Abstract
Purpose
Prostate cancer foci have a characteristic feature in magnetic resonance imaging (MRI). We aimed to assess the clinical value of MRI before prostate biopsy in prostate cancer detection.
Materials and Methods
From March 2009 to June 2010, 154 patients were enrolled in this study. A total of 51 patients with a clinical suspicion of prostate cancer underwent prostate MRI by a 3T scanner before transrectal ultrasound (TRUS)-guided biopsies. A total of 103 patients with a clinical suspicion of prostate cancer underwent prostate MRI after biopsies. The sensitivity, specificity, and positive predictive value (PPV) were evaluated. In addition, tumor location of pathologic findings and ADC mapping on MRI were matched and compared.
Results
The sensitivity of MRI before and after biopsy was 84.8% and 92.4%, respectively. The PPV of MRI before and after biopsy was 75.7% and 92.4%, respectively. The MRI location match percentage before and after biopsy was 89.3% and 94.1%, respectively.
Conclusions
Compared with other previous reports, our results show that the prostate cancer detection sensitivity of MRI is on the rise. Furthermore, MRI before prostate biopsy can provide more information by which to identify prostate cancer during prostate biopsy and thus reduce the false-negative rate.
Keywords: Biopsy, MRI, Prostate cancer
INTRODUCTION
Prostate cancer is the third leading cause of death after lung cancer and colorectal cancer in Europe. The incidence of prostate cancer is 30 in 100,000 males [1]. According to the American Cancer Society, prostate cancer is the third leading cause of death among all cancer deaths in males, and in 2007, a total of 218,890 new prostate cancer patients were diagnosed and 27,050 patients died of prostate cancer [2]. As the number of prostate cancer patients has increased, the diagnosis, assessment of disease stage, and treatment methods have improved greatly.
Transrectal prostate biopsy is a useful method for prostate cancer diagnosis and lesion site assessment [3]. However, the procedure entails discomfort and has risks for hemorrhage and prostatitis afterwards. In addition, if the cancer lesions are small, biopsy may not be performed accurately [4-6].
Magnetic resonance imaging (MRI) is a useful tool for prostate cancer diagnosis and disease staging workup. Particularly, at present, it is widely used to assess the location and border of tumors as well as to determine the level of infiltration to adjacent tissues. Nevertheless, if MRI is performed after transrectal prostate biopsy, due to hemorrhaging within tissues, it may be difficult to assess the location of cancer lesions and to determine the range and border of the tumor; consequently, the size of the lesion may be underestimated or overestimated [7-9]. However, in the past 20 years, with the introduction of diffusion-weighted imaging, dynamic contrast-enhanced MRI, and MR spectroscopy, prostate cancer can be diagnosed more accurately and effectively [10-17].
In the present study, we examined whether MRI that was performed before prostate biopsy is of help in the diagnosis of prostate cancer.
MATERIALS AND METHODS
From March 2009 to June 2010, the study was conducted on 154 patients who underwent prostate biopsy and MRI. Among the 154 patients, 51 patients underwent MRI before prostate biopsy (group 1) and 103 patients underwent MRI after prostate biopsy (group 2).
In all patients, digital rectal examination (DRE), serum prostate-specific antigen (PSA) measurement, transrectal prostate ultrasonography (TRUS), and MRI were performed at our outpatient clinic. DRE and TRUS examination were performed by a single experienced urologist.
1. Prostate biopsy protocol
Before the biopsy procedure, oral quinolone agents were administered to all patients to prevent infection caused by the procedures. All patients assumed the left down lateral decubitus position with their knees brought up toward their chest, and a transrectal ultrasound probe was inserted through the anus. The prostate was examined by 7 MHz ultrasound. The peripheral zone mid-gray image of the prostate was optimized. Axial assessment from the base to the apex and sagittal planes assessment were done. Prostate biopsy was performed by using a standard spring-loaded biopsy device. According to the extended 10 to 12 core biopsy, it was performed on the bilateral base, midgland, and apex of the peripheral zone. If hypoechoic lesions were detected within an echo view, biopsy samples were obtained. In addition, targeted biopsies were performed in the abnormal regions of the MRI. Biopsy samples were labeled according to their location and were immediately stored.
