Summary
To segregate chromosomes properly, the cell must prevent merotely, an error that occurs when a single kinetochore is attached to microtubules emanating from both spindle poles. Recent evidence suggests that cooperation between Pcs1/Mde4 and condensin complexes plays an important role in preventing merotely.
Main Text
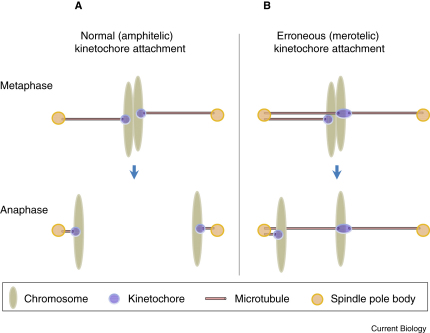
Accurate segregation of the genetic material during cell division requires that sister kinetochores attach to microtubules emanating from opposite spindle poles. Merotelic kinetochore orientation is an error in which a single kinetochore is attached to microtubules emanating from both spindle poles [1,2]. If a merotelically attached kinetochore remains uncorrected, it causes the chromatid to lag on the anaphase spindle, hindering its poleward segregation (Figure 1). It is important to understand how cells prevent and correct merotelic kinetochore attachments because merotely represents a major mechanism of aneuploidy in mitotic cells and is the primary mechanism of chromosomal instability in cancer cells [3–8]. Several proteins have been implicated in correcting or preventing merotelic attachments, including condensin and the fission yeast Pcs1/Mde4 complex, a homolog of the budding yeast monopolin complex [9–13]. Two recent studies provide important insights into how Pcs1/Mde4 and condensin prevent merotelic kinetochore attachments [14,15].
Figure 1.
Chromosome segregation during mitosis.
(A) In order to segregate chromosomes properly, sister kinetochores must attach to microtubules emanating from opposite spindle poles (amphitelic kinetochore attachment). (B) Merotelic kinetochore attachment is an error in which a single kinetochore is attached to microtubules emanating from both spindle poles. During anaphase, a merotelically attached chromatid lags behind and its timely segregation is hindered.
Previous studies suggested that both the Csm1/Lrs4 monopolin subcomplex in the budding yeast Saccharomyces cerevisiae and its counterpart Pcs1/Mde4 in the fission yeast Schizosaccharomyces pombe act at kinetochores as molecular clamps which lock together microtubule attachment sites. While the Pcs1/Mde4 complex clamps together microtubule attachment sites on a single kinetochore in order to prevent merotelic attachments, the Csm1/Lrs4 complex clamps together microtubule binding sites from sister kinetochores during meiosis I in order to establish mono-orientation (attachment of sister kinetochores to microtubules emanating from the same pole) [11,16]. Although this model was consistent with the experimental data and nicely explained the mutant phenotype observed in cells lacking Csm1/Lrs4 or Pcs1/Mde4, it was rather speculative. A strong argument in favour of the ‘clamp’ model came only recently from the structural analysis of the Csm1/Lrs4 complex. Corbett et al. [15] showed that the Csm1/Lrs4 complex has a distinctive V-shaped structure, with two pairs of kinetochore-binding domains positioned about 10 nm apart. Thus, a plausible model for Csm1/Lrs4-mediated mono-orientation of sister kinetochores is that these two pairs of kinetochore-binding domains bind across sister kinetochores, bringing them so close together that they effectively prevent bi-orientation (attachment of sister kinetochores to microtubules emanating from the opposite poles). Moreover, Corbett et al. [15] showed that the S. pombe Pcs1/Mde4 complex has the same general architecture as Csm1/Lrs4, suggesting that both Csm1/Lrs4 and its S. pombe counterpart Pcs1/Lrs4 may function as molecular clamps or crosslinkers at kinetochores.
