Abstract
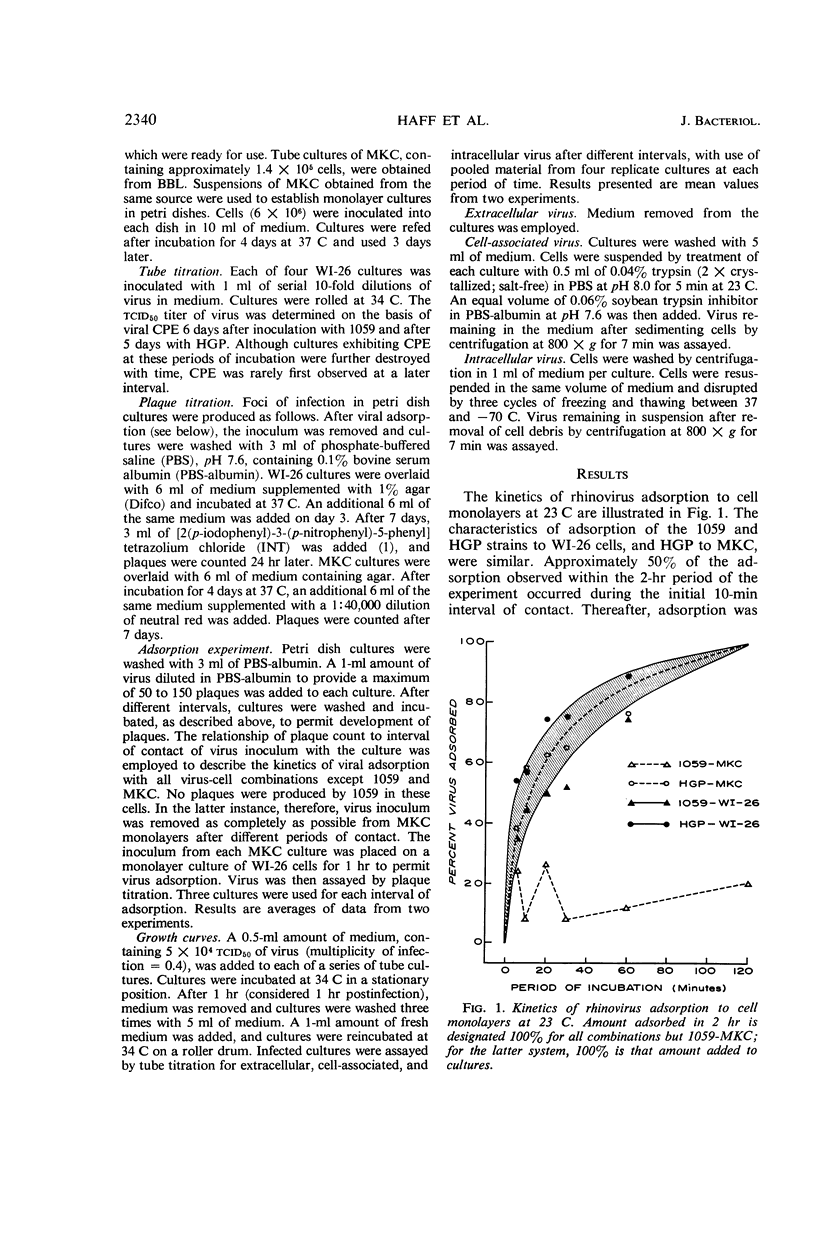
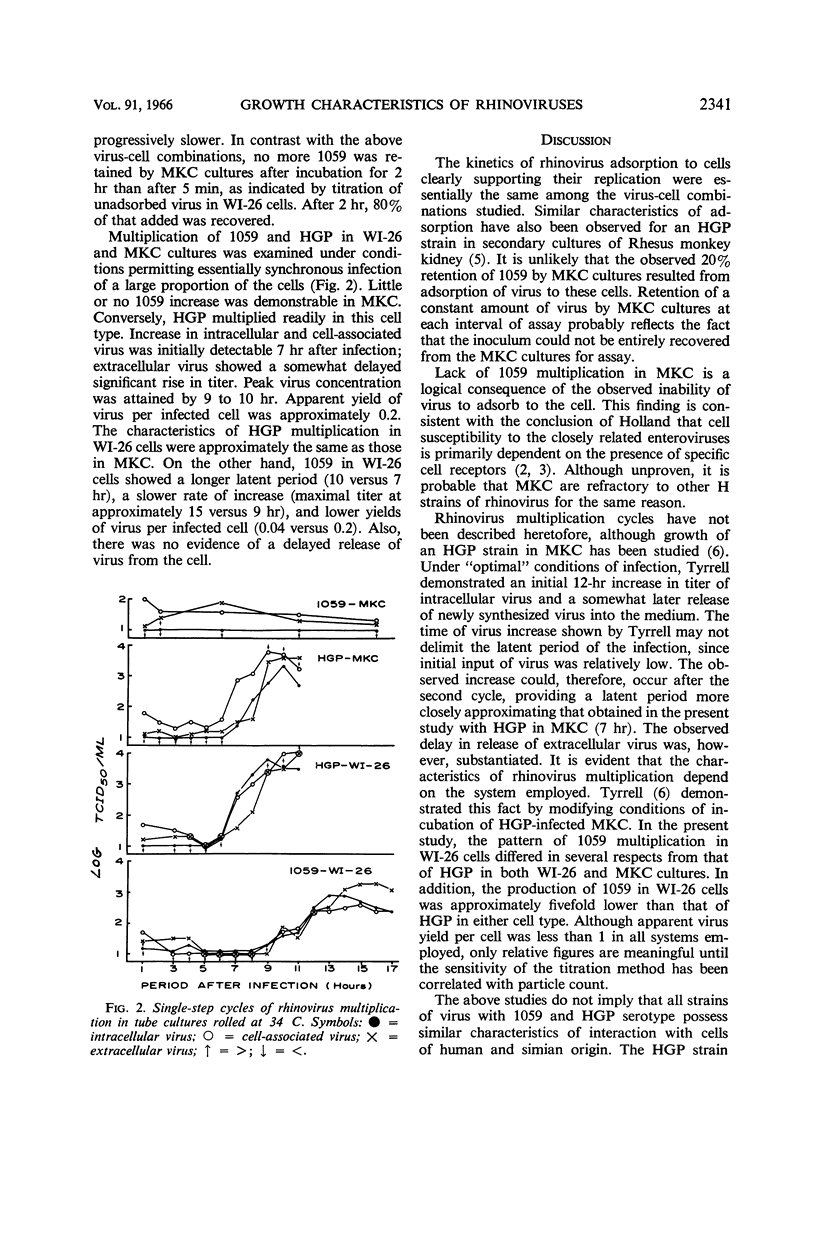
Haff, R. F. (Smith Kline & French Laboratories, Philadelphia, Pa.), B. Wohlsen, E. E. Force, and R. C. Stewart. Growth characteristics of two rhinovirus strains in WI-26 and monkey kidney cells. J. Bacteriol. 91:2339–2342. 1966.—Viruses with 1059 and HGP serotype and with human and monkey host range characteristics, respectively, were employed. Adsorption kinetics of the 1059 and HGP strains to WI-26 cells, and HGP to Green African monkey kidney cells (MKC), were similar. Fifty per cent of the virus was adsorbed to cell monolayers within 10 min; adsorption was essentially complete by 2 hr. The 1059 strain failed to adsorb to MKC, at least to an appreciable extent. Lack of receptors for adsorption of 1059 accounts for the inability of this cell to support multiplication of the virus. It is probable that MKC are refractory to infection with other H strains of rhinovirus for the same reason. Single-step multiplication cycles have been described for the HGP strain in WI-26 and MKC cultures and for the 1059 strain in WI-26 cells. In both cells, HGP exhibited a latent period of 7 hr. Increase of intracellular and cell-associated virus appeared somewhat prior to that of extracellular virus. Maximal titers were