Abstract
Background
Injectable calcium sulfate is a clinically proven osteoconductive biomaterial, and it is an injectable, resorbable and semi-structural bone graft material. The purpose of this study was to validate the clinical outcomes of injectable calcium sulfate (ICS) grafts as compared with those of a demineralized bone matrix (DBM)-based graft for filling in contained bony defects created by tumor surgery.
Methods
Fifty-six patients (41 males and 15 females) with various bone tumors and who were surgically treated between September 2003 and October 2007 were included for this study. The patients were randomly allocated into two groups, and either an ICS graft (28 patients) or a DBM-based graft (28 patients) was implanted into each contained defect that was developed by the surgery. The radiographic outcomes were compared between the two groups and various clinical factors were included for the statistical analysis.
Results
When one case with early postoperative pathologic fracture in the DBM group was excluded, the overall success rates of the ICS and DBM grafting were 85.7% (24/28) and 88.9% (24/27) (p > 0.05), respectively. The average time to complete healing was 17.3 weeks in the ICS group and 14.9 weeks in the DBM group (p > 0.05). Additionally, the ICS was completely resorbed within 3 months, except for one case.
Conclusions
Although the rate of resorption of ICS is a concern, the injectable calcium sulfate appears to be a comparable bone graft substitute for a DBM-based graft, with a lower cost, for the treatment of the bone defects created during surgery for various bone tumors.
Keywords: Injectable calcium sulfate, Demineralized bone matrix, Bone tumor
Bone grafting is an essential procedure in musculoskeletal tumor surgery. After curettage or aspiration of various bone tumors, a large contained defect is usually generated. Autogenous bone has been considered the gold standard for grafting to this defect because it shows optimum skeletal incorporation. Nevertheless, complications to the donor site and its limited supply has led to the pursuit of alternative grafting materials such as allografts that include demineralized bone matrix (DBM) and various synthetic bone grafts.1,2)
Since Dreesman3) first reported the use of calcium sulfate to obliterate the bone cavities caused by tuberculosis, it has been used in the form of plaster of Paris (CaSO4) for the treatment of bone defects for more than a century. From 1996, medical grade calcium sulfate, which has minimal trace elements and it provides a uniform α crystalline structure with a predictable in vivo resorption rate, has been available.1) Calcium sulfate cement has been demonstrated to be an osteoconductive and biocompatible material without any adverse biologic reactions that occur after the implantation.4,5) Recently, an injectable and moldable form of calcium sulfate has been developed, and it offers many advantages when used to completely fill contained defects that have complex geometric shapes. Its resorption can be monitored radiographically because it is radiopaque. It hardens in situ and provides initial mechanical strength comparable to that of polymethyl-methacrylate (PMMA), which makes early weight-bearing possible even when it is grafted in the lower extremities.6,7) It also can be drilled through and then receive adjunctive hardware. However, to the best of our knowledge, no clinical or radiographic comparative study on injectable calcium sulfate (ICS) has been reported, especially in the area of musculoskeletal tumor surgery.
The purpose of the current study was to assess the efficacy of ICS compared to that of a DBM-based graft as a bone graft substitute for the treatment of musculoskeletal tumors. We hypothesized that ICS would be an effective substitute comparable to a DBM-based graft for treating surgically created bone defects.
METHODS
Study Design
Between September 2003 and October 2007, 62 consecutive patients underwent prospectively designed surgical treatment by the same surgeon (JHO). Those patients with symptoms and either active benign or low grade malignant bone tumor diagnosed on plain radiograph and magnetic resonance imaging (MRI) and that required a bone-grafting procedure to fill a contained defect after curettage or aspiration of bone tumors were included in this study. Those patients with uncontrolled diabetes, alcoholism, peripheral vascular disease, systemic inflammatory disease, use of systemic steroids, pathologic fracture through the tumor and high grade malignant bone tumor were excluded.
