Abstract
A previous randomized clinical trial based on self-determination theory (SDT) and consistent with the Public Health Service (PHS) Guideline for Treating Tobacco Use and Dependence demonstrated that an intensive intervention could change autonomous self-regulation and perceived competence which in part facilitated long-term tobacco abstinence. The current article describes a pragmatic comparative effectiveness trial of three SDT-based intensive tobacco-dependence interventions. Eligible participants are randomized to one of three treatment conditions designed to facilitate long-term maintenance of tobacco abstinence, namely, Community Care (CC), which includes the 6-month SDT-based intervention previously shown to promote autonomous self-regulation, perceived competence, medication use, and tobacco abstinence; Extended Need Support (ENS), which extends the 6-month SDT-based intervention to 12 months and trains an important other to provide support for smokers’ basic psychological needs; and Harm Reduction (HR), which provides extended need support and recommends medication use for participants who do not want to stop smoking completely within 30 days but who are willing to reduce their cigarette use by half. The primary outcome is 12-month prolonged abstinence from tobacco, which is assessed one year following termination of treatment (two years post-randomization). Secondary outcomes include 7- and 30-day point prevalence tobacco abstinence, number of days using smoking-cessation medication, change in autonomous self-regulation and perceived competence, and perceived need support from important others.
Keywords: autonomy, perceived competence, pragmatic comparative effectiveness trial, self-determination theory, tobacco abstinence
Introduction
In 2003, the National Institutes of Health funded 21 studies to examine long-term maintenance of health-behavior change, via the Health Maintenance Consortium. Herein, we describe interventions based on self-determination theory (SDT) [1–4] and designed to facilitate long-term tobacco abstinence, which will be examined in a pragmatic comparative effectiveness trial called the Smoker’s Health Project (SHP).
Importance of Developing Effective Interventions for Maintenance of Tobacco Abstinence
Despite the many behavioral, pharmacological, and public-policy efforts to promote smoking cessation, tobacco use remains the leading cause of preventable death in the US[5]. Underscoring the importance of tobacco abstinence, people who stop smoking live longer and have better quality of life than those who continue to smoke [6–7]. The Public Health Service (PHS) Guideline for Treating Tobacco Use and Dependence recommends that intensive treatment include at least four contacts of more than 10 minutes each, with total contact time exceeding 30 minutes; provision of medical advice about the health risks of smoking; a description of the health benefits of cessation and available pharmacotherapy; and delivery of additional counseling [8–9]. Intensive interventions have been shown to enhance tobacco abstinence and improve health, are cost effective relative to other standard medical treatments, and provide a mortality benefit to those who receive them [8, 10–12]. Nonetheless, most people who stop smoking relapse within one year following termination of treatment, thus highlighting the importance of understanding the factors involved in maintenance of tobacco abstinence [8].
Most theories of health behavior operationalize maintenance as sustained change over time, although such change is often measured while the intervention or some other schedule of reinforcement remains operative. With its applications to health behavior, SDT speaks to the issue of maintenance and specifies that true maintenance occurs only after the intervention has ended completely. Most theories fail to address mechanisms through which maintenance occurs [13]. In contrast, SDT proposes that both autonomous self-regulation (ASR) and perceived competence (PC) facilitate maintenance of health-behavior change [14], as maintenance is more likely to occur when people feel volitional to integrate new behaviors (e.g., not using tobacco to cope with stress) and use medication to relieve withdrawal symptoms and/or lower risk directly. Before describing the SHP interventions in detail, we provide a brief overview of SDT and how it guided the development of our previous randomized clinical trial for smokers [15–16], which formed a foundation for the current pragmatic comparative effectiveness trial.
