Abstract
Background
Acute infective conjunctivitis is a common problem in primary care, traditionally managed with topical antibiotics. A number of clinical trials have questioned the benefit of topical antibiotics for patients with acute infective conjunctivitis
Aim
To determine the benefit of antibiotics for the treatment of acute infective conjunctivitis in primary care and which subgroups benefit most
Design
An individual patient data meta-analysis
Method
Relevant trials were identified and individual patient data gathered for meta-analysis and subgroup analysis
Results
Three eligible trials were identified. Individual patient data were available from all primary care trials and data were available for analysis in 622 patients. Eighty per cent (246/308) of patients who received antibiotics and 74% (233/314) of controls were cured at day 7. There was a significant benefit of antibiotics versus control for cure at seven days in all cases combined (risk difference 0.08, 95% confidence interval (CI) = 0.01 to 0.14). Subgroups that showed a significant benefit from antibiotics were patients with purulent discharge (risk difference 0.09, 95% CI = 0.01 to 0.17) and patients with mild severity of red eye (risk difference 0.10, 95% CI = 0.02 to 0.18), while the type of control used (placebo drops versus nothing) showed a statistically significant interaction (P=0.03)
Conclusion
Acute conjunctivitis seen in primary care can be thought of as a self-limiting condition, with most patients getting better regardless of antibiotic therapy. Patients with purulent discharge or a mild severity of red eye may have a small benefit from antibiotics. Prescribing practices need to be updated, taking into account these results
Keywords: antibacterial agents, conjunctivitis, family practice, meta-analysis
INTRODUCTION
Acute infective conjunctivitis is a common problem in the primary care setting, accounting for up to 1% of GP consultations in the UK.1,2 Standard treatment for acute infective conjunctivitis has traditionally been with topical antibiotics.3 There was little evidence from primary care on which to base treatment decisions until 2005, when three trials based on primary care populations were published.4∼6 These trials confirmed a high rate of resolution in untreated cases and a limited effect of antibiotics within the primary care setting. Subsequently, clinical guidelines have been updated to limit the use of antibiotics.7 In addition, the differentiation between a viral and bacterial cause is difficult on clinical grounds, and it is generally impractical to request and await microbiology results before initiating treatment.8
Since 2005, GPs have responded to evidence by reducing prescribing rates for acute infective conjunctivitis, but over-the-counter availability of chloramphenicol in the UK has resulted in a 48% increase in the use of topical chloramphenicol.9 Reliable identification of subgroups that will benefit from antibiotics is important for guiding prescribing practice in both primary care and pharmacies. Previous studies have not been large enough for reliable subgroup analysis. Individual patient data meta-analysis has been shown to be an efficient method for subgroup analysis when only a limited number of trials are available.10
This study conducted a meta-analysis using individual patient data, with the aim of assessing the overall benefit of antibiotics as well as the benefit in different subgroups of patients with acute infective conjunctivitis in a primary care setting
METHOD
Selection of studies
The Cochrane Central Register of Controlled Trials (CENTRAL), Embase, MEDLINE, and PubMed were searched for randomised controlled trials up to and including April 2010. Methodological filters were used for identifying randomised controlled trials (RCTs) in Embase11 and MEDLINE;12 no other limits or filters were used. The following search terms were used: (keyword conjunctivitis, bacterial) or (acute or infect* or bacteria*) conjunctiv*) and (keyword anti-bacterial agents) or (antibiotic*). Trials were eligible for inclusion if they were carried out within the primary care setting and were randomised, comparing antibiotic with placebo or no treatment.
A total of 332 potentially relevant trials were identified; 325 of these were excluded on review of titles and abstracts by two independent assessors; seven were retrieved for full text review, and where clarification was needed the authors were contacted; three of these articles met the inclusion criteria. Authors of individual trials were contacted and asked for their raw data. The following data were requested from each trial investigator: outcome on day 7, culture results, age, symptom diaries or GP records, presence of purulent discharge, and severity of red eye.
