Abstract
Cyanamide is an allelochemical produced by hairy vetch (Vicia villosa Roth.). Its phyotoxic effect on plant growth was examined on roots of onion (Allium cepa L.) bulbs. Water solution of cyanamide (2–10 mM) restricted growth of onion roots in a dose-dependent manner. Treatment of onion roots with cyanamide resulted in a decrease in root growth rate accompanied by a decrease in accumulation of fresh and dry weight. The inhibitory effect of cyanamide was reversed by its removal from the environment, but full recovery was observed only for tissue treated with this chemical at low concentration (2–6 mM). Cytological observations of root tip cells suggest that disturbances in cell division may explain the strong cyanamide allelopathic activity. Moreover, in cyanamide-treated onion the following changes were detected: reduction of mitotic cells, inhibition of proliferation of meristematic cells and cell cycle, and modifications of cytoskeleton arrangement.
Keywords: Allelopathy, Cell division, Cyanamide, Microtubules, Onion, Root growth
Introduction
Cyanamide (CA), named also hydrogen cyanamide (CN2H2), is an organic compound commonly applied in agriculture. It has been produced worldwide since early twentieth century, and in its form of calcium salt (CaCN2) has been used as synthetic fertilizer. It was demonstrated that cyanamide calcium salt, when applied to soil, may be hydrolyzed into active CA after contact with water molecules and then is converted into urea inter alia by cyanamide hydratase (Cah) produced by the fungus Myrothecium verrucaria (Maier-Greiner et al. 1991). Therefore, it may be considered as a source of inorganic nitrogen. Except of its fertilizing role, it is used as pesticide, fungicide, or nematocide (Zhang et al. 2005). CA was also applied for defoliation of cotton plants in order to facilitate harvesting of the bolls (Steller and Morgan 1965). Its more recent applications are mainly breaking dormancy of fruits (e.g. grape, apricot, apple), buds (Shulman et al. 1983, 1986; Halaly et al. 2008; Perez et al. 2008; Ophir et al. 2009) or seeds of grape or oil palm (Spiegel-Roy et al. 1987; Jiménez et al. 2008), or improving rooting in some plant species, e.g. apple, peach, kiwi, or grapevine (Guevara et al. 2008 and references herein).
Cyanamide was first found in hairy vetch (Vicia villosa subs. varia Roth.), in all of its vegetative organs including leaves, stems, roots, and in small amount in seed endosperm. The CA concentration in shoots was about 369–498 μg g−1 FW, whereas in roots and endosperm was lower than 170 μg g−1 FW (Kamo et al. 2003). Hairy vetch is an annual cover crop in a no-till cropping system used in Japan and USA as additional nitrogen source in soil; it also prevents evaporation of water and soil erosion (Inderjit and Asakawa 2001). Examination of about 550 species demonstrated that CA is a rare compound and besides hairy vetch it is produced only in bird vetch (Vicia cracca L.) reaching concentration 3,460–3,579 μg g−1 FW (Kamo et al. 2006) and in leaves of Robinia pseudoacaccia (at concentration about 440–460 μg g−1 FW) (Kamo et al. 2008).
Hairy vetch, as cover crop is known to suppress growth of many weeds. Its toxicity may be attributed to a high CA concentration. It was demonstrated that crude extract from hairy vetch leaves and stems containing 1.3 ppm of CA inhibited radicle growth of lettuce (Lactuca sativa L.) by 40% (Kamo et al. 2003). Moreover, it was established that inhibitory effect of hairy vetch on weed growth was not caused by phenolic compounds (Inderjit and Asakawa 2001).
Allelopathy is defined as plant to plant, plant to microorganism, and microorganism to microorganism interaction by chemicals belonging to the secondary metabolites (allelochemicals). Allelopathic compounds are released into the environment and usually provoke inhibitory effects in acceptor organisms (Gniazdowska and Bogatek 2005). Allelopathy is one of the most important phenomenon influencing natural ecosystems; it may be also considered as a strategy of interference in competitive crop cultivars (Bhowmik and Inderjit 2003). Many allelochemicals, produced by numerous species, are already known but in most cases, mechanism of their action remains unknown. In past decades, big efforts have been made to elucidate physiological effects of allelochemicals in acceptor plants. The goal for focusing research on target structure/processes of known allelochemicals is a possibility of taken advantage of allelopathy for natural weed management such as, for example, a natural herbicide.
Physiological effects of allelochemicals have been well determined only in a few cases, e.g. for juglone, (±)-catechin, sorgoleone or some phenolic compounds (Hejl and Koster 2004; Field et al. 2006; Ding et al. 2007; Chobot et al. 2009). They mainly include inhibition of photosynthesis and respiration, inhibition of enzymes activities, or modification in tissue water content. Little is known about cytological alterations induced by allelochemicals. There are only a few papers describing modifications in morphology and structure of plant cells after treatment with allelochemicals. Benzoic acid increased vacuolization, number of ER, and number of Golgi structures and induced changes in plastid structure in mustard (Brassica juncea L.) roots (Kaur et al. 2005). Sorgoleone inhibited division in bean (Phaseolus vulgaris L.) root tip cells (Hallak et al. 1999). It has also been shown that sorgoleone affected mitosis decreasing the percentage of cells in prophase, metaphase, and telophase. Gallic acid led to a destruction of microtubule assembly in Arabidopsis thaliana (L.) (Rudrappa et al. 2007). There are also limited data on impact of allochemicals on plant morphology. Chon et al. (2002) examined anatomical responses of alfalfa (Medicago sativa L.) roots to coumarins. It has been reported that coumarins caused seminal root enlargement, increased volume of vascular cylinder, and inhibited cell division (Chon et al. 2002). Coumarins (1.3 mM) modified also mitochondria structure in onion roots: condensation of electron-dense matrix and appearance of membrane protrusions (Kupidłowska et al. 1994).
Plant growth is dependent inter alia on proper cell division in root or stem tips. It is also known that proper cytoskeleton orientation that accompanies cell divisions is crucial for correct proceeding of mitosis (Wade 2009). Cell divisions are in turn prerequisite for root growth as newly formed cells are the only factors of the organ elongation via increasing their dimensions (mainly in line with long axis of the root; Ding et al. 2008; Wasteneys and Ambrose 2009). Rudrappa et al. (2007) found that water extract (20%, v/v) from noxious weed (Phragmites australis Cav.) caused disruption in root cell microtubule network in A. thaliana. But no evidence of impact of allelochemicals treatment on microtubule organization during cell divisions has been found.
The objective of the present study was to investigate the mode of action of CA during inhibition of root growth of onion (Allium cepa L.) bulbs. We performed cytological observations of root tip cells that allowed us to analyze modifications of cell division and cytoskeleton arrangement and analyzed the cell cycle activity by flow cytometry. We demonstrated that restriction in elongation growth of onion roots in reaction to CA is due to severe modification in root cell division and disturbances in formation of the cytoskeleton. Knowledge on the mode of action of allelochemicals (e.g. CA), which suppress growth of neighboring plants in field conditions may be useful in the future if we would like to use allelopathy as an ecological weed management tool for controlling crop productivity.
