Abstract
Background
Long-term use of benzodiazepines (BZDs) is common. Not only is such use ineffective, but it also has several risks in addition to dependence, and remains a significant problem among the older population
Aim
To systematically review randomised controlled trials that evaluate the effectiveness of minimal interventions to reduce the long-term use of BZDs in primary care.
Design and setting
Systematic review and meta-analysis of randomised controlled trials in UK general practices.
Method
Cochrane Central, MEDLINE, and Embase (1967–2010) were searched for trials of minimal interventions (such as a single letter or one consultation from a GP) for patients in primary care with long-term (>3 months) BZD use. Pooled risk differences were calculated with 95% confidence intervals.
Results
From 646 potentially relevant abstracts, three studies (615 patients) met all the inclusion criteria. The pooled risk ratio showed a significant reduction/cessation in BZD consumption in the minimal intervention groups compared to usual care (risk ratio [RR] = 2.04, 95% confidence interval [CI] = 1.5 to 2.8, P<0.001; RR = 2.3, 95% CI = 1.3 to 4.2, P = 0.008) respectively. Two studies also reported a significant proportional reduction in consumption of BZD from baseline to 6 months in intervention groups compared to the control group. The secondary outcome of general health status was measured in two studies; both showed a significant improvement in the intervention group.
Conclusion
A brief intervention in the form of either a letter or a single consultation by GPs, for long-term users of BZD, is an effective and efficient strategy to decrease or stop their medication, without causing adverse consequences.
Keywords: benzodiazepines, cessation of treatment, long term care, patient education as topic, primary care, reduction
INTRODUCTION
Long-term use of benzodiazepines (BZDs) is common for anxiety disorders, insomnia, and alcohol withdrawal, as adjuvant therapy in schizophrenia and depression, and as muscle relaxants. Their short-term benefits are well recognised, but their long-term use has risks in addition to dependence: daytime somnolence, blunted reflexes, memory impairment, and an increased risk of falls and hip fractures in older people. In a recent review comparing sedatives with placebo, cognitive events were 4.8 times, adverse psychomotor events 2.6 times, and reports of daytime fatigue 3.8 times more common, respectively.1 Hence, long-term BZD use is usually inappropriate, and these drugs should be used only in limited circumstances, for short periods.2,3
The most common long-term use is for sleeping problems; at least 10% of adults complain of significant insomnia, with incident use of a hypnotic drug increasing with age.4 In the community, hypnotic use in those aged over 65 years is at least double (14%) that of younger people.5 It is even higher in older patients attending medical practices, with 26% of women and 6% of men using sleep medications.6 In North America and the UK, 5% to 33% of older people have been prescribed a BZD or a BZD receptor agonist (zolpidem, zopiclone, zaleplon) for sleep problems.7,8
While long-term use is becoming less common, it remains a significant problem. In a study of patients in south-east London, 1.5% of men and 3.5% of women were found to be taking BZDs every day, and an estimated quarter of a million people in the UK took them for over 7 years.9 In Australia, of the total Pharmaceutical Benefits Scheme (PBS) medicines obtained by doctor-shoppers, 36% were BZDs.10 Despite an overall fall in BZD prescriptions internationally over the last 20 years, a substantial number of long-term users receive regular prescriptions, especially for hypnotics.10–15 In 2009 in the UK, there were still 4.2 million benzodiazepine items (used to treat insomnia) dispensed, at a cost of £19 million.15
Long-term use is not only ineffective, but is also associated with several undesirable side effects. The risk of adverse events, particularly falls and cognitive impairment, is higher in older people; and the benefits of these drugs may not be justified. Thus, reduction in the long-term use of BZDs in primary care settings is important. As has been demonstrated in several trials, withdrawal of BZDs should be flexible, and a key element is a slow step-down process of about 25% reduction each week. Trials have shown successful reduction with many interventions, but most are resource intensiveand require several clinical visits. In some trials, a simple letter was used as the only intervention, which may be more widely applicable. Therefore, this study aimed to systematically review randomised controlled trials that evaluated the effectiveness of minimal intervention to reduce or cease the long-term use of BZDs in adults in primary care.
How this fits in
This review found that long-term benzodiazepine (BZD) use could be reduced by ‘minimal interventions’ — a simple tailored letter or consultation. The trials are consistent, but also show that various enhancements do not increase effectiveness. For the tailored letter, the ‘number needed to post’ is about 12 for one additional person to cease BZD use. Widespread use of this strategy would be clinically and economically worthwhile.
