Abstract
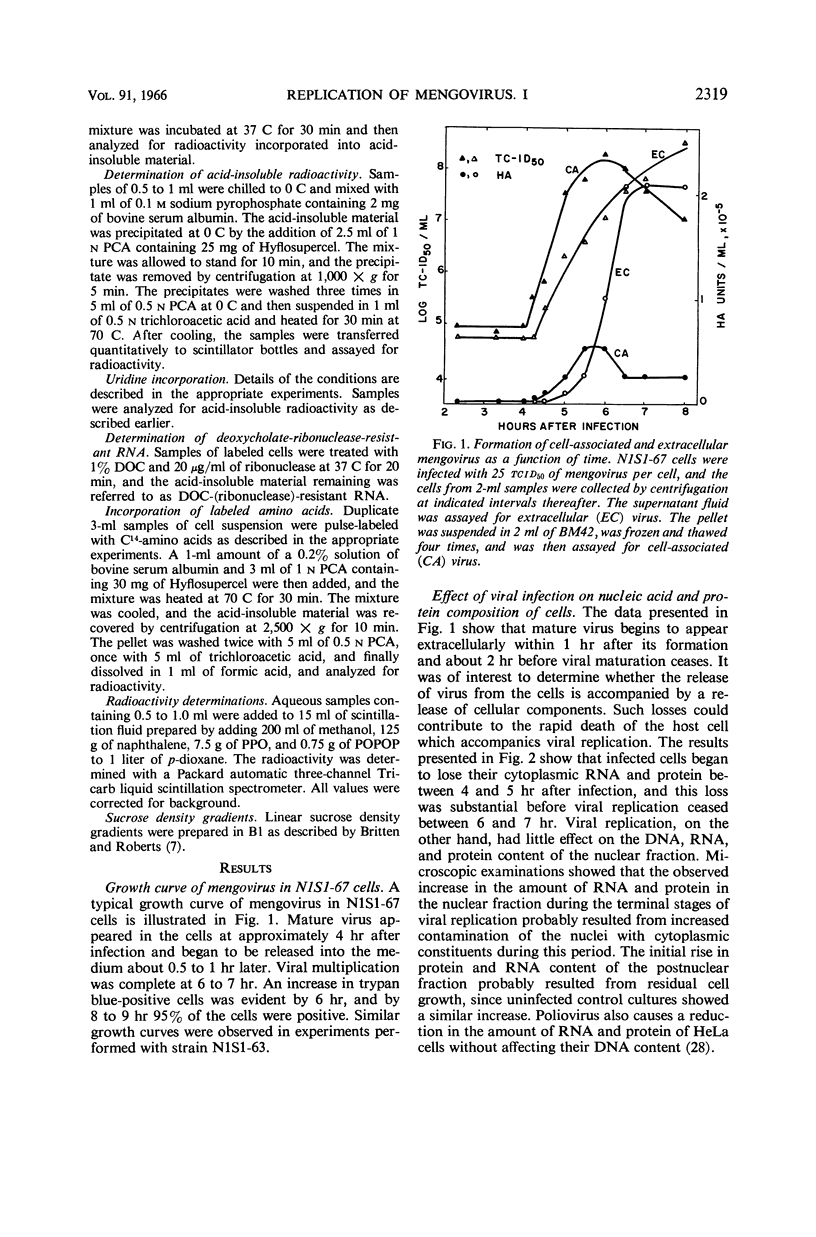
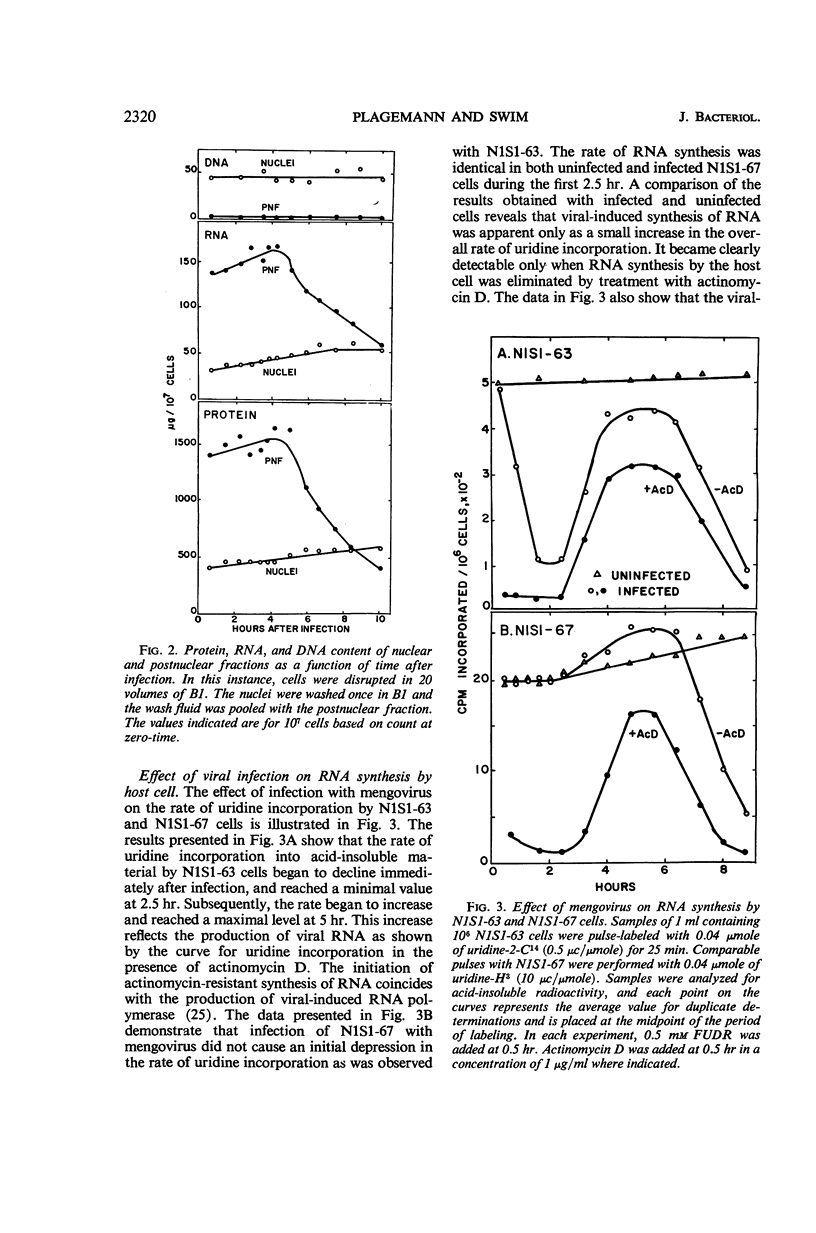
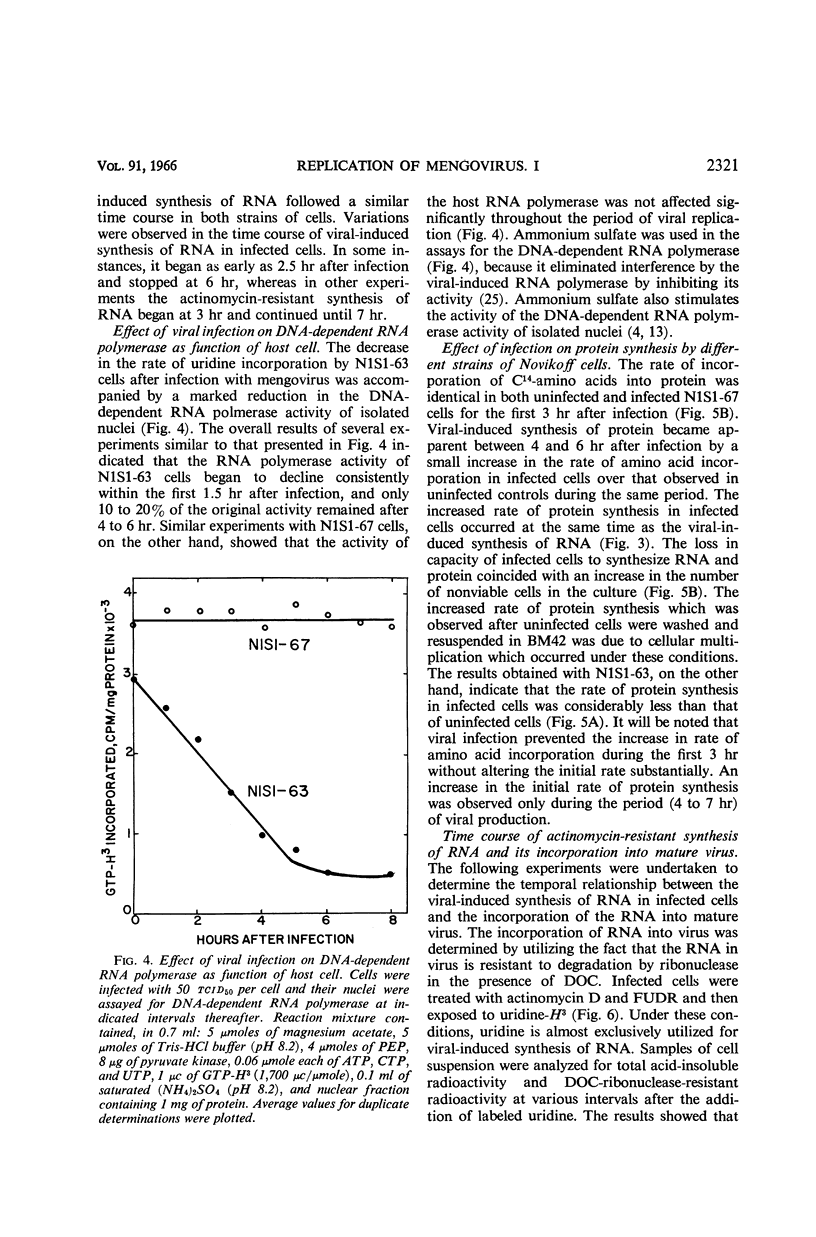
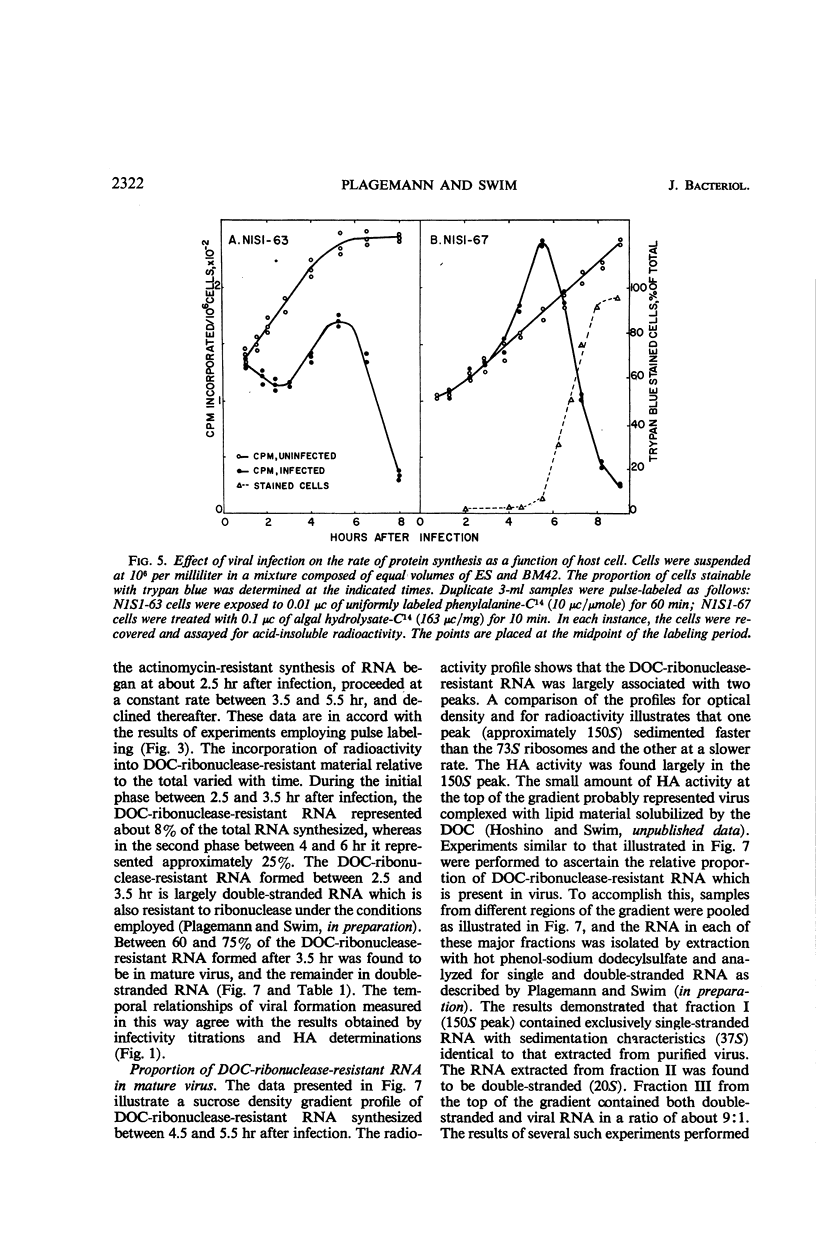
Plagemann, Peter G. W. (Western Reserve University, Cleveland, Ohio), and H. Earle Swim. Replication of mengovirus. I. Effect on synthesis of macromolecules by host cell. J. Bacteriol. 91:2317–2326. 1966.—The replication of mengovirus was studied in two strains of Novikoff (rat) hepatoma cells propagated