Materials and methods
Plant material
Onion bulbs were grown in 100 ml pots in distilled water in light at 20°C for 72 h. The bulbs were then transferred (day 0) to the distilled water (control) or to CA aqueous solutions at various concentrations: 2, 6 and 10 mM. Culture of onion bulbs was carried on in 100 ml pots for additional 6 days. Length, fresh weight (FW), dry weight (DW) of roots, and root growth rate (RGR) were determined after 0, 1 and 6 days of culture. Roots of bulbs continuously grown in water were used as control. DW of roots was determined using scales-drier RADWAG 50G, Radom, Poland. Roots were cut off and dried at 105°C to 3% water content.
Recovery effect after CA treatment
After 1 day culture in CA solutions (2, 6 and 10 mM), the bulbs were transferred into pots filled with fresh distilled water and cultured for additional 5 days. Length, FW, DW of roots, and RGR were determined at the beginning of CA treatment and after 1 and 6 days of culture.
Hydrogen peroxide (H2O2) concentration
H2O2 determination in freshly harvested roots of onion bulbs (control and treated by CA) was done according to Velikova et al. (2000). Roots (0.5 g) including meristematic, elongation, and differentiation zone were homogenized with 0.1% (w/v) cold TCA in ice. The obtained extract was centrifuged at 15,000g for 15 min at 4°C. The supernatant (0.5 ml) was added to 0.5 ml 10 mM potassium phosphate buffer (pH 7.0) and freshly prepared 1.0 ml 1 M KI in 10 mM potassium phosphate buffer (pH 7.0). The H2O2 concentration was determined using spectrophotometer Shimadzu UV 1700 by measurement of the absorption at 390 nm.
In situ localisation of superoxide anion
Roots including meristematic, elongation, and differentiation zone were incubated in 3 mM nitroblue tetrazolium (NBT) (Sigma-Aldrich) in 10 mM Tris–HCl buffer (pH 7.4) at room temperature for 15 min (Gniazdowska et al. 2010). Superoxide anion was visualized as deposit of dark blue insoluble formazan compounds (Beyer and Fridovich 1987).
Mitotic index
Mitotic index and changes in chromosome morphology were determined using the acetocarmine squash method. After 1-day treatment with CA, distal fragments of roots (1 cm long) were cut off and fixed in Carnoy’s fixative (3:1, v/v, glacial acetic acid–ethanol) for 24 h. Next, roots were washed three times for 15 min in 70% ethanol and incubated with acetocarmine (1% carmine in 45% acetic acid) at 30°C for 20 min, then transferred onto the slide. Two-millimeter-long root tips were cut off and a drop of acetocarmine was added. The tips were heated until cell walls were macerated and then squashed. The mitotic index was counted after 1-day treatment with CA. All root tip cells were counted and results were calculated as percentage of each phase in relation to control. Changes in chromosome morphology and mitotic index were observed under light microscope Olympus AX70 PROVIS and photographed using programs View Finder and Studio Lite (Olympus). For each experiment, ten onion root tips from five biological repetitions were analysed.
Cell cycle
After 1-day incubation in water, in the CA solutions, and after recovery, meristematic zone (about 2 mm long) of onion roots was cut off. Five root tips from various onions formed a sample. Samples were prepared as previously described (Sliwinska and Łukaszewska 2005). For each sample, the DNA content in 8,000–10,000 nuclei was measured using a Partec CCA flow cytometer (Partec, Münster, Germany). Analyses were performed on five biological replicates. Histograms were analyzed using a DPAC v.2.2 computer program (Partec) and the percentage of the nuclei of particular DNA contents as well as the G2/G1 ratios were calculated.
Immunolocalization of microtubule cytoskeleton
As an important component of the cytoskeleton in the cell cycle, microtubule assemblies in meristematic cells were investigated using an immunocytological method. Material for immunolocalization studies was prepared essentially according to de Almeida Engler et al. (2001). In brief, after 1-day treatment with CA (2, 6 or 10 mM) the 0.5 cm long root tips were fixed for 3 h in a fixative [4% paraformaldehyde, 0.25% glutaraldehyde in microtubule stabilizing buffer (MSB), pH 6.9]. After rinsing in MSB (twice) and phosphate buffered saline (PBS) the samples were dehydrated in an ethanol series supplemented with 10 mM dithiothreitol (DTT) and finally embedded in butyl-methyl methacrylate (BMM). Next, the samples were sectioned longitudinally and 3.5 μm thick sections were transferred to slides coated with poly-l-lysine. After removing resin roots tips were rehydrated in PBS.
For blocking unspecific epitopes, sections were incubated in 0.1 M NH4Cl in PBS for 5 min and rinsed twice in PBS for 5 min, then incubated for 45 min in 1% bovine serum albumin (BSA) at 20°C. After washing twice with PBS for 5 min, sections were incubated with monoclonal mouse anti-α-tubulin IgG (clone B-5-1-1, Sigma-Aldrich) diluted 1:150 in PBS containing 1% BSA for 45 min at 37°C. As a negative control, 1% BSA in PBS solution was applied in this step. After incubation with anti-α-tubulin IgG sections were washed three times with PBS for 15 min and incubated with the BSA solution three times for 10 min. Then sections were incubated in darkness with goat anti-mouse IgG conjugated with Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA) diluted 1:200 in the BSA solution for 1 h at 37°C. After three rinses with PBS for 15 min, sections were incubated in 2,4-diamidino-2-phenylindole (DAPI) (0.3 μg ml−1) for 15 min, rinsed with PBS twice for 5 min, and mounted in 1% glycerol. Fluorescence was examined with Olympus AX70 PROVIS microscope equipped with FITC filter. Images were acquired using following programs: View Finder and Studio Lite (Olympus). Each experiment was repeated at least five times and data from representative, individual experiments are presented.
Statistics
Data were analyzed using the StatGraphics 5.1 Software. Mean values were computed for each experiment and mean differences were calculated using Tukey’s studentized range test. Standard errors (SE) were also provided to indicate the variations associated with the particular mean values.
Results
CA modifies growth of onion bulb roots
Roots of control (untreated) onion bulbs grew well during the whole period of culture (Table 1). At the beginning of the experiment the length of roots of control bulbs was about 8 mm and increased to 23 mm after additional 6 days. Roots of bulbs treated with CA were shorter than control, after 6 days their length was ca. 16 or 9 mm for 2 and 6 mM CA, respectively (Table 1). The effect of CA on length of roots was dose dependent in lower concentration of CA (2–6 mM). No significant differences in root length, were observed in roots treated with 6 and 10 mM CA.
Table 1.