2. Prostate MRI protocol
For prostate MRI, the 3-Tesla MR image system (A Tim System MAGNETOM Verio; SIEMENS, Germany) was used. No enema, drug administration, or other pretreatments were performed except that the patients fasted for 8 hours before MRI. The images were assessed by two radiologists with more than 15 years of experience. They discussed the radiologic findings with each other and confirmed the report after discussion. The four sequences evaluated in this study were axial T1WI, T2WI, DWI, and ADC map. Imaging parameters for the axial T1-weighted fast spin-echo (FSE) images were repetition/echo time (TR/TE) 600/13 ms, echo train length (ETL) 3, number of excitations (average) 3, matrix size 320×224, plain resolution 0.7×0.5 mm, slice thickness 3 mm (Voxel size: 0.7×0.5×3), and acquisition time 3 min 23 seconds. Axial T2-weighted FSE images were TR/TE 3,060/102 ms, ETL 21, number of excitations (average) 3, matrix size 324×320, plain resolution 0.7×0.5 mm, slice thickness 3 mm (Voxel size: 0.7×0.5×3), and acquisition time 2 min 33 seconds. Axial diffusion-weighted images (DWI) were TR/TE 7,300/98 ms, average 6, ETL(-), matrix size 106×77, plain resolution 1.8×1.4mm, slice thickness 3 mm (Voxel size: 1.8×1.4×3), and acquisition time 5 min 29 s, with free breath. DWI was obtained by using diffusion gradients with three b values (0, 500, and 800 s/mm2) along the orthogonal three directions of the motion-probing gradients. The ADC map was automatically created from the DWIs acquired at b factors of 500 and 800 s/mm2. The sensitivity encoding (GRAPPA) reduction factor was two. The slice thickness was 3 mm for all sequences (Sag T2 BLADE; 4 mm). The interslice gap was 0 mm in all sequences. The field of view was 18×18 cm for sagittal sequences and 15×15 cm for axial sequences.
3. Pathological specimens
After fixing the prostatectomy specimens in formalin, the whole prostate glands were serially blocked into approximately 4 to 6 mm thick (5 mm average) sections from the apex to the base in the transverse planes. These specimens were then submitted in entirety for paraffin embedding as whole mounts. The average number of blocks of a radical prostatectomy specimen was 36.3 (range, 30-43) per case. After paraffin embedding, micro-sections were placed on glass slides and stained with hematoxylin-eosin. For all cases, the same pathologist assessed both the biopsy samples and the radical prostatectomy specimens, while also evaluating any correlations between them. For an individual cancer focus, the pathological evaluation included the cancer location, Gleason score, histologic type, tumor size, extracapsular involvement, seminal vesicle invasion, lymphatic/vascular invasion, lymph node metastasis, perineural/prostatic urethral invasion, and surgical margin involvement. When multiple cancer foci were present in one case, several large lesions were chosen for the pathological evaluation.
4. Image analysis and statistical analysis
The specimen was prepared as serial blocks and examined histologically in the department of pathology. Two pathologists clearly marked the site where prostate cancer was located, and the result of the prostate biopsy and MRI findings of the site were compared.
Statistical analysis was carried out by using sensitivity, specificity, and positive predictive value.
RESULTS
The total number of patients was 154. Of these, 51 patients underwent MRI before biopsy (group 1), and 103 patients underwent MRI after biopsy (group 2).
The mean age of group 1 was 67.16±6.38 years, the mean PSA value was 14.16±17.66 ng/ml, the mean prostate size was 42.98±22.70 ml, and the mean number of prostate biopsy cores was 10.24±0.65. The mean age of group 2 was 69.09±7.55 years, the mean PSA value was 16.60±23.07 ng/ml, the mean prostate size was 37.28±16.28 ml, and the mean number of prostate biopsy cores was 10.08±0.38 (Table 1).
TABLE 1.