Self-Determination Theory and Its Applications to Health Behavior
SDT is a macro-theory of human motivation, emotion, and personality in social contexts. With its organismic perspective, SDT posits a natural tendency toward psychological growth, physical health, and social wellness that is supported by satisfaction of the basic psychological needs for autonomy, competence, and relatedness. Autonomy refers to the experience that behavior is volitional and reflectively endorsed, and is measured on the treatment self-regulation questionnaire. SDT is the only empirically derived theory of motivation in which autonomy is a central focus, and much research from SDT has examined its correlates in the health-care domain [14]. Competence refers to the experience of feeling able to achieve a desired outcome (e.g., to stop smoking). Relatedness refers to the experience of genuine care and concern from, and trust in, important others (e.g., health-care providers, spouses, friends).
In a previous trial, we designed and tested an intensive tobacco-dependence intervention based on SDT and consistent with the PHS Guideline that was shown to predict tobacco abstinence at 6-, 18-, and 30-months post-randomization, as mediated by change in ASR, PC, and aspirations for physical health [15–19]. The SHP will compare two augmented SDT-based interventions intended to facilitate long-term tobacco abstinence to a standard intervention previously shown to be superior to community care. We reasoned that the salubrious effects of our previous trial might be augmented by extending the duration of the intervention to provide a longer period of need support, by teaching important others to support smokers’ basic psychological needs, and by recommending medication use for participants who do not want to stop smoking completely within 30 days but who are willing to reduce their cigarette use by half.
Importance of Internalization for Maintenance of Health-Behavior Change
According to SDT, maintenance of a new health behavior requires that people endorse the value of, and develop the requisite skills for, change [14]. Internalization refers to the natural, active process whereby a behavior that was initially prompted by external sources is regulated with an experience of autonomy and an accompanying sense of competence [20]. However, the process of internalization can function more or less effectively and thus behavioral regulations vary in the extent to which they are self-determined [21]. People perceive themselves to be autonomous when their behavior is experienced with a sense of volition and choice, whereas people perceive themselves to be controlled when they experience pressure or coercion to think, feel, or behave in certain ways. For example, people who smoke are said to be autonomously self-regulated if they attempt to stop smoking because it is personally important to them (identified regulation) or congruent with deeply held values and aspirations (integrated regulation). In contrast, people who smoke are said to be controlled if they attempt to stop because their health-care provider or spouse pressures them to do so (external regulation) or because they pressure themselves to do so through guilt or shame (introjected regulation).
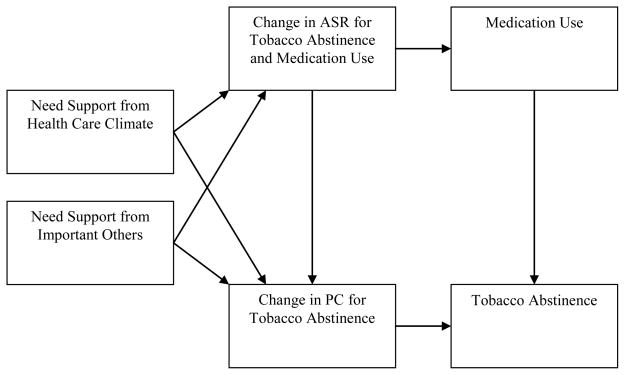
The process of internalization is supported by satisfaction of the basic psychological needs for autonomy, competence, and relatedness. Health-care providers and important others can support smokers’ basic psychological needs by following the guidelines listed in Table 1, which are consistent with SDT and the PHS Guideline. Guided by the SDT model of health-behavior change, Figure 1 depicts the path model that will be tested in this trial. Randomized clinical trials in the domains of physical activity, nutrition, weight loss, and dental hygiene have shown that social-contextual factors that support need satisfaction promote internalization of ASR, PC, aspirations for physical health, and maintenance of health-behavior change [14, 19, 22–25].
Table 1.
Autonomy Support
|
Competence Support
|
Relatedness Support
|
Figure 1.
The hypothesized path model depicting associations among measured constructs that will be tested in the SHP.
Notes. ASR = Autonomous self-regulation. PC = Perceived competence.