Root length (mm) of control (non-treated) onion bulbs, bulbs treated with CA continuously for 1 or 6 days and after recovery phenomenon (1 day CA + 5 days H2O)
| 0 | 1 day | 6 days | 1 day CA + 5 days H2O | |
|---|---|---|---|---|
| Root length (mm) | ||||
| Control | 7.9 ± 0.23 | 19.43 ± 0.24a | 22.97 ± 0.26a | – |
| CA 2 mM | – | 12.62 ± 0.16b | 16.23 ± 0.31b | 37.04 ± 0.71a |
| CA 6 mM | – | 8.84 ± 0.48c | 8.86 ± 0.24c | 17.50 ± 0.58b |
| CA 10 mM | – | 7.93 ± 0.49c | 8.01 ± 0.65c | 12.20 ± 0.36c |
Values for particular treatment (in columns, ±SE) followed by different small letter are significantly different at P = 0.05, N = 100 (Tukey’s test)
The effect of short (1 day) treatment with CA was reversed by 5-day-long culture in water, but was dependent on CA concentration (Table 1). Recovery effect after treatment with CA at low (2 mM) concentration led to stimulation of root growth. Roots of onion bulbs treated with 2 mM CA and transferred to water for additional 5 days were approximately 61% longer as compared with control (Table 1). Roots of onion bulbs treated 24 h with 6 mM CA and transferred to water grew well; length of roots after 5 days was only ca. 5 mm shorter than controls. Short-term treatment of roots with CA at high (10 mM) concentration resulted in irreversible modification of root growth; after 5 days of culture in water roots were twice as short as control roots (Table 1).
Fresh and dry weight of roots of control non-treated onion bulbs increased continuously during experiment (Table 2). FW of control roots was approximately 83 mg at the beginning of culture and increased twice, up to 152 mg after 6 days. Similarly, DW of control roots was 9 mg on day 0, and after 6 days it increased for more than 6 mg (Table 2). CA treatment significantly decreased FW of roots, just after the first day of the experiment. The inhibitory effect of CA on FW and DW of roots was dose dependent (Table 2). The strongest effect of CA treatment on FW rate was observed in onions treated with 10 mM CA. After 6 days of exposure to 10 mM CA, both FW and DW of roots decreased significantly to 63 and 3.3 mg, respectively.
Table 2.
Fresh weight (FW) and dry weight (DW) of roots of onion bulbs cultured in water (control) or bulbs treated with CA (2–10 mM) continuously for 1 or 6 days and after recovery phenomenon (1 day CA + 5 days H2O)
| 0 | 1 day | 6 days | 1 day CA + 5 days H2O | |
|---|---|---|---|---|
| FW (mg) | ||||
| Control | 83 ± 4 | 140 ± 6.12a | 152 ± 4.12a | 175 ± 4.00a |
| CA 2 mM | – | 117 ± 13.21b | 121 ± 14.32b | 283 ± 13.17b |
| CA 6 mM | – | 89 ± 2.15c | 91 ± 6.54c | 163 ± 5.02c |
| CA 10 mM | – | 83 ± 3.18d | 63 ± 5.76d | 123 ± 2.46d |
| DW (mg) | ||||
| Control | 9 ± 0.23 | 11 ± 0.52a | 15.2 ± 0.17a | 15.3 ± 0.16a |
| 2 mM | – | 10 ± 0.70a | 9.4 ± 0.26b | 20 ± 0.47b |
| 6 mM | – | 9 ± 0.23b | 8.5 ± 0.25c | 12 ± 0.36c |
| 10 mM | – | 8.5 ± 0.39c | 3.3 ± 0.09d | 11 ± 0.17d |
Values for particular treatment (in columns, ±SE) followed by different small letter are significantly different at P = 0.05, N = 10 (Tukey’s test)
Recovery effect of CA treatment resulted in increased roots FW (Table 2). The best recovery effect on FW of roots was observed for onions cultured in 2 mM CA; FW increased in about 217% in comparison with day 0 (Table 2).
Cyanamide induced similar modification in root DW (Table 2), with the only exception of DW after 1-day treatment with 2 mM CA, which was not significantly different as compared with the control (Table 2).
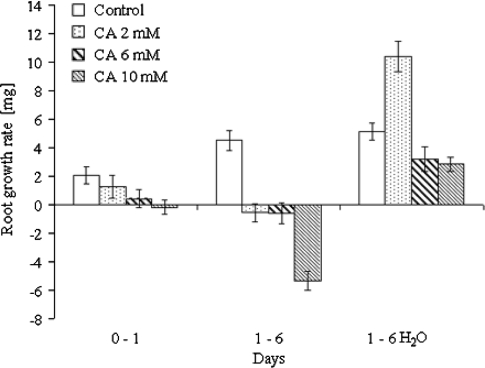
Dynamics of RGR was determined as DW increase in periods of 0–1 day and 1–6 days. RGR of control (non-treated) bulbs increased continuously during the culture (Fig. 1). In CA-treated onion bulbs RGR was significantly lower in comparison with the control at the initial period of experiment (0–1 days), and was dose dependent. CA at the highest concentration (10 mM) completely halted increase of root DW (Fig. 1). This effect was even more visible as CA treatment was prolonged between first and 6th day (Fig. 1). The strongest inhibitory effect on RGR was detected after 10 mM CA; approximately 12 mg of dry weight loss in comparison with control was observed (Fig. 1).
Fig. 1.
Root growth rate determined as DW increase in periods of 0–1 day and 1–6 days and after recovery period. Onion bulbs were cultured in water (control) or continuously in CA solution (2–10 mM). The recovery effect of CA was determined as described in “Materials and methods”. Values are presented as the mean (±SE), n = 10
Recovery effect revealed that inhibition of dry weight accumulation by CA was partially reversible. Roots treated with 6 and 10 mM CA were characterized by positive RGR but they did not reach values of control roots. The strongest effect on RGR was observed for recovery after 2 mM CA; RGR values were approximately twice as high as the values of controls (Fig. 1).
CA induces oxidative stress in roots of onion bulbs
Effect of CA on H2O2 accumulation
H2O2 concentration in the tissue was determined after root treatment with CA. The concentration of H2O2 in control roots in water was constant during the culture period and reached the value about 35 μmol g−1FW (Table 3). In onion roots cultured in CA at the lowest (2 mM) concentration, the H2O2 content was even lower than that in control roots mainly after 1 day of treatment. CA (6 mM) led to approximately twofold increase in H2O2 concentration in roots treated for 6 days (Table 3). The highest H2O2 concentration was measured in roots exposed to 10 mM CA for only 1 day. Prolonged CA application decreased the H2O2 concentration probably due to induction of tissue degradation.
Table 3.