Basic patient characteristics
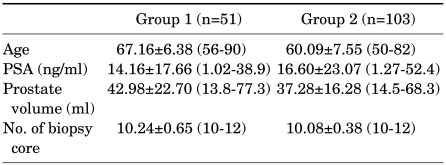
In group 1, 36 patients were diagnosed as having prostate cancer by prostate biopsy, and the remaining 15 patients were diagnosed as having benign prostatic hyperplasia. Of 37 patients who were diagnosed as having prostate cancer by MRI, 31 patients were definitely diagnosed as having prostate cancer by prostate biopsy. The remaining patients were found to have benign prostatic hyperplasia or chronic inflammation. Of 31 patients who were definitely diagnosed as having prostate cancer, the site of the MRI lesion matched in 28 patients. Of 14 patients who were diagnosed as having a low possibility of prostate cancer by MRI, 5 patients were diagnosed as having prostate cancer and the remaining 9 patients were diagnosed as having benign prostatic hyperplasia or chronic inflammation by biopsy (Fig. 1).
FIG. 1.
Study design and schematic flow of group 1.
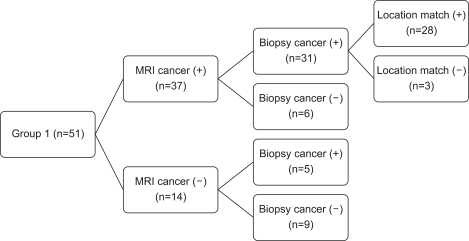
In group 2, 93 patients were diagnosed as having prostate cancer by prostate biopsy, and the remaining 10 patients were diagnosed as having benign prostatic hyperplasia. Of 93 patients who were diagnosed as having prostate cancer by MRI, 86 patients were definitely diagnosed as having prostate cancer by prostate biopsy. The remaining 7 patients were found to have benign prostatic hyperplasia or chronic inflammation. Of 86 patients who were definitely diagnosed as having prostate cancer, the site of the MRI lesion matched in 81 patients. Of 10 patients who were diagnosed as having a low possibility of prostate cancer by MRI, 7 patients were diagnosed as having prostate cancer and the remaining 3 patients were diagnosed as having benign prostatic hyperplasia or chronic inflammation by biopsy (Fig. 2).
FIG. 2.
Study design and schematic flow of group 2.
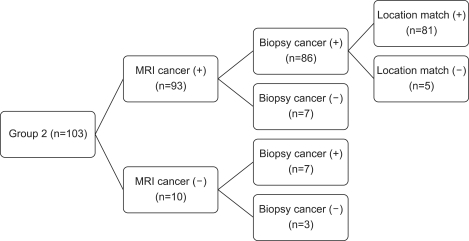
Cases in which the location of the primary lesions matched were limited to the cases whose ADC map and the location confirmed by the pathologic results were identical (Fig. 3).
FIG. 3.
(A) A 74-year-old male with prostate cancer. The PSA level was 7.3 ng/ml, GS was 7. The radical prostatectomy specimen showed one clear cancer foci. (B) The MRI images show a focal, round, low signal intensity area in the left peripheral zone.
In group 1, the sensitivity for prostate cancer detection was 84.8%, the positive predictive value was 75.7%, and the matching rate of the prostate cancer location was 89.3%. In group 2, the sensitivity for prostate cancer detection was 92.4%, the positive predictive value was 92.4%, and the matching rate of the prostate cancer location was 94.1%.
DISCUSSION
Recently, PSA screening tests have been widely performed for the early diagnosis of prostate cancer. As a result, for patients with an elevated PSA value, patients who require additional examinations such as TRUS and prostate biopsy are on the rise. Consequently, early prostate cancer with small lesions and early stage disease is being abundantly detected [18,19].
Basically, prostate cancer is diagnosed according to PSA values and the pathologic results of (transrectal ultrasonography-guided) prostate biopsy. The purpose of prostate biopsy is to confirm prostate cancer lesions as well as to assess the location of cancer, lesion size, and grade. The extended 10 to 12 core biopsy that is currently performed at many medical institutions examines the posterior direction of the peripheral zone. It thus has been reported that approximately 30% of cancer lesions located in the anterior peripheral zone, the lateral part, the transition zone, and the midline preurethral anterior fibromuscular stroma may be overlooked [20,21]. In addition, due to the sampling error that may occur during the specimen collection stage, the assessment of the location of cancer lesions has limited sensitivity [5]. Diverse sensitivities of prostate cancer detection by transrectal prostate biopsy have been reported, from approximately 50% to 75%. It has been reported that the sensitivity of diagnosis could be raised up to 95% by use of extended 10 to 12 core biopsy [22]. Although prostate biopsy shows high accuracy for the detection of cancer lesions, a small number of patients are still diagnosed as being negative by biopsy [23].