Overview of the Smoker’s Health Project (SHP)
Research Objectives
The first goal of the SHP is to determine whether extending the duration of the intervention from six to 12 months would enhance ASR, PC, medication use, and maintenance of tobacco abstinence. The second goal is to examine whether important others can learn to support smokers’ basic psychological needs and thereby facilitate long-term tobacco abstinence. In our previous trial that intervened on multiple cardiovascular risk factors, autonomy support from important others enhanced ASR, PC, and tobacco abstinence, and reduced percent calories from fat among those with high cholesterol, but no intervention has been shown to increase need support from important others [26]. The third goal is to determine whether recommending medication use for participants who do not want to stop smoking completely within 30 days but who are willing to reduce their cigarette use by half would enhance ASR, PC, and tobacco abstinence. Studies have shown that offering medications to those who reduce their cigarette use by half may facilitate tobacco abstinence [9], but research is needed to determine whether such a recommendation would enhance ASR and PC. The fourth goal is to provide additional support for the PHS Guideline, as clinical recommendations based on systematic literature reviews, meta-analyses, and expert panel discussions require empirical support, but few such guidelines are actually tested in effectiveness or pragmatic trials. The fifth goal is to support autonomy, which along with enhancing well-being and reducing social injustice has been elevated to the highest level of biomedical ethics and medical professionalism [27–29]. Because this trial measures ASR for tobacco abstinence and medication use, its findings will contribute to a growing literature on interventions designed to support autonomy as a clinical goal, in and of itself. Overall, the SHP is designed to examine the effectiveness of two augmented SDT-based interventions—both place greater emphasis on medication use and need support, and one uses a smoking-reduction approach—relative to our previously validated treatment that is now offered in the community.
Study Design
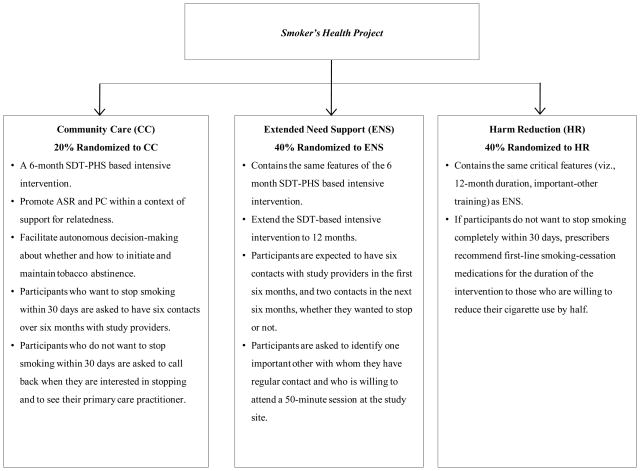
The SHP (for study design, see Figure 2) is a comparative effectiveness trial of two augmented SDT-based interventions relative to a standard intervention that included the 6-month SDT-PHS based intervention previously shown to facilitate long-term tobacco abstinence [15–16, 18]. We decided against inclusion of a no-treatment control group for the following reasons:
Figure 2.
Study design of the Smoker’s Health Project.
When randomization in the SHP began, the 6-month SDT-based intervention was supported by New York State and Excellus BlueCross BlueShield for those who wanted to stop smoking completely within 30 days, and thus had become part of usual care in the region.
It would be unethical not to offer the 6-month SDT-based intervention to those willing to be in the study because the treatment was shown to be superior to the historical control group, and because quality and length of life are increased by smoking cessation [28, 30].
If this treatment, which was available in the community, was not offered to potential participants, then few would enroll in the study.
Because the CC condition in the SHP was nearly equivalent to the 6-month SDT-based intervention from our previous trial [15–16, 18], we could compare the three SHP interventions to the two groups from our previous trial without increasing risk to those randomized to CC. Indeed, the PHS Guideline [8] noted that no-treatment control groups limit the ability to determine a trial’s efficacy. It would thus be important to include our previous intervention as a CC condition to determine the efficacy of the two augmented SDT-based interventions compared to the new CC standard.
