Abstract
Arterial compliance, the inverse of arterial stiffness, is a prognostic indicator of arterial health. Central and peripheral arterial compliance decrease with acute cold stress and may increase post exercise when exercise-induced elevations in core temperature are likely still present. Increased blood flow through the conduit arteries associated with elevated core temperature increases shear stress which in turn releases nitric oxide and other endothelial derived factors. These changes, in conjunction with supportive in vitro data, suggest that elevated core temperature may indirectly increase central and peripheral arterial compliance (i.e., decrease arterial stiffness). The purpose of this study was to test the hypothesis that increased core temperature decreases central and peripheral arterial stiffness, as measured with pulse wave velocity (PWV). Using Doppler ultrasound, carotid-femoral (central) and carotid-radial (peripheral) arterial PWVs were measured from eight subjects (age 37 ± 11 years; mass 68.8 ± 11.1 kg; height 171 ± 3 cm) before and during passive heat-stress induced increases in core temperature of 0.47 ± 0.05, 1.03 ± 0.12, and 1.52 ± 0.07°C (i.e., baseline, 0.5, 1.0, and 1.5°C, respectively). Changes in PWV were evaluated with a one-way repeated measures ANOVA. When analyzed as group means, neither central (677 ± 161, 617 ± 72, 659 ± 74, and 766 ± 207 cm/s; P=0.12) nor peripheral (855 ± 192, 772 ± 95, 759 ± 49, and 858 ± 247 cm/s; P=0.56) PWV changed as core temperature increased from baseline to 0.5, 1.0, and 1.5°C, respectively. However, individual changes in central (average r = −0.89, P < 0.05) and peripheral (average r = −0.93, P < 0.05) PWV with heat stress were significantly correlated with normothermic baseline PWV. In conclusion, these data suggest that the magnitude by which heat stress reduced PWV was predicated upon normothermic PWV, with the individuals having the highest normothermic PWV being most responsive to the heat stress-induced reductions in PWV.
Keywords: hyperthermia, compliance, distensibility, pulse wave velocity
INTRODUCTION
Arterial compliance, the inverse of arterial stiffness, is a prognostic indicator of arterial health (Laurent et al., 2006). The acute effect of exercise on arterial compliance is dependent on exercise duration, intensity, and mode. Thirty minutes of moderate intensity (e.g., 65% VO2max) aerobic exercise leads to a transient increase in central and peripheral compliance that returns to baseline after 60 min post-exercise (Kingwell et al., 1997; Heffernan et al., 2007). However, with short duration aerobic exercise, increases in peripheral arterial compliance are localized to the exercising limbs (Naka et al., 2003; Sugawara et al., 2003). High intensity, short-duration anaerobic exercise leads to acute decreases in central compliance, while peripheral compliance either increases or does not change (Heffernan et al., 2007; Rakobowchuk et al., 2009). The mechanisms responsible for these varying effects of exercise on arterial compliance are unclear.
It is well known that moderate intensity exercise can increase body temperature as much as 1.0°C in as little as 30 min (Saltin & Hermansen, 1966; Gregson et al., 2002), while high intensity and short duration anaerobic exercise can increase body temperature, but not always (Deschenes et al., 1998; Watson et al., 2005). It is possible that the transient and inconsistent changes in arterial compliance following various types of exercise may be associated with differences in core body temperature during these exercise bouts.
Recent evidence suggest that cold stress causes acute decreases in central and peripheral compliance that may be the result (or cause) of increases in blood pressure or from sympathetic nervous system activation (Hess et al., 2009). However it is unknown if the reverse occurs; that is, whether heat stress increases vascular compliance. In support of this hypothesis, direct heating of isolated iliac arteries increases vessel compliance, although these vessels were heated to a non-physiological temperature of 60°C (Mitchel et al., 1994). It remains unknown whether a more physiological heating stimulus similarly increases arterial compliance.
Passive heat stress increases blood flow through large conduit arteries (i.e., aorta, brachial, and femoral arteries) secondary to increases in cardiac output and reductions in systemic vascular resistance, which in turn increases shear stress (Kellogg et al., 2003). Increased shear stress increases nitric oxide and/or cytochrome-related hyperpolarizing factors, which are known vasodilators and modulators of vascular tone, arterial elasticity, and arterial compliance (Kinlay et al., 2001; Sugawara et al., 2007; Bellien et al., 2010). Therefore it stands to reason that passive heat stress, through a direct affect of temperature on the vessels as shown in vitro (Mitchel et al., 1994) and/or the release of vasodilator substances associated with increased shear stress, may increase arterial compliance and thus reduce arterial stiffness. To that end, the purpose of this study was to test the hypothesis that increases in core temperature via passive heat-stress decreases arterial stiffness.
