Abstract
Context
Uterine decidualization is critical to embryonic implantation and sustained pregnancy.
Objective
To evaluate the role of gap junction intercellular communications and connexin (Cx) proteins in the morphological and biochemical differentiation of decidualized human endometrial stromal cell (ESC) cultures.
Design
Translational cell biological study.
Setting
Academic medical center.
Patients
Endometrial tissue was provided by five healthy reproductive age women on no hormonal medication, undergoing laparoscopy in the early proliferative phase of the menstrual cycle.
Interventions
Endometrial biopsy under general anesthesia, establishment and decidualization of ESC with 10 nM 17β-estradiol, 100 nM progesterone and 0.5 mM dibutyryl cAMP (E/P/c), and manipulation of gap junctions in vitro via a combination of pharmacological or transgenic approaches.
Main Outcome Measures
Decidualized ESC evaluated morphologically for epithelioid transformation, gap junctions by dye diffusion and Cx43, prolactin, VEGF and IL-6 expression by RT-PCR, Western and ELISA methods.
Results
Cx43 accumulation and functional gap junctions between decidualized ESC increase concomitantly with morphological differentiation following E/P/c treatment. Disruption of gap junctions using pharmacological inhibitors or Cx43 shRNA prevents morphological differentiation and inhibits prolactin and VEGF secretion. By contrast, IL-6 secretion from decidualized ESC is augmented by both approaches.
Conclusions
The findings suggest that decidualized ESC function as a coordinated secretory organ to regulate embryonic implantation via intercellular cooperation mediated by gap junctions. When adjacent cells can communicate through these junctions, decidual prolactin and VEGF secretion appears to be optimized for vascular development of the placental bed. Conversely, when intercellular communications are disrupted, angiogenesis is impaired and an inflammatory state is induced.
Keywords: gap junction intercellular communication, connexin, prolactin, VEGF, interleukin-6
INTRODUCTION
Invasive hemochorial placentation, as observed in mice, rats, subhuman primates and women, triggers an endometrial stromal response referred to as decidualization. This is defined as the differentiation of endometrial mesenchyme to decidual stroma with distinct morphological and biochemical characteristics. Decidualized stromal cells accumulate glycogen and take on a plump, epithelioid appearance. Ultrastructurally, club-shaped lamellar processes between adjacent cells interdigitate extensively and form clustered microdomains called gap junctions (Lawn et al., 1971) that allow adjacent cells to directly share ions and small molecules <1 kD in size (Yamasaki et al., 1999). In rodents, endometrial stromal gap junctions are absent until a deciduogenic stimulus is provided (Orlando-Mathur et al., 1996). Gap junctions are found in almost all mammalian tissues except circulating blood cells and adult skeletal muscle and are comprised of tetrahelical, membrane-spanning connexin (Cx) proteins ranging in size from 26 to 56 kD that surround an aqueous pore. The human genome encodes 21 Cx family proteins (Söhl & Willecke, 2003), of which the predominant Cx isoform in endometrial stroma is 43 kD (Cx43). This protein also is referred to as “gap junction α-1 protein,” especially in microarray annotation lists (Piao et al., 2006). In mouse and rat decidua, Cx43 is upregulated by estrogen (E) and expressed prior to placental invasion (Pauken & Lo, 1995; Grümmer et al., 1996). The temporospatial expression of Cx43 in human endometrium is controversial. Jahn et al. (1995) observed maximal Cx43 staining in endometrial stromal cells during the E-dominant periovulatory period, whereas Granot et al. (2000) noted an increase in Cx43 during the mid-late secretory phase when both E and progesterone (P) levels are elevated. Human endometrial stromal cells in culture express functional Cx43 proteins and generate gap junction intracellular connections (Tanmahasamut & Sidell, 2005).
To identify steroid-regulated gene networks with functional relevance to embryonic implantation, we utilized a model in which implantation is activated by a single administration of E to ovariectomized, P-primed pregnant mice (Mantena et al., 2006). Gene expression profiling using Affymetrix microarrays (430 2.0 Array) revealed that Cx43 increased markedly after hormone induction in the delayed implantation model (Bagchi & Bagchi, unpublished results). Cx43 protein expression in mouse uterus during normal pregnancy, was undetectable by immunohistochemistry in undifferentiated stromal cells during the preimplantation period. However, on day 5 of pregnancy, within 12 h of the initiation of implantation, a marked induction in Cx43 protein expression was observed in the primary decidual zone immediately surrounding the implanting embryo (Laws et al., 2008). Moreover, in a conditional knockout of the Cx43 gene in adult mouse uterus, created by employing the Cre-LoxP strategy under the control of the progesterone receptor (PR) promoter (Kim et al., 2008), mice with deleted Cx43 alleles exhibited severe fertility defects associated with failure to express decidual markers. An unexpected finding was that the uterine decidua in these mice also failed to undergo angiogenesis characteristic of the peri-implantation period (Laws et al., 2008). The goal of the current study was to evaluate, independently of embryonic development, the significance of Cx43 and gap junctions in primary human endometrial stromal cells subjected to in vitro decidualization. Our findings indicate that Cx43 and functional gap junctions are required for the morphological and biochemical changes induced during endometrial stromal cell differentiation, including effects on angiogenic and proinflammatory proteins.
MATERIALS AND METHODS
Tissues and cells
Source of human tissues
Endometrial tissue specimens were obtained from patients undergoing laparoscopy for tubal sterilization after providing written informed consent under a study protocol approved by the institutional review board at Emory University School of Medicine. Five healthy ovulatory women, who had not received hormonal therapy for at least three months before surgery, were recruited. Endometrial biopsies were collected under sterile conditions and transported to the laboratory on ice in phosphate buffered saline (PBS). All endometrial biopsies were performed in the early proliferative cycle phase to minimize effects from endogenous luteal phase progesterone, and histology was consistent with the subjects’ menstrual dating. Specimens used in the current study were obtained from women without evidence of endometriosis or other pelvic pathology.
Human endometrial cell culture conditions and in vitro decidualization
Endometrial stromal cells (ESC) were prepared from endometrial biopsies, as we have described previously (Ryan et al., 1994). After collagenase digestion, glandular epithelial cells were separated from ESC and debris by filtration through narrow gauge sieves. ESC were subcultured at least twice to eliminate contamination by macrophages or other leukocytes and were used before the sixth passage for all experiments. In prior studies from our laboratory, we extensively characterized ESC cultures prepared using this protocol and confirmed that they are more than 95% pure and retain functional estrogen and progesterone receptors, as well as other phenotypic endometrial markers for at least five passages in vitro (Ryan et al., 1994). The cells were cultured in 24-well plates or 6 cm dishes depending on the requirements of the assays, as described below.
ESC were grown to 60–80% confluence in phenol red-free medium (DMEM/Ham’s F-12, cat# 10-092cv, CellGro, Manassas, VA) supplemented with 5% charcoal-stripped fetal calf serum and then treated with a combination of 10 nM 17β-estradiol, 100 nM progesterone and 0.5 mM dibutyryl cAMP (decidualization cocktail, “E/P/c”) or solvent control. Half of the culture medium was removed and replaced every two days for up to 8 d, and after treatment the cultures were allowed to incubate for an additional 48 h before the morphology of the cells was assessed, and prolactin was measured in the supernatant (ELISA kits from Alpha Diagnostic International, San Antonio, TX) as a biochemical marker of decidualization (Ramathal et al., 2010). Figures reflect representative experiments performed in triplicate and each experiment was repeated a minimum of three independent times with similar results.
Protein and mRNA analyses
Western blot analyses
Western blot analyses were performed on whole-cell extracts obtained from direct dissolution of cells by vortexing in cell extraction buffer (cat# FNN0011, BioSource Intl., Camarillo, CA), followed by protein determination using a bicinchoninic acid protein assay kit (Sigma Chemical Co.). Protein (30 μg) from cells treated with medium (control), or E/P/c was loaded onto 12% SDS-PAGE gels, then transferred to PVDF membranes and blocked with 5% skim milk in PBS. Total Cx43 was detected using the rabbit polyclonal anti-Cx43 antibody (1:1000 dilution, cat# 3512, Cell Signaling Technology, Danvers, MA), then incubated with secondary antibody linked to horseradish peroxidase and visualized by chemiluminescent detection. Cx26 was detected using rabbit antibodies (O-24, 1:1000 dilution) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Secreted prolactin and VEGF protein isoforms were assayed in cell culture supernatants that were concentrated 100-fold using Amicon Ultracel-3K centrifugal filters. Prolactin was detected using mouse monoclonal antibodies (A-7, 1:500 dilution, cat# 46698, Santa Cruz); VEGF was detected with mouse monoclonal anti-VEGF antibody (C-1, 1:500 dilution, cat# SC-7269, Santa Cruz). The immunoreactive bands were visualized by the Enhanced Chemiluminescence (ECL) System (Amersham Pharmacia Biotech, Piscataway, NJ). Blots were washed, reprobed with mouse monoclonal anti-human β-actin antibodies (1:1000 dilution, cat# 31430, Sigma, St. Louis, MO), and developed in an identical manner to ensure even loading. Molecular weight standards were used to verify the identity of immunopositive bands. In Fig. 1A, MagicMark XP® Western protein standards from Invitrogen (cat# LC5602) were utilized and these revealed faint signals following secondary antibody and ECL reactions. In the other Western blots, SeeBlue Plus2® pre-stained standards (cat# LC5925, Invitrogen), which are not detected by ECL, were used to calibrate the migration of proteins on the original gel and the size of each band is indicated in the figure.