The type of grafting was decided by permuted block randomization at the time of surgery. Patients were randomly allocated into two groups, and either an ICS-based graft (32 patients) or DBM-based graft (30 patients) was placed in each contained defect that was developed by the surgery. During the follow-up after the surgery, 6 patients (10% of the enrolled patients) were excluded from this study. Two patients were diagnosed with high grade malignant bone tumor by the final histological examination; wide resection was performed immediately after the final diagnosis. The remaining 4 patients were lost during the follow-up. Finally, the ICS group consisted of 28 patients and the DBM-based grafting group consisted of 28 patients in this study (90% of the initially enrolled patients). This study was carried out with the approval of the institutional review board of the authors' hospital, and written informed consent was requested and obtained from all the patients.
Operative Procedure
Bone tumor, except for a simple bone cyst (SBC), was exposed via an appropriate sized cortical window according to the size of the tumor. Then the intraosseous tumor was removed by using large and small curettes until no tumorous tissue was seen grossly. Next, the entire bony margin within the cavity was further removed with a high-speed burr under direct vision and fluoroscopy. All the overhanging bony ledges were removed to ascertain the presence of any hidden tumor. The curetted tissue was sent to the Department of Pathology for frozen sectioning and the permanent pathologic diagnosis. When aggressive benign bone tumor and low grade malignant bone tumor were suspected according to the previous radiographic study or the frozen sections, adjuvant treatment with 99% anhydrous alcohol was applied for 10 minutes.8) After suctioning, extensive saline irrigation was performed to rinse out the alcohol. The volume of the contained defect was measured by the amount of irrigation solution that was used to fill each defect.
In the ICS group, minimally-invasive injectable graft (MIIG 1.1.5, Wright Medical Technology Inc., Arlington, TN, USA) was mixed for 30 seconds to a smooth consistency using the manufacture's vacuum mixer. The bone defect was filled within another one minute by using an injection syringe that contained ICS. We avoided overfilling or pressurizing the defect site. Subsequently, we allowed five minutes to set the ICS. More recently, a newer product of MIIG was developed (MIIG X3, Wright Medical Technology Inc.). Thus, we started using the newer version of MIIG from January 2007. It has 160% higher compressive strength than the previous product.9) It also provides a longer working time; 2.5 minutes were allowed to inject this material into the bony defect and 11 minutes to set the ICS. In one case of an intertrochanteric lesion of the proximal femur, prophylactic fixation with compression hip screw was performed after the setting of the ICS (Table 1, No. 19).
Table 1.
Demographic Data of the Patients
SBC: simple bone cyst, M-CS: myxoid chondrosarcoma, OF: ossifying fibroma, ABC: aneurismal bone cyst, FD: fibrous dysplasia, CB: chondroblastoma, IO-lipoma: intraosseous lipoma, L-CS: low grade chondrosarcoma, L-OSA: low grade osteosarcoma, IO-ganglion: intraosseous ganglion, EC: enchondroma, NOF: nonossifying fibroma, GCT: giant cell tumor, OO: osteoid osteoma, LCH: Langerhans cell histiocytosis, 115: minimally-invasive injectable graft (MIIG) 1.1.5, X3: MIIGX3, Allo: allogenic bone graft, Auto: autologous bone graft, ICS: injectable calcium sulfate, DBM: demineralized bone matrix, BM: bone marrow.
In the DBM group, a manufactured DBM (Orthoblast II, Integra OrthoBiologics Inc., Irvine, CA, USA) syringe was applied to the bone defect area and injection was performed. When the volume of the contained defect was measured to be more than 5 mL, the cancellous allograft or autograft was mixed with DBM and grafted due to the additional osteoconductive property. Concerning pathologic fracture during the early postoperative periods, 10) physical activity was restricted in the DBM group for 4 weeks in cases with an upper extremity lesion and for 6 weeks in those patients with a lower extremity lesion. In one case of intertrochanteric lesion of the proximal femur, prophylactic fixation with compression hip screw was performed after the curettage of the intraosseous lipoma (Table 1, No. 52).