How this fits in
Chloramphenicol eye drops are available to patients over the counter in the UK, despite lack of guidelines on which patients, if any, will benefit from antibiotics. A number of clinical trials have questioned the benefit of topical antibiotics for acute conjunctivitis, but individually they have been too small to carry out a subgroup analysis. This individual patient data meta-analysis shows that most patients with acute infective conjunctivitis will get better without antibiotics. Patients with purulent discharge and a mild severity of red eye may get some benefit from antibiotics.
Outcome measures
The main outcome measure was cure at day 7. Bacterial culture positivity at presentation was used as a secondary outcome measure to identify features that predicted positive bacterial growth. The reason forusing positive bacterial growth as a secondary outcome measure is that it has been previously shown that there is a stronger treatment effect in patients with a positive bacterial culture,4 and this is more useful in a clinical setting where bacterial culture is rarely undertaken. Cure at day 7 was defined as no remaining symptoms recorded in patient diaries at day 7 for trials using diaries, otherwise according to GP record on day 7 stating complete resolution of conjunctivitis.
For trials using diaries, patients with missing data at day 7 were counted as cured at day 7 if their last diary record showed them as cured (equivalent to last value carried forward). Analysis of diaries showed that relapse rates after ‘cure’ were very low (<5%), and hence this imputation is reasonable. Patients with no diary information and missing data at day 7 were treated as missing, and sensitivity analyses assuming (a) all cured and (b) none cured were carried out to assess their impact on the results.
Positive bacterial culture was defined as growth of pathogenic bacteria from the study eye. Pathogenic bacteria were taken as Haemophilus influenzae or Streptococcus pneumonia, with Moraxella catarrhalis included as a bacterial pathogen in children (0-18 years) and significant growth of Staphylococcus aureus included for one
Potential effect modifiers
Potential effect modifiers for subgroup analysis were chosen according to literature and current guidelines, as well as available data. Current guidelines suggest prescribing antibiotics where the conjunctivitis is severe, or in children where they may be excluded from school or child care.7 Previous literature has suggested predictors of bacterial-positive conjunctivitis that is likely to benefit from antibiotics are increased severity of redness,15 and purulent discharge. 16∼18 Therefore, the potential effect modifiers chosen were age (<5 years/>5 years but <18 years), culture positivity (positive/negative for pathogenic bacteria), severity of red eye (mild/moderate or severe), and purulent discharge (yes/no). Research nurse orGP records at the initial visit were used to document these predictors.
Statistical analysis
All trial data were checked for consistency, and any questions resolved with the principal investigators. Intention-to-treat analysis was used. Datasets were combined into an SPSS file and results obtained from cross-tabulations. These results were entered in the Cochrane Review Manager software RevMan 5.0 to calculate pooled estimates of the effect, 95% confidence intervals (CIs) for the pooled effect, and heterogeneity levels [I2] for each subgroup across studies. Both risk differences and risk ratios were used as summary measures forthese calculations (fixed effect models used in all).
To assess if the effect of antibiotics was changed by any of the potential effect modifiers (age, positive culture, severity of the eye, and purulent discharge), a fixed-effects conditional logistic regression analysis was used to calculate the interaction. For this model, the dependent variable was cure at day 7 (yes/no), with independent variables given by randomisation group (antibiotics yes versus no), the effect modifier (for example, age <5 years/>5 years but <18 years), and an interaction term (age × randomisation group). These models were fitted in STATA using the command xtlogit, with trial as the indexing variable to account for differences within trials. To explore the potential predictors of a positive culture, diagnostic summary measures were calculated (sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and the odds ratio [OR] of the likelihood ratios) from the simple frequency data (not accounting for trial differences). An adjusted OR was calculated to take account of trial differences, using a fixed-effect conditional logistic regression model similar to the one used to assess the effect interaction, but in this case with a positive culture as the dependent variable (outcome).
RESULTS
The search yielded three RCTs conducted in the primary care setting.4–6 The data of all three were available. The study characteristics are shown in Table 1.