METHOD
This review included primary care studies that were randomised controlled trials, with ‘minimal intervention’ for BZD withdrawal. Minimal intervention was defined as a letter, self-help information, or short consultation with a GP. These explained:
concern over the patient's long-term use of hypnotics;
their potential side effects; and
advice for patients to gradually reduce or cease their BZD, with less likelihood of withdrawal symptoms.
The patients included in the trials were adults aged over 18 years (males orfemales) who had long-term benzodiazepine usage (>3 months). Case series, review papers, and duplicate publications were excluded.
Search methods
The Cochrane Central Register of Controlled Trials, MEDLINE and Embase databases (January 1967 to August 2010) were searched. The following medical subject heading (MeSH) search terms were used: [exp]“Substance-Related Disorders” AND [exp] benzodiazepines AND (withdraw* OR discontinu* OR reduc* OR letter OR quit) AND ((Clinical Trial[ptyp] OR Randomized Controlled Trial[ptyp])), with adapted versions for Cochrane and Embase. There were no language or publication restrictions. The references of all identified studies and of relevant systematic reviews were checked. Two review authors and an expert librarian carried out the search.
Data extraction and management
Two review authors independently reviewed and selected trials from searches. Two authors then assessed the trials, rated the study quality, and extracted relevant data. Disagreements were resolved through discussion with the third author Trial authors were contacted to request missing data or to clarify methods where needed.
The aspects of trial quality assessed were: quality of randomisation; quality of blinding (allocation concealment); and analysis by intention-to-treat (ITT).
Data were extracted using a standardised form. Information included: age and sex of participants; treatment setting; average initial dose of BZDs; number of participants; whether analysis was by ITT; randomisation method; exclusion criteria; outcomes in relation to BZD use; and general health status (for example, measured by a 12-item General Health Questionnaire [GHQ] and/or Short Form [36] Health Survey [SF-36]); adverse effects of withdrawal; and other medicines being used, including those with potential drug interactions.
Meta-analytic calculations were done with Meta-Analyst, with risk ratios (RRs) calculated using a random effects model. A pooled risk difference was also estimated, to allow a number needed to treat (NNT) to be calculated.
RESULTS
Of 646 potentially relevant abstracts retrieved, 25 relevant abstracts were selected for detailed evaluation by two reviewers. Fifteen potentially useful full-text articles were retrieved for furthere valuation, and only three papers that fulfilled the main inclusion criteria were finally included for review.16–18 Two of these trials were three-arm studies that included two active interventions. Hence, five interventions were available (Figure 1).
Figure 1.
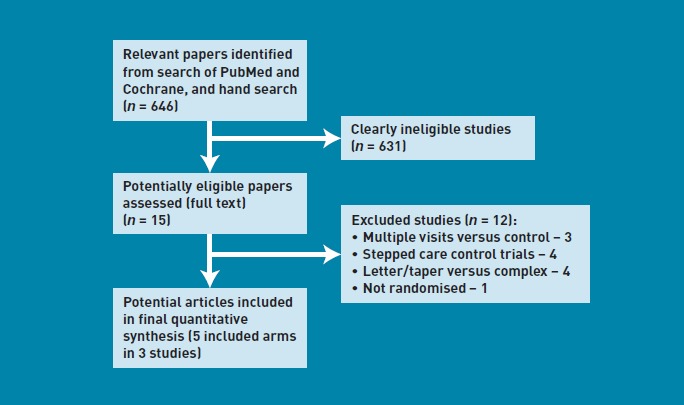
Flowchart of search and study selection.
The studies (Table 1) had a high proportion of women (>60%), and participants' mean age was above 60 years. The BZD dosage in all included trials was expressed in terms of diazepam equivalents, either5 mg or 10 mg. The numbers of patients lost to follow-up in all three studies were low. No difference was found in withdrawal rates between studies of different treatment modalities.
Table 1.