In such a case, when a prostate biopsy of a patient with an elevated PSA is negative, urologists face a dilemma [24,25]. Many indexes of prostate cancer applying the PSA value have been reported. Although such indexes show the risk or probability for the presence of prostate cancer, the lesion site cannot be assessed by the application of indexes [26,27]. In addition, it has been suggested that biopsy be performed again on such patients, and to perform biopsy on more sites; nonetheless, such patients experience increased cost and an increased morbidity rate [28].
MRI differs from other radiological diagnosis methods such as X-ray or computed tomography, because it does not engage ionized radiation and thus is not harmful to the body. The plane images can be reconstructed as 3D images, and contrast and resolution are superior to computed tomography. In addition, images can be constructed at various angles; thus, it is useful for examining intra-pelvic organs such as the prostate. According to previous reports, the sensitivity of prostate cancer detection by MRI is approximately 55% to 65%, and the positive predictive value has been reported to be approximately 80% to 85%. Due to the development of advanced technical methods such as diffusion-weighted imaging, dynamic-contrast-enhanced MRI, and MR spectroscopy, the sensitivity is on the rise [5,29,30].
In both group 1 and group 2, the sensitivity for detecting prostate cancer was shown to be higher than in other older studies. One reason for this difference may be that PSA and other clinical information of the patients were shared by the radiologists; thus, during the interpretation, the information could be used as reference data. Hence, the possibility of bias may be considered. In addition, it is thought that the techniques applying MRI were greatly improved in comparison with the past, and the development of advanced equipment may be of help to raise sensitivity. This could be confirmed by the difference in the sensitivity for detecting prostate cancer between group 1 and group 2. The sensitivity and positive predictive value of group 1 were lower than those of group 2. This may be because in the group 2 cases, images were assessed after the pathologic diagnosis. In group 1, the images were assessed before the pathologic diagnosis.
Transrectal prostate biopsy is a useful method for diagnosing prostate cancer and for assessing the location of lesion, and it has been considered to be the standard assessment for detecting nonsurgical cancer locations [3]. However, the procedure is uncomfortable, and it carries a risk for hemorrhage and prostatitis after the procedure. Furthermore, if the lesion is small, biopsy may not be performed accurately [4-6]. Regarding sampling errors, that is pointed out to be a limitation of prostate biopsy. It could be overcome to a certain degree by TRUS-guided biopsy; nonetheless, for intra-prostatic, tiny, focal prostate cancer lesions, it is not at a level comparable to the sensitivity of MRI.
Magnetic resonance imaging is a useful method for diagnosing prostate cancer and for determining disease stages. Particularly, it has been widely used to assess the location and border of tumors and the depth of infiltration to adjacent organs. Its sensitivity and specificity are continuously being improved [30]. However, when MRI is performed after prostate biopsy, due to hemorrhages within tissues, it is difficult to determine the location of lesions, the range, and the cancer margin border, and thus the size of the lesion may be underestimated or overestimated [7-9]. But, as confirmed in our study, until now, the sensitivity of MRI that is performed before prostate biopsy is not great enough to replace prostate biopsy.
Accuracy, low cost, reduced morbidity rate of other diseases or complications, and noninvasiveness are the best conditions for tumor diagnosis if possible. MRI is still an expensive diagnostic tool. However, it provides higher resolution and contrast images of suspicious lesions with 3D reconstruction and it makes prostate biopsy more accurate, thus reducing the false-negative rate and morbidity rate. As observed in our study, MRI performed before prostate biopsy is anticipated to be of help in the diagnosis of prostate cancer and the determination of the area of biopsy. We suggest that additional studies on more patients are required in the future.
CONCLUSIONS
The sensitivity, the positive predictive value, and the lesion location match rate of MRI performed before prostate biopsy were lower than that of MRI performed after biopsy. Nonetheless, the possibility of the biopsy result influencing the reading of the MRI cannot be ruled out. In comparison with other previous reports, our results suggest that the sensitivity of prostate cancer detection by MRI is on the rise. We thus conclude that MRI as a screening tool to diagnose prostate cancer has become more useful. In addition, MRI before prostate biopsy can provide more information by which to identify prostate cancer during prostate biopsy, which is anticipated to be of help in reducing the false-negative rate.