H2O2 concentration in roots of control (non-treated) onion bulbs and onion bulbs cultured continuously in 2–10 mM CA
| 0 | 1 day | 6 days | |
|---|---|---|---|
| H2O2 (μmol g−1 FW) | |||
| Control | 96.36 ± 3.19 | 35.71 ± 2.25a | 35.21 ± 7.60a |
| 2 Mm | – | 17.32 ± 2.02b | 33.13 ± 2.40a |
| 6 mM | – | 25.43 ± 3.64c | 51.11 ± 2.72b |
| 10 mM | – | 153.38 ± 1.54d | 65.09 ± 8.16c |
Values for particular treatment (in columns, ±SE) followed by different small letter are significantly different at P = 0.05, N = 10 (Tukey’s test)
Visualization of superoxide anion in roots of onion bulbs
In situ accumulation of O2 − in control roots of onion bulbs or roots treated by CA is presented in Fig. 2. At the whole-tissue level, in roots of onion bulbs cultured in water, an accumulation of formazan after 1 day occurred mostly at the elongation and differentiation zones, while after additional 5 days dark staining was detected rather in root tips. Homogeneous accumulation of formazan in roots treated with 10 mM CA was observed just after 1 day of exposure to the allelochemicals. In roots treated with 6 mM CA accumulation of formazan was also observed after just 1 day and mainly in the elongation and differentiation zones, similarly as in control plants. Only minor staining was detected in root tips (Fig. 2). Prolonged (6 days) treatment with the highest (10 mM) concentration of CA resulted in degradation of the tissue, and no NBT staining was visualized.
Fig. 2.
Visualization of superoxide anion in roots of control (non-treated) onion bulbs and onion bulbs cultured continuously in 2–10 mM CA
CA induces alterations in mitotic activity of root tip cells
Mitotic index in cells of onion root tips is modified by CA
Cyanamide modified the proportions of phases of mitosis depending on the concentration of the allelochemicals. The percentage of dividing cells decreased gradually in root tips treated with CA, and this effect was dose dependent (Table 4). CA caused a decrease of the all phase index. The strongest effect was observed in roots cultured in 10 mM CA, where only telophases were observed (Table 4).
Table 4.
Mitotic index in root meristem of control (non-treated) and CA treated onion bulbs 1 day after CA application
| Dividing cells | Phases of mitosis | ||||
|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||
| Control | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| 2 mM | 14.44 ± 3.23b | 13.11 ± 2.11b | 20.73 ± 3.12b | 13.44 ± 2.76b | 10.03 ± 3.10b |
| 6 mM | 2.19 ± 0.74c | 2.60 ± 0.97c | 2.78 ± 0.76c | 0.77 ± 0.15c | 0.79 ± 0.10c |
| 10 mM | 0.65 ± 0.27d | 0.00d | 0.00d | 0.00d | 4.50 ± 1.02d |
Data are expressed as percentage of control
Values for particular treatment (in columns, ±SE) followed by different small letter are significantly different at P = 0.05, N = 10 (Tukey’s test)
Effect of CA on mitotic activity of root tip cells
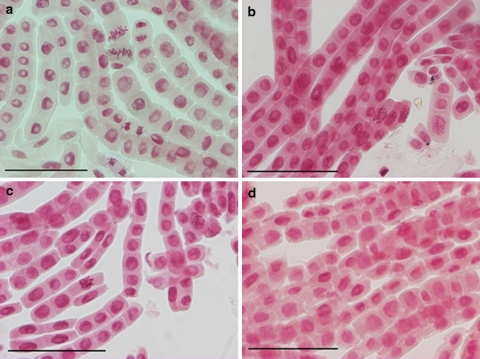
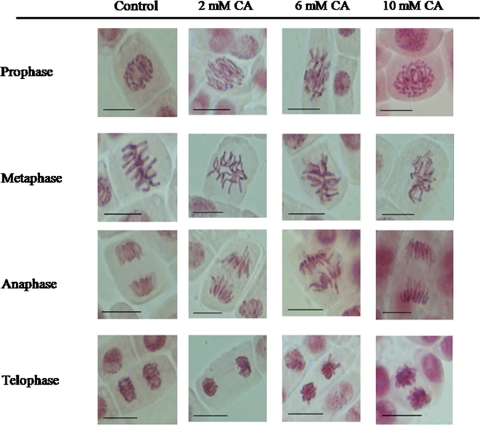
Cyanamide induced severe modification in cell division of onion roots. In control, non-treated roots every phase of mitosis and appropriate mitotic figures were observed (Fig. 3). In the prophase, chromatin condensation and formation of chromosomal territories were detected. The metaphase was represented as metaphase plate with thickened chromosomes with pointed centromers and chromatides. In the anaphase, two groups of chromosomes were observed that moved in orderly way; at the telophase, chromatids reached the pools and formed oval nuclei (Fig. 4). Influence of 2 or 6 mM CA on mitosis was manifested mainly by alterations in formation of the metaphase plate. No metaphase plate was observed in cells of roots growing in cyanamide solution (Fig. 4). Chromosomes were dispersed over the entire cell. The structure of chromosomes was also affected by CA and resulted in chromosome shortening, condensation. CA at the highest concentration (10 mM) arrested the mitotic activity in roots cells; thus only telophases were seen (Fig. 4).
Fig. 3.
Cells from the meristematic zone of onion root tips. Roots were treated with CA for 1 day. Control (a) and treatment with 2 mM CA (b), 6 mM CA (c), and 10 mM CA (d). Bars 100 μm
Fig. 4.
Phases of mitosis in control and 1-day CA-treated root tip cells. Bars 20 μm
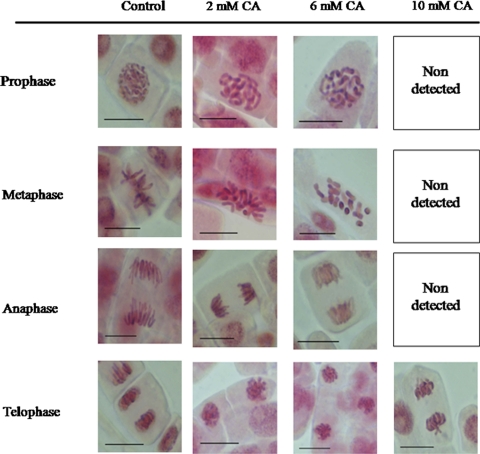
Effect of CA treatment on mitosis was fully reversible (Fig. 5). All phases of mitosis were frequent in root tip cells after recovery of the treatment with 2 and 6 mM CA as well as 10 mM CA. Chromosomes did not always form the correct metaphasal plate but were localized in the center of the cell and in horizontal position. The structure of chromosomes was similar as in the control root tip cells, with thin chromatids, and apparent centromers. Correct prophases and telophases after recovery experiment were observed (Fig. 5).
Fig. 5.
Phases of mitosis in control and 1-day CA-treated root tips and recovered 1 day in water. Bars 20 μm
Effect of CA on the cell cycle
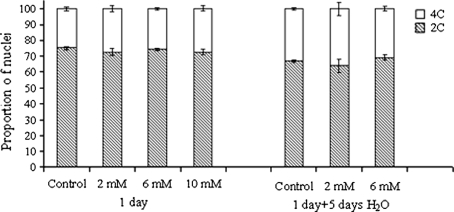
Root tips of all investigated onion bulbs contained nuclei with 2C (G1/G0 phase of the cell cycle) and 4C DNA (G2 phase of the cell cycle; Fig. 6), altbeit in different proportions depending on the treatment. In the root tips of the bulbs incubated for 1 h in CA the proportion of 4C nuclei was about 1–1.5% higher than in those incubated in water. The differences were more distinct after further 5 days in water (recovery), when in the tips treated with 2 and 6 mM CA the proportion of 4C nuclei was 3% higher and 2% lower than in the control, respectively. The G2/G1 ratio significantly increased during this period in all treated and non-treated bulb root tips (Table 5). It reached the highest value in those treated with 2 mM CA. No data were determined for recovery after 10 mM CA.