in vitro. The replicative cycle in both strains required 6.5 to 7 hr. Infection resulted in a marked depression of ribonucleic acid (RNA) and protein synthesis by strain N1S1-63. Inhibition of RNA synthesis was reflected by a decrease in the deoxyribonucleic acid (DNA)-dependent RNA polymerase activity of isolated nuclei. Mengovirus had no effect on either protein or RNA synthesis or on the DNA-dependent RNA polymerase activity of a second strain, N1S1-67. The time course of viral-induced synthesis of RNA by cells was studied in cells treated with actinomycin D. It was first detectable between 2.5 and 3 hr after infection and continued until 6.5 to 7 hr. The formation of mature virus was estimated biochemically by measuring the amount of RNA synthesized as a result of viral infection which was resistant to degradation by ribonuclease in the presence of deoxycholate. Approximately 70% of the deoxycholate-ribonuclease-resistant RNA was located in mature virus, and the remainder was double-stranded. The formation of mature virus began about 45 min after viral-directed (actinomycin-resistant) synthesis of RNA was detectable in the cell, and only about 18 to 20% of the total RNA synthesized was incorporated into virus. Release of virus from cells began about 1 hr after maturation was first detectable. Release of virus from cells was accompanied by a loss of a large proportion of their cytoplasmic RNA and protein.