Footnotes
The authors have nothing to disclose.
References
- 1.Levi F, Lucchini F, Negri E, Boyle P, La Vecchia C. Leveling of prostate cancer mortality in Western Europe. Prostate. 2004;60:46–52. doi: 10.1002/pros.20058. [DOI] [PubMed] [Google Scholar]
- 2.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 3.Horndalsveen Berild G, Nielsen K. Accuracy in Core Biopsy of the Prostate. An autopsy study. Urol Int. 1986;41:276–278. doi: 10.1159/000281217. [DOI] [PubMed] [Google Scholar]
- 4.Loch T, Eppelmann U, Lehmann J, Wullich B, Loch A, Stockle M. Transrectal ultrasound guided biopsy of the prostate: random sextant versus biopsies of sono-morphologically suspicious lesions. World J Urol. 2004;22:357–360. doi: 10.1007/s00345-004-0462-4. [DOI] [PubMed] [Google Scholar]
- 5.Wefer AE, Hricak H, Vigneron DB, Coakley FV, Lu Y, Wefer J, et al. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol. 2000;164:400–404. [PubMed] [Google Scholar]
- 6.Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243:28–53. doi: 10.1148/radiol.2431030580. [DOI] [PubMed] [Google Scholar]
- 7.White S, Hricak H, Forstner R, Kurhanewicz J, Vigneron DB, Zaloudek CJ, et al. Prostate cancer: effect of postbiopsy hemorrhage on interpretation of MR images. Radiology. 1995;195:385–390. doi: 10.1148/radiology.195.2.7724756. [DOI] [PubMed] [Google Scholar]
- 8.Chelsky M, Schnall MD, Seidmon EJ, Pollack H. Use of endorectal surface coil magnetic resonance imaging for local staging of prostate cancer. J Urol. 1993;150:391–395. doi: 10.1016/s0022-5347(17)35490-3. [DOI] [PubMed] [Google Scholar]
- 9.Schnall MD, Imai Y, Tomaszewski J, Pollack HM, Lenkinski RE, Kressel HY. Prostate cancer: local staging with endorectal surface coil MR imaging. Radiology. 1991;178:797–802. doi: 10.1148/radiology.178.3.1994421. [DOI] [PubMed] [Google Scholar]
- 10.Costouros NG, Coakley FV, Westphalen AC, Qayyum A, Yeh BM, Joe BN, et al. Diagnosis of prostate cancer in patients with an elevated prostate-specific antigen level: role of endorectal MRI and MR spectroscopic imaging. AJR Am J Roentgenol. 2007;188:812–816. doi: 10.2214/AJR.06.0165. [DOI] [PubMed] [Google Scholar]
- 11.Reinsberg SA, Payne GS, Riches SF, Ashley S, Brewster JM, Morgan VA, et al. Combined use of diffusion-weighted MRI and 1H MR spectroscopy to increase accuracy in prostate cancer detection. AJR Am J Roentgenol. 2007;188:91–98. doi: 10.2214/AJR.05.2198. [DOI] [PubMed] [Google Scholar]
- 12.Kozlowski P, Chang SD, Jones EC, Berean KW, Chen H, Goldenberg SL. Combined diffusion-weighted and dynamic contrast-enhanced MRI for prostate cancer diagnosis--correlation with biopsy and histopathology. J Magn Reson Imaging. 2006;24:108–113. doi: 10.1002/jmri.20626. [DOI] [PubMed] [Google Scholar]
- 13.Tanimoto A, Nakashima J, Kohno H, Shinmoto H, Kuribayashi S. Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging. 2007;25:146–152. doi: 10.1002/jmri.20793. [DOI] [PubMed] [Google Scholar]
- 14.Desouza NM, Reinsberg SA, Scurr ED, Brewster JM, Payne GS. Magnetic resonance imaging in prostate cancer: the value of apparent diffusion coefficients for identifying malignant nodules. Br J Radiol. 2007;80:90–95. doi: 10.1259/bjr/24232319. [DOI] [PubMed] [Google Scholar]
- 15.Shimofusa R, Fujimoto H, Akamata H, Motoori K, Yamamoto S, Ueda T, et al. Diffusion-weighted imaging of prostate cancer. J Comput Assist Tomogr. 2005;29:149–153. doi: 10.1097/01.rct.0000156396.13522.f2. [DOI] [PubMed] [Google Scholar]