Fig. 6.
Changes in 2C (G1/G0 phase), 4C (G2 phase) in nuclei of onion root tip cells after 1-day CA treatment and after recovery (1 day in CA and 5 days in water). Values are presented as the mean (±SE), n = 5
Table 5.
The G2/G1 ratio in the cells of onion root tips treated with CA and after recovery
| 1 day | 1 day CA + 5 days H2O | |
|---|---|---|
| G2/G1 ratio | ||
| Control | 0.335 ± 0.021a B* | 0.494 ± 0.014 b** A |
| 2 mM | 0.385 ± 0.041 B | 0.604 ± 0.103 a A |
| 6 mM | 0.350 ± 0.011 B | 0.449 ± 0.036 b A |
| 10 mM | 0.385 ± 0.033 | – |
Values are presented as the mean (±SE), N = 5
ano significant difference
*values for particular CA concentration followed by different capital letter (in lines) are significantly different at P = 0.05 (Tukey’s test)
**values for particular treatment (in columns) followed by different small letter are significantly different at P = 0.05 (Tukey’s test)
CA modifies the cytoskeleton arrangement
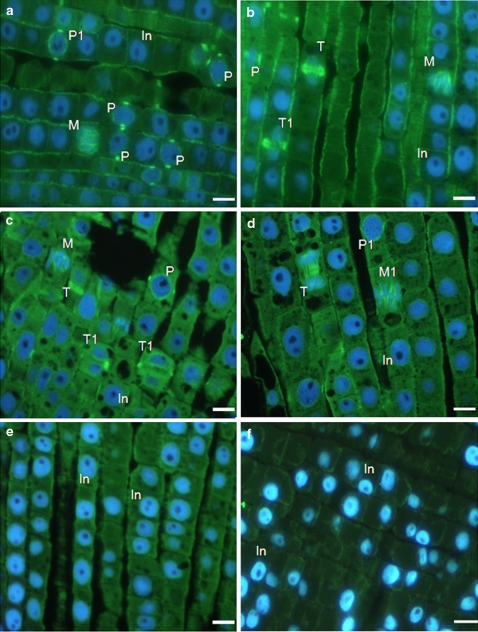
Experiments focused on the mitotic index revealed that in CA-treated plants chromatin condensation and chromosome formation took place but no metaphase plate was established. This suggests that cyanamide may influence those cytoskeleton assemblies which are responsible for concerted chromosome movement during karyokinesis. To analyze this, an immunocytological approach was used to search for specific assemblies of microtubules characteristic for consecutive mitotic phases in meristematic cells of control and CA-treated root tips. Control experiments (with no primary antibody applied) resulted in no green signal on sections (data not shown) thus proving validity of the method. While applying full protocol for immunolabeling (with primary antibody), it was observed that in control (non-treated) plants all mitotic stages were present and the accompanying microtubule arrangement was visualized: preprophase band, mitotic spindle as well as phragmoplast (Fig. 7a, b). In sections from roots treated with 2 mM CA no pronounced differences in comparison with control could be seen. Typical microtubule arrays related to successive karyokinetic stages and to cytokinesis were observed (Fig. 7c, d). In contrast, incubation with higher concentrations of CA (6 and 10 mM) resulted in evident disappearance of the characteristic cytoskeleton patterns like mitotic spindle or phragmoplast as well as mitotic figures (meant as chromosome assemblies) (Fig. 7e, f). On the last photographs immunolocalization reveals only faint, dispersed green coloration which may be ascribed to globular tubulin or thin, single microtubules and no massive cytoskeleton assemblies looking like, e.g. preprophase band could be found.
Fig. 7.
Immunofluorescent detection of microtubule arrays in meristematic cells of onion root tips (longitudinal sections). Controls (a, b) and treatment with 2 mM CA (c, d), 6 mM CA (e) and 10 mM CA (f) for 1 day. Microtubules are visible as green coloration due to Alexa Fluor 488 fluorescence. Blue coloration of nuclei is due to counterstaining with DAPI. In cell in interphase, M cell in prometaphase/metaphase with mitotic spindle, M1 cell in metaphase with mitotic spindle, P cell in prophase with preprophase band, P1 cell in prophase with preprophase band and emerging mitotic spindle, T cell in telophase with phragmoplast, T1 cell in late telophase with declining phragmoplast. Bars 20 μm
Discussion
The mode of action of allelochemicals usually involves alteration in one of crucial physiological processes, e.g. photosynthesis, respiration, water or nutrient transport (Weir et al. 2004; Gniazdowska and Bogatek 2005). Inhibition of any of them results in disturbances in plant growth and development. We have demonstrated, for the first time, CA toxicity during onion bulb root growth. The inhibitory effect of CA on onion root growth was dose dependent (Tables 1, 2). A similar restriction in plant growth after treatment with specific particular allelochemicals was detected by many authors (e.g. Politycka and Mielcarz 2007; Batish et al. 2008). Cinnamic acid at a concentration of 0.05 mM inhibited growth of cucumber (Cucumis sativus L.) roots by 30% and at 0.25 mM by 55% (Ding et al. 2007). Benzoic acid at concentration 68, 150, and 250 μg g−1 of soil caused 30, 59, and 81% inhibition of mustard root growth, respectively (Kaur et al. 2005). In our experiment, 6-day-long treatment with 10 mM CA aqueous solution totally blocked elongation growth in onion roots (Table 1). Kamo et al. (2003) showed that CA at a concentration of 10 μM inhibited lettuce (Lactuca sativa L.) radicle growth by up to 40%. The comparison of the above data indicates that CA effect on plant growth depends not only on the concentration but also on the susceptibility of the acceptor plant species.
The recovery effect of allelopathic stress was not often reported (Oracz et al. 2007). In our experiment, reversible effect of CA treatment (2–10 mM) on onion root growth was observed after short-term exposure to 2 mM CA, in that treated roots (grown in CA and then in water) were even longer than controls (Table 1). This is in line with the observation of Ding et al. (2010) demonstrating that allelochemicals at low concentration could act as plant growth stimulators. The authors report that 40 μM robdopsin B significantly promoted root length of lettuce. Similarly, increase in root length of sorghum [Sorghum bicolor L. (Moench)] seedlings cultured in water extract of senna (Cassia angustifolia Vahl.) was detected (Hussain et al. 2007).