Table 1.
Characteristics of principal trials
| Everitt et al, 20066 | Rietveld et al, 20054 | Rose et al, 20055 | |
|---|---|---|---|
| Setting | UK Primary care | The Netherlands Primary care | UK Primary care |
| Participants | 307 adults and children | 181 adults | 326 children aged 6 months to 12 years |
| Study design | Open, factorial, randomised control trial | Double-blind, randomised placebo-controlled trial | Double-blind, randomised placebo-controlled trial |
| Intervention | Immediate chloramphenicol eye drops versus delayed chloramphenicol eye drops versus no eye drops | Fusidic acid gel versus placebo | Chloramphenicol 0.5% versus placebo |
| Determination of cure at day 7 | Patient assessed cure as recorded in a daily diary | GP assessed at day 7 | Parent assessed cure as recorded in a daily diary |
For the trial by Rietveld et al,4 data were available for 163 of the 181 patients initially randomised. Forthe trial by Rose et al,5 data were available for 317 of the 326 patients initially randomised. The aim of this meta-analysis was to compare antibiotic therapy to placebo or no antibiotic therapy, and therefore the delayed antibiotic arm of the study by Everitt et al (n = 109) was excluded from the meta-analysis.6 From the remaining 198 patients in the trial by Everitt et al, data were available for 142. This gives a total number of 622 patients included in this meta-analysis.
Combining data from all three trials, 80% (246/308) of patients who received antibiotics and 74% (233/314) of controls were cured at day 7. The risk difference between antibiotic and control groups was 0.08 (95% CI = 0.01 to 0.14), giving a number needed to treat of 13.
Table 2 shows the effect of antibiotics on the number of patients cured at day 7 for different subgroups. The effect of using no placebo in the control group is compared to the placebo control. The subgroups that significantly benefited from antibiotics were those with a purulent discharge and those with a mild severity of red eye. The type of control used (placebo or no drops) showed a statistically significant interaction. The trial that used no placebo showed a significant effect of antibiotics compared to control (risk difference [RD] = 0-23, 95% CI = 0.08 to 0.37), while the combination of the two trials that used a placebo showed a non-significant effect of antibiotics compared to control (RD = 0-03, 95% CI = -0-04 to 0-11). Figure 1 shows the RDs between antibiotic and no antibiotic group for cure at day 7 for each subgroup. The level of heterogeneity across the trials is indicated in Figure 1 by I2.
Table 2.
Subgroup analysis for the outcome ‘cured at day 7’
| Numbers | Antibiotic group, n/N | Control group,a n/N | RD (95% CI) | NNT | RR (95% CI) | P-value for interactionb | |
|---|---|---|---|---|---|---|---|
| All cases | 622 | 246/308 | 223/314 | 0.08 (0.01 to 0.14) | 13 | 1.11 (1.02 to 1.21) | |
| Type of control | 622 | ||||||
| Placebo | 480 | 185/235 | 181/245 | 0.03 (−0.04 to 0.11) | 34 | 1.05 (0.95 to 1.15) | |
| Non-placebo | 142 | 61/73 | 42/69 | 0.23 (0.08 to 0·37) | 5 | 1.40 (1.13 to 1.73) | 0.03 |
| Culture result | 547 | ||||||
| Negative | 255 | 92/127 | 89/128 | 0.02 (−0.09 to 0.12) | 50 | 1.02 (0.88 to 1.19) | |
| Positive | 292 | 119/141 | 113/151 | 0.08 (−0.01 to 0.17) | 13 | 1.11 (0.99 to 1.24) | 0.33 |
| Discharge | 619 | ||||||
| Non-purulent | 266 | 94/126 | 93/140 | 0.08 (−0.03 to 0.19) | 13 | 1.12 (0.96 to 1.31) | |
| Purulent | 353 | 151/181 | 127/172 | 0.09 (0.01 to 0.17) | 12 | 1.12 (1.00 to 1.25) | 0.72 |
| Severity | 599 | ||||||
| Mild redness | 365 | 158/186 | 134/179 | 0.10 (0.02 to 0.18) | 10 | 1.13 (1.02 to 1.25) | |
| Moderate or severe redness | 234 | 77/110 | 77/124 | 0.06 (−0.06 to 0.18) | 17 | 1.10 (0.91 to 1.33) | 0.40 |
| Age, years | 384 | ||||||
| <5 | 287 | 125/145 | 112/142 | 0.07 (−0.01 to 0.16) | 15 | 1.09 (0.98 to 1·22) | |
| 5–18 | 97 | 42/49 | 39/48 | 0.04 (−0.10 to 0.18) | 25 | 1.05 (0.88 to 1.24) | 0.74 |
Control group refers to placebo or no antibiotic group according to trial.