METHODS
Subjects
Three males and five females (n = 8) participated in this study; subjects’ mean ± SD age, mass and height were 37 ± 11 years, 68.8 ± 11.1 kg, and 171 ± 3 cm. Subjects were excluded if they were smokers, taking medications, hypertensive (resting systolic blood pressure >139 mmHg), obese (body mass index >30 kg/m2), or had any self-reported cardiovascular, metabolic or neurological diseases. Although otherwise healthy, a lack of biochemistry data to confirm health status is a recognized limitation of the study. Subjects refrained from alcohol and exercise 24 h, food 4 h, and caffeine 12 h before the study. Each subject had a urine specific gravity of <1.028 prior to testing. Written informed consent was obtained from all subjects before participating in this study. Study procedures and the informed consent were approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas.
Instrumentation and measurements
Each subject was dressed in a water-perfused, tube-lined suit (Med-Eng, Ottawa, Canada) that covered the entire body, except the head, face, hands, feet, and one forearm. The water-perfused suit permitted the control of skin and core temperature by changing the temperature of the water perfusing the suit. Core temperature was measured from an ingestible pill telemetry system (HQ, Palmetto, FL). The pill was ingested immediately on arrival at the laboratory, which was ~1 h before the onset of data collection and ~2 hours before the onset of the heat stress. Mean skin temperature was measured via the weighted average of six thermocouples attached to the skin (Taylor et al., 1989). Heart rate was obtained from an electrocardiogram (HP Patient Monitor, Agilent, Santa Clara, CA) interfaced with a cardiotachometer (CWE, Ardmore, PA). Arterial blood pressure was measured by auscultation of the brachial artery via electrosphygmomanometry (SunTech, Raleigh, NC). The blood pressure cuff was placed directly on the skin, underneath the water-perfused suit. Mean arterial blood pressure (MAP) was calculated as 1/3 pulse pressure + diastolic pressure.
Arterial stiffness was measured by pulse wave velocity (PWV) using Doppler ultrasound (LOGIQ e, GE Healthcare, Milwaukee, Wisconsin) (Laurent et al., 2006). PWV is the preferred method to evaluate arterial compliance (Laurent et al., 2006) given that PWV is proportional to the inverse of the square root of compliance; thus, PWV decreases as arterial compliance increases (Bramwell & Hill, 1922b, a). PWV was measured with Doppler ultrasound and calculated as the distance between measurement sites divided by the time delay between the two waveforms. Central PWV was calculated from the carotid and femoral arteries, while peripheral PWV was calculated from carotid and radial arteries (Laurent et al., 2006). All PWV measures were performed on the left side of the body with consistent probe location being assured by marking the skin. Because only one Doppler ultrasound probe was used, the R-wave of the ECG was used as a reference point to calculate the time delay between waveforms. Specifically, pulse transit time was calculated by subtracting the time between the peak of the R-wave and the foot of the carotid pulse from the time between the peak of the R-wave and the foot of the distal pulse (i.e., femoral for central PWV and radial for peripheral PWV) for at least ten consecutive cardiac cycles (Laurent et al., 2006). A potential limitation to the design is that the foot of the pulse wave was identified visually (versus computer aided) at the point of the systolic upstroke (Heffernan et al., 2007). Since measurements between sites occurred within a short time frame (~5 min), it is unlikely that there were any differences in the left ventricle isovolumic period between measurements, which would have otherwise affected measured pulse transit times (Laurent et al., 2006). Carotid and femoral measurement order was randomized between subjects. Distance between arterial measurement sites was calculated by subtracting the distance from the carotid location to the sternal notch from the distance between the sternal notch and the femoral or radial site (Laurent et al., 2006).