Dose-dependent restriction of onion root growth by CA resulted in alterations in fresh and dry weight accumulation (Table 2). That phenomenon did not occur due to low absorption of water by the roots, since CA insignificantly reduced water osmotic potential. Ten milli Molar CA aqueous solution was characterized by osmotic potential ψ s = −0.15 MPa (data not shown). Therefore, another explanation for the disruption of plant growth by tested allelochemicals shall be considered. There are many examples of growth restriction and decrease of plant weight in response to allelochemicals. Bogatek et al. (2006) observed that various concentrations of aqueous sunflower leaf extracts induced alterations in fresh and dry weight accumulation in mustard seedling. (−)-Catechin caused approximately 50% inhibition of dry weight accumulation in festuca (Festuca idahoensis Elmer) and 60% in koeleria (Koeleria micrantha Lam.) seedlings (Bais et al. 2003). Gallic acid reduced fresh weight of A. thaliana seedling in approximately 80% (Rudrappa et al. 2007).
Dose-dependent effects on RGR were observed after onion root treatment with CA at all tested concentrations. After 6 days of culture, RGR of roots treated with CA were lower than in control (Fig. 1). Additionally, in CA solution at the highest concentration the turning brown and rotting of onion roots was detected (data not shown). Similar observations were reported for lettuce seedlings treated with robdosin B from Isodon japonica (Burm. f) at concentrations of 120–200 μM (Ding et al. 2010).
Visible restriction of plant root growth may result from alterations in cell division and/or cell ultrastructure. CA induced modification in root tip cells division pattern (Fig. 3). Cytotoxic effect of that compound was dose dependent and included a reduction of the mitotic index. CA decreased or totally inhibited (at 10 mM concentration) the number of cells at mitosis (Table 4). Comparable results were reported for lettuce seedlings treated with leukamenin E, which induced chromosome stickiness, chromosome bridges, multipolar divisions or C-colchicine metaphase (Ding et al. 2008). In our material, shortening and condensation of chromosomes after CA application may indicate alteration in chromatin organization. It may be related to quantity or quality of histones or other proteins responsible for proper chromatin arrangement (Jin et al. 2008). However, according to Sánchez-Moreiras et al. (2008), not all allelochemicals cause chromosomal abnormalities or such observations are rare to date. Culture of lettuce seedling in 1 mM BOA (2(3H)-benzoxazolinone) decreased the number of cells in mitosis but did not affect chromosomal structure and orientation (Sánchez-Moreiras et al. 2008). In our experiment, decrease of frequency of each phase of mitosis and presence of chromosomes diffused within the whole cell in onion root tips treated with CA (Fig. 4) suggest that the allelopatic compound might influence the mitotic spindle organization. Hallak et al. (1999) found that 0.1 mM sorgoleone treatment of bean plants induced a number of colchicine metaphases; similar results were obtained in our material. Those authors suggested that the allelochemical impact on mitosis could be the effect of depolymerization of microtubules which blocks the formation of the mitotic spindle. In our study no mitotic figures were found after onion root treatment with high CA concentrations (6 and 10 mM). In addition, we noted that CA severely hampered formation of microtubule arrays involved in karyokinesis (Fig. 7), which may result either from depolymerization of microtubules or from hyperstabilization of these structures in late interphase cell so that no tubulin heterodimers for building structures like a mitotic spindle were available. Lack of this spindle could explain abnormal metaphases and also confirms our other cytological observations.
Treatment of A. thaliana with gallic acid (50 μM) totally disrupted microtubule orientation, not only in the root tip (Rudrappa et al. 2007). It was suggested that microtubule reorganization and network disruption may be strongly correlated with accumulation of reactive oxygen species (ROS) leading to damage of microtubule protein (Rudrappa et al. 2007). Recently, it was proven that alteration in ROS production or accumulation in different root zones of A. thaliana might severely influence root growth (Tsukagoshi et al. 2010). A correlation between growth rate, localization of transition zone (meristem to elongation zone) and relative distribution of different ROS (O2 − vs. H2O2) in the meristematic and elongation zone was demonstrated. In addition, specific ROS balance in root may be regulated by the newly identified transcription factor UPB1, which independently of auxin and cytokinin signaling could regulate peroxidase activity (Tsukagoshi et al. 2010). Induction of oxidative stress (expressed as overproduction of ROS) by allelochemicals seems to be a general phenomenon reported previously for many plants (Bais et al. 2003; Oracz et al. 2007). Our data also confirmed such a tendency. CA treatment resulted in overaccumulation of ROS both O2 − and H2O2. NBT staining intensity indicated O2 − accumulation in elongation and differentiation zone of roots of onion bulbs at the beginning of CA treatment, and then also in root tips (Fig. 2). Mild allelopathic stress induced by 6 mM CA led to double, as compared with control, accumulation of H2O2, while strong stress (10 mM CA) resulted in even lethal enhancement of H2O2 production (Table 3). It was well correlated with the decrease of viability (50%) of onion root cells in reaction to increased concentration of CA applied in the experiment (data not shown). It is possible that CA-mediated inhibition of onion root growth may be due to alterations in O2 − and H2O2 gradient resulting in the movement of the transition zone from cellular proliferation to differentiation. Unfortunately, we cannot simply compare H2O2 and O2 − distribution between meristematic and elongation zones in roots of onion bulbs, as in our experiment H2O2 was determined in relatively long fragments of the root, including cells from both zones. We may suspect that H2O2 accumulated mostly in elongation and differentiation zone, as Córdoba-Pedregosa et al. (2007) reported a non-homogenous accumulation of this metabolite in roots, increasing toward the onion bulb base. It should be mentioned that a rise in H2O2 level in response to CA was observed also in grapevine buds during dormancy breakage (Perez et al. 2008). Moreover, in this tissue CA inhibited also catalase activity (Perez and Lira 2005) and lowered its transcript level just after 1 day of treatment (Halaly et al. 2008). We may suggest that relatively low concentrations of H2O2 in onion roots treated with 2 mM CA may result from the induction of the cellular antioxidative system, mainly activity of catalase (CAT) and glutathione reductase (GR) similarly as it was detected for sunflower allelochemicals (Oracz et al. 2007). In addition, it was demonstrated that in grapevine buds during dormancy removal CA enhanced gene expression of GR and ascorbate peroxidase (APX) (Halaly et al. 2008).
The inhibitory effect of CA on the mitotic index (Table 4) in onion root tip cells may be the cause of arresting the cell cycle. Measurement of nuclear DNA content in root tip cells showed no significant differences after CA treatment (Table 5). This suggests that not only mitosis but also DNA synthesis was inhibited. As CA at higher concentration (6, 10 mM) had a more pronounced effect on the mitotic index, it elucidates the question whether CA caused the damage of DNA. Similar structure of interphase nuclei in control and CA-treated cells (Fig. 3) appears not to support this speculation. In some cases stress factors such as oxidative stress could provoke cell cycle retardation at G1/S and/or G2/M phases and contribute to reduction in mitotic index (Den Boer and Murray 2000). In our experiment, the most probable explanation of alterations in cell division seems to be the blockage of transition at the G2/M checkpoint caused by CA, especially since such arresting of cells ability to enter mitosis by allelochemicals was reported previously. Treatment of cucumber roots with 0.25 mM phenylcarboxylic acid resulted in inhibition of CycB gene expression, a marker gene specific to the G2/M phase (Zhang et al. 2009).
