one was euthanized due to infection with simian varicella virus infection 18 months post-TBI,4 and the two others are described herein as case Nos. 1 and 2.
Case No. 1 was clinically stable for 3 years, at which point a routine blood collection identified anemia and thrombocytopenia. This progressed to neutropenia and eventually pancytopenia. Case No. 2 was clinically stable for 2 years, after which routine blood collection identified anemia and thrombocytopenia. Therapy over a number of weeks in both cases included numerous plasma rich and whole blood transfusions, broad-spectrum antibiotics and nutritional support. The animals did not respond to therapy, and euthanasia was elected.
Prominent gross findings included poor body condition, and case No. 1 also had multicentric hemorrhage including into the gastrointestinal tract and abdomen (Fig. 1), fibrinous to fibrous peritonitis, and hepatomegaly with fibrosing cholecystitis.
Figure 1.
Gross photograph of case No. 1 demonstrating abdominal hemorrhage.
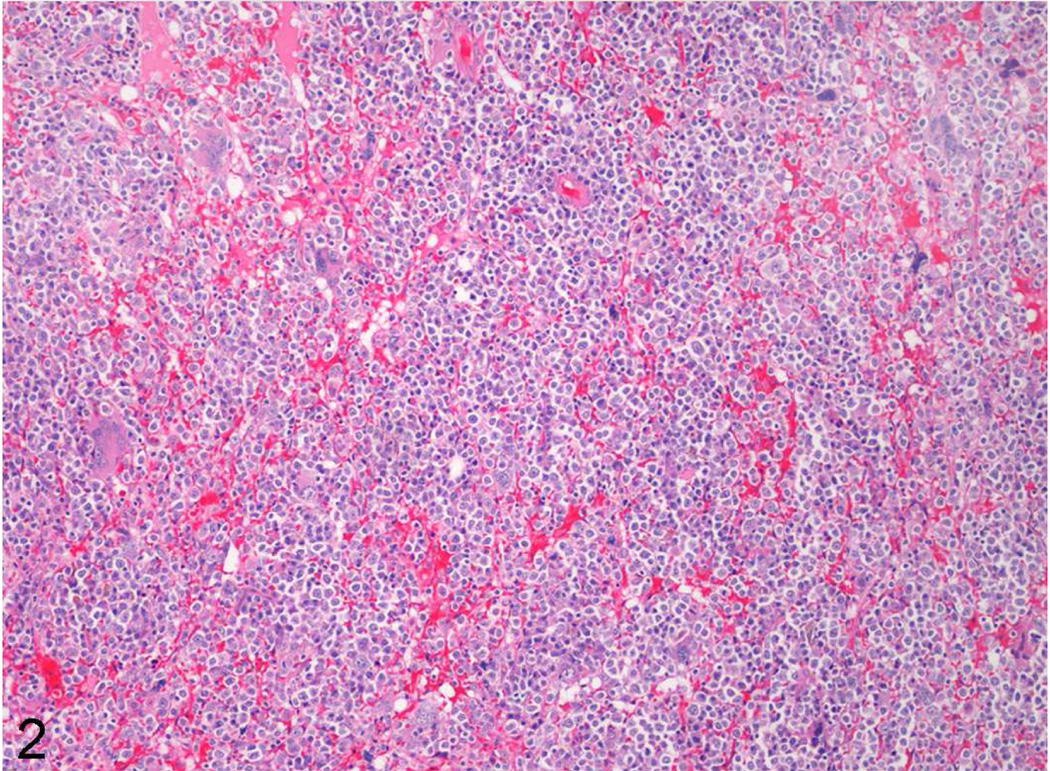
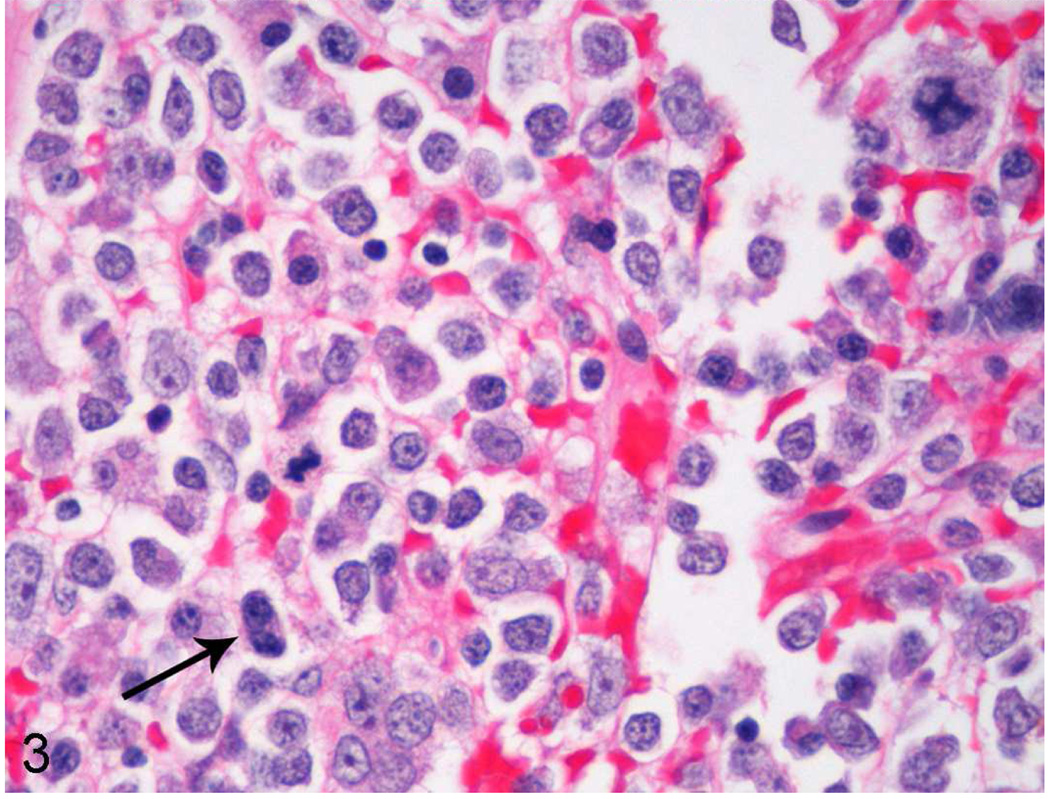
Tissue and organ samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 3–5 microns, and stained routinely with hematoxylin and eosin. Histology in both cases identified highly cellular bone marrow composed of predominately immature hematopoietic cells of all lineages though with a predominance of myeloid blast cells (Figs. 2, 3). Blast cells sometimes exhibited irregular, large nuclei with scattered irregular mitotic figures, and occasional binucleated cells (Fig. 3). Maturation arrest was present in all lineages however rare maturation was seen in the erythroid lineage. Additionally, there was multifocal, single cell necrosis/apoptosis. In case No. 1, other prominent histologic findings included