The SHP is a pragmatic trial, as it includes smokers who are typically excluded from efficacy trials, measures medication use that is allowed to vary rather than tries to maximize medication use, and minimizes harm to those randomized to CC by offering the 6-month SDT-based intervention [31–32]. There is no run-in period prior to randomization, which is often used to maximize motivation for adhering to the protocol and medications prescribed through attrition of those less motivated before randomization. Rather, participants are randomized at their initial visit after completing informed consent and baseline questionnaires, which reflects actual practice where patients accept or decline treatment within the first visit or two. The SHP is a cessation-induction trial, as it includes participants regardless of whether or not they want to stop smoking [33]. Participants with a history of chemical dependence, depression, or anxiety are accepted in the study because the SHP is designed to reach a large percentage of adult smokers.
Recruitment, Honoraria, and Study Outcomes
Participants are eligible if they have smoked at least 100 cigarettes in their lifetime, have smoked five or more cigarettes per day during the four weeks prior to enrollment, are at least 18 years of age, and can read and speak English. Participants are recruited using IRB-approved newspaper, bus, and radio advertisements; signs in medical facilities; and mailings to patients in local physicians’ practices. Importantly, participants are eligible whether or not they intend to stop smoking. Participants are excluded for any of the following reasons: pregnancy, psychotic illness, too sick to participate, life expectancy of less than two years, and do not plan to live in the area for two years. Participants are paid $100 for completion of the study, and payment is pro-rated based on the percentage completed. Participants do not pay for counseling or prescription services. However, they do pay for medications and medication co-pays, either out-of-pocket or through their health insurance, and are eligible for reimbursement of $150 from a grant provided to the SHP by the American Lung Association in Rochester, New York.
The primary outcome is 12-month prolonged abstinence from tobacco, which is assessed one year following termination of treatment (two years post-randomization) [33–34]. Biochemical validation of the 12-month prolonged abstinence from tobacco measure is not possible due to the short half life of cotinine (36 hours) and the brief time that carbon monoxide takes to normalize after the last cigarette (8 hours) [35]. Secondary outcomes include 7- and 30-day point prevalence (7dPP and 30dPP) tobacco abstinence at six, 12, 18 and 24 months post-randomization, number of days using smoking-cessation medication, change in ASR for tobacco abstinence and medication use during the first 12 months of the study, change in PC during the first 12 months of the study, and perceived need support from important others. Although the SHP protocol recommends that participants receive 4–6 phone/office visits during the intervention, and they are encouraged to keep these visits, care is taken to avoid coercing participants into complying with an exact number of contacts. Participants are able to schedule additional visits during the intervention if desired.
The SHP Interventions
Community Care (CC)
The CC condition is a 6-month SDT-based intensive intervention that is consistent with the PHS Guideline and designed to promote ASR and PC within a context of support for relatedness through unconditional positive regard. The goal of CC is to facilitate autonomous decision-making about whether and how to initiate and maintain tobacco abstinence. Once the smoker indicates willingness to stop, the counselor teaches skills building, problem solving, and provides information on medications available as per the PHS Guideline recommendations [8]. In addition, smokers who indicate they wish to taper off cigarettes are taught a tapering protocol [36] as a way to support ASR and PC. Participants who indicate that they want to stop smoking within 30 days are asked to have six contacts over six months with study providers. Those who indicate that they do not want to stop smoking within 30 days are asked to call back when they are interested in stopping, as required by the locally supported programs.
Extended Need Support (ENS)
The ENS provides all that the CC condition participants receive and extends the SDT-PHS based intensive intervention to 12 months, during which participants are expected to have six contacts with study providers in the first six months (at least one contact with a prescriber), and two contacts in the next six months. Participants in both augmented SDT-based interventions are asked to have the same number of contacts with study providers regardless of whether they intend to stop smoking. Participants are asked to identify one important other (non-health-care professional) with whom they have regular contact and who is willing to attend a 50-minute session at the study site. Family and friends can be either autonomy supportive or controlling, and have more daily interaction with participants than do health care practitioners [26, 37]. During their visit, important others are asked to consider a behavior they want to change and how they would want to be treated if they were to make that change, which is designed to elicit a set of need-supportive behaviors. The important others are asked to record these behaviors on a worksheet during their visit (see Appendix A) and take the list home after the session. Once these behaviors are elicited and discussed, important others are asked to relate to the participant in that way. Important others are also asked to watch a DVD with the participant, which educates them on the health effects of smoking, the health benefits of tobacco abstinence, the highly addictive nature of nicotine, the effects and side effects of smoking-cessation medication, typical withdrawal symptoms, and the expected intensity and duration of withdrawal symptoms (6–12 weeks).