All data presented suggest that the allelopathic effect of CA on onion root growth is a complex phenomenon. It includes induction of oxidative stress, reduction of the number of mitotic cells, inhibition of proliferation of meristematic cells and cell cycle with modifications of the cytoskeleton arrangement. In general, we postulate that CA-induced onion root growth inhibition is related mostly to disturbances in cell division. To get deeper insight into that mechanism further research is necessary. Especially helpful would be the elucidation at the molecular level at which checkpoint of cell cycle the allelochemicals may act.
Acknowledgments
Part of the work was financed by Polish Ministry of Science and Higher Education Grant No N N310 067739. We thank Marek Bogatek for the English correction of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- APX
Ascorbate peroxidase
- BMM
Butyl-methyl methacrylate
- BOA
2(3H)-benzoxazolinone
- BSA
Bovine serum albumin
- CA
Cyanamide
- Cah
Cyanamide hydratase
- CAT
Catalase
- DAPI
2,4 Diamidino-2-phenylindole
- DTT
Dithiothreitol
- DW
Dry weight
- FW
Fresh weight
- GR
Glutathione reductase
- MSB
Microtubule stabilizing buffer
- NBT
Nitroblue tetrazolium
- PBS
Phosphate buffered saline
- ROS
Reactive oxygen species
- RGR
Root growth rate
References
- Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science. 2003;301:1377–1380. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- Batish DR, Singh HP, Kaur S, Kohli RK, Yadav SS. Caffeic acid affects early growth and morphogenic response of hypocotyl cuttings of mung bean (Phaseolus aureus) J Plant Physiol. 2008;165:297–305. doi: 10.1016/j.jplph.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Beyer WF, Fridovich I. Assaying for superoxide oxidase dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Bhowmik PC, Inderjit J. Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot. 2003;22:661–671. doi: 10.1016/S0261-2194(02)00242-9. [DOI] [Google Scholar]
- Bogatek R, Gniazdowska A, Zakrzewska W, Oracz K, Gawroński SW. Allelopathic effects of sunflower extracts on mustard seed germination and seedling growth. Biol Plant. 2006;50:156–158. doi: 10.1007/s10535-005-0094-6. [DOI] [Google Scholar]
- Chobot V, Huber C, Trettenhahn G, Hadacek F. (±)-Catechin: chemical weapon, antioxidant, or stress regulator? J Chem Ecol. 2009;35:980–996. doi: 10.1007/s10886-009-9681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chon S-U, Choi S-K, Jung S, Jang H-G, Pyo B-S, Kim S-U. Effects of alfalfa leaf extracts and phenolic allelochemicals on early seedling growth and root morphology on alfalfa and barnyard grass. Crop Prot. 2002;21:1077–1082. doi: 10.1016/S0261-2194(02)00092-3. [DOI] [Google Scholar]
- Córdoba-Pedregosa MC, Villalba JM, Córdoba F, González-Reyes JA. Changes in growth pattern, enzymatic activities related to ascorbate metabolism, and hydrogen peroxide in onion roots growing under experimentally increased ascorbate content. J Plant Regul. 2007;26:341–350. doi: 10.1007/s00344-007-9021-8. [DOI] [Google Scholar]
- de Almeida Engler J, De Groodt R, Van Montagu M, Engler G. In situ hybridization to mRNA of Arabidopsis tissue sections. Methods. 2001;23:325–334. doi: 10.1006/meth.2000.1144. [DOI] [PubMed] [Google Scholar]
- Den Boer BGW, Murray JAH. Triggering the cell cycle in plants. Trends Cell Biol. 2000;10:245–250. doi: 10.1016/S0962-8924(00)01765-7. [DOI] [PubMed] [Google Scholar]
- Ding J, Sun Y, Xiao CL, Shi K, Zhou YH, Yu JQ. Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J Exp Bot. 2007;58:3765–3773. doi: 10.1093/jxb/erm227. [DOI] [PubMed] [Google Scholar]
- Ding L, Qi L, Jing H, Li J, Wang W, Wang T. Phytotoxic effects of leukamenin E (an ent-kaurene diterpenoid) on root growth and root hair development in Lactuca sativa L. seedlings. J Chem Ecol. 2008;34:1492–1500. doi: 10.1007/s10886-008-9556-6. [DOI] [PubMed] [Google Scholar]
- Ding L, Jing H, Qin B, Qi L, Li J, Wang T, Liu G. Regulation of cell division and growth in roots of Lactuca sativa L. seedlings by the ent-kaurene diterpenoid rabdosin B. J Chem Ecol. 2010;36:553–563. doi: 10.1007/s10886-010-9783-5. [DOI] [PubMed] [Google Scholar]
- Field B, Jordan F, Osbourn A. First encounters–deployment of defense-related natural products by plants. New Phytol. 2006;172:193–207. doi: 10.1111/j.1469-8137.2006.01863.x. [DOI] [PubMed] [Google Scholar]
- Gniazdowska A, Bogatek R. Allelopathic interactions between plants. Multi site action of allelochemicals. Acta Physiol Plant. 2005;27:395–407. doi: 10.1007/s11738-005-0017-3. [DOI] [Google Scholar]
- Gniazdowska A, Krasuska U, Czajkowska K, Bogatek R. Nitric oxide, hydrogen cyanide and ethylene are necessary in the control of germination and undisturbed development of young apple seedlings. Plant Growth Regul. 2010;61:75–84. doi: 10.1007/s10725-010-9452-2. [DOI] [Google Scholar]
- Guevara E, Jiménez VM, Herrera J, Bangerth F. Effect of hydrogen cyanamide on the endogenous hormonal content of pea seedlings (Pisum sativum L.) Braz J Plant Physiol. 2008;20:159–163. doi: 10.1590/S1677-04202008000200008. [DOI] [Google Scholar]
- Halaly T, Pang X, Batikoff T, Crane O, Keren A, Venkateswari J, Ogrodovitch A, Sadka A, Lavee S, Or E. Similar mechanisms might be triggered by alternative external stimuli that induce dormancy release in grape buds. Planta. 2008;228:79–88. doi: 10.1007/s00425-008-0720-6. [DOI] [PubMed] [Google Scholar]
- Hallak AMG, Davide LC, Souza IF. Effects of sorghum (Sorghum bicolor L.) root exudates on the cell cycle of the bean plant (Phaseolus vulgaris L.) root. Genet Mol Biol. 1999;22:95–99. doi: 10.1590/S1415-47571999000100018. [DOI] [Google Scholar]
- Hejl AM, Koster KL. Juglone disrupts root plasma membrane H+-ATPase activity and impairs water uptake, root respiration, and growth in soybean (Glycine max) and corn (Zea mays) J Chem Ecol. 2004;30:453–471. doi: 10.1023/B:JOEC.0000017988.20530.d5. [DOI] [PubMed] [Google Scholar]
- Hussain A, Siddiqui SU, Khalid S, Jamal A, Qayyum A, Ahmad Z. Allelopathic potential of senna (Cassia angustifolia Vahl.) on germination and seedling characters of some major cereal crops and their associated grassy weeds. Pak J Bot. 2007;39:1145–1153. [Google Scholar]