Operative Procedure for SBC
For patients with SBC, the procedures were guided by fluoroscopy. Two 10-gauge Peverty needles (Kyungwon Medical, Seoul, Korea) were inserted proximally and distally into each cavity. If a cyst was multi-locular, then the procedures were performed separately in each cavity. The diagnosis of a SBC was confirmed by the aspiration of the straw-colored fluid. The contents of the cyst were further aspirated, and then cystography proceeded to confirm the full visualization of each cyst. The cyst was irrigated with normal saline to wash offthe contrast medium, and either ICS or DBM was injected within another one minute into the defect. As for the ICS group, the same procedures were applied as those for an injection after the curettage. In one patient whose cyst was located near the lesser trochanter, prophylactic fixation with reconstruction nail was performed after injection of the ICS. In the DBM group, autogenous bone marrow was aspirated from the anterior iliac crest using an 11-gauge needle (Angiotech, Wheeling, IL, USA). Multiple sites were punctured to obtain 9 to 18 mL of bone marrow through a single skin-puncture site, with taking no more than 2 to 3 mL from each site.11) The DBM was then mixed with aspirated bone marrow and it was injected through the Peverty needles. The volume of the cyst was measured by the preoperative radiography and using the formula (π/6 × length × width × height).12) Physical activity was restricted in the DBM group for 4 weeks for the cases with a humeral lesion and for 6 weeks in those with a lower extremity lesion.
Radiographic Assessment
Postoperative follow-up was scheduled at 2, 6, and 12 weeks and then every 3 months after the surgery until bony union happened. The assessment was based on the radiographic findings and the presence of complications. The degree of calcium sulfate resorption was determined by the anteroposterior (AP) and lateral views at each follow-up. The time to complete resorption was recorded. We defined the time to healing as the period that was required to achieve bony trabeculation visualized in the osseous defect on the AP and lateral views.
Statistical Analysis
The results of the ICS graft were compared with those of the DBM-based graft in terms of the overall success rate and the time to healing. Additional factors such as age, gender, the location of the tumor, additional fixation and the volume of the defect were also evaluated to determine the efficacy of the treatment. Student's t-test was used for the continuous variables and chi-squared test was used to evaluate categorical variables. Fisher's exact test was used to compare the two groups with a smaller sample size (n < 5). Linear regression analysis was used to determine the correlation between the volume of the defect and the time to graft resorption or the time to bone healing. We performed all the statistical analyses with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical Findings
The patients' medical records were reviewed for the demographic and perioperative data (Table 1). The overall patient population consisted of 41 males and 15 females. Their mean age was 30.4 years (range, 2 to 64 years) and the mean follow-up time was 17.3 months (range, 3 to 46 months). Each group was compared in terms of the patient demographics, the location of the tumor, additional fixation and the volume of the defect, but no statistical difference was observed between the two groups (Table 2).
Table 2.
Details of the ICS-based Grafting Group and the DBMbased Grafting Group
Values are presented as number (%) unless otherwise stated.
ICS: injectable calcium sulfate, DBM: demineralized bone matrix.
The Success Rate
One case of pathologic fracture was excluded in calculating the overall success rate, which occurred on the 11th postoperative day in the DBM group (Table 1, No. 55). As a result, the overall success rates of the ICS and DBM groups were 85.7% (24/28) and 88.9% (24/27), respectively. The success rates of the ICS and DBM groups of SBC patients were 87.5% (7/8) and 62.5% (5/8), respectively. As for the bone tumors other than SBC, the success rates of the ICS and DBM were 85.0% (17/20) and 100% (19/19) in each group. There was no statistical difference between the two groups in each disease category (Table 3).
Table 3.
Comparison of the Success Rate
ICS: injectable calcium sulfate, DBM: demineralized bone matrix, SBC: simple bone cyst.
To verify whether any factor had an influence on the success rates of the procedures, we compared the patients in the ICS group with those in the DBM group in terms of age, gender, the location of the tumor, additional fixation and the volume of the defect. However, none of these factors were found to statistically affect the success rates of the bone healing in either group.