P-value for interaction calculated using a fixed-effect logistic regression model with trial used as indexing variable. A significant P-value is less than 0.05. NNT = number needed to treat. RD = risk difference. RR = risk ratio.
Figure 1.
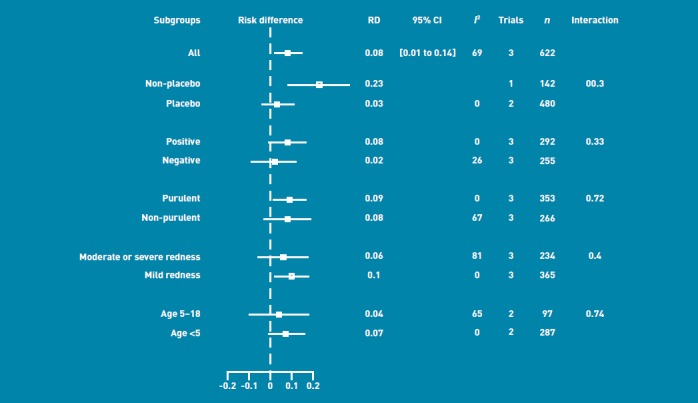
Risk differences (RD) with 95% confidence intervals (CI) for the effect of antibiotic versus no antibiotic for the outcome ‘cured at day 7’.
Sensitivity analyses based on the assumptions that all missing data were for patients who were (a) cured or (b) not cured showed a reduction of effect in the no-placebo group (Everitt et al trial only) when missing data were treated as not cured (RD = 0.14; 95% CI = 0 to 0.28). This is more consistent with the null hypothesis than in the primary analysis. The rest of the results were robust to the choice of imputed values for the missing data.
Table 3 shows the predictors of a positive culture result at presentation. Specificity and sensitivity are shown, as are the positive and negative likelihood ratios. The predictive values of combined factors are also shown.
Table 3.
Predictors of a positive culture
| Odds ratio | ||||||||
|---|---|---|---|---|---|---|---|---|
| Culture positive, n/N | Culture negative, n/N | Sensitivity, % | Specificity, % | LR+ | LR− | Unadjusted, LR+/LR− | Adjusteda (95% CI) | |
| Purulent discharge | 233/311 | 110/281 | 75 | 61 | 1.91 | 0.41 | 4.6 | 1.7 (1.08 to 2.58) |
| Severity (moderate+) | 100/295 | 131/277 | 34 | 53 | 0.72 | 1.25 | 0.6 | 1.4 (0.89 to 2.12) |
| Age <5 years | 239/273 | 45/100 | 88 | 55 | 1.95 | 0.23 | 8.6 | 7.9 (4.60 to 13.61) |
| Purulent discharge and moderate+ | 66/293 | 49/276 | 23 | 82 | 1.27 | 0.94 | 1.4 | 1.3 (0.80 to 1.96) |
| Purulent discharge and age <5 years | 204/271 | 32/99 | 75 | 68 | 2.33 | 0.37 | 6.4 | 5.9 (3.54 to 9.77) |
| Moderate+ and age <5 years | 58/255 | 9/97 | 23 | 91 | 2.45 | 0.85 | 2.9 | 3.2 (1.50 to 6.98) |
| Purulent discharge and moderate+ and age <5 years | 51/253 | 7/97 | 20 | 93 | 2.79 | 0.86 | 3.3 | 3.0 (1.31 to 7.07) |
Adjusted odds ratio obtained using a fixed-effect logistic regression model with trial used as indexing variable. LR = likelihood ratio.