- Inderjit, Asakawa C (2001) Nature of interference potential of hairy vetch (Vicia villosa Roth) to radish (Raphanus sativus L.): does allelopathy play any role? Crop Prot 20:261–265
- Jiménez VM, Guevara E, Herrera J, Alizaga R, Bangerth F. Changes in hormone concentrations during dormancy release of oil palm (Elaeis guineensis) seeds. Seed Sci Tech. 2008;36:575–587. [Google Scholar]
- Jin W, Lamb JC, Zhang W, Kolano B, Birchler JA, Jiang J. Histone modifications associated with both A and B chromosomes of maize. Chromosome Res. 2008;16:1203–1214. doi: 10.1007/s10577-008-1269-8. [DOI] [PubMed] [Google Scholar]
- Kamo T, Hiradate S, Fujii Y. First isolation of natural cyanamide as a possible allelochemical from hairy vetch Vicia villosa. J Chem Ecol. 2003;29:275–283. doi: 10.1023/A:1022621709486. [DOI] [PubMed] [Google Scholar]
- Kamo T, Sato M, Kato K, Hiradate S, Nakajima E, Fujii Y, Hirota M. Quantification of cyanamide contents in herbacous plants. Biosci Biotechnol Biochem. 2006;70:2310–2312. doi: 10.1271/bbb.60171. [DOI] [PubMed] [Google Scholar]
- Kamo T, Endo M, Sato M, Kasahara R, Yamaya H, Hiradate S, Fujii Y, Hirai N, Hirota M. Limited distribution of natural cyanamide in higher plants: occurrences in Vicia villosa subsp. varia, V. cracca, and Robinia pseudo-acacia. Phytochemistry. 2008;69:1166–1172. doi: 10.1016/j.phytochem.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Kaur H, Inderjit, Kaushik S (2005) Cellular evidence of allelopathic interference of benzoic acid to mustard (Brassica juncea L.) seedling growth. Plant Physiol Biochem 43:77–81 [DOI] [PubMed]
- Kupidłowska E, Dobrzyńska K, Parys E, Zobel AM. Effect of coumarin and xanthotoxin on mitochondria structure, oxygen uptake, and succinate dehydrogenase activity in onion root cells. J Chem Ecol. 1994;20:2471–2480. doi: 10.1007/BF02036184. [DOI] [PubMed] [Google Scholar]
- Maier-Greiner UH, Obermaier-Skrobranek BMM, Estermaier LM, Kammerloher W, Freund C, Wulfing C, Burkert UI, Matern DH, Breuer M, Eulitz M, Kufrevioglu OI, Hartmann GR. Isolation and properties of a nitrile hydratase from the soil fungus Myrothecium verrucaria that is highly specific for the fertilizer cyanamide and cloning of its gene. Proc Natl Acad Sci USA. 1991;88:4260–4264. doi: 10.1073/pnas.88.10.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir R, Pang X, Halaly T, Venkateswari J, Lavee S, Galbraith D, Or E. Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene-ABA interplay and cell enlargement. Plant Mol Biol. 2009;71:403–423. doi: 10.1007/s11103-009-9531-9. [DOI] [PubMed] [Google Scholar]
- Oracz K, Bailly C, Gniazdowska A, Come D, Corbineau F, Bogatek R. Induction of oxidative stress by sunflower phytotoxins in germinationg mustard seeds. J Chem Ecol. 2007;33:251–264. doi: 10.1007/s10886-006-9222-9. [DOI] [PubMed] [Google Scholar]
- Perez FJ, Lira W. Possible role of catalse in post-dormancy bud break in grapevines. J Plant Physiol. 2005;162:301–308. doi: 10.1016/j.jplph.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Perez F, Vergara R, Rubio S. H2O2 is involved in the dormancy-breaking effect of hydrogen cyanamide in grapevine buds. Plant Growth Regul. 2008;55:149–155. doi: 10.1007/s10725-008-9269-4. [DOI] [Google Scholar]
- Politycka B, Mielcarz B. Involvement of ethylene in growth inhibition of cucumber roots by ferulic and p-coumaric acids. Allelopathy J. 2007;19:451–460. [Google Scholar]
- Rudrappa T, Bonsall J, Gallagher JL, Seliskar DM, Bais HP. Root-secreted allelochemical in the noxious weed Phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J Chem Ecol. 2007;33:1898–1918. doi: 10.1007/s10886-007-9353-7. [DOI] [PubMed] [Google Scholar]
- Sánchez-Moreiras AM, de la Pena TB, Reigosa MJ. The natural compound benzoxazolin-2(3H)-one selectively retards cell cycle in lettuce root meristems. Phytochemistry. 2008;69:2172–2179. doi: 10.1016/j.phytochem.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Shulman Y, Nir G, Fanberstein L, Lavee S. The effect of cyanamide on the release from dormancy of grapevine buds. Scientia Hort. 1983;19:97–104. doi: 10.1016/0304-4238(83)90049-3. [DOI] [Google Scholar]
- Shulman Y, Nir G, Lavee S. Oxidative processes in bud dormancy and the use of hydrogen cyanamide in breaking dormancy. Acta Hort. 1986;179:141–148. [Google Scholar]
- Sliwinska E, Łukaszewska E. Polysomaty in growing in vitro sugar-beet (Beta vulgaris L.) seedlings of different ploidy level. Plant Sci. 2005;168:1067–1074. doi: 10.1016/j.plantsci.2004.12.003. [DOI] [Google Scholar]
- Spiegel-Roy P, Shulman Y, Ashbel E. Effect of cyanamide in overcoming grape seed dormancy. HortScience. 1987;22:208–210. [Google Scholar]
- Steller WA, Morgan PW. Determination of cyanamide residues on ginned cottonseed. J Agr Food Chem. 1965;13:329–330. doi: 10.1021/jf60140a011. [DOI] [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- Wade RH. On and around microtubules: an overview. Mol Biotechnol. 2009;43:177–191. doi: 10.1007/s12033-009-9193-5. [DOI] [PubMed] [Google Scholar]
- Wasteneys GO, Ambrose JC. Spatial organization of plant cortical microtubules: close encounters of the 2D kind. Trends Cell Biol. 2009;19:62–71. doi: 10.1016/j.tcb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Weir TL, Park S, Vivanco JM. Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol. 2004;7:472–479. doi: 10.1016/j.pbi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Zhong WQ, Widholm JM. Expression of fungal cyanamide hydratase in transgenic soybean detoxifies cyanamide in tissue culture and in planta to provide cyanamide resistance. J Plant Physiol. 2005;162:1064–1073. doi: 10.1016/j.jplph.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gu M, Xia X, Shi K, Zhou Y, Yu J. Effects of phenylcarboxylic acids on mitosis ednoreduplication and expression of cell cycle-related genes in roots of cucumber (Cucumis sativus L.) J Chem Ecol. 2009;35:679–688. doi: 10.1007/s10886-009-9642-4. [DOI] [PubMed] [Google Scholar]