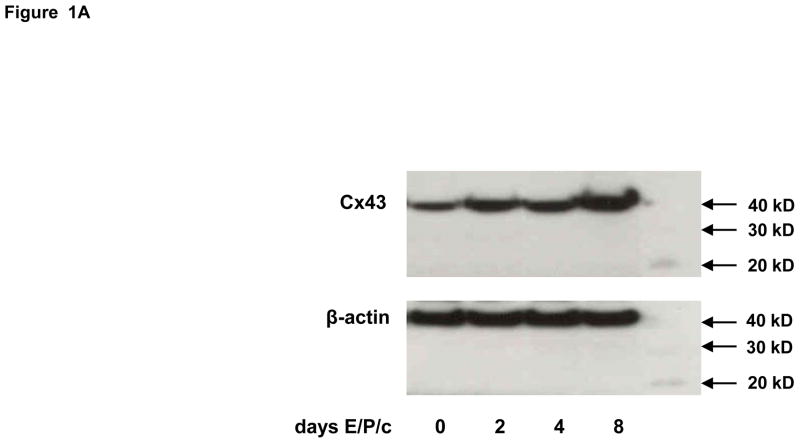
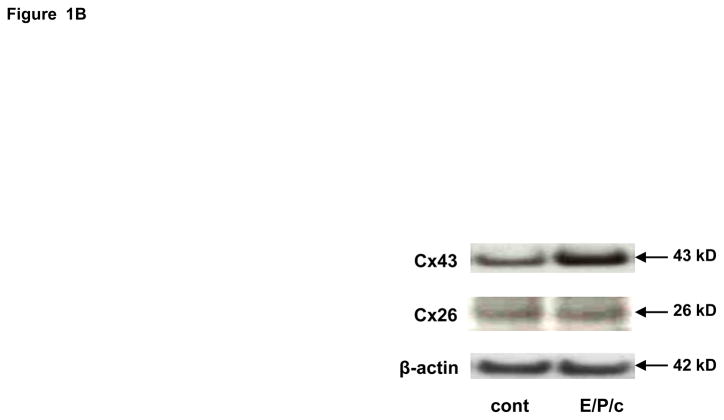
Figure 1.
(A) Time course of Cx43 induction. Endometrial stromal cells (ESC) were cultured for 0, 2, 4 and 8 days in the presence of 10 nM estradiol, 100 nM progesterone and 0.5 mM dibutyryl cAMP (E/P/c). Western blotting as described allowed quantification of Cx43 (43 kD MW) and β-actin (42 kD MW). Molecular weight standards, faintly ECL positive, are shown in the right lane. (B) ESC cultured for 8 days in the absence (cont) or presence of hormones (E/P/c) as above were lysed and probed for Cx43, Cx26 and β-actin, with corresponding molecular weights as indicated at right..
ELISA
Prolactin in ESC-conditioned medium was quantified using a commercial ELISA kit (Alpha Diagnostic International, San Antonio, TX). The assay was linear over a concentration of 1–100 ng/ml. All assays were performed in triplicate and each experiment was repeated a minimum of three times with cells derived from different subjects. Results are reported as ng/106 cells/48 h. VEGF protein secreted into ESC-conditioned media was measured using a commercial ELISA kit (Human VEGF Quantikine, R&D Systems, Minneapolis, MN) that was validated previously in our laboratory (Peeters et al., 2005). The assay was sensitive to VEGF levels of 15 pg/ml and linear from 15–900 pg/ml. Results are reported as pg/106 cells/48 h. Interleukin (IL)-6 concentrations in ESC-conditioned media were analyzed using commercially available ELISA kits from R&D Systems. The assay has a sensitivity of 200 pg/ml and is linear from 0.2–200 ng/ml. Results are reported as pg/106 cells/48 h. ELISA plates were read at 450 nm wavelength using an HtII ELISA plate reader (Anthos Labtec Instruments, Salzburg, Austria) with the correction wavelength set at 570 nm. All assays were performed in triplicate and each experiment was repeated a minimum of three times with cells derived from different subjects.
RNA isolation, RT-PCR, and real-time PCR
Total RNA from tissues was extracted using PureLink™ Micro-to-midi Total RNA Purification Systems (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. Concentration and purity of the total RNA samples were measured using the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE). cDNA was synthesized using Superscript™ First-Strand Sythesis System for RT-PCR (Invitrogen) according to the manufacturer’s methodology and used as template for conventional and real-time PCR assays. Amplicons were visualized on 1% agarose gels containing 0.2 μg/μl ethidium bromide. A 100-bp ladder (Invitrogen) was used as a size standard. Primers for amplification were synthesized by Operon (Alameda, CA) based on the following sequences: Cx43 sense 5′-TACCATGCGACCAGTGGTGCGCT-3′ and Cx43 antisense GAATTCTGGTTATCATCGGGGAA-3′ (292-bp amplicon); VEGF sense 5′-GCACCCATGGCAGA-3′ and antisense 5′-GCTGCGCTGATAGA-3′(72 bp amplicon); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense 5′-GAGTCAACGGATTTGGTCGT-3′ and antisense 5′-GACAAGCTTCCCGTTCTCAG-3′ (185-bp amplicon). For real-time quantitative PCR, we used 2× SYBR Green Supermix (Bio-Rad, Hercules, CA) and followed the vendor’s guidelines with some modifications. A total reaction volume of 20 μl contained 10 μl 2× SYBR Green, 1 μl 50 mM MgCl2 and 1 μl primer mix. For Cx43 and VEGF, 5 μl cDNA were used, and 3 μl cDNA were used for GAPDH. Samples were processed using Opticon Monitor3 software (Bio-Rad Hercules, CA) under the following conditions: one denaturation cycle of 95°C, 15 min followed by 32 amplification cycles of 95°C, 10 sec, 60°C, 15 sec, and 72°C, 40 sec. PCR was run in an Opticon2 real-time thermocycler (Bio-Rad) as described elsewhere (Sidell et al., 2010). The data were analyzed after normalization to GAPDH, using the formula 2ΔΔct, where ct is the cycle threshold. The following formula was used to arrive at the fold decrease in Cx43 and VEGF mRNA level for each sample:
Where C=ct for Cx43, VEGF or GAPDH mRNA measured in control samples, and T=ct for Cx43, VEGF or GAPDH mRNA quantified in treated samples. Melting curve analysis of each sample reveal sharp, single amplicon peaks and these were supplemented with agarose gel electrophoresis of randomly selected samples to confirm the success of amplification reactions. Fluorescence spectra were recorded during the annealing phase of each reaction. Second derivative analysis of the amplification curves established the threshold cycles for each sample.
Functional assays of gap junctions
Pharmacological blockers of gap junctions
Two established, chemically unrelated gap junction-blockers were selected for these studies (Schiller et al., 2001; Contreras et al., 2002). 18α-glycyrrhetinic acid (AGA) and octanol (OcOH) were obtained from Sigma (St. Louis, MO). The two compounds were evaluated in preliminary studies over wide concentration ranges of 30–1000 μM and 0.1–3.0 mM, respectively, and optimal concentrations (without evidence of cell toxicity) were established. The compounds were concurrently added with the hormone cocktail (or control medium) and included in the incubation medium for up to 8 days during in vitro decidualization and had no effects on cell viabililty under the optimized conditions of the assays reported here (50 μM and 1.5 mM, respectively). Equimolar concentrations of glycyrrhizic acid (GA, 50 μM) and ethanol (EtOH, 1.5 mM), non-inhibitory glycoside and alcohol, respectively, likewise showed no toxic effects and served as controls for each of the two gap junction blocking compounds.