Harm Reduction (HR)
The HR condition contains the same critical features (viz., 12-month duration, important-other training) as ENS. Additionally, if during the initial interview participants indicate that they do not want to stop smoking completely within 30 days, or if they try to stop, fail, and do not want to make another attempt within 30 days, prescribers recommend first-line smoking-cessation medications for the duration of the intervention to those who are willing to reduce their cigarette use by half [38–46]. Participants are informed that there is no evidence that reducing cigarette use improves health, but doing so may increase confidence for stopping. Drug side effects and potential toxicities are discussed, and participants are asked whether they want to use a medication. The Federal Drug Administration approved the use of the medication protocol in this trial.
Training in Need Support
SHP practitioners include a tobacco-dependence counselor, nurse, health educator, and health psychologist, and SHP prescribers include a physician assistant and primary care internist. All SHP providers receive approximately 25 hours of training to deliver the interventions in an autonomy-supportive manner. Practitioners review the PHS Guideline, attend didactic training on tobacco dependence, view training tapes on motivational interviewing, read the Smoker’s Health Manual, observe four clinical interviews, and conduct four clinical interviews in the presence of a supervisor. Once the supervisor and practitioner agree that the intervention can be administered efficaciously, the practitioner is able to see participants independently. On-going supervision occurs every 2–4 weeks throughout the study.
Practitioners are trained to consider participants’ autonomy as the primary clinical goal. It is often thought that autonomy support involves increasing the behavioral outcome (e.g., tobacco abstinence or medication use), but this is not necessarily the case, as autonomy support involves non-judgmental acceptance of patients’ reluctance to stop smoking or use medication. Thus, a clinical encounter is considered successful if the patient has greater clarity about whether to make a change attempt and, if so, can develop a behavioral and pharmacological plan to facilitate tobacco abstinence. This secondary outcome is represented by change in ASR and PC. Practitioners are taught to assume that all participants, regardless of whether they want to stop, are ambivalent about smoking. Those who do not want to stop may experience conflict between the known health risks of smoking and their inherent tendency toward health, whereas those who do want to stop may experience conflict between their inherent tendency toward health and the consequences of nicotine addiction and withdrawal. Said differently, practitioners are trained to assume that smokers may feel conflicted about their enjoyment of smoking and the risks that smoking poses, and to elicit and acknowledge such conflict by reflecting it to the patient. Finally, practitioners are taught to give participants the responsibility to decide whether or not they want to stop smoking and/or use medications, and to provide a clear recommendation of tobacco abstinence for all participants because smoking cessation increases quality and length of life.
Support for Autonomy
The following components of the interventions are designed to support autonomy:
Eliciting and acknowledging participants’ perspectives and life aspirations
Practitioners ask participants about their experiences regarding smoking and stopping, as well as their views on the health consequences of smoking and the health benefits of stopping. Participants are asked to identify several important life aspirations and indicate how smoking may help and/or hinder their attaining these goals [47]. This discussion, which has been shown to facilitate long-term tobacco abstinence, is intended to help participants and understand how tobacco use fits into the participant’s life before considering an attempt to stop smoking and determine how best to make that change [19]. This exploration of values is intended to facilitate integrated self-regulation—a form of autonomous self-regulation.