Time to Resorption/Healing
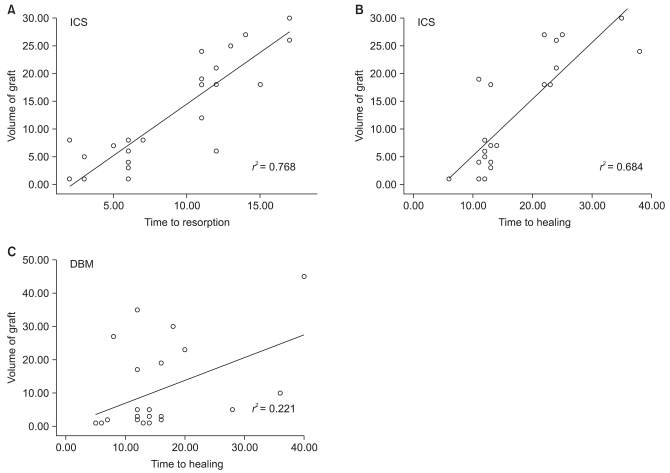
The mean time to healing was 17.3 weeks (range, 6 to 38 weeks) in the ICS group and 14.9 weeks (range, 5 to 40 weeks) in the DBM group (p > 0.05). Additionally, the ICS graft was completely resorbed within 3 months except for one case with pathologic fracture. All of the defects healed in a centripetal fashion; the more peripherally placed calcium sulfate was resorbed first and the more central portion was the last to disappear (Fig. 1). The average time to resorb was 8.9 weeks (range, 2 to 17 weeks). The rate of resorption and healing of ICS depended on the volume of the defect, but the rate of healing in the DBM group did not show linearity (Fig. 2).
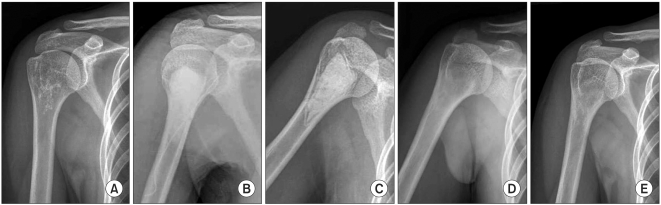
Fig. 1.
The serial radiographs of a 55-year-old female patient show (A) intramedullary calcified bone tumor in the proximal humeral metaphysis preoperatively. The follow-up radiographs taken (B) immediately after the operation, (C) 6 weeks postoperatevely and, (D) 12 weeks postoperatively show the gradual resorption of the injectable calcium sulfate. (E) The final radiograph shows skeletal incorporation.
Fig. 2.
Linear regression analysis demonstrated that the volume of the injectable calcium sulfate (ICS) graft was correlated with (A) the time to resorption and (B) the time to healing. On the contrary, (C) the volume of the demineralized bone matrix (DBM)-based graft was not correlated with the time to healing.
Complications
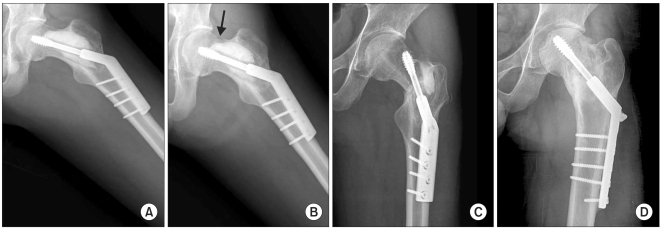
There were two cases of pathologic fracture in the ICS group. The first patient had been treated for low grade chondrosarcoma on the diaphysis of the humerus (Table 1, No. 14). We performed curettage and ICS grafting as described earlier without prophylactic fixation. She complained of a pain after slipping down at 12 weeks postoperatively; the plain radiograph showed an undisplaced fracture through the grafting region with complete absorption of the ICS. We treated her conservatively with an armsling, and bony union was achieved 2 months later. We regarded this case as a failed healing because the fracture occurred after the resorption of the ICS. The second patient had fibrous dysplasia on his left proximal femur and mainly on the intertrochanteric region (Table 1, No. 19). We performed curettage through the bony window on the lateral cortex of the proximal femur and ICS was grafted. After the setting period of the ICS, we reamed through the calcium sulfate for internal fixation to the femoral head. However, it was very difficult to ream and make a hole for the lagging screw; it took as much as 30 minutes to complete this procedure. Fortunately, we succeeded to fix the lagging screw internally through the canal. After the operation, he complained of a sustained pain on his left hip; we continued to recommend to him to walk with a crutch. The follow-up plain radiograph showed a cortical breakage on the superior femur neck at 6 months postoperatively, and pathologic fracture with an incomplete resorption of ICS was evident 10 months postoperatively. We performed curettage of the remnant calcium sulfate and a salvage procedure with internal fixation and autogenous bone grafting (Fig. 3). No evidence of tumor recurrence was identified.