Dual-label cell coupling assay
Donor ESC were double labeled with the green fluorescent dye calcein (gap junction-permeable, Molecular Probes, Carlsbad, CA) and the red fluorescent dye Di-I (gap junction-impermeable, Molecular Probes) as described previously (Laws et al., 2008). The labeled cells were washed and then placed in contact with unlabeled ESC in a monolayer at a ratio of 1:8. Dye transfer was visualized by fluorescence microscopy after 2 hours at 37 C. The cells that fluoresce both green (calcein) and red (Di-I) are the dual-loaded donor cells, whereas those fluorescing only green (typically more diffuse in intensity) indicate originally unlabeled ESC in the monolayer, now demonstrating functional gap junction coupling. The latter cells were quantified per high-power field in six random fields per 6 cm plate and the mean numbers of two independent observers are presented. These experiments were performed after 4 days of E/P/c treatment, when Cx43 upregulation was approximately half-maximal (Fig. 1A).
Transgenic assays
Transient transfection of VEGF promoter construct
A 3019-bp human VEGF promoter-firefly luciferase reporter vector containing the complete 5′ UTR was constructed in pGL2 as described previously (Mueller et al., 2000). ESC were seeded at a density of 4 × 104 cells per well in six-well dishes and grown to 75% confluence. After 8 d of incubation in the absence or presence of E/P/c, cells were transiently transfected for another 24 h with 3 μl of Lipofectamine 2000 lipofection reagent (Invitrogen, Carlsbad, CA) as described previously (Sidell et al., 2010). The preparation of cell extracts and measurement of luciferase activities were carried out using the Dual-Luciferase Reporter kit according to recommendations of the manufacturer (Promega Corp., Madison, WI). Data are presented as mean (± SEM) relative light units (RLU)/103 cells.
Cx43 shRNA knockdown experiments
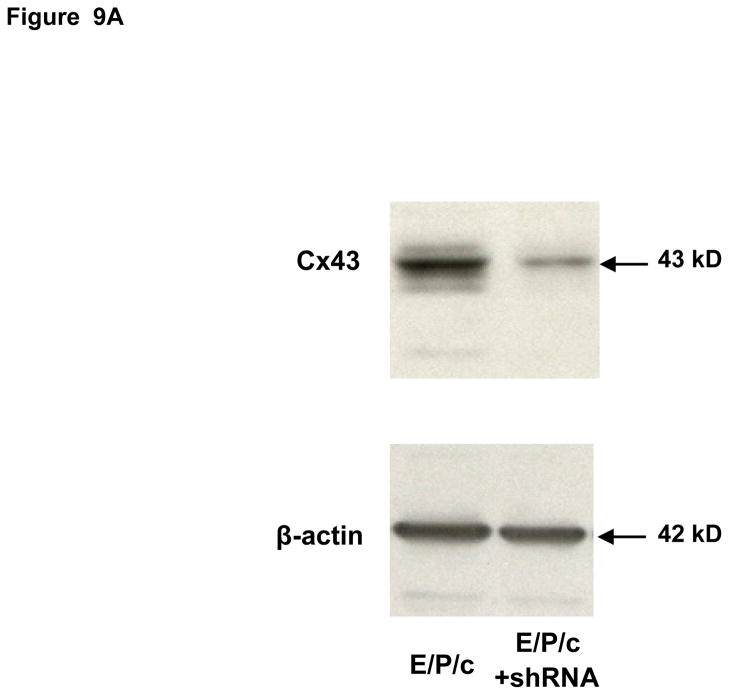
Stable knockdown of Cx43 was accomplished using shRNA. To obtain the desired constructs we screened five lentiviral DNA expression vector sets, each of which contained 58 nucleotide shRNA duplexes that targeted human Cx43 (Sigma). The shRNA vectors were cotransfected with helper plasmids VSVG and delta 8.9 into HEK293T cells using Lipofectamine™ 2000 (Invitrogen) for lentivirus production. Empty vector (pLKO.1-puro) served as a negative control. Virus-containing media from HEK293T cells were collected 48 h after transfection and mixed with 8 μg/ml of polybrene for infection of ESC. HEK293T cells were replenished with fresh medium for another 24 h, and the virus-containing medium was collected for a second infection of ESC. 24 hrs after the second infection, cells were grown in DMEM/F12 medium containing 10% fetal bovine serum for 48 h prior to selection with puromycin (5 μg/ml). Uninfected ESC cells were used as a selection control. Cells were maintained in puromycin for 8 days, after which Cx43 protein expression was determined by Western blotting. The cells with optimal knockdown (~75% Cx43 protein reduction but unaffected β-actin expression; Fig. 9A) were used for the experiments presented (Sigma: TRCN0000059776, Clone ID NM_000165.2-977s1c1).
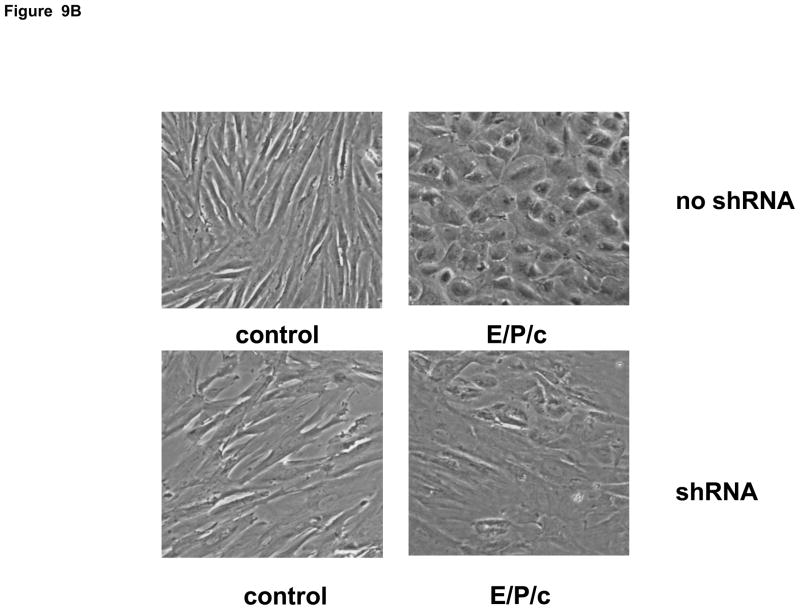
Figure 9.