Experimental Protocol
After instrumentation, subjects were supine for approximately 30 min prior to normothermic measures so that body fluid shifts had stabilized (Pivarnik et al., 1986). Water at 34°C was perfused through the suit during this period. After this resting period, central and peripheral PWV were obtained. Subjects were then exposed to a passive heat stress by perfusing 49°C water through the suit. Measures of PWV began just prior to reaching a 0.5, 1.0, and 1.5°C elevation in core temperature from pre heat-stress baseline; this corresponded to 36 ± 5, 60 ± 11, and 77 ± 15 min of heat stress, respectively. Femoral measures occurred while briefly exposing the measurement site to room temperature (~26°C); while the carotid and radial measurement sites were exposed to room temperature throughout testing. Blood pressure was measured following the last PWV at each time point.
Statistical Analysis
Heart rate and skin and core temperature were sampled at 50 Hz via a data-acquisition (Biopac System, Santa Barbara, CA). These data were averaged at each time point over the period in which PWV measures occurred. Data were analyzed using SigmaStat 3.11 (Chicago, IL). A one-way repeated measures analysis of variance (ANOVA) was used to examine cardiovascular and thermoregulatory differences across core body temperatures. Post-hoc Bonferroni follow-up t-tests were conducted if a significant main effect was identified. A Pearson Product Moment Correlation was calculated for the change in heart rate with heat stress versus change in central and peripheral PWV. A Pearson Product Moment Correlation also was calculated for the change in central and peripheral PWV versus the respective normothermic baseline PWV. All data are reported as mean ± SD. Significance was set at P < 0.05.
The within-subject coefficients of variation for the time between the peak of the R-wave and the foot of the carotid, femoral, and radial artery pulse wave over ten cardiac cycles were 3.3, 2.1, and 1.4%, respectively. When subjects’ baseline PWV were examined on two different occasions on the same day, the coefficient of variation was 5.7%, and the correlation coefficient between measures was 0.86, a value similar to others (Heffernan et al., 2007). With an alpha set 0.05, a power of 0.8, and standard deviation of 44 cm/s, eight subjects were sufficient to detect a 55 cm/s difference in PWV, a similar acute change observed by others (Kingwell et al., 1997).
RESULTS
Core temperature increased 0.47 ± 0.05, 1.03 ± 0.12, and 1.52 ± 0.07°C from baseline to each measurement period (P < 0.05). Likewise skin temperature and heart rate increased over time (P < 0.05; Table 1). The only change in mean arterial pressure occurred from baseline to +0.5°C (P < 0.05). Diastolic blood pressure decreased over the same temperature change (P< 0.05; Table 1).
Table 1.
Thermal and hemodynamic responses at normothermia and after subjects’ core temperatures were increased approximately 0.5, 1.0, and 1.5°C.
| Normothermia | +0.5°C | +1.0°C | +1.5°C | |
|---|---|---|---|---|
| Mean Skin Temperature (°C) | 34.2 ± 0.6 | 38.0 ± 0.4* | 38.6 ± 0.4* | 38.9 ± 0.3 |
| Core Temperature (°C) | 37.16 ± 0.16 | 37.63 ± 0.15* | 38.18 ± 0.20* | 38.67 ± 0.18* |
| Mean Arterial Pressure (mmHg) | 82 ± 5 | 77 ± 5* | 81 ± 5 | 81 ± 6 |
| Systolic Blood Pressure (mmHg) | 111 ± 10 | 109 ± 9 | 115 ± 10 | 115 ± 11 |
| Diastolic Blood Pressure (mmHg) | 68 ± 4 | 62 ± 6* | 64 ± 3 | 63 ± 6 |
| Heart Rate (beats/min) | 58 ± 6 | 84 ± 11* | 99 ± 10* | 108 ± 13* |
Significant difference from the prior temperature stage (P < 0.05).