Key features of included BZD-withdrawal trials
| Study characteristics | Bashir et al, 199416 | Cormack et al, 199417 | Heather et al, 200418 |
|---|---|---|---|
| Numberof participants | 109 | 222 | 284 |
| Setting; patients | UK general practice; patients with long-term BZD use | UK general practice; patients with long-term BZD use | UK general practice; patients with long-term BZD use |
| Follow-up | 6 months | 6 months | 6 months |
| Interventions | One consultation from GP+ self-help bookleta to reduce gradually/stop drug | Group 1: single letter from GPb; group 2: letterb + 4-monthly information sheetsa on reducing medication and coping with withdrawal | Group 1: letter from GP to reduce gradually/stop drugb; group 2: consultation and self-help bookleta |
| Comparison | Allowed to continue usual dose | Allowed to continue usual dose | Allowed to continue usual dose |
| Outcome measures | |||
| BZD usage | Prescription records | Prescription records | Prescription records |
| Health questionnaire | GHQ | – | GHQ and SF-36 |
| Methodological quality of studies | |||
| Adequate randomisation? | Yes (used date of birth with baseline equality) | Yes | Yes |
| Baseline similarity? | No | Unclear | Yes |
| Groups treated equally? | Yes | Unclear | Unclear |
| Loss to follow-up | 3.7% (BZD reduction), 15% (GHQ) | 5.8% | 3.9% (BZD reduction), 30% (GHQ) |
| Outcomes measures blind or objective? | Unblinded but objective prescription records | Unblinded but objective prescription records | Unblinded but objective prescription records |
Sample not provided
sample provided.
BZD = benzodiaze epines. GHQ = general health questionnaire.
SF = short-form.
Reduction or cessation of benzodiazepine use
All three studies reported statistically significant reductions in BZD consumption with the minimal intervention group compared to control groups (Figure 2). Effect sizes varied minimally between studies: there was no significant heterogeneity [P = 0.0 %, P = 0.59). The pooled results indicated a relative benefit increase, with twice the reduction in BZD consumption in the intervention groups — either letter or letter and short consultation groups — compared to the control group (RR = 2.04, 95% confidence interval [CI] = 1.5 to 2.8, P<0.001). In the pooled analysis of cessation of BZD use (Figure 3), the intervention group appeared to be superior, with twice the rate of cessation of the usual care group. The RR for cessation in BZD use was 2.3 (95% CI = 1.3 to 4.2, P = 0.008). There was no significant heterogeneity [P = 0.0%, P = 0.79). To allow calculation of a NNT, the pooled risk difference was also calculated, which was a difference of 0.08 (95% CI = 0.03 to 0.13), and hence the NNT was 12.
Figure 2.
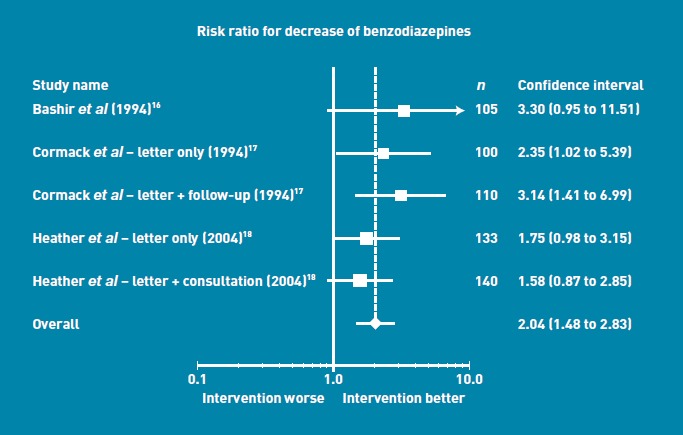
Risk ratio for decrease of benzodiazepine use.
Figure 3.
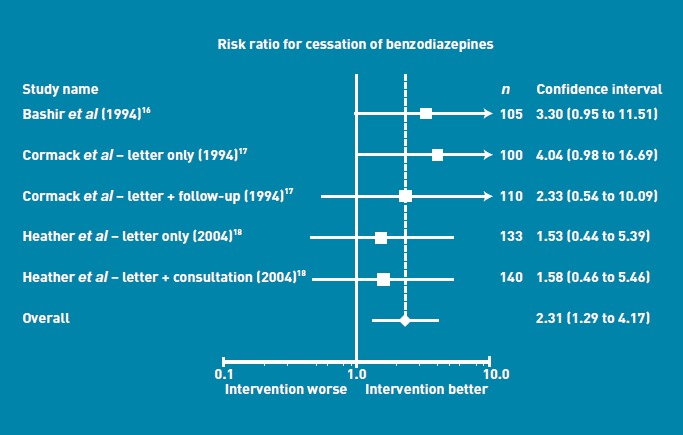
Risk ratio for cessation of benzodiazepines.
Of the three studies, two reported the proportional BZD reduction from baseline to 6-month follow-up period.17,18 There was an observed 20–35% reduction in the intervention group compared to the control group (10–15%). The standard deviation of proportional reduction was only available for two studies,17,18 and is summarised in Table 2.