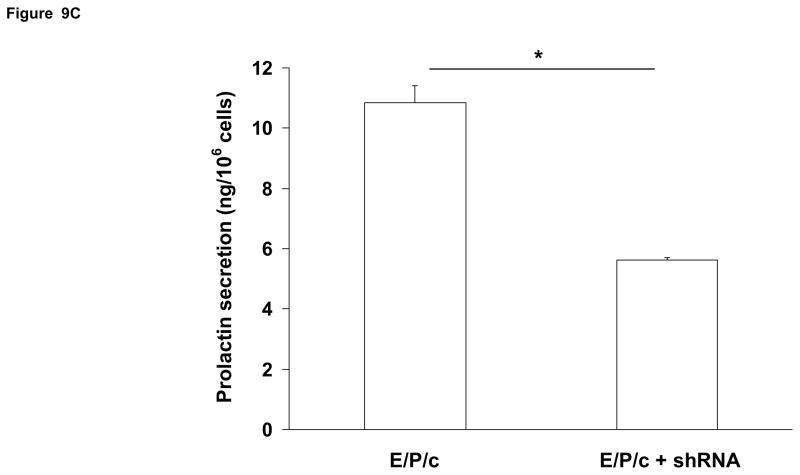
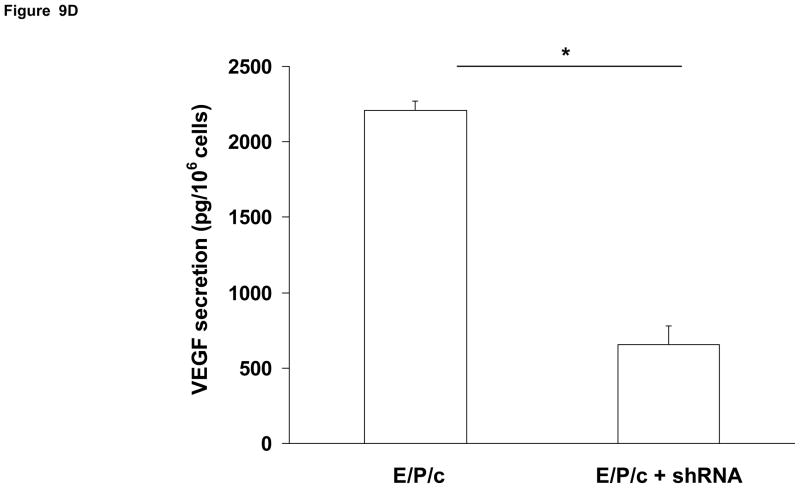
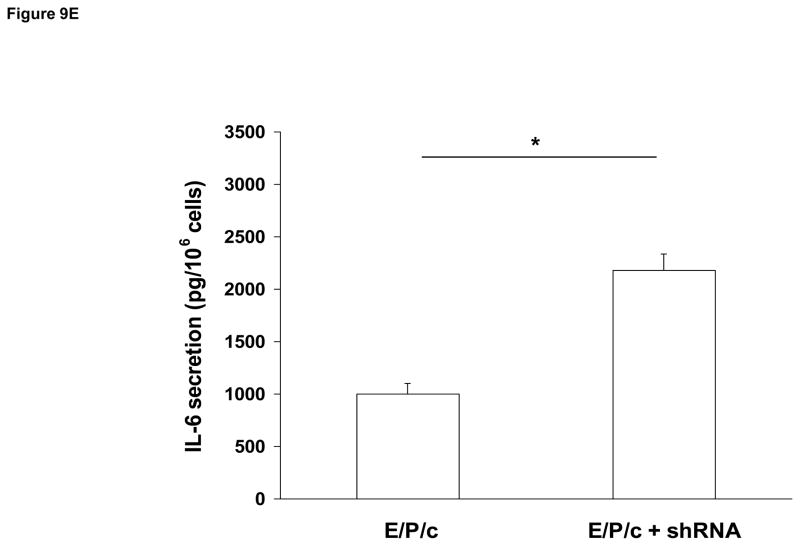
(A) Lentivirus shRNA knock-down of Cx43 protein. ESC cultured for 8 days with hormones (E/P/c) in the presence of Cx43 shRNA (+shRNA) show marked reduction in Cx43 protein relative to β-actin expression, as detected by Western blotting. (B) Attenuated morphological decidualization of ESC. (Upper panel) ESC cultured for 8 days without (control) or with (E/P/c) hormones in the absence of Cx43 shRNA (no shRNA) show classical morphological changes associated with in vitro decidualization. (Lower panel) ESC cultured for 8 days under the same conditions in the presence of Cx43 shRNA, show marked attenuation of differentiated phenotype. Magnification x 400. Cx43 shRNA also reduced prolactin (C) and VEGF (D) secretion in E/P/c-treated ESC, whereas cells cultured for 8 days with hormones in the presence of Cx43 shRNA (+shRNA) showed increased IL-6 production (E) (*P<0.05, t-test).
Statistical analyses
All of the data presented are representative assays performed in triplicate and each result was replicated in a minimum of three independent ESC isolates from different subjects. The data were normally distributed (Kolmogorov-Smirnov test) and are expressed as means ± SEM. When multiple comparisons were made the Bonferroni correction was employed. Significant differences were accepted when Student’s t test (two-tailed analyses) yielded P <0.05 between individual groups.
RESULTS
In vitro decidualization of ESC is associated with Cx43 upregulation
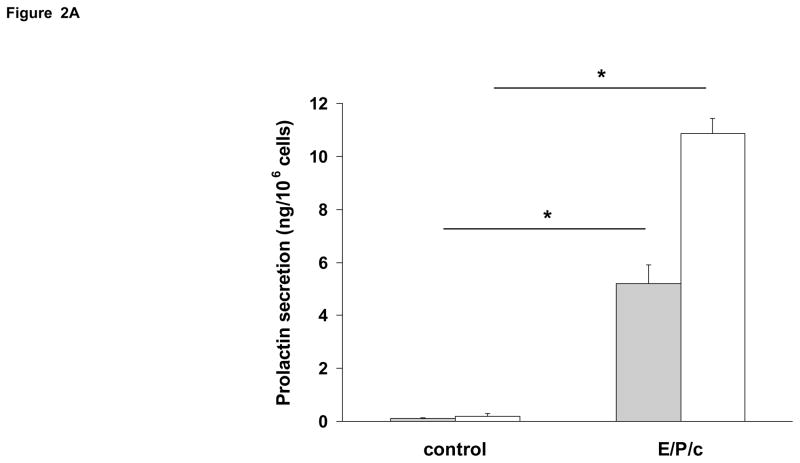
Incubation of ESC with E/P/c induced a progressive increase in Cx43 expression (Fig. 1A), which was approximately half-maximal at 4 d. By contrast, Cx26 was expressed at lower constitutive levels and did not show the hormonal modulation observed in the predominant Cx43 band (Fig 1B). In concert with the observed effects on Cx43, there were concomitant temporal increases in prolactin and VEGF secretion (Figs. 2A and 2B, respectively).
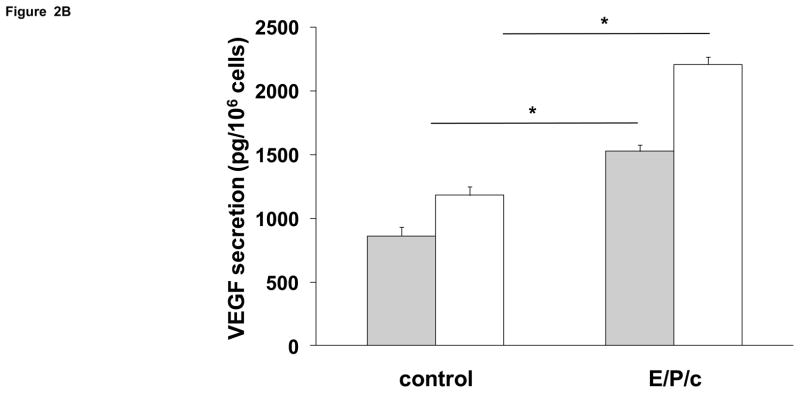
Figure 2.
Time course of biochemical differentiation of ESC exposed to E/P/c. (A) Prolactin and (B) VEGF secretion both increased following hormone stimulation in vitro. Grey histograms indicate protein secretion after 4 d incubation without (control) and with hormones (E/P/c) and white histograms show the protein concentrations after 8 d incubation (*P<0.05, t-test).
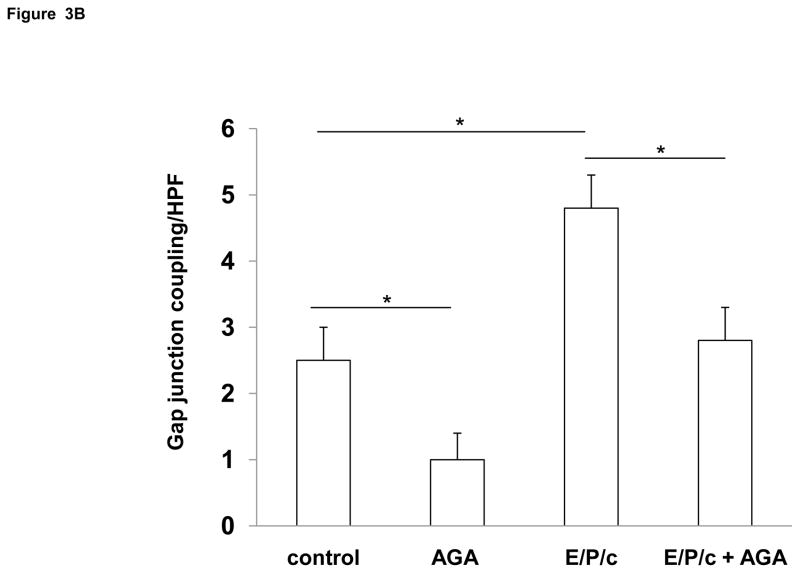
Decidualization increases gap junction coupling in ESC and inhibitors of gap junctions impair decidualization
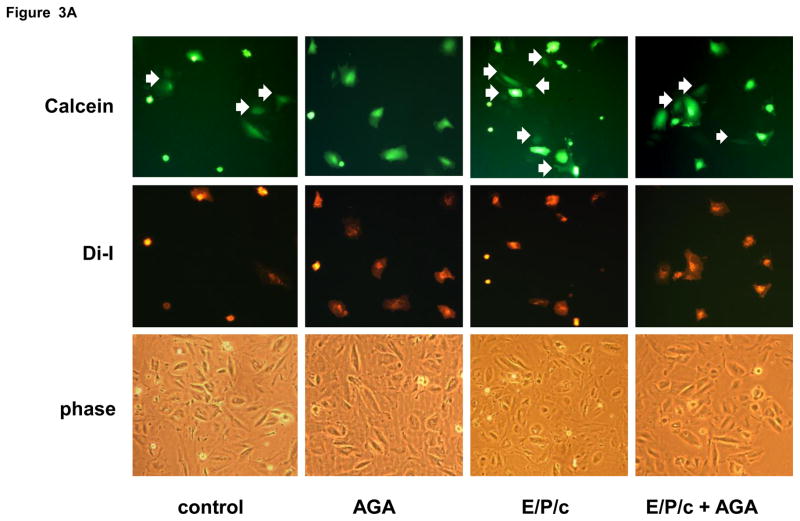
To determine if Cx43 upregulation was associated with a functional increase in gap junctions, we utilized a dye diffusion assay. Donor ESC, labeled with permeable (calcein) and impermeable (Di-I) fluorescent dyes, were incubated on monolayers of unlabeled ESC for 4 hours. Diffusion of calcein dye (green) from donor to recipient cells indicates the presence of gap junctions (white arrows in Fig. 3A), whereas Di-I dye (red) remains within donor cells. Treatment for 4 days with E/P/c increased gap junction coupling by ~60% (P<0.05) above control levels (Fig. 3B). We also employed well characterized pharmacological inhibitors of gap junctions to test whether the latter functionally mediate decidualization of ESC. The addition of 50 μM AGA reduced gap junction coupling in control ESC by ~40% and in E/P/c-treated cells by ~35% (P<0.05, Fig. 3B).