DISCUSSION
Summary
The individual patient data meta-analysis of antibiotic use for acute conjunctivitis in primary care shows that there is a small overall significant effect of antibiotics versus control, with a number needed to treat of 13. However, most patients recovered by day 7 whether they received antibiotics or not. Taking only the two trials that used a placebo control, there was no overall significant effect of antibiotics versus control. Patient subgroups identified as gaining a benefit from antibiotic were those with purulent discharge and mild severity of red eye. Predictors of bacterial culture positivity at presentation were purulent discharge and age less than 5 years.
It was found that patients with a mild severity of red eye were more likely to benefit from antibiotics compared to those with a moderate or severe red eye. This could be because viral and allergic causes of conjunctivitis, as well as alternative diagnoses such as episcleritis, may give a more dramatically red eye.17 It may also be that in patients with only mild red eye, the diagnosis of acute conjunctivitis hinges more on the presence of more specif ic signs and symptoms, such as purulent discharge.
Previous studies have described purulent discharge as an indicator of a bacterial cause,15-18 with the presumption that this would help clinicians decide which patients would benefit from antibiotics. In this study, purulent discharge did predict benefit from antibiotics, and also predicted a positive bacterial culture. However, bacterial culture positivity was not an indicator of benefit from antibiotics in this study. This may reflect the inaccuracy of cultures, particularly in the primary care setting where transport times may confound the results. It may also be due to an insufficient sample size. However, in either case, the effect is likely to be small and the findings demonstrate that even when the cause of conjunctivitis is bacterial, most patients will get better without the use of antibiotics.
As well as cure rate at day 7, which was the main outcome measure in this study, it is also important to know whether antibiotics may shorten the duration of symptoms. To address this, survival analyses were carried out on the two datasets using patient diaries. Results were consistent with those reported in this meta-analysis, with no difference in recovery time for the trial by Rose et al,5 but a clear difference in the trial by Everitt et al,6 which used no placebo.
Strengths and limitations
A key strength of this study is that using the individual patient data from three trials yielded 622 patients, giving the study greater power than any of the individual studies, and enabling subgroup analysis. There was a low level of heterogeneity across the studies, allowing the data to be combined. However, there are also some limitations. The quality of the three studies included was variable. All three studies detailed their randomisation techniques. The trials by Rose et al5 and Rietveld et al4 were blinded and used adequate masking. In the Rose et al trial,5 nine of the 326 patients were lost with respect to 7-day follow-up. In the Rietveld et al trial,4 18 of the 181 patients were lost to follow-up. In the Everitt et al trial,6 there were no data on cure at day 7 for 56 of the 198 patients. It should also be noted that a large number of patients (30%) in the control arm of the Everitt et al trial went on to receive antibiotics.
Two of the studies included in this meta-analysis used a placebo in the control arm, while one did not. Figure 1 shows a significant effect of antibiotics versus control in the trial using no placebo, but not in the two trials using a placebo. This highlights the need for using a placebo in randomised controlled drug trials, but also suggests that there could be a hygiene or irrigation effect of putting non-antibiotic drops into the eye. This is an interesting finding and would be an area for further research. Although a lack of evidence prohibits clear guidelines here, cleaning the eyes is a cheap and simple procedure and could be part of management advice given by GPs.
All the studies here were carried out in primary care populations. This therefore limits the implications of this study to unselected primary care populations.