Table 2.
Reduction in benzodiazepine use across studies
| Bashir et al, 199416 | Cormack et al, 199417 | Heather et al, 200418 | ||||||
|---|---|---|---|---|---|---|---|---|
| Letter | Control | Letter | Letter + follow-up | Control | Letter | Letter + consultation | Control | |
| Number | 50 | 55 | 65 | 75 | 69 | 88 | 95 | 89 |
| Decreased BZD | 9 | 3 | 24 | 37 | 11 | 36 | 35 | 21 |
| Ceased BZD | 9 | 3 | 15 | 10 | 4 | 9 | 10 | 6 |
| Proportional BZD reduction over 6 months | 0.32 | 0.37 | 0.1 | 0.24 | 0.22 | 0.16 | ||
| Standard deviation of reduction | 0.43 | 0.41 | 0.44 | 0.93 | 0.94 | 0.93 | ||
BZD = benzodiazepine.
Secondary outcome
In the study by Bashir et al, at the 6-month follow-up period, the proportion of patients who suffered from psychiatric morbidity, according to the GHQ, was 11% lower in the intervention compared to the control group (3%).6 The intervention group that reported reduction in BZD usage showed a modest psychiatric improvement over the trial period, according to the GHQ. In comparison, the control group had unchanged qualitative and quantitative symptoms over the 6 months. In the study by Heather et al, there was no significant difference between study groups on the SF-36 overall scores.18 However, there was a significant difference in changes on the SF-36 subscore (‘mental’) in patients who had undergone a true reduction and those who had not (χ2 = 7.0; P = 0.008), with ‘true reducers’ showing a mean increase of 5.4, compared to a decline of 2.2 in those who were not ‘true reducers’. Similarly, there was no significant change in GHQ-total score between follow-up and initial assessment, nor any significant differences between groups in relation to changes in somatic symptoms, anxiety, or insomnia (on GHQ subscales). There was a modest correlation (ρ = 0.20; P = 0.011) between changes in BZD intake and changes in GHQ-B (anxiety and insomnia).
DISCUSSION
Summary
The three trials identified in this review found that a simple letter intervention could reduce BZD usage in older patients on long-term BZDs. The effect was substantial, with one cessation of benzodiazepines for every 12 letters sent. There appeared to be no additional advantage in either self-help information ora short consultation with a GP
Strengths and limitations
There were some minor flaws in all trials: one study had weak randomisation methods and some baseline inequality; all had minor loss to follow-up. However, these flaws seem unlikely to explain the size of the effects seen. A fourth paper was excluded because of lower quality, but showed similar results.19 However, the consistency of the results across different measures and different studies is reassuring.
Comparison with existing literature
This review is consistent with a previous review, which found that a number of interventions are able to reduce BZD use.20 Most of these interventions, however, involved levels of skill and resource usage that would not be feasible for widespread use. The letter interventions used were similar in the trials, and were adapted from the first study by Cormack et al.17 In their letters, both Cormack et al and Heather et al explained why BZDs should not be continued for prolonged periods. However, the recommended method of discontinuation was slightly different (Appendix 1). The main barrier to implementation will be GP practices' ability to generate a list of patients for whom the letter is appropriate. However, when this is not possible, GPs could consider simply giving the letter to appropriate patients when they consult.
Implications for practice and research
Given the problems of cognitive impairment and falls induced by BZDs and other hyposedatives, the routine and widespread use of this simple letter intervention appears warranted. While only a modest percentage of patients will reduce or cease their BZD, the minimal effort required suggests it would have a high benefit-to-effort ratio. However, further research is needed to find methods to enhance the impact of such a minimal intervention.
Acknowledgments
We thank Elaine Beller (Bond University) for statistical advice and Nick Heather for providing letter details in their study.
Appendix 1. Key elements of primary care practitioners' letters to reduce hypnotic use
Explain your concern over the individual patient's long-term use of a hypnotic/s — ideally name the specific drug(s) and possibly the extent of use over a defined period.
Highlight potential side effects when taken over a prolonged period.
Ask the patient to consider a reduction in their use.
Include advice on how to feasibly, gradually, and safely reduce or cease use.
OR
Include advice on how to gradually reduce or cease use in a manner that is not only feasible but can also decrease the likelihood of withdrawal symptoms.
Invite the patient to discuss the issue further with you.