Figure 3.
(A) Dye diffusion studies to assess functional gap junctions. Donor ESC doubly labeled with gap junction permeable (calcein, green) and impermeable (diI, red) dyes were cultured with unlabeled recipient ESC as described in Materials and Methods. White arrows indicate diffusion of calcein from donor to recipient cells. Similarities in confluence of the variously treated cultures is demonstrated by phase contrast (phase) microscopy in the lower panel. Original magnification × 200. (B) Quantification of the number of gap junctions (indicated by calcein diffusion, above) per high power field (HPF) indicates that 18α-glycyrrhetinic acid (AGA) inhibited gap junction coupling under control and hormone-treated conditions. In addition, 4 days of E/P/c treatment resulted in a significant increase in functional coupling compared to control (*P<0.05, t-test). The figure reflects a selected representative experiment.
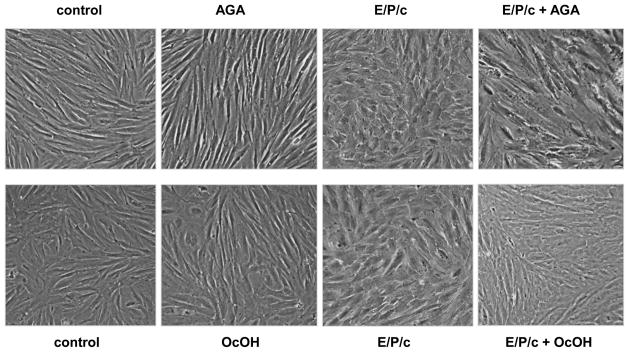
AGA and OcOH partially reverse morphological and biochemical decidualization in ESC
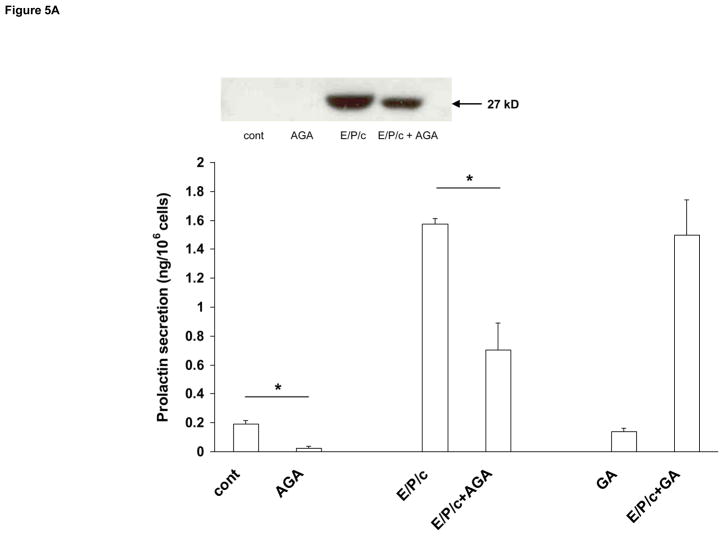
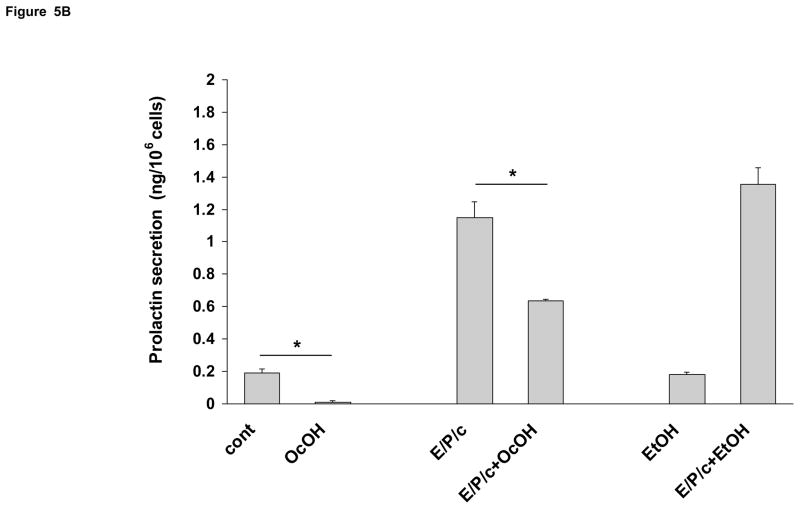
Having established that we could block ESC gap junctions pharmacologically, cell morphology was assessed in the absence or presence of 50 μM AGA or 1.5 mM OcOH. These concentrations were selected following pilot dose-response experiments undertaken to identify optimal drug concentrations that were not toxic to the cell cultures, and they correspond well to previous reports in other cell types (Schiller et al., 2001; Contreras et al., 2002). Under control conditions the inhibitors had no apparent effects on cell morphology; however, in E/P/c-treated ESC, the inclusion of either gap junction blocker partially prevented the epithelioid changes induced after 8 d of hormone exposure, when morphological transformation is maximal (Fig. 4). Biochemical decidualization was confirmed by measuring prolactin secretion from ESC. Under control conditions, ESC secreted very low concentrations into conditioned media. Prolactin was undetectable in Western blots (Fig. 5, inset) and only reached concentrations of ~0.2 ng/106 cells as measured by ELISA. However, after 8 d exposure to E/P/c, prolactin secretion was induced ~8-fold. Incubation in the presence of AGA (50 μM) reduced both basal and hormone-induced prolactin by >50% (P<0.02). By contrast, the non-inhibitory glycoside GA (50 μM) did not reduce basal or hormone-induced prolactin (Fig. 5A). OcOH (1.5 mM) inhibited basal and E/P/c-induced prolactin by ~30% (P<0.05). EtOH (1.5 mM), a non-inhibitory alcohol, had no effect on prolactin levels in basal or hormone-treated conditions (Fig. 5B).
Figure 4.
Gap junction inhibitors block morphological differentiation of hormone-treated ESC. In ESC cultured for 8 days in the absence of hormones (control), neither AGA nor octanol (OcOH) affected the fibroblastic appearance of the cultures (left panels). However, in cells treated with E/P/c for 8 days, co-incubation with AGA or OcOH attenuated the epithelioid morphological changes observed with E/P/c alone. Magnification × 400.
Figure 5.
Gap junction inhibitors block biochemical differentiation of ESC. Prolactin secretion as detected by ELISA and expressed as ng/106 cells, was used as an indicator of ESC decidualization. ESC cultured for 8 days in the absence of hormones (cont.) had very low levels of prolactin production, which were significantly upregulated in the presence of E/P/c. (A) AGA inhibited both basal and E/P/c-stimulated prolactin secretion (*P<0.05, t-test), whereas the non-inhibitory glycoside, glycyrrhizic acid (GA), had no effects on basal or stimulated prolactin secretion. The inset shows prolactin in the supernatants as detected by Western blotting. The latter assay was less sensitive than the ELISA but shows similar trends. The figure reflects a selected representative experiment.
(B) OcOH also reduced control and E/P/c-stimulated prolactin secretion, whereas an equimolar concentration of EtOH had no effect (*P<0.05, t-test). The figure reflects a selected representative experiment.