Comparison with existing literature
A previous Cochrane Review of the use of antibiotics for conjunctivitis also showed a just significant benefit of antibiotics overall.19 However, this review included studies from secondary care as well as primary care and included some older studies that were not thought to be of high quality. Three more-recent studies, not carried out in primary care, have shown a significant effect of antibiotics for conjunctivitis.20–22 It is difficult to compare the present studies with these three recent trials in secondary care, as their focus was microbiological rather than clinical. All three trials confined their analyses to culture-positive patients, and hence excluded over half the randomised patients. The spectrum of illness seen in secondary care, and the focus on microbiological cure, is likely to account for these differences.
The small effect of antibiotics in acute conjunctivitis that has been shown here is similar to that found in systematic reviews looking at sore throat and otitis media.23,24 Recent evidence also suggests that the use of antibiotics for acute otitis media may increase the recurrence rate.25 The National Institute for Health and Clinical Excellence has drawn up guidelines to limit the use of antibiotics for self-limiting respiratory tract infections in primary care.26,27 In light of the present findings, similar guidelines need to be drawn up for the use of antibiotics in acute infective conjunctivitis. These results support the recent statement that it was a mistake to make chloramphenicol available over the counter because of the low efficacy of the drug in treating conjunctivitis.28
Implications for practice
This individual patient data meta-analysis demonstrates that topical antibiotics are of limited benefit in acute infective conjunctivitis and most patients will get better without them. There is a limited set of patients who may benefit from antibiotics, including patients with purulent discharge and patients with mild severity of red eye. However, even in these groups the benefit of antibiotics is limited. Judicious use of antibiotics is important to reduce the risks of antibiotic resistance in the population. Prescribing practices and over-the-counter policies need to be updated to reflect these findings. Furthermore, patient expectations of antibiotic use need to be addressed, as they are likely to be a strong driving force behind prescribing decisions.
Funding
No funding was received for this work.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that they have no conflicts of interests that may be relevant to the submitted work.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Dart J. Eye disease at a community health centre. BMJ. 1986;293(6560):1477–1480. doi: 10.1136/bmj.293.6560.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonnell P. How do general practitioners manage eye disease in the community? Br J Ophthalmol. 1988;72(10):733–736. doi: 10.1136/bjo.72.10.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everitt H, Little P. How do GPs diagnose and manage acute infective conjunctivitis? AGP survey. Fam Pract. 2002;19(6):658–660. doi: 10.1093/fampra/19.6.658. [DOI] [PubMed] [Google Scholar]
- 4.Rietveld R, Riet G, Bindels P, et al. The treatment of acute infectious conjunctivitis with fusidic acid: a randomised controlled trial. Br J Gen Pract. 2005;55(521):924–930. [PMC free article] [PubMed] [Google Scholar]
- 5.Rose P, Harnden A, Brueggemann A, et al. Chloramphenicol treatment for acute infective conjunctivitis in children in primary care: a randomised double-blind placebo-controlled trial. Lancet. 2005;366(9479):37–43. doi: 10.1016/S0140-6736(05)66709-8. [DOI] [PubMed] [Google Scholar]
- 6.Everitt H, Little P, Smith P. A randomised controlled trial of management strategies for acute infective conjunctivitis in general practice. BMJ. 2006;333(7563):321–324. doi: 10.1136/bmj.38891.551088.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NICE. Clinical summary: acute infective conjunctivitis. London: National Institute for Health and Clinical Excellence; Clinical Knowledge Summaries. http://cks.library.nhs.uk/conjunctivitis_infective/management/quick_answers/scenario_acute_infective_conjunctivitis#-304649 (accessed 3 May 2011) [Google Scholar]