Full text
PDF

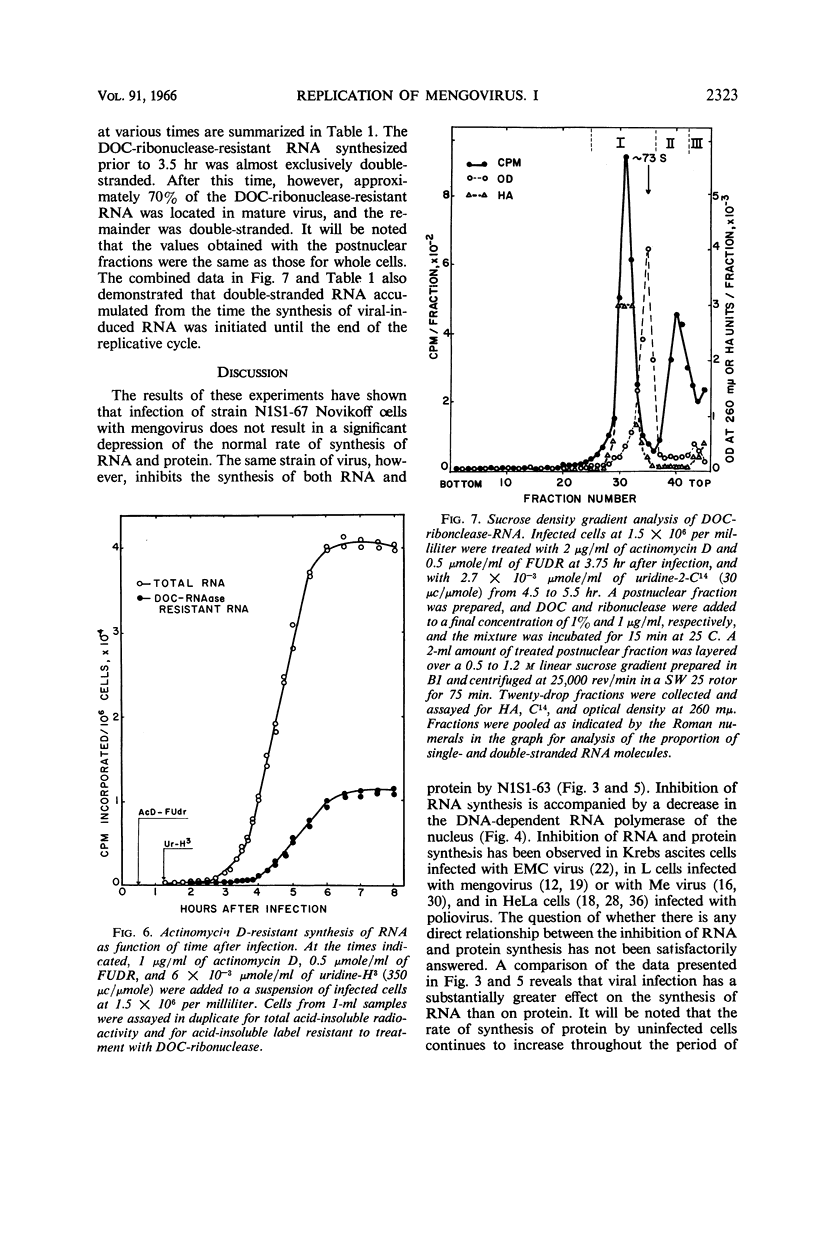
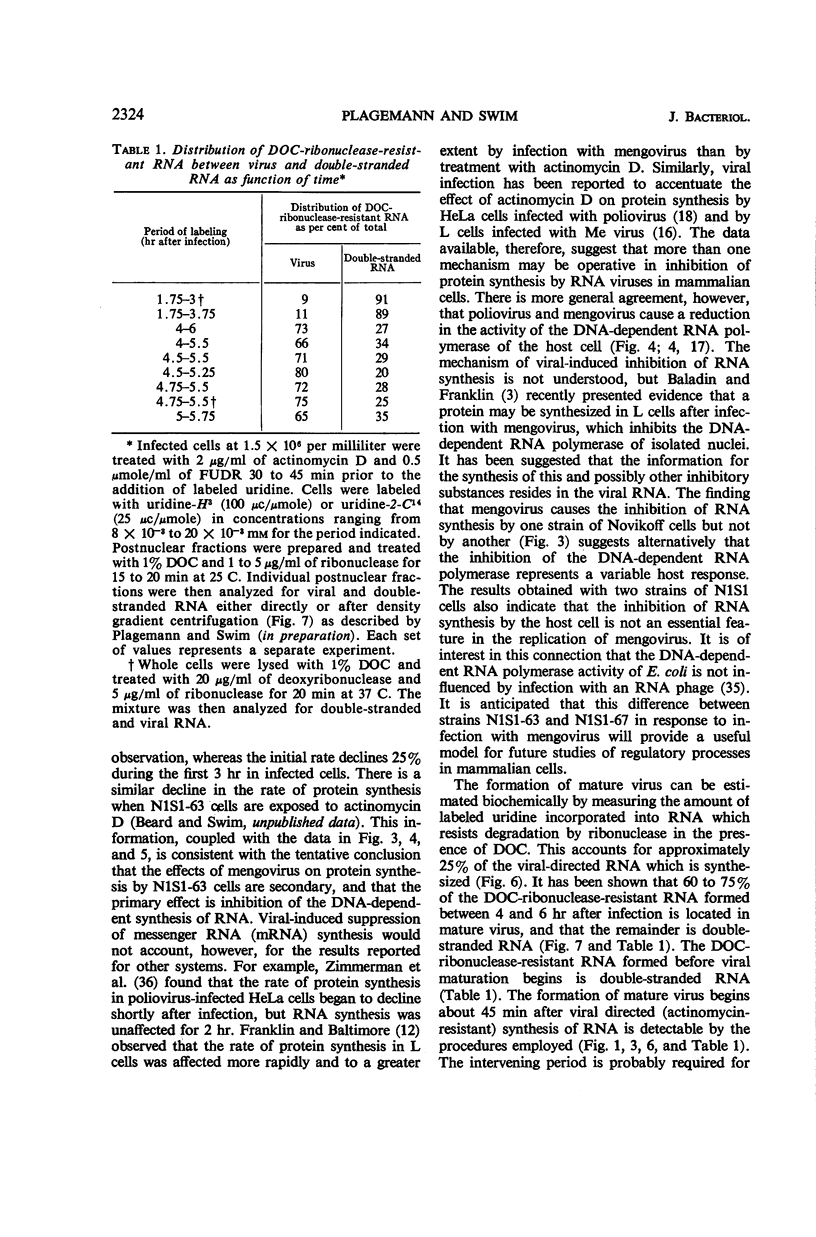


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTIMORE D., FRANKLIN R. M. A NEW RIBONUCLEIC ACID POLYMERASE APPEARING AFTER MENGOVIRUS INFECTION OF L-CELLS. J Biol Chem. 1963 Oct;238:3395–3400. [PubMed] [Google Scholar]
- BALTIMORE D., FRANKLIN R. M. Preliminary data on a virus-specific enzyme system responsible for the synthesis of viral RNA. Biochem Biophys Res Commun. 1962 Nov 27;9:388–392. doi: 10.1016/0006-291x(62)90021-9. [DOI] [PubMed] [Google Scholar]
- BALTIMORE D., FRANKLIN R. M. The effect of Mengovirus infection on the activity of the DNA-dependent RNA polymerase of L-cells. Proc Natl Acad Sci U S A. 1962 Aug;48:1383–1390. doi: 10.1073/pnas.48.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandin I. G., Franklin R. M. The effect of mengovirus infection on the activity of the DNA-dependent RNA polymerase of L-cells. II. Preliminary data on the inhibitory factor. Biochem Biophys Res Commun. 1964 Feb 18;15(1):27–32. doi: 10.1016/0006-291x(64)90097-x. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- FAULKNER P., MARTIN E. M., SVED S., VALENTINE R. C., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 2. The isolation, crystallization and chemical characterization of mouse encephalomyocarditis virus. Biochem J. 1961 Sep;80:597–605. doi: 10.1042/bj0800597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- GOLDBERG I. H. Ribonucleic acid synthesis in nuclear extracts of mammalian cells grown in suspension culture; effect of ionic strength and surface-active agents. Biochim Biophys Acta. 1961 Jul 22;51:201–204. doi: 10.1016/0006-3002(61)91042-3. [DOI] [PubMed] [Google Scholar]