Fig. 3.
The anteroposterior radiographs of a 21-year-old man with fibrous dysplasia of his left femur neck show (A) the three months postoperative view after the curettage and injectable calcium sulfate grafting with prophylactic fixation. (B) Six months postoperatively, he complained of continuous pain on his left hip, and the radiograph showed the cortical breakage on the superior aspect of the femur neck (arrow). (C) Ten months postoperatively, complete fracture occurred in spite of activity restriction. Note that the calcium sulfate is not completely resorbed. (D) The final radiograph after curettage of the remnant calcium sulfate and internal fixation with autogenous bone grafting.
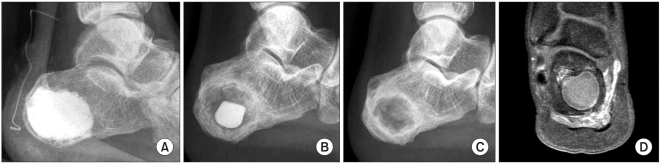
There were two patients whose lesions failed to heal. The lesions were located in the calcaneus; one was myxoid chondrosarcoma and the other was SBC (Table 1, No. 4, 8). The patient with chondrosarcoma, who we previously reported on as a case report,13) had a sustained pain after the operation. Plain radiograph showed complete resorption of the ICS, and MRI revealed cystic change in the grafting area (Fig. 4). In the other patient with SBC, persistence of a cyst was evident on the plain radiograph. In each patient, the time period to complete resorption of ICS was measured to be 6 weeks and 2 weeks, respectively.
Fig. 4.
The radiographs showed a 20-year-old man who had undergone surgery for myxoid chondrosarcoma that arose from the calcaneus (A, B). (A) The immediate postoperative view shows the status of injectable calcium sulfate injection. At the follow-up, the calcium sulfate was (B) gradually resorbed in a centripetal fashion, and (C) it was completely resorbed at 6 weeks postoperatively. (D) The magnetic resonance imaging taken 6 months postoperatively shows a peripherally enhanced cystic lesion within the calcaneus, and this indicated failure of healing.
There were three patients whose lesion failed to heal after the DBM-based grafting. All of the three patients were diagnosed as having SBC, two on the humerus and one on the femur. One patient in the DBM group with a wound infection was treated successfully with debridement and administration of intravenous antibiotics (Table 1, No. 3).
DISCUSSION
The advantages of an autograft are well established even though there is a dearth of research with level I evidence. An autograft has been considered the gold standard for the management of osseous defects because it is usually well incorporated into the surrounding bone with both osteoconductive and osteoinductive biologic capacity.14) Despite its effectiveness, several limitations do exist when using autografts; its acquisition requires an additional incision, the operative time, intraoperative bleeding and postoperative morbidity especially prolonged pain are increased. In addition, postoperative infection, pathologic fracture at the donor site, formation of neuroma, growth impairment in infants and children, the limited quantities and the variable quality of the harvested autograft are also the negative factors.2,15) Th is is why surgeons have sought a material that could be effectively used instead of autogenous bone.
In the present study, we examined the performance of ICS as a bone-graft substitute to fill the contained bone defects. The radiographic results showed the comparable success rate with the DBM-based graft. The mean time to healing in the ICS group was 17.3 weeks, which tended to be more delayed than the DBM group, but this was without statistical difference. Resorption of ICS was predictable in a timely fashion and this depended on the volume of the defect. All but one of the ICS grafts were completely resorbed within 3 months. The patients did not experience any pathologic fracture during the early postoperative period, which was attributed to the initial mechanical strength that was comparable to PMMA.16) The current results agree with the results reported by Kelly et al.17) In their multicentered study, the radiographic results showed that 99% of the calcium sulfate had been resorbed and 88% of the defect was filled with trabeculated bone at 6 months postoperatively. Clayer18) reported that calcium sulfate cement was resorbed completely within 8 weeks in his case series with aneurismal bone cysts, and Clayer found favorable early clinical and radiological responses with a low complication rate. Gitelis et al.19) also reported that the calcium sulfate was a reasonable alternative to an autogenous bone graft for treating benign bone lesions.