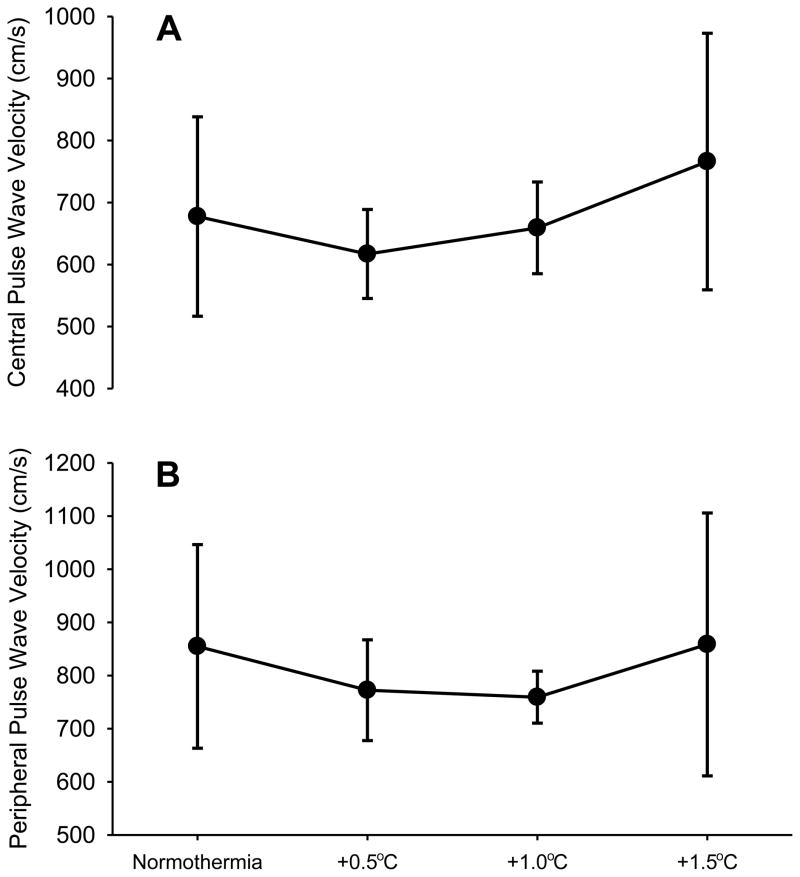
Averaged central PWV did not change from baseline to 0.5, 1.0, nor 1.5°C increases in core temperature (P = 0.12, Figure 1A). Similarly, averaged peripheral PWV did not change between these core body temperatures (P = 0.56; Figure 1B). When analyzed as percent change from baseline, the results were consistent with absolute values (data not shown). Correlation analysis confirmed that changes in central (r = 0.04, P = 0.84) and peripheral (r = 0.20, P = 0.28) PWV were independent of core temperature. Increased variability in PWV between subjects was due to one subject whose responses were greater than 2SD from the mean. When those observations were removed and the data re-analyzed, the findings were consistent with that reported with the entire group and thus this individual’s data were included throughout.
Figure 1.
Mean ± SD central (A) and peripheral (B) pulse wave velocity at baseline normothermia and after core temperature increases of 0.5, 1.0, and 1.5°C. There were no significant differences across temperatures.
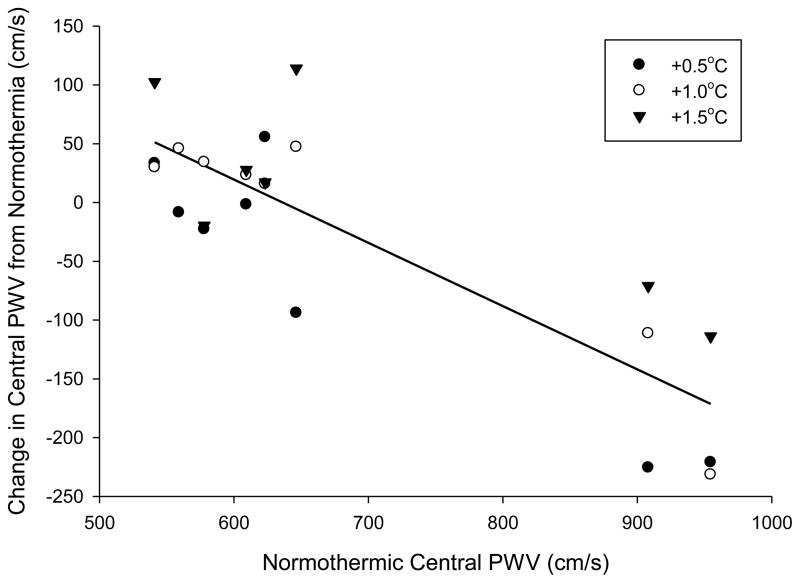
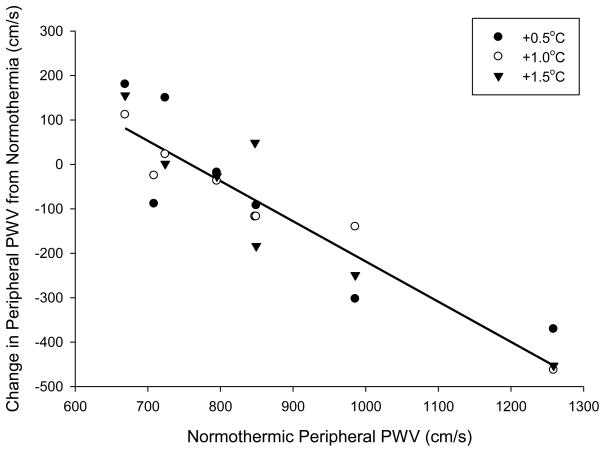
There were no significant correlations between the change in heart rate and change in central (r = 0.10, P = 0.640) or peripheral (r = 0.02, P = 0.94) PWV. However, the change in central PWV when core temperature was increased 0.5°C (r = −0.93, P < 0.001), 1.0°C (r = −0.94, P < 0.001), and 1.5°C (r = −0.80, P = 0.03) was significantly correlated with normothermic central PWV (Figure 2). Likewise the change in peripheral PWV when core temperature was increased 0.5°C (r = −0.88, P = 0.004), 1.0°C (r = −0.97, P < 0.001), and 1.5°C (r = −0.93, P = 0.002) was significantly correlated with normothermic peripheral PWV (Figure 3).
Figure 2.
Correlation between central pulse wave velocity (PWV) at normothermia versus the change in central PWV after core temperature increases of 0.5, 1.0, and 1.5°C. The correlation was significant at +0.5°C (r = −0.93, P < 0.001), +1.0°C (r = −0.94, P < 0.001), and +1.5°C (r = −0.80, P = 0.03). Line of best fit is the average of all temperature responses. The addition of an outlier datapoint with a baseline PWV of 559 cm/s and change in PWV at +1.5°C of 652 cm/s does not change the significance of the statistical outcome.
Figure 3.
Correlation between peripheral pulse wave velocity (PWV) at normothermia versus the change in peripheral PWV after core temperature increases of 0.5, 1.0, and 1.5°C. The correlation was significant at +0.5°C (r = −0.88, P = 0.004), +1.0°C (r = −0.97, P < 0.001), and +1.5°C (r = −0.93, P = 0.002). Line of best fit is the average of all temperature responses. The addition of an outlier datapoint with a baseline PWV of 709 cm/s and change in PWV at +1.5°C of 737 cm/s yields a non-significant correlation (r = −0.53, P = 0.18).