- HARRIS M. Growth measurements on monolayer cultures with an electronic cell counter. Cancer Res. 1959 Nov;19:1020–1024. [PubMed] [Google Scholar]
- HARUNA I., NOZU K., OHTAKA Y., SPIEGELMAN S. AN RNA "REPLICASE" INDUCED BY AND SELECTIVE FOR A VIRAL RNA: ISOLATION AND PROPERTIES. Proc Natl Acad Sci U S A. 1963 Nov;50:905–911. doi: 10.1073/pnas.50.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSEN P., VERWOERD D. W. STUDIES ON THE MULTIPLICATION OF A MEMBER OF THE COLUMBIA SK GROUP (ME VIRUS) IN L CELLS. III. ALTERATION OF RNA AND PROTEIN SYNTHETIC PATTERNS IN VIRUS-INFECTED CELLS. Virology. 1963 Dec;21:617–627. doi: 10.1016/0042-6822(63)90235-6. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. Inhibition of DNA-primed RNA synthesis during poliovirus infection of human cells. Biochem Biophys Res Commun. 1962 Dec 19;9:556–562. doi: 10.1016/0006-291x(62)90125-0. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., PETERSON J. A. NUCLEIC ACID AND PROTEIN SYNTHESIS DURING POLIOVIRUS INFECTION OF HUMAN CELLS. J Mol Biol. 1964 Apr;8:556–575. doi: 10.1016/s0022-2836(64)80011-5. [DOI] [PubMed] [Google Scholar]
- HOMMA M., GRAHAM A. F. SYNTHESIS OF RNA IN L CELLS INFECTED WITH MENGO VIRUS. J Cell Physiol. 1963 Oct;62:179–192. doi: 10.1002/jcp.1030620207. [DOI] [PubMed] [Google Scholar]
- KELLY R. B., GOULD J. L., SINSHEIMER R. L. THE REPLICATION OF BACTERIOPHAGE MS2. IV. RNA COMPONENTS SPECIFICALLY ASSOCIATED WITH INFECTION. J Mol Biol. 1965 Mar;11:562–575. doi: 10.1016/s0022-2836(65)80011-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN E. M., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. IV. The localization of metabolic changes within subcellular fractions of Krebs II mouse-ascites-tumour cells infected with