Calcium sulfate acts as an osteoconductive matrix that provides a scaffold for a new bone to grow.4) It allows for in-growth of blood vessels and osteogenic cells. In addition, it resorbs as a newly formed bone remodels and it restores the original anatomic features and structural properties.20) Peltier and Jones21) asserted that osteoconduction requires the bone-graft substitute to have a resorption rate similar to the rate of new-bone formation. Owing to the recent advances in producing calcium sulfate as a bone graft substitute, such as the process that allows minimizing the trace elements and making them into an extremely uniform crystalline pattern, the rate of its resorption is consistent with that of the new bone growth.22) However, some authors have criticized calcium sulfate for its rapid resorption, which happens before the bone tissue has had time to grow into the defect.17,19,23) Likewise, the speed of resorption was too fast to form new bone ingrowth in two calcaneal lesions in the current study at six and two weeks, respectively, which seemed to be the cause of the cyst formation. As another reason, we attributed this failure to the poor blood supply to this region. From this point of view, conflicting results have been presented regarding the successful use of calcium sulfate graft. Walsh et al.5) reported that the complete healing of the defect was not observed with calcium sulfate alone, and they hypothesized that the calcium sulfate acts as a barrier to additional bone infiltration. In contrast, by spectral analysis, Yi et al.7) found evidence that the calcium sulfate cement resorption was paralleled with bone ingrowth. Bucholz1) reported positive results when calcium sulfate was used as an osteoconductive bone void filler for the expansion of grafts and for the filling of bone graft harvest sites. They also reported that there was no generation of undesirable dissolution products during this process. Likewise, the resorption rate of the ICS seems to be paralleled with the rate of bone ingrowth in most of the cases of the current study. However, we recommend more careful use of the ICS for optimal bone healing, and especially in large or distal lesion of the extremities where the blood supply is poor. In addition, hardened calcium sulfate can be drilled through, but it cannot be easily reamed through. Excessive reaming through ICS may cause thermal injury to the surrounding tissues, which may harm the process of bone ingrowth. The present study also demonstrated that complete resorption of calcium sulfate may not indicate complete bone healing, and the mechanical strength of the grafted area could be weaker than the initial strength after complete resorption of calcium sulfate has been identified.
We also investigated the effectiveness of DBM as another alternative to an autograft in the present study. DBM was prepared from a freeze-dried allograft with a method that may preserve some of the osteoinductive and osteoconductive properties.24) When it is mixed into a glycerin carrier, this gives the material a gel-like consistency that can be injected through large gauge needles. Previous studies have identified that DBM grafting demonstrated healing that was comparable25) or superior26) to the results achieved by the standard iliac bone graft. In the current study, complete healing of contained defects occurred in 24 of 27 patients (88.9%). In the SBC patients, DBM and autogenous bone marrow were combined to form one composite graft to enhance the osteoinductive properties. The success rate with one injection was 62.5% (5/8) which was comparable to a previous study.27) As for the five patients who had a defect larger than 5 mL, the composite graft with a morsilized autograft or an allograft was performed to impart additional osteoinductive and osteoconductive properties to the composite graft, and the results were satisfactory with a success rate of 100% when excluding the case with early postoperative fracture. Despite these good results, DBM has some limitations for its use; it is expensive and it may carry the risk of disease transmission as it is a human product. Most DBM preparations lack substantial mechanical properties, and there is no significant improvement in mechanical strength during the early stages of healing.10,28) Also, the osteoinductive potential can vary depending on the donor29) and can be influenced by the tissue processing.30)
The present study has several limitations. First, we did not have a control group with standard autogenous iliac bone grafts, although there has been no prospective randomized controlled study for autogenous grafts to validate their effectiveness. Instead, we had tried to make the DBM group have optimal healing capacity with additional auto- or allograft and bone marrow. Second, we did not perform histological or mechanical studies for the healed tissues after the ICS grafting. Histomorphometric analysis or mechanical testing for assessing the compressive strength during each healing period would make it possible to observe more comparative results. Third, we cannot suggest the cut-offvalue to define a 'large' defect as associated with the healing capacity of ICS. A larger study population would be needed for this type of analysis. Last, the exact timing of bony incorporation was difficult to determine on plain radiographs. The addition of CT scans would allow more accurate evaluation of the healing status.