DISCUSSION
In vitro evidence suggests that direct heating of isolated arteries increases arterial compliance (Mitchel et al., 1994). Given these findings, coupled with the observation that increases in shear stress are capable of modulating arterial elasticity (Kinlay et al., 2001), we tested the hypothesis that elevations in core temperature decrease central and peripheral arterial stiffness. Averaged central and peripheral arterial stiffness were unaffected by increases in core temperature up to 1.5°C. However, individual changes in central and peripheral arterial stiffness were negatively correlated with baseline normothermic arterial stiffness; that is, the largest decreases in arterial stiffness with heating occurred in the individuals with the highest baseline stiffness.
Arterial elasticity, and thus stiffness, has passive and active elements. The structural composition of the artery wall provides the passive component, and it is unlikely that moderate heat stress (or other acute perturbations) affects this component. The active component is related to arterial tone that can be changed by acute perturbations (Kinlay et al., 2001). The present data show that changes in central and peripheral arterial stiffness during passive heating are predicated on baseline stiffness. Individuals with increased normothermic arterial stiffness had a greater decrease in stiffness when hyperthermic versus those with lower baseline arterial stiffness (Figures 2 and 3). It is unknown why hyperthermia affects vessels differently depending on baseline tone. It may be that the reserve to further increase vascular compliance in an already compliant bed is diminished, resulting in the greatest increases in vascular compliance to heat stress in the more stiff vessels. It also is possible that shear stress related factors leading to increased compliance are countered by artery stiffening due to increases in sympathetic activity known to occur with heat stress (Niimi et al., 1997; Cui et al., 2002; Cui et al., 2004). This hypothesis is supported by findings that increases in sympathetic activity are associated with decreases in arterial compliance (Swierblewska et al., 2010). However, given the present findings, if this hypothesis is correct then modulation of arterial stiffness by sympathetic activity would be different between individuals having higher and lower normothermic PWV. Although not measured in the present study, it may also be that the magnitude of the elevation in sympathetic activity to heat stress was greater in the individuals with the highest normothermic arterial compliance.