transmural, chronic-active cholecystitis associated with gram-positive cocci, secondary chronic-active hepatitis and peritonitis, multicentric hemorrhage and systemic lymphoid depletion. Both cases also had diffuse testicular atrophy. Finally, case No. 2 also had extramedullary hematopoiesis present in numerous tissues and organs including lymph nodes, spleen, thymus, liver, lungs, and adrenal glands.
Figure 2.
Low magnification of bone marrow from case No. 1 demonstrating diffuse hypercellularity with rare to no differentiation.
Figure 3.
High magnification of bone marrow from case No. 1 revealing predominance of blast cells with maturation arrest, and an irregular mitotic figure in the upper right corner. Nuclei are sometimes irregular, and a binucleated cell is present (arrow). The only identifiable mature cells are scattered plasma cells or maturing cells of the erythroid lineage.
Changes in the bone marrow; hyperplasia with maturation arrest and cellular dysplasia; are best classified as myelodysplasia.8 Myelodysplasia can progress to leukemia,8 which may have occurred if this animal was not euthanized. Indeed, case No. 2 was previously reported as developing leukemia based on in-vitro antemortem analysis of bone marrow aspirates.10 The authors feel that this animal is now better classified as myelodysplasia based on evaluation of all findings including histological examination of bone marrow. Myelodysplasia resulted in the described blood dyscrasias, which led to thrombocytopenia, anemia, and in case No. 1 leukopenia. In case No. 1, consequences of the blood dyscrasias resulted in multicentric hemorrhage, and immunosuppression with development of opportunistic bacterial cholecystitis, hepatitis and peritonitis. Testicular atrophy in both cases were chronic consequences of TBI.