- 8.Rietveld RP, ter Riet G, Bindels PJ, et al. Predicting bacterial cause in infectious conjunctivitis: cohort study on informativeness of combinations of signs and symptoms. BMJ. 2004;329(7459):206–210. doi: 10.1136/bmj.38128.631319.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis H, Mant D, Scott C, et al. Topical antibiotic use for acute infective conjunctivitis:relative impact of clinical evidence and over the counter prescribing. Br J Gen Pract. 2009;59(569):897–900. doi: 10.3399/bjgp09X473132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koopman L, van derHeijden GJ, Glasziou P, et al. A systematic review of analytical methods used to study subgroups in (individual patient data) meta-analyses. J Clin Epidemiol. 2007;60(10):1002–1009. doi: 10.1016/j.jclinepi.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc. 2006;94(1):41–47. [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins PT, Green S. Cochrane handbook for systematic reviews of interventions. http://www.cochrane-handbook.org (accessed 3 May 2011) [Google Scholar]
- 13.Gigliotti F, Williams W, Hayden F, et al. Etiology of acute conjunctivitis in children. J Paediatr. 1981;98(4):531–536. doi: 10.1016/s0022-3476(81)80754-8. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A, Brinder J, Nazar-Stewart V. Acute conjunctivitis in childhood. J Paediatr. 1993;122(1):10–14. doi: 10.1016/s0022-3476(05)83479-1. [DOI] [PubMed] [Google Scholar]
- 15.Granet D. Allergic rhinoconjunctivitis and differential diagnosis of the red eye. Allergy Asthma Proc. 2008;29(6):565–574. doi: 10.2500/aap.2008.29.3170. [DOI] [PubMed] [Google Scholar]
- 16.BenEzra D. Current practice: diagnosis and treatment in primary healthcare. Allergy. 1995;50(21 Suppl):30–33. doi: 10.1111/j.1398-9995.1995.tb04254.x. discussion 34-38. [DOI] [PubMed] [Google Scholar]
- 17.Weber CM, Eichenbaum JW. Acute red eye. Differentiating viral conjunctivitis from other, less common causes. Postgrad Med. 1997;101(5):185–186. 189–192, 195–196. doi: 10.3810/pgm.1997.05.246. [DOI] [PubMed] [Google Scholar]
- 18.Jackson WB. Differentiating conjunctivitis of diverse origins. Surv Ophthalmol. 1993;38(Suppl):91–104. doi: 10.1016/0039-6257(93)90034-5. [DOI] [PubMed] [Google Scholar]
- 19.Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006;(2):CD001211. doi: 10.1002/14651858.CD001211.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Karpecki P, DePaolis M, Hunter JA, et al. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: a multicenter, prospective, randomized, double masked, vehicle-controlled, 5-day efficacy and safety study. Clin Ther. 2009;31(3):514–526. doi: 10.1016/j.clinthera.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Abelson MB, Heller W, Shapiro AM, et al. Clinical cure of bacterial conjunctivitis with azithromycin 1%: vehicle-controlled, double-masked clinical trial. Am J Ophthalmol. 2008;145(6):959–965. doi: 10.1016/j.ajo.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Tepedino ME, Heller WH, Unser DW, et al. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr Med Res Opin. 2009;25(5):1159–1169. doi: 10.1185/03007990902837919. [DOI] [PubMed] [Google Scholar]
- 23.Glasziou PP, Del Mar CB, Sanders S, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2004;(1):CD000219. doi: 10.1002/14651858.CD000219.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;(4):CD000023. doi: 10.1002/14651858.CD000023. [DOI] [PubMed] [Google Scholar]
- 25.Bezakova N, Damoiseaux RA, Hoes AW, et al. Recurrence up to 3.5 years after antibiotic treatment of acute otitis media in very young Dutch children: survey of trial participants. BMJ. 2009;338:b2525. doi: 10.1136/bmj.b2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institute for Health and Clinical Excellence. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. London: National Institute for Health and Clinical Excellence; 2008. http://www.nice.org.uk/nicemedia/pdf/CG69FullGuideline.pdf (accessed 3 May 2011) [PubMed] [Google Scholar]
- 27.Butler C. Commentary: Controversies in NICE guidance on antibiotic prescribing for self limiting respiratory tract infections in primary care. BMJ. 2008;337:a656. doi: 10.1136/bmj.a656. [DOI] [PubMed] [Google Scholar]
- 28.Scott G. Overthe counter chloramphenicol eye drops. BMJ. 2010;340:c1016. doi: 10.1136/bmj.c1016. [DOI] [PubMed] [Google Scholar]