Hyperthermia increases blood flow through the skin and associated conduit arteries, which would increase shear stress and promote the release of nitric oxide and/or cytochrome-related hyperpolarizing factors (Kinlay et al., 2001; Bellien et al., 2010); both proposed to decrease arterial stiffness (Kellogg et al., 1998; Kinlay et al., 2001; Kellogg, 2006; Kooijman et al., 2008). Although unlikely, we cannot exclude the possibility that in the less stiff more compliant vessels while normothermic, the magnitude of the release of nitric oxide and/or cytochrome-related hyperpolarizing factors was not adequate, or the arteries were not sufficiently sensitive to these substances to cause changes in arterial tone to heat stress.
By definition, vessel compliance relates the change in volume for a given change in pressure. A more compliant vessel will have a greater increase in volume for a given increase in pressure, while the opposite will be the case for a less compliant vessel (O’Rourke et al., 2002). Because of the curvilinear nature of the compliance curve, the operating point on the compliance curve influences the effect of an acute perturbation on vessel compliance. For example, cold stress acutely decreases compliance in older individuals, who have a decreased baseline arterial compliance compared to their younger counterparts (Hess et al., 2009). Similarly, the effect of heat stress on arterial stiffness was dependent on normothermic values. Although we did not have adequate power to statistically compare the effects of age, it is possible the correlation between baseline stiffness and changes in PWV with heating was related to aging, given that as a group older individuals have decreased arterial compliance while normothermic (Vaitkevicius et al., 1993). Nevertheless, based upon the present findings older individuals with decreased baseline compliance may have a greater capacity to increase compliance with heating.
Acute changes in arterial compliance and blood pressure are inter-related, such that blood pressure changes may lead to, or be the result of, changes in arterial compliance (Kinlay et al., 2001). For example, an acute perturbation such as cold stress increases blood pressure which occurs in concert with an acute decrease in arterial compliance (Hess et al., 2009). Changes in peripheral and central arterial compliance were not observed in subjects in which cold stress minimally affected blood pressure (Hess et al., 2009). Although heat stress resulted in a small drop in mean arterial pressure (Table 1), it is unlikely that the magnitude of this decrease was sufficient to influence PWV (Hess et al., 2009). Further, at greater increases in core temperature (i.e., +1.0 and +1.5°C), when mean arterial pressure was similar to baseline, average PWV remained unchanged, and the correlation between baseline PWV and change in PWV was independent of degree of heat stress.
The current findings have important implications for future research. First, these data support the notion that changes in arterial compliance immediately following exercise (Kingwell et al., 1997) have the potential to be dependent on internal temperature. Second, technology such as beat-to-beat measures of cardiac output with Modelflow assumes arterial compliance is not changing by an acute perturbation (Wesseling et al., 1993). We recently observed that on average Modelflow underestimates cardiac output during heat stress (Shibasaki et al., 2011). Based on the present findings, the source of this error may be related to inter-subject variability in heat stress-induced changes in aortic compliance, with the individuals having the highest normothermic vascular stiffness perhaps being the ones with the largest changes in aortic compliance to heat stress and thus the greatest error in the Modelflow readings.
Limitations to the interpretation of the data
Whole-body heat stress causes pronounced increases in heart rate (see Table 1). Increases in heart rate via cardiac pacing independently increase PWV, indicative of decreased arterial compliance (Liang et al., 1999; Lantelme et al., 2002). Alternatively, decreases in arterial compliance that occur during conditions that cause tachycardia may be related to increases in sympathetic neural activity that contributed to the tachycardic response; although this effect would not explain the aforementioned effects of cardiac pacing on arterial compliance. Equally possible is that increased heart rate shortens the time available for vessel recoil and leads to vessel stiffening and thus decreased compliance (Lantelme et al., 2002). Given these observations, it is possible that in the present study heat stress consistently decreased arterial stiffness in all subjects, but this response was masked and/or offset by the effect of increases in heart rate on recoil time reducing compliance; the net effect being no change in arterial stiffness. However, tachycardia-induced changes in compliance occur when heart rate is greater than 120 beats per minute (Callaghan et al., 1984), which is less than the average heart rates in the current study (Table 1). These findings, coupled with an absence of a correlation between change in heart rate and changes in central and peripheral PWV, it is unlikely that heat stress induced increases in heart rate influenced PWV.