In conclusion, injectable calcium sulfate appears to be an effective bone graft substitute that is comparable for a DBM-based graft, with a lower cost, for the treatment of the bone defects created during surgery for various bone tumors.
However, when the defect size is considerably large or when the blood supply to the lesion seems to be poor, ICS should be used with more caution.
ACKNOWLEDGEMENTS
The authors thank Ms. Hye Ran Kim and Ms. Sang Mi Shim for their support for the data collection and patient recruitment. Th is study was supported by a grant No. 06-2006-004 from the Red Medical Co., Korea. The research expense was approved by the IRB of Seoul National University Bundang Hospital.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002;(395):44–52. doi: 10.1097/00003086-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Dreesmann H. Ueber knochenplombierung. Beitr Klin Chir. 1892;9:804–810. [Google Scholar]
- 4.Blaha JD. Calcium sulfate bone-void filler. Orthopedics. 1998;21(9):1017–1019. doi: 10.3928/0147-7447-19980901-31. [DOI] [PubMed] [Google Scholar]
- 5.Walsh WR, Morberg P, Yu Y, et al. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop Relat Res. 2003;(406):228–236. doi: 10.1097/01.blo.0000030062.92399.6a. [DOI] [PubMed] [Google Scholar]
- 6.Yu B, Han K, Ma H, et al. Treatment of tibial plateau fractures with high strength injectable calcium sulphate. Int Orthop. 2009;33(4):1127–1133. doi: 10.1007/s00264-008-0611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi X, Wang Y, Lu H, Li C, Zhu T. Augmentation of pedicle screw fixation strength using an injectable calcium sulfate cement: an in vivo study. Spine (Phila Pa 1976) 2008;33(23):2503–2509. doi: 10.1097/BRS.0b013e318184e750. [DOI] [PubMed] [Google Scholar]
- 8.Oh JH, Yoon PW, Lee SH, Cho HS, Kim WS, Kim HS. Surgical treatment of giant cell tumour of long bone with anhydrous alcohol adjuvant. Int Orthop. 2006;30(6):490–494. doi: 10.1007/s00264-006-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkoff SM, Sanders JC, Jasper LE. The effect of the monomer-to-powder ratio on the material properties of acrylic bone cement. J Biomed Mater Res. 2002;63(4):396–399. doi: 10.1002/jbm.10258. [DOI] [PubMed] [Google Scholar]
- 10.Wingerter S, Tucci M, Bumgardner J, Benghuzzi H. Evaluation of short-term healing following sustained delivery of osteoinductive agents in a rat femur drill defect model. Biomed Sci Instrum. 2007;43:188–193. [PubMed] [Google Scholar]
- 11.Batinic D, Marusic M, Pavletic Z, et al. Relationship between differing volumes of bone marrow aspirates and their cellular composition. Bone Marrow Transplant. 1990;6(2):103–107. [PubMed] [Google Scholar]
- 12.Shin KH, Moon SH, Suh JS, Yang WI. Tumor volume change as a predictor of chemotherapeutic response in osteosarcoma. Clin Orthop Relat Res. 2000;(376):200–208. doi: 10.1097/00003086-200007000-00027. [DOI] [PubMed] [Google Scholar]
- 13.Kwon JW, Choi JA, Kwack KS, Oh JH, Chung JH, Kang HS. Myxoid chondrosarcoma in the calcaneus: a case report with MR imaging findings. Skeletal Radiol. 2007;36(Suppl 1):S82–S85. doi: 10.1007/s00256-006-0199-9. [DOI] [PubMed] [Google Scholar]
- 14.Bloemers FW, Blokhuis TJ, Patka P, Bakker FC, Wippermann BW, Haarman HJ. Autologous bone versus calciumphosphate ceramics in treatment of experimental bone defects. J Biomed Mater Res B Appl Biomater. 2003;66(2):526–531. doi: 10.1002/jbm.b.10045. [DOI] [PubMed] [Google Scholar]