AGA and OcOH inhibit hormone-induced VEGF secretion in decidualized ESC
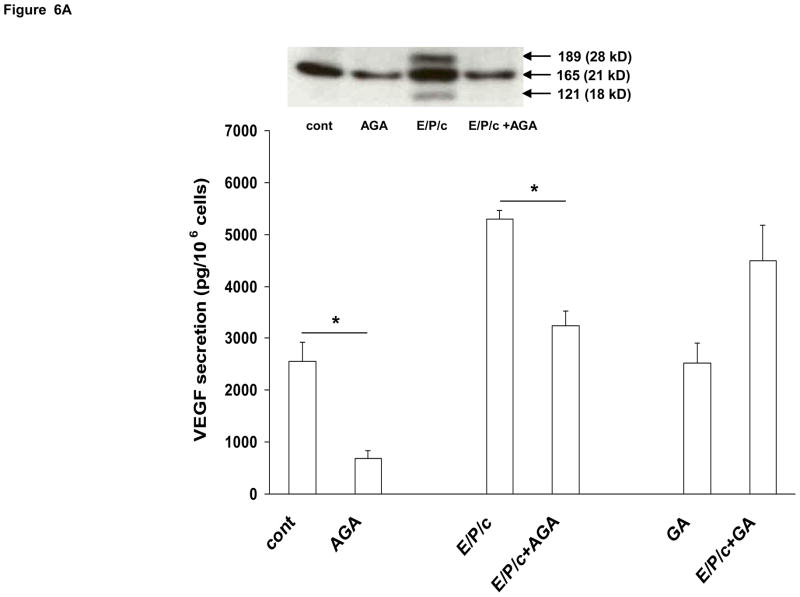
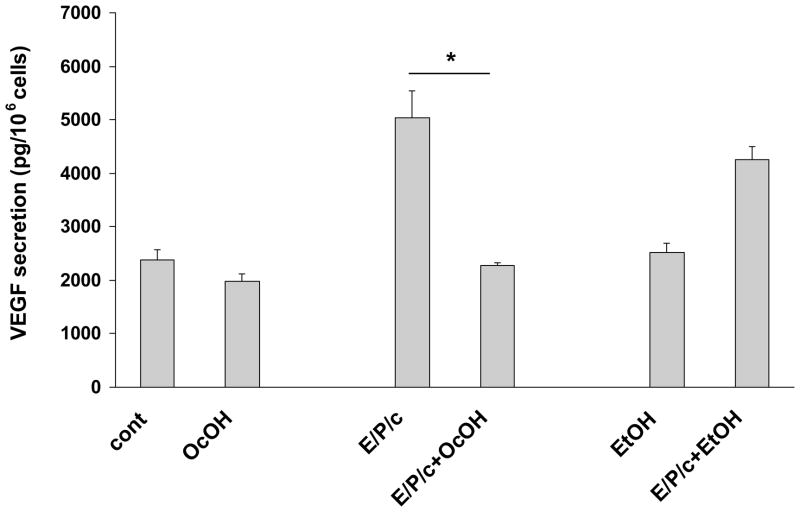
Our previous study identified VEGF as a sensitive marker of endometrial stromal differentiation in vivo and in vitro (Laws et al., 2008). In the current experiments we also evaluated the effects of gap junction blockade on ESC VEGF secretion. AGA inhibited VEGF secretion into the conditioned media ~70% under control conditions, and ~50% in E/P/c-treated cells (P<0.05). The non-inhibitory glycoside GA had no effects (Fig. 6A). Western blotting of the cell supernatants (Fig. 6A, inset) indicated that all three major human VEGF isoforms (28, 24 and 18 kD, corresponding to 189, 165 and 121 amino acid peptides, respectively) were reduced by AGA. OcOH inhibited E/P/c-induced VEGF by ~60% (P<0.05), but had little effect on basal VEGF secretion. EtOH, a non-inhibitory alcohol, had no effect on VEGF levels in basal or hormone-treated conditions (Fig. 6B).
Figure 6.
Gap junction inhibitors block biochemical markers of angiogenesis in decidualized ESC. VEGF secretion as detected by ELISA and expressed as pg/106 cells, was significantly upregulated in cells cultured for 8 days with E/P/c relative to cells without hormone treatment (cont). (A) AGA significantly blunted VEGF production in control and E/P/c-treated cells (*P<0.05, t-test), whereas the non-inhibitory glycoside, GA, had no effects on VEGF secretion. The inset shows VEGF in the supernatants as detected by Western blotting and indicates that all three major protein isoforms (corresponding to 189, 165 and 121 aa) are upregulated by E/P/c and inhibited by AGA. The figure reflects a selected representative experiment. (B) OcOH also reduced E/P/c-stimulated VEGF secretion, whereas an equimolar concentration of EtOH had no effect (*P<0.05, t-test). The figure reflects a selected representative experiment.
AGA-induced inhibition of VEGF expression in decidualized ESC is mediated, in part, by reduced mRNA accumulation
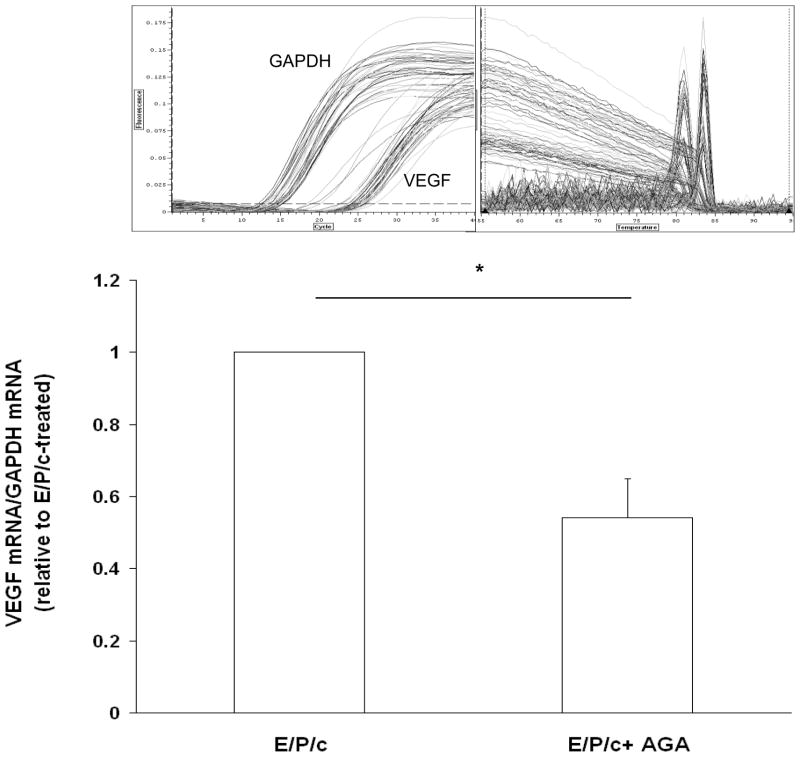
To begin to understand mechanistically how gap junction blockade results in the reduction of secreted VEGF, we quantified VEGF mRNA in decidualized ESC in the absence or presence of AGA. Real-time RT-PCR revealed a ~50% decrease in steady-state VEGF mRNA accumulation in the presence of the gap junction inhibitor (Fig. 7). Preliminary results failed to show inhibitory effects of E/P/c + AGA (50 μM) relative to E/P/c alone on transcriptional activation of a full-length human VEGF-luciferase reporter construct (Mueller et al., 2000) (38±8 RLUvs. 34±10 RLU/103 cells, respectively) implying that post-transcriptional regulation by AGA may be involved in the reduction of VEGF transcripts.
Figure 7.
Inhibitory effects of AGA on VEGF production are partly mediated at the level of mRNA metabolism. Real-time RT-PCR was used to quantify VEGF mRNA transcripts in decidualized ESC. Incubation with E/P/c for 8 days in the presence of AGA resulted in ~50% reduction in steady-state VEGF mRNA. The inset reveals the PCR amplification profiles and melting curve analyses, showing precise, single peaks for the amplified cDNAs.