In case No. 2, previously published data on stem cell analyses from bone marrow aspirates obtained antemortem during the clinical phase of demise and evaluated in-vitro revealed, in addition to overexpression of HOXB4, dysregulated expression of oncogenes and a block in myeloid differentiation.10 Additionally, knockdown of HOXB4 in a leukemic canine cell line that also was overexpressing HOXB4 restored myeloid differentiation in-vitro, suggesting overexpression of HOXB4 was critical to induction of the blood dyscrasias.10 In case Nos. 1 and 2, multiple integration sites were identified on in-vitro evaluation of marrow stem cells.10 These findings, in combination with the experimental history, clinicopathologic changes and histologic changes, suggest that myelodysplasia in both cases was due to a combination of insertional mutagenesis from the retroviral vector and resultant overexpression of HOXB4.1–3,7,9,10 Finally, in the four macaques that had successful reconstitution with marrow cells genetically modified to overexpress HOXB4 using a gammaretroviral vector,9 two animals (case Nos. 1 and 2) developed myelodysplasia.
Experience with animal models and humans undergoing retroviral mediated gene therapy indicate that integration sites are non-random, yet in most cases the integration event itself is a neutral event.1–3,5–7,9,10 However, non-random integration can affect gene expression adversely, and this results in a risk that altered gene expression with or without subsequent mutations in transduced cells can result in clonal expansion or neoplasia.1–3,5,7,10 This risk, of course, is significantly increased if the therapeutic gene incorporated in the viral vector can give a growth advantage to transduced cells, such as with the gene encoding HOXB4.7,9,10 As such, the design of retroviral vectors and of therapeutic genes is critical to minimize these risks, and the use of animal models is crucial to evaluate the safety of gene therapy before human trials.5,10 Despite adverse consequences of retroviral-mediated gene therapy as described in this report, the future of gene therapy still holds promise as methodologies continue to evolve to identify and ameliorate risks of non-random insertions and of specific therapeutic genes.
Acknowledgements
The authors wish to thank Mac Durning, Trevor Pierce, and the veterinary and husbandry staff of the Washington National Primate Research Center for providing excellent technical support. This work was supported by the Washington National Primate Research Center NIH/NCRR grant P51 RR000166, and Fred Hutchinson Cancer Research Center NIH grants HL53750, HL84345, DK56465, and DK47754.
References
- 1.Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional Oncogenesis in 4 Patients after Retrovirus-Mediated Gene Therapy of SCID-X1. J Clin Invest. 2008;118(9):3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacein-Bey-Abina S, Von Kalle C, Schmidt v, et al. LMO2-Associated Clonal T Cell Proliferation in Two Patients after Gene Therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 3.Howe SJ, Mansour MR, Schwarzwaelder K, et al. Insertional Mutagenesis Combined with Acquired Somatic Mutations causes Leukemogenesis following Gene Therapy of SCID-X1 Patients. J Clin Invest. 2008;118(9):3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hukkanen RR, Gillen M, Grant R, et al. Simian Varicella Virus in Pigtailed Macaques (Macaca nemestrina): Clinical, Pathologic, and Virologic Features. Comp Med. 2009;59(5):482–487. [PMC free article] [PubMed] [Google Scholar]
- 5.Neinhuis AW, Dunbar CE, Sorrentino BP. Genotoxicity of Retroviral Integration in Hematopoietic Cells. Mol Ther. 2006;13(6):1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Ott MG, Schmidt M, Schwarzwaelder K, et al. Correction of X-Linked Chronic Granulomatous Disease by Gene Therapy, Augmented by Insertional Activation of MDS1-EV11, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 7.Schiedlmeier B, Klump H, Will E, et al. High-level Ectopic HOXB4 Expression Confers a Profound In Vivo Competitive Growth Advantage on Human Cord Blood CD34+ Cells, but Impairs Lymphomyeloid Differentiation. Blood. 2002;101(5):1759–1768. doi: 10.1182/blood-2002-03-0767. [DOI] [PubMed] [Google Scholar]
- 8.Vergilio J, Bagg A. Myelodysplastic Syndromes; Contemporary Biologic Concepts and Emerging Diagnostic Approaches. Am J Clin Pathol. 2003;119 suppl 1:S58–S77. doi: 10.1309/T8DF-XEXM-YLHW-51DG. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XB, Beard BC, Beebe K, et al. Differential Effects of HOXB4 on Nonhuman Primate Short- and Long-Term Repopulating Cells. PLoS Med. 2006;3(5):0687–0698. doi: 10.1371/journal.pmed.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang XB, Beard BC, Trobridge GD, et al. High Incidence of Leukemia in Large Animals after Stem Cell Gene Therapy with a HOXB4-Expressing Retroviral Vector. J Clin Invest. 2008;118(4):1502–1510. doi: 10.1172/JCI34371. [DOI] [PMC free article] [PubMed] [Google Scholar]