encephalomyocarditis virus. Biochem J. 1961 Dec;81:514–520. doi: 10.1042/bj0810514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B. A transplantable rat liver tumor induced by 4-dimethylaminoazobenzene. Cancer Res. 1957 Nov;17(10):1010–1027. [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. II. General properties of the viral-induced ribonucleic acid polymerase. J Bacteriol. 1966 Jun;91(6):2327–2332. doi: 10.1128/jb.91.6.2327-2332.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH E., FRANKLIN R. M., SHATKIN A. J., TATUMEL Action of actinomycin D on animal cells and viruses. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1238–1245. doi: 10.1073/pnas.48.7.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., LOCKART R. Z., Jr, SEBRING E. D. Alterations in HeLa cell metabolism resulting from poliovirus infection. Virology. 1959 Oct;9:244–259. doi: 10.1016/0042-6822(59)90118-7. [DOI] [PubMed] [Google Scholar]
- SWIM H. E., PARKER R. F. Effect of pluronic F68 on growth fibroblasts in suspension on rotary shaker. Proc Soc Exp Biol Med. 1960 Jan;103:252–254. doi: 10.3181/00379727-103-25477. [DOI] [PubMed] [Google Scholar]
- SWIM H. E., PARKER R. F. Stable tissue culture media prepared in dry form. J Lab Clin Med. 1958 Aug;52(2):309–311. [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., SIMON L., OCHOA S. Induction by an RNA phage of an enzyme catalyzing incorporation of ribonucleotides into ribonucleic acid. Proc Natl Acad Sci U S A. 1963 Mar 15;49:407–414. doi: 10.1073/pnas.49.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN E. F., HEETER M., DARNELL J. E. RNA synthesis in poliovirus-infected cells. Virology. 1963 Mar;19:400–408. doi: 10.1016/0042-6822(63)90080-1. [DOI] [PubMed] [Google Scholar]