Although the clamp model is now supported by structure–function analyses, it has been challenged by a recent study from Tada et al. [14], who showed that the role of Pcs1 and Mde4 is to recruit condensin to kinetochores and proposed that condensin at kinetochores clamps together microtubule attachment sites. Chromatin immunoprecipitation experiments clearly showed that kinetochore condensin localization is diminished in pcs1Δ cells and, notably, artificial targeting of condensin to kinetochores largely suppressed the growth defect and halved the incidence of lagging chromosomes in pcs1Δ cells [14]. These observations are consistent with previous studies showing that both Pcs1/Mde4 and condensin are important for preventing merotelic attachments [10,11,16] and that in budding yeast, monopolin proteins Csm1 and Lrs4 are required for recruitment of condensin to ribosomal DNA [17]. However, other studies showed that condensin associates with kinetochores independently of Csm1 and Lrs4 [18] and that condensin is not an obligate component of a system preventing merotelic attachments in vertebrate kinetochores [19]. These apparent discrepancies in the literature further underscore the importance of the Tada et al. [14] study. Crucially, the work of Tada et al. raises the following key question. Does the Pcs1/Mde4 complex act as a microtubule site clamp (Figure 2A), or does it prevent merotelic attachments solely by recruiting condensin to kinetochores (Figure 2B)? Although Tada et al. nicely showed that Pcs1 and Mde4 act as a condensin recruiter at kinetochores and that this is an important mechanism for preventing merotelic attachments [14], further experiments are needed to establish whether condensin recruitment is the only role of the Pcs1/Mde4 complex in preventing merotely, or whether Pcs1/Mde4 also functions as a microtubule site clamp, as suggested by previous studies (Figure 2) [11,15,16]. Elegant experiments in which kinetochore condensin was specifically inactivated by proteolytic cleavage showed that this disturbed the structure of centromeric chromatin, and frequent separation of core and pericentromeric domains was observed [14]. It is likely that this defect contributes to the high incidence of merotelic attachments observed in condensin mutant cells; therefore, it will be important to determine whether the absence of Pcs1 or Mde4 leads to a similar phenotype. Moreover, the distinct V-shape structure of the monopolin complex makes important predictions about its putative clamping function [15]. Using this structure as a guide, mutations that prevent the clamping ability should be designed and tested in vivo. Finally, in order to extend the current studies to other organisms, it will be important to identify counterparts of the fission yeast Pcs1/Mde4 complex in higher eukaryotes. Although structural and sequence analyses showed that the Pcs1/Mde4 complex shares similar features with the conserved kinetochore complex Spc24/Spc25 [12,15], it is not known whether in higher eukaryotes the Spc24/Spc25 complex took over the function of the Pcs1/Mde4 or whether there are true homologs of Pcs1/Mde4 which have not been identified yet.
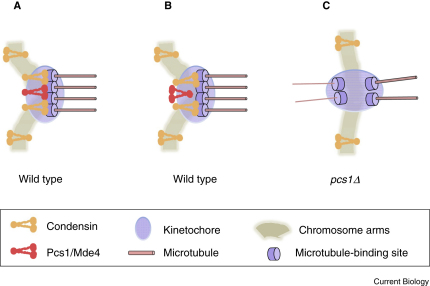
Figure 2.
Models of how condensin and Pcs1/Mde4 might function to prevent merotelic attachments.
(A) Pcs1/Mde4 prevents merotelic attachments by loading condensin on kinetochores and by clamping microtubule attachment sites. Both Pcs1/Mde4 and condensin function as a microtubule site clamp. (B) Pcs1/Mde4 prevents merotelic attachments solely by loading of condensin on kinetochores. Condensin clamps together microtubule attachment sites in order to prevent merotelic kinetochore orientation. (C) In the absence of Pcs1 (or Mde4), the kinetochore pool of condensin is strongly reduced, leading to high frequencies of merotelic attachments.
In summary, mounting evidence suggests that both the Pcs1/Mde4 and condensin complexes are required to prevent merotely. There are two models of how Pcs1 and Mde4 prevent merotelic kinetochore attachments. Whereas the clamp model suggests that Pcs1/Mde4 complex itself acts as a molecular clamp which locks together microtubule attachment sites (Figure 2A), an alternative model suggests that the Pcs1/Mde4 complex prevents merotely indirectly by recruiting condensin to kinetochores and that condensin acts as a molecular clamp which locks together microtubule attachment sites (Figure 2B). These two models are not mutually exclusive and it is possible that both Pcs1/Mde4's clamping activity and its role as a condensin recruiter are required to efficiently prevent merotelic kinetochore attachments. Further studies are needed to unveil molecular details of how kinetochore pools of Pcs1/Mde4 and condensin complexes prevent merotely. Given the importance of this process for our understanding of how cells ensure faithful segregation of chromosomes, it is likely that this will continue to be an area of intense research in the future.