- 16.Morgan VA, Kyriazi S, Ashley SE, DeSouza NM. Evaluation of the potential of diffusion-weighted imaging in prostate cancer detection. Acta Radiol. 2007;48:695–703. doi: 10.1080/02841850701349257. [DOI] [PubMed] [Google Scholar]
- 17.Haider MA, van der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, Lockwood G, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007;189:323–328. doi: 10.2214/AJR.07.2211. [DOI] [PubMed] [Google Scholar]
- 18.Aus G, Bergdahl S, Lodding P, Lilja H, Hugosson J. Prostate cancer screening decreases the absolute risk of being diagnosed with advanced prostate cancer--results from a prospective, population-based randomized controlled trial. Eur Urol. 2007;51:659–664. doi: 10.1016/j.eururo.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Oberaigner W, Horninger W, Klocker H, Schönitzer D, Stühlinger W, Bartsch G. Reduction of prostate cancer mortality in Tyrol, Austria, after introduction of prostate-specific antigen testing. Am J Epidemiol. 2006;164:376–384. doi: 10.1093/aje/kwj213. [DOI] [PubMed] [Google Scholar]
- 20.Lemaitre L, Puech P, Poncelet E, Bouyé S, Leroy X, Biserte J, et al. Dynamic contrast-enhanced MRI of anterior prostate cancer: morphometric assessment and correlation with radical prostatectomy findings. Eur Radiol. 2009;19:470–480. doi: 10.1007/s00330-008-1153-0. [DOI] [PubMed] [Google Scholar]
- 21.Bouyé S, Potiron E, Puech P, Leroy X, Lemaitre L, Villers A. Transition zone and anterior stromal prostate cancers: zone of origin and intraprostatic patterns of spread at histopathology. Prostate. 2009;69:105–113. doi: 10.1002/pros.20859. [DOI] [PubMed] [Google Scholar]
- 22.Haffner J, Lemaitre L, Puech P, Haber GP, Leroy X, Jones JS, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;22 doi: 10.1111/j.1464-410X.2011.10112.x. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 23.Rietbergen JB, Kruger AE, Hoedemaeker RF, Bangma CH, Kirkels MJ, Schröder FH. Repeat screening for prostate cancer after 1-year followup in 984 biopsied men: clinical and pathological features of detected cancer. J Urol. 1998;160:2121–2125. doi: 10.1097/00005392-199812010-00046. [DOI] [PubMed] [Google Scholar]
- 24.Ellis WJ, Brawer MK. Repeat prostate needle biopsy: who needs it? J Urol. 1995;153:1496–1498. [PubMed] [Google Scholar]
- 25.Keetch D, Catalona W, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994;151:1571–1574. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 26.Woodrum DL, Brawer MK, Partin AW, Catalona WJ, Southwick PC. Interpretation of free prostate specific antigen clinical research studies for the detection of prostate cancer. J Urol. 1998;159:5–12. doi: 10.1016/s0022-5347(01)63996-x. [DOI] [PubMed] [Google Scholar]
- 27.Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542–1547. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 28.Scattoni V, Zlotta A, Montironi R, Schulman C, Rigatti P, Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol. 2007;52:1309–1322. doi: 10.1016/j.eururo.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu T, Nishie A, Ro T, Tajima T, Yamaguchi A, Kono S, et al. Prostate cancer detection: the value of performing an MRI before a biopsy. Acta Radiol. 2009;50:1080–1088. doi: 10.3109/02841850903216718. [DOI] [PubMed] [Google Scholar]
- 30.Park SY, Kim JJ, Kim TH, Lim SH, Han DH, Park BK, et al. The role of endorectal magnetic resonance imaging in predicting extraprostatic extension and Seminal vesicle invasion in clinically localized prostate cancer. Korean J Urol. 2010;51:308. doi: 10.4111/kju.2010.51.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]