- 15.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graftharvest donor site morbidity: a statistical evaluation. Spine (Phila Pa 1976) 1995;20(9):1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Rohmiller MT, Schwalm D, Glattes RC, Elalayli TG, Spengler DM. Evaluation of calcium sulfate paste for augmentation of lumbar pedicle screw pullout strength. Spine J. 2002;2(4):255–260. doi: 10.1016/s1529-9430(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 17.Kelly CM, Wilkins RM, Gitelis S, Hartjen C, Watson JT, Kim PT. The use of a surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res. 2001;(382):42–50. doi: 10.1097/00003086-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Clayer M. Injectable form of calcium sulphate as treatment of aneurysmal bone cysts. ANZ J Surg. 2008;78(5):366–370. doi: 10.1111/j.1445-2197.2008.04479.x. [DOI] [PubMed] [Google Scholar]
- 19.Gitelis S, Piasecki P, Turner T, Haggard W, Charters J, Urban R. Use of a calcium sulfate-based bone graft substitute for benign bone lesions. Orthopedics. 2001;24(2):162–166. doi: 10.3928/0147-7447-20010201-19. [DOI] [PubMed] [Google Scholar]
- 20.Peltier LF. The use of plaster of Paris to fill defects in bone. Clin Orthop. 1961;21:1–31. [PubMed] [Google Scholar]
- 21.Peltier LF, Jones RH. Treatment of unicameral bone cysts by curettage and packing with plaster-of-Paris pellets. J Bone Joint Surg Am. 1978;60(6):820–822. [PubMed] [Google Scholar]
- 22.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84(3):454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Hu G, Xiao L, Fu H, Bi D, Ma H, Tong P. Study on injectable and degradable cement of calcium sulphate and calcium phosphate for bone repair. J Mater Sci Mater Med. 2010;21(2):627–634. doi: 10.1007/s10856-009-3885-z. [DOI] [PubMed] [Google Scholar]
- 24.Sampath TK, Muthukumaran N, Reddi AH. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci U S A. 1987;84(20):7109–7113. doi: 10.1073/pnas.84.20.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiedeman JJ, Garvin KL, Kile TA, Connolly JF. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics. 1995;18(12):1153–1158. doi: 10.3928/0147-7447-19951201-05. [DOI] [PubMed] [Google Scholar]
- 26.Gepstein R, Weiss RE, Hallel T. Bridging large defects in bone by demineralized bone matrix in the form of a powder: a radiographic, histological, and radioisotope-uptake study in rats. J Bone Joint Surg Am. 1987;69(7):984–992. [PubMed] [Google Scholar]
- 27.Rougraff BT, Kling TJ. Treatment of active unicameral bone cysts with percutaneous injection of demineralized bone matrix and autogenous bone marrow. J Bone Joint Surg Am. 2002;84(6):921–929. doi: 10.2106/00004623-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Iwata H, Sakano S, Itoh T, Bauer TW. Demineralized bone matrix and native bone morphogenetic protein in orthopaedic surgery. Clin Orthop Relat Res. 2002;(395):99–109. doi: 10.1097/00003086-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Traianedes K, Russell JL, Edwards JT, Stubbs HA, Shanahan IR, Knaack D. Donor age and gender effects on osteoinductivity of demineralized bone matrix. J Biomed Mater Res B Appl Biomater. 2004;70(1):21–29. doi: 10.1002/jbm.b.30015. [DOI] [PubMed] [Google Scholar]
- 30.Takikawa S, Bauer TW, Kambic H, Togawa D. Comparative evaluation of the osteoinductivity of two formulations of human demineralized bone matrix. J Biomed Mater Res A. 2003;65(1):37–42. doi: 10.1002/jbm.a.10345. [DOI] [PubMed] [Google Scholar]