References
- 1.Cimini D. Detection and correction of merotelic kinetochore orientation by Aurora B and its partners. Cell Cycle. 2007;6:1558–1564. doi: 10.4161/cc.6.13.4452. [DOI] [PubMed] [Google Scholar]
- 2.Gregan J., Polakova S., Zhang L., Tolic-Norrelykke I.M., Cimini D. Merotelic kinetochore attachment: causes and effects. Trends Cell Biol. 2011;21:374–381. doi: 10.1016/j.tcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cimini D., Howell B., Maddox P., Khodjakov A., Degrassi F., Salmon E.D. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhoum S.F., Thompson S.L., Manning A.L., Compton D.A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 2009;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganem N.J., Godinho S.A., Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silkworth W.T., Nardi I.K., Scholl L.M., Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS One. 2009;4:e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson S.L., Compton D.A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka K., Hirota T. Chromosome segregation machinery and cancer. Cancer Sci. 2009;100:1158–1165. doi: 10.1111/j.1349-7006.2009.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stear J.H., Roth M.B. Characterization of HCP-6, a C. elegans protein required to prevent chromosome twisting and merotelic attachment. Genes Dev. 2002;16:1498–1508. doi: 10.1101/gad.989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samoshkin A., Arnaoutov A., Jansen L.E., Ouspenski I., Dye L., Karpova T., McNally J., Dasso M., Cleveland D.W., Strunnikov A. Human condensin function is essential for centromeric chromatin assembly and proper sister kinetochore orientation. PLoS One. 2009;4:e6831. doi: 10.1371/journal.pone.0006831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregan J., Riedel C.G., Pidoux A.L., Katou Y., Rumpf C., Schleiffer A., Kearsey S.E., Shirahige K., Allshire R.C., Nasmyth K. The kinetochore proteins Pcs1 and Mde4 and heterochromatin are required to prevent merotelic orientation. Curr. Biol. 2007;17:1190–1200. doi: 10.1016/j.cub.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rumpf C., Cipak L., Schleiffer A., Pidoux A., Mechtler K., Tolic-Norrelykke I.M., Gregan J. Laser microsurgery provides evidence for merotelic kinetochore attachments in fission yeast cells lacking Pcs1 or Clr4. Cell Cycle. 2010;9:3997–4004. doi: 10.4161/cc.9.19.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Losada A., Hirano T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 2005;19:1269–1287. doi: 10.1101/gad.1320505. [DOI] [PubMed] [Google Scholar]
- 14.Tada K., Susumu H., Sakuno T., Watanabe Y. Condensin association with histone H2A shapes mitotic chromosomes. Nature. 2011;474:477–483. doi: 10.1038/nature10179. [DOI] [PubMed] [Google Scholar]
- 15.Corbett K.D., Yip C.K., Ee L.S., Walz T., Amon A., Harrison S.C. The monopolin complex crosslinks kinetochore components to regulate chromosome-microtubule attachments. Cell. 2010;142:556–567. doi: 10.1016/j.cell.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabitsch K.P., Petronczki M., Javerzat J.P., Genier S., Chwalla B., Schleiffer A., Tanaka T.U., Nasmyth K. Kinetochore recruitment of two nucleolar proteins is required for homolog segregation in meiosis I. Dev. Cell. 2003;4:535–548. doi: 10.1016/s1534-5807(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 17.Johzuka K., Horiuchi T. The cis element and factors required for condensin recruitment to chromosomes. Mol. Cell. 2009;34:26–35. doi: 10.1016/j.molcel.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Brito I.L., Yu H.G., Amon A. Condensins promote coorientation of sister chromatids during meiosis I in budding yeast. Genetics. 2010;185:55–64. doi: 10.1534/genetics.110.115139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro S.A., Gatlin J.C., Dong Y., Joglekar A., Cameron L., Hudson D.F., Farr C.J., McEwen B.F., Salmon E.D., Earnshaw W.C. Condensin regulates the stiffness of vertebrate centromeres. Mol. Biol. Cell. 2009;20:2371–2380. doi: 10.1091/mbc.E08-11-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]