attained by 9 to 10 hr. In contrast, initial increase of 1059 in WI-26 cells occurred after 10 hr. Titer rose to peak level 15 hr after infection. Yield of 1059 in WI-26 cells was also fivefold lower than that of HGP in either cell system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOPER P. D. Tetrazolium salts as stains for animal virus plaque assays. Virology. 1959 Apr;7(4):469–470. doi: 10.1016/0042-6822(59)90077-7. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The location and nature of enterovirus receptors in susceptible cells. J Exp Med. 1961 Aug 1;114:161–171. doi: 10.1084/jem.114.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- PARSONS R., TYRRELL D. A. A plaque method for assaying some viruses isolated from common colds. Nature. 1961 Feb 25;189:640–642. doi: 10.1038/189640a0. [DOI] [PubMed] [Google Scholar]
- TYRRELL D. A., BYNOE M. L., HITCHCOCK G., PEREIRA H. G., ANDREWES C. H. Some virus isolations from common colds. I. Experiments employing human volunteers. Lancet. 1960 Jan 30;1(7118):235–237. doi: 10.1016/s0140-6736(60)90166-5. [DOI] [PubMed] [Google Scholar]
- TYRRELL D. A., BYNOE M. L. Some further virus isolations from common colds. Br Med J. 1961 Feb 11;1(5223):393–397. doi: 10.1136/bmj.1.5223.393. [DOI] [PMC free article] [PubMed] [Google Scholar]