The relationship of PWV to arterial compliance is described by the Bramwell-Hill equation where arterial compliance= 1/(PWV^2 * blood viscosity) (Bramwell & Hill, 1922b), and thus changes in blood viscosity have the potential to affect arterial compliance measures independent of PWV. That said, there is no known evidence to suggest that the relatively minor increase in blood temperature with passive heating (i.e. 1.5°C) is sufficient to decrease blood viscosity to the point where physiological significant increases in arterial compliance occur in the absence of changes in PWV. Secondly, the loss of hypotonic sweat throughout heating may increase blood density sufficient to increase viscosity, which would counter a direct affect of increases in blood temperature in potentially decreasing blood viscosity (du Nouy, 1929; Merrill et al., 1963). Therefore it is reasonable to assume that in this study PWV changes reflected arterial compliance.
It is recognized that passive heating is not the same as exercise-induced increases in core body temperature. However the present protocol increased core body temperature 1.5°C, an increase similarly observed after ~30 min of treadmill exercise at 70% VO2max in 22°C, 37% relative humidity (Gregson et al., 2002). It is possible that changes in arterial stiffness following similar exercise (Kingwell et al., 1997; Heffernan et al., 2007) are a due to a combination of metabolites produced during exercise, increased core body temperature, and baseline arterial stiffness.
In conclusion, using PWV as a measure of arterial stiffness, the present data show that core temperature increases up to 1.5°C above baseline with passive heating do not affect average peripheral or central arterial stiffness from a sample of individuals of varying ages. However, the subjects with the greatest normothermic arterial stiffness generally were the ones with the greatest decreases in stiffness to the heat stress. The present findings support in vitro observations where pronounced, and non-physiological, heating of arteries increased arterial compliance. Secondly, these observations suggest elevations in core temperature may, in part, explain previously observed post-exercise increases in arterial compliance. Lastly, prior observations of inaccuracies in Modelflow derived cardiac output may be due to the variable changes in arterial stiffness that occur during heat stress (Shibasaki et al., 2011).
Acknowledgments
We would like to thank the volunteers for their time. The assistance of Kim Hubing, MS and Jena Langlois, RN is appreciated. This study was supported by NIH Grants HL61388 & HL84072.
References
- Bellien J, Favre J, Iacob M, Gao J, Thuillez C, Richard V, Joannides R. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension. 2010;55:674–680. doi: 10.1161/HYPERTENSIONAHA.109.142190. [DOI] [PubMed] [Google Scholar]
- Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proceedings of the Royal Society of Medicine. 1922a;93:298–306. [Google Scholar]
- Bramwell JC, Hill AV. Velocity of transmission of the pulse-wave and elasticity of arteries. Lancet. 1922b;i:891–892. [Google Scholar]
- Callaghan FJ, Babbs CF, Bourland JD, Geddes LA. The relationship between arterial pulse-wave velocity and pulse frequency at different pressures. Journal of medical engineering & technology. 1984;8:15–18. doi: 10.3109/03091908409032067. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R252–258. doi: 10.1152/ajpregu.00337.2001. [DOI] [PubMed] [Google Scholar]
- Cui J, Zhang R, Wilson TE, Crandall CG. Spectral analysis of muscle sympathetic nerve activity in heat-stressed humans. American journal of physiology. Heart and circulatory physiology. 2004;286:H1101–H1106. doi: 10.1152/ajpheart.00790.2003. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Kraemer WJ, Bush JA, Doughty TA, Kim D, Mullen KM, Ramsey K. Biorhythmic influences on functional capacity of human muscle and physiological responses. Med Sci Sports Exerc. 1998;30:1399–1407. doi: 10.1097/00005768-199809000-00008. [DOI] [PubMed] [Google Scholar]
- du Nouy PL. The viscosity of blood serum, as a function of temperature. the Journal of general physiology. 1929;12:363–377. doi: 10.1085/jgp.12.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson WA, Drust B, Cable NT. The effects of pre-warming on the metabolic and thermoregulatory responses to prolonged submaximal exercise in moderate ambient temperatures. European Journal of Applied Physiology. 2002;2002:526–533. doi: 10.1007/s00421-002-0580-x. [DOI] [PubMed] [Google Scholar]