Funding
Paul Glasziou is supported by an NHMRC Australia Fellowship (no. 527500).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Glass J, Lanctôt KL, Herrmann N, et al. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee on the Review of Medicines. Systematic review of the benzodiazepines. Guidelines for data sheets on diazepam, chlordiazepoxide, medazepam, clorazepate, lorazepam, oxazepam, temazepam, triazolam, nitrazepam, and flurazepam. Committee on the Review of Medicines. BMJ. 1980;280(6218):910–912. doi: 10.1136/bmj.280.6218.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyrer PJ. Benzodiazepines on trial. BMJ. 1984;288(6424):1101–1102. doi: 10.1136/bmj.288.6424.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morin CM, Bastien C, Guay B, et al. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161(2):332–342. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- 5.Ohayon MM, Caulet M. Psychotropic medication and insomnia complaints in two epidemiological studies. Can J Psychiatry. 1996;41(7):457–464. doi: 10.1177/070674379604100711. [DOI] [PubMed] [Google Scholar]
- 6.Hohagen F, Käppler C, Schramm E, et al. Prevalence of insomnia in elderly general practice attenders and the current treatment modalities. Acta PsychiatrScand. 1994;90(2):102–108. doi: 10.1111/j.1600-0447.1994.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 7.Aparasu RR, Mort JR, Brandt H. Psychotropic prescription use by community-dwelling elderly in the United States. J Am Geriatr Soc. 2003;51(5):671–677. doi: 10.1034/j.1600-0579.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- 8.Craig D, Passmore AP, Fullerton KJ, et al. Factors influencing prescription of CNS medications in different elderly populations. Pharmacoepidemiol Drug Saf. 2003;12(5):383–387. doi: 10.1002/pds.865. [DOI] [PubMed] [Google Scholar]
- 9.Petursson H, Lader M, editors. Dependence on tranquillizers. Maudsley Monographs 28. Oxford: Oxford University Press; 1984. [Google Scholar]
- 10.Bruno R. Benzodiazepine and pharmaceutical opiate misuse and their relationship to crime: an examination of illicit prescription drug markets in Hobart — Tasmanian Technical Report. Melbourne: Turning Point Alcohol and Drug Centre; 2004. [Google Scholar]
- 11.Chaplin S. Benzodiazepine prescribing. Lancet. 1988;1(8577):120–121. doi: 10.1016/s0140-6736(88)90319-4. [DOI] [PubMed] [Google Scholar]
- 12.Petursson H. An international perspective on benzodiazepine abuse. In: Hallstrom C, editor. Benzodiazepine dependence. Oxford: Oxford University Press; 1993. Chapter 12. [Google Scholar]
- 13.Taylor S, McCracken CF, Wilson KC, et al. Extent and appropriateness of benzodiazepine use: results from an elderly urban community. Br J Psychiatry. 1998;173:433–448. doi: 10.1192/bjp.173.5.433. [DOI] [PubMed] [Google Scholar]
- 14.Wilcock M, MacKenzie I, Bolt J. County-wide survey of the repeat prescription of hypnotics. Br J Psychiatry. 1999;174:277. doi: 10.1192/s0007125000262089. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Clinical Excellence. NICE implementation uptake report: Zaleplon, Zolpdem and Zopiclone for the short-term management of insomnia. Technology Appraisal 77. London: National Institute for Health and Clinical Excellence; 2010. [Google Scholar]
- 16.Bashir K, King M, Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br J Gen Pract. 1994;44(386):408–412. [PMC free article] [PubMed] [Google Scholar]
- 17.Cormack MA, Sweeney KG, Hughes-Jones H, et al. Evaluation of an easy, cost-effective strategy for cutting benzodiazepine use in general practice. Br J Gen Pract. 1994;44(378):5–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Heather NA, Bowie A, Ashton H, et al. Randomized controlled trial of two brief interventions against long-term benzodiazepine use: outcome of intervention. Addict Res Theory. 2004;12:141–154. [Google Scholar]
- 19.Cormack MA, Owens RG, Dewey ME. The effect of minimal interventions by general practitioners on long-term benzodiazepine use. J R Coll Gen Pract. 1989;39(327):408–411. [PMC free article] [PubMed] [Google Scholar]
- 20.Oude Voshaar RC, Couvee JE, van Balkom AJ, et al. Strategies for discontinuing long-term benzodiazepine use. Meta-analysis. Br J Psychiatry. 2006;189:213–220. doi: 10.1192/bjp.189.3.213. [DOI] [PubMed] [Google Scholar]