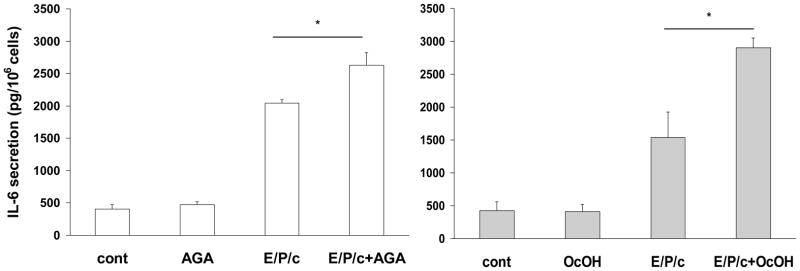
AGA and OcOH stimulate hormone-induced IL-6 secretion in decidualized ESC
Several immunomodulatory cytokines, including IL-6, are produced at the fetomaternal interface by human secretory endometrium and deciduas (Tabibzadeh et al., 1995). We noted that in vitro decidualization of ESC resulted in a ~5-fold increase in IL-6 secretion (Fig. 8). Under control conditions, AGA (50 μM) had no effect on IL-6 secretion into the conditioned media, however in E/P/c-treated cells, AGA further stimulated IL-6 by ~30% (P<0.05; Fig. 8A). OcOH (1.5 mM) stimulated E/P/c-induced IL-6 ~2-fold (P<0.02; Fig. 8B), but also had no effect on basal IL-6 secretion. The non-inhibitory glycoside (GA, 50 μM) and alcohol (EtOH, 1.5 mM) each had no effect on IL-6 levels in basal or hormone-treated conditions (data not shown).
Figure 8.
Gap junction inhibitors stimulate biochemical markers of inflammation in decidualized ESC. IL-6 secretion as detected by ELISA and expressed as pg/106 cells, was significantly upregulated in cells cultured for 8 days with E/P/c relative to cells without hormones (cont). Moreover, co-incubation with gap junction inhibitors AGA (left panel) or OcOH (right panel) further stimulated IL-6 secretion in decidualized ESC (*P<0.05, t-test). The figure reflects a selected representative experiment.
Confirmation of gap junction role in decidualization using transgenic approach
As the previous experiments utilized pharmacological inhibitors to establish a relationship between gap junctions and decidual transformation, we undertook confirmatory studies by infecting ESC with Cx43 shRNA lentiviral constructs to knock-down gap junction formation. In cells exposed to E/P/c for 8 days, Cx43 shRNA virus reduced Cx43 protein by ~75% (Fig. 9A). Infection of ESC with empty virus had no effects (data not shown). After 8 d under control conditions, Cx43 shRNA had negligible effects on ESC morphology, but dramatically reduced the decidual phenotype in cells exposed to E/P/c for 8 days (Fig. 9B). Cx43 shRNA reduced E/P/c-stimulated prolactin secretion by ~50% (Fig. 9C, P<0.05) and VEGF secretion by ~75% (Fig. 9D, P<0.02). As observed with the pharmacological gap junction inhibitors, Cx43 shRNA provoked a ~220% increase in IL-6 secretion in E/P/c-stimulated ESC (Fig. 9E, P<0.02). These data confirm that Cx43 is necessary for the morphological and biochemical differentiation of ESC during hormone-induced decidualization and suggest that gap junctions mediate the decidualized phenotype.
DISCUSSION
Decidualization is critical to early placentation and embryo survival in eutherian species. In mice and rats, the morphogenetic changes associated with decidualization are accompanied by profound biochemical responses, including the upregulation of prolactin-like proteins (PLP) (Alam et al., 2007). In the baboon, prolactin, IGFBP-1, and -smooth muscle actin are upregulated (Fazleabas et al., 2004) and in women, endometrial decidualization is accompanied by the massive upregulation and secretion of prolactin (Daly et al., 1983) and IGFBP-1 (Koistinen et al, 1986). In the current study we have verified that VEGF also is induced during primary human ESC decidualization. The physiological significance of decidual prolactin and VEGF are incompletely understood, but both proteins have potent angiogenic activities (Ferrara et al., 1996; Clapp et al., 1998; Struman et al., 1999) and targeted disrupution of Cx43 in the mouse uterus inhibited the expression of these proteins and impaired endometrial angiogenesis in vivo (Laws et al., 2008). We hypothesized that transformation of fibroblastic endometrial stromal cells into an organized decidual endocrine unit requires the de novo establishment of selective cell-cell communication networks, which are mediated in part via Cx43 biosynthesis and gap junction formation, in anticipation of embryonic implantation. This report focuses on the modulation of decidual protein expression in differentiating human endometrial stromal cells by molecules that regulate gap junctions.
For successful pregnancy, vascular development needs to occur on both sides of the maternal-fetal interface. The remarkable finding of Ferrara et al. (1996), that haplodeficiency for VEGF led to embryonic lethality in mice, underscores the fetal requirement for this angiogenic factor. However, normal maternal production of VEGF is likewise necessary for decidual vascularization (Jauniaux et al., 2006). Even if miscarriage is averted, failure of normal angiogenic growth factor production in early pregnancy is associated with subsequent pregnancy complications including preeclampsia and fetal growth restriction (Taylor et al., 2003; Levine et al., 2004).
As noted above, the decidual reaction induces Cx43 and transforms fibroblastic endometrial stromal cells into an epithelioid phenotype with new morphogenetic and functional properties. Of particular interest to us are reports in other systems of the association of Cx43 and gap junction expression with angiogenesis. Cx43 knockout mice die at birth with epicardial vascular apoptosis and a meta-analysis of murine knock-out models has placed connexins as key modulators in a network of VEGF regulatory genes (Argraves & Drake, 2005). Recent studies from our group indicate that conditional knock-out of Cx43 in the mouse uterus and Cx43 knockdown in telomerase-immortalized human ESC (Krikun et al., 2001) led to reduced prolactin and VEGF expression (Laws et al., 2008). The complementary pharmacological and transgenic approaches used in this report to inhibit gap junctions in primary human ESC confirm that biomarkers of angiogenesis are reduced.
IL-6 is a 26-kD Th2 cytokine synthesized by uterine epithelial and stromal cells, particularly during the implantation period (Tabibzadeh et al., 1995; Salamonsen et al., 2000). A broadly accepted, albeit oversimplified, model for T-helper (Th) cell modulation during implantation proposes that pregnancy causes a shift in the balance of uterine cytokine biosynthesis from a predominant Th1, or “cellular” immune phenotype, to a Th2, or “humoral” immune pattern (Chaouat, 2007). Th2 cytokines, such as IL-6 induced at the fetomaternal interface, are believed to modulate immune tolerance of the embryonic allograft (Saito, 2000). Our baseline data are consistent with this observation, suggesting that decidualization per se induces IL-6 upregulation. However, unlike prolactin and VEGF, gap junction blockade by AGA or OcOH further upregulated IL-6 secretion in decidualized ESC. Knockdown of Cx43 with shRNA showed a similar stimulatory effect. While this may seem counterintuitive to reduced decidual function by gap junction blockade, there is evidence that excessive IL-6 production is detrimental to murine placentation (Zenclussen et al., 2003) and increased decidual IL-6 mRNA and protein production was observed in cases of preeclampsia (Lockwood et al., 2008). Interestingly, data from the mouse indicate that prolactin negatively regulates IL-6 expression in decidual cells (Bao et al., 2007). Hence, the observed increase in IL-6 following AGA, OcOH or shRNA treatment may be a consequence of reduced prolactin expression noted in the presence of gap junction inhibitors.
In conclusion, we have demonstrated that ESC differentiation into decidual cells is associated with concomitant upregulation of Cx43 and functional gap junction intercellular communications. Blockade of gap junctions using pharmacological inhibitors (AGA or OcOH), or inhibition of Cx43 by shRNA, impairs morphological differentiation. This effect is accompanied by a dramatic reduction of prolactin secretion, the quintessential marker for biochemical ESC decidualization. VEGF, a critical angiogenic product of the decidual cell, also is inhibited when gap junctions are disturbed. By contrast, secretion of the immunomodulatory cytokine IL-6 is enhanced when gap junctions are blocked. Our findings suggest a new model of the uterine decidua as a coordinated secretory organ that regulates embryonic implantation via intercellular cooperation mediated by gap junctions. When adjacent cells communicate through these junctions, decidual prolactin and VEGF secretion appears to be optimized for vascular development of the placental bed. Conversely, when intercellular communications are disrupted, angiogenesis is impaired and an inflammatory state is induced. We currently are attempting to identify the small molecules that presumably traverse these important gap junctions, as they should provide key insights into uterine function and novel targets for fertility regulation.
Highlights.
Gap junctions and connexin (Cx) proteins appear to facilitate decidual differentiation
Human endometrial stromal cells were differentiated in vitro by incubation with hormones
Pharmacological gap junction inhibitors and Cx43 siRNA knockdown blocked decidual morphology
Prolactin, IGFBP-1 and VEGF were reduced, whereas IL-6 was increased by gap junction blockade
Gap junctions maintain decidual function and prevent inflammation in endometrial stromal cells
Acknowledgments
Financial Support: This research was supported by the Eunice Kennedy Shriver NICHD through R01-HD55379 and cooperative agreement U54 HD55787 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research
The authors thank Kara Barrett, RN of the Human Uterine Biology program, Emory University Department of Gynecology and Obstetrics, for her assistance in subject recruitment and regulatory compliance.