- Heffernan KS, Collier SR, Kelly EE, Jae SY, Fernhall B. Arterial stiffness and baroreflex sensitivity following bouts of aerobic and resistance exercise. Int J Sports Med. 2007;28:197–203. doi: 10.1055/s-2006-924290. [DOI] [PubMed] [Google Scholar]
- Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol. 2009;107:1076–1082. doi: 10.1152/japplphysiol.00605.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100:1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol. 2003;94:1971–1977. doi: 10.1152/japplphysiol.00826.2002. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Berry KL, Cameron JD, Jennings GL, Dart AM. Arterial compliance increases after moderate-intensity cycling. Am J Physiol. 1997;273:H2186–2191. doi: 10.1152/ajpheart.1997.273.5.H2186. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–1053. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol. 2008;586:1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. doi: 10.1161/01.hyp.0000019132.41066.95. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Liang YL, Gatzka CD, Du XJ, Cameron JD, Kingwell BA, Dart AM. Effects of heart rate on arterial compliance in men. Clin Exp Pharmacol Physiol. 1999;26:342–346. doi: 10.1046/j.1440-1681.1999.03039.x. [DOI] [PubMed] [Google Scholar]
- Merrill EW, Gilliland ER, Cokelet G, Shin H, Britten A, Wells RE., Jr Rheology of human blood, near and at zero flow. Effects of temperature and hematocrit level. Biophys J. 1963;3:199–213. doi: 10.1016/s0006-3495(63)86816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel JF, Fram DB, Aretz TA, Gillam LD, Woronick C, Waters DD, McKay RG. Effect of low-grade conductive heating on vascular compliance during in vitro balloon angioplasty. Am Heart J. 1994;128:21–27. doi: 10.1016/0002-8703(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Naka KK, Tweddel AC, Parthimos D, Henderson A, Goodfellow J, Frenneaux MP. Arterial distensibility: acute changes following dynamic exercise in normal subjects. American journal of physiology. Heart and circulatory physiology. 2003;284:H970–978. doi: 10.1152/ajpheart.00529.2002. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–444. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- Pivarnik JM, Goetting MP, Senay LC., Jr The effects of body position and exercise on plasma volume dynamics. Eur J Appl Physiol Occup Physiol. 1986;55:450–456. doi: 10.1007/BF00422750. [DOI] [PubMed] [Google Scholar]
- Rakobowchuk M, Stuckey MI, Millar PJ, Gurr L, Macdonald MJ. Effect of acute sprint interval exercise on central and peripheral artery distensibility in young healthy males. Eur J Appl Physiol. 2009;105:787–795. doi: 10.1007/s00421-008-0964-7. [DOI] [PubMed] [Google Scholar]
- Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. Journal of Applied Physiology. 1966;21:1757–1762. doi: 10.1152/jappl.1966.21.6.1757. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Bundgaard-Nielsen M, Seifert T, Secher NH, Crandall CG. Modelflow underestimates cardiac output in heat stressed individuals. Am J Physiol Reg. 2011;300:R486–491. doi: 10.1152/ajpregu.00505.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara J, Komine H, Hayashi K, Yoshizawa M, Yokoi T, Otsuki T, Shimojo N, Miyauchi T, Maeda S, Tanaka H. Effect of systemic nitric oxide synthase inhibition on arterial stiffness in humans. Hypertens Res. 2007;30:411–415. doi: 10.1291/hypres.30.411. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Otsuki T, Tanabe T, Maeda S, Kuno S, Ajisaka R, Matsuda M. The effects of low-intensity single-leg exercise on regional arterial stiffness. Jpn J Physiol. 2003;53:239–241. doi: 10.2170/jjphysiol.53.239. [DOI] [PubMed] [Google Scholar]
- Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Boutouyrie P, Somers VK, Narkiewicz K. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens. 2010;28:979–984. doi: 10.1097/hjh.0b013e328336ed9a. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- Watson G, Judelson DA, Armstrong LE, Yeargin SW, Casa DJ, Maresh CM. Influence of diuretic-induced dehydration on competitive sprint and power performance. Med Sci Sports Exerc. 2005;37:1168–1174. doi: 10.1249/01.mss.0000170100.93279.be. [DOI] [PubMed] [Google Scholar]
- Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]