Footnotes
Disclosure Summary: The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jie Yu, Department of Gynecology and Obstetrics, Emory University, Atlanta, GA 30322.
Juanjuan Wu, Department of Gynecology and Obstetrics, Emory University, Atlanta, GA 30322.
Indrani C. Bagchi, Department of Veterinary Biosciences, University of Illinois Urbana/Champaign, Urbana, IL 61802.
Milan K. Bagchi, Department of Molecular and Integrative Physiology, University of Illinois Urbana/Champaign, Urbana, IL 61801.
Neil Sidell, Department of Gynecology and Obstetrics, Emory University, Atlanta, GA 30322.
Robert N. Taylor, Department of Gynecology and Obstetrics, Emory University, 1639 Pierce Drive, Suite 4217-WMB, Atlanta, GA 30322.
References
- Alam SM, Konno T, Dai G, Lu L, Wang D, Dunmore JH, Godwin AR, Soares MJ. A uterine decidual cell cytokine ensures pregnancy-dependent adaptations to a physiological stressor. Development. 2007;134:407–415. doi: 10.1242/dev.02743. [DOI] [PubMed] [Google Scholar]
- Argraves WS, Drake CJ. Genes critical to vasculogenesis as defined by systematic analysis of vascular defects in knockout mice. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:875–884. doi: 10.1002/ar.a.20232. [DOI] [PubMed] [Google Scholar]
- Bao L, Tessier C, Prigent-Tessier A, Li F, Buzzio OL, Callegari EA, Horseman ND, Gibori G. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology. 2007;148:2326–2334. doi: 10.1210/en.2006-1643. [DOI] [PubMed] [Google Scholar]
- Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol. 2007;29:95–113. doi: 10.1007/s00281-007-0069-0. [DOI] [PubMed] [Google Scholar]
- Clapp C, López-Gómez FJ, Nava G, Corbacho A, Torner L, Macotela Y, Dueñas Z, Ochoa A, Noris G, Acosta E, Garay E, Martínez de la Escalera G. Expression of prolactin mRNA and of prolactin-like proteins in endothelial cells: evidence for autocrine effects. J Endocrinol. 1998;158:137–144. doi: 10.1677/joe.0.1580137. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Sáez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly DC, Maslar IA, Riddick DH. Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol. 1983;145:672–678. doi: 10.1016/0002-9378(83)90572-0. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Kim JJ, Strakova Z. Implantation: embryonic signals and the modulation of the uterine environment--a review. Placenta. 2004;25 (suppl A):S26–S31. doi: 10.1016/j.placenta.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Granot I, Dekel N, Bechor E, Segal I, Fieldust S, Barash A. Temporal analysis of connexin43 protein and gene expression throughout the menstrual cycle in human endometrium. Fertil Steril. 2000;73:381–386. doi: 10.1016/s0015-0282(99)00531-2. [DOI] [PubMed] [Google Scholar]
- Grümmer R, Reuss B, Winterhager E. Expression pattern of different gap junction connexins is related to embryo implantation. Int J Dev Biol. 1996;40:361–367. [PubMed] [Google Scholar]
- Jahn E, Classen-Linke I, Kusche M, Beier HM, Traub O, Grümmer R, Winterhager E. Expression of gap junction connexins in the human endometrium throughout the menstrual cycle. Hum Reprod. 1995;10:2666–2670. doi: 10.1093/oxfordjournals.humrep.a135764. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28:1770–1782. doi: 10.1128/MCB.01556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinen R, Kalkkinen N, Huhtala ML, Seppälä M, Bohn H, Rutanen EM. Placental protein 12 is a decidual protein that binds somatomedin and has an identical N-terminal amino acid sequence with somatomedin-binding protein from human amniotic fluid. Endocrinology. 1986;118:1375–1378. doi: 10.1210/endo-118-4-1375. [DOI] [PubMed] [Google Scholar]
- Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145:2291–2296. doi: 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- Lawn AM, Wilson EW, Finn CA. The ultrastructure of human decidual and predecidual cells. J Reprod Fertil. 1971;26:85–90. doi: 10.1530/jrf.0.0260085. [DOI] [PubMed] [Google Scholar]
- Laws MJ, Taylor RN, Sidell N, DeMayo FJ, Lydon JP, Gutstein DE, Bagchi MK, Bagchi IC. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development. 2008;135:2659–2668. doi: 10.1242/dev.019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Yen CF, Basar M, Kayisli UA, Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G, Schatz F. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol. 2008;172:1571–1579. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantena SR, Kannan A, Cheon YP, Li Q, Johnson PF, Bagchi IC, Bagchi MK. C/EBP beta is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci USA. 2006;103:1870–1875. doi: 10.1073/pnas.0507261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA. 2000;97:10972–10977. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando-Mathur CE, Bechberger JF, Goldberg GS, Naus CC, Kidder GM, Kennedy TG. Rat endometrial stromal cells express the gap junction genes connexins 26 and 43 and form functional gap junctions during in vitro decidualization. Biol Reprod. 1996;54:905–913. doi: 10.1095/biolreprod54.4.905. [DOI] [PubMed] [Google Scholar]
- Pauken CM, Lo CW. Nonoverlapping expression of Cx43 and Cx26 in the mouse placenta and decidua: a pattern of gap junction gene expression differing from that in the rat. Mol Reprod Dev. 1995;41:195–203. doi: 10.1002/mrd.1080410210. [DOI] [PubMed] [Google Scholar]
- Peeters LL, Vigne JL, Tee MK, Zhao D, Waite LL, Taylor RN. PPAR gamma represses VEGF expression in human endometrial cells: implications for uterine angiogenesis. Angiogenesis. 2005;8:373–379. doi: 10.1007/s10456-005-9027-4. [DOI] [PubMed] [Google Scholar]
- Piao M, Mori D, Satoh T, Sugita Y, Tokunaga O. Inhibition of endothelial cell proliferation, in vitro angiogenesis, and the down-regulation of cell adhesion-related genes by genistein. Combined with a cDNA microarray analysis. Endothelium. 2006;13:249–266. doi: 10.1080/10623320600903940. [DOI] [PubMed] [Google Scholar]
- Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28:17–26. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–649. doi: 10.1210/jcem.78.3.8126136. [DOI] [PubMed] [Google Scholar]
- Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol. 2000;47:87–103. doi: 10.1016/s0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Dimitriadis E, Robb L. Cytokines in implantation. Semin Reprod Med. 2000;18:299–310. doi: 10.1055/s-2000-12567. [DOI] [PubMed] [Google Scholar]
- Schiller PC, D’Ippolito G, Balkan W, Roos BA, Howard GA. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone. 2001;28:362–369. doi: 10.1016/s8756-3282(00)00458-0. [DOI] [PubMed] [Google Scholar]
- Sidell N, Feng Y, Hao L, Wu J, Yu J, Kane MA, Napoli JL, Taylor RN. Retinoic acid is a cofactor for translational regulation of vascular endothelial growth factor in human endometrial stromal cells. Mol Endocrinol. 2010;24:148–160. doi: 10.1210/me.2009-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söhl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10:173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- Struman I, Bentzien F, Lee H, Mainfroid V, D’Angelo G, Goffin V, Weiner RI, Martial JA. Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA. 1999;96:1246–1251. doi: 10.1073/pnas.96.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibzadeh S, Kong QF, Babaknia A, May LT. Progressive rise in the expression of interleukin-6 in human endometrium during menstrual cycle is initiated during the implantation window. Hum Reprod. 1995;10:2793–2799. doi: 10.1093/oxfordjournals.humrep.a135793. [DOI] [PubMed] [Google Scholar]
- Tanmahasamut P, Sidell N. Up-regulation of gap junctional intercellular communication and connexin43 expression by retinoic acid in human endometrial stromal cells. J Clin Endocrinol Metab. 2005;90:4151–4156. doi: 10.1210/jc.2004-0663. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Krutovskikh V, Mesnil M, Tanaka T, Zaidan-Dagli ML, Omori Y. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III. 1999;322:151–159. doi: 10.1016/s0764-4469(99)80038-9. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Blois S, Stumpo R, Olmos S, Arias K, Malan Borel I, Roux ME, Margni RA. Murine abortion is associated with enhanced interleukin-6 levels at the feto-maternal interface. Cytokine. 2003;24:150–60. doi: 10.1016/j.cyto.2003.08.002. [DOI] [PubMed] [Google Scholar]