Providing a clear rationale for change
Practitioners provide a clear rationale for change based on participants’ reasons for stopping, the health risks of smoking, and the health benefits of stopping. Practitioners present an individualized 10-year absolute risk for developing coronary artery disease as a natural frequency to each participant [48]. For example, Mr. Smith, who has a 10-year absolute risk of 10%, is told that 1 in 10 individuals like him is expected to have a heart attack, need bypass surgery or angioplasty, die suddenly from a heart attack, or develop chest pain (angina) from too little blood flow to the heart within the next 10 years. Mr. Smith is then asked how he feels about his level of risk, and his response is reflected back to him. Next, he is told that if he stopped smoking permanently, his risk for having a cardiovascular event would be reduced by half within one year and that only 1 in 20 individuals with that risk is expected to have a cardiovascular event. Finally, Mr. Smith is asked how he feels about that change in risk, and his response is reflected back to him. This elicitation of and reflection on the participant’s perspective about the health risks smoking poses is intended to facilitate internalization.
Providing effective options for change
Practitioners provide each participant with a clear recommendation to stop smoking so as to improve health, extend length of life, and improve quality of life. Practitioners recommend behavioral counseling and medication use for those participants without contraindications. Providing participants with choice among effective options for change is intended to enhance autonomy, yet practitioners are mindful not to offer an overwhelming list of options. Practitioners give a clinical recommendation if participants have difficulty making a decision about treatment options, or if participants solicit their advice.
Supporting self-initiations for change
Practitioners solicit participants’ input on whether or not they want to stop smoking. If participants are not ready to stop, practitioners acknowledge this and ask them to return in two months for further discussion. If participants are ready to stop, practitioners ask them about how they want to proceed with the health-behavior change. For instance, many participants have used medications in the past with varying levels of success. Practitioners explore these experiences and ask the participants if they prefer a particular medication over another. If yes, practitioners provide instructions on effective use and side effects of the chosen drug, and answer any related questions. A recommendation is provided only if requested by participants. Importantly, an office or phone follow-up is scheduled (typically within 1–3 days) to discuss how the method is working in an open, non-judgmental manner.
Minimizing pressure and control
Practitioners explore participants’ perspectives before giving a clinical recommendation and acknowledge when participants do not want to use treatment or schedule a visit within a particular time. Both the SHP protocol and informed consent form indicate that participants need not intend to stop smoking or make a stop attempt during the intervention in order to be enrolled. After the intervention, participants are referred to their primary-care provider for ongoing care.
Support for Competence
Support for competence is intended to enhance participants’ perceptions that they can attain their behavior-change goals and increase the actual percentage of those who succeed. From an SDT perspective, only those who feel volitional (autonomous) to make a change attempt are expected to benefit from competence support [14]. Thus, SHP practitioners are trained to focus predominantly on supporting autonomy and relatedness until participants indicate an intent to stop smoking, after which competence is supported directly along with autonomy and relatedness. Teaching participants how to make a change attempt may be met with resistance if they are not ready to learn such approaches. If participants request information on how to stop smoking before establishing an intention to change, practitioners answer those requests directly, as refusing to do so may be perceived as controlling and/or judgmental by participants.
The following components of the interventions are designed to support competence:
Offering effectance-relevant feedback
Practitioners give participants information on how cigarettes work (rapid delivery of nicotine by the tars), the nature of nicotine addiction (short half-life of tobacco, duration and expected pattern of withdrawal symptoms), effects of tobacco use on weight (appetite suppression, increased metabolic rate), and how medications work to relieve withdrawal symptoms (anxiety, constipation, craving, dysphoric mood, headaches, increased appetite, irritability, trouble concentrating). Providing this information in a non-judgmental manner is intended to enhance ASR and PC, which can avoid undermining need satisfaction and eliciting reactance toward the intervention.
Helping to establish a behavior-change plan
Practitioners work with participants to establish a plan to stop smoking and manage withdrawal symptoms. Using a PHS worksheet, those who are willing to make an attempt to stop smoking specify a stop date, select a medication to manage withdrawal symptoms, receive skills-building and problem-solving training, ask others for support, and arrange a follow-up meeting with a study practitioner.
Support for Relatedness
The following components of the interventions are designed to support relatedness:
Relating to participants in an empathic, non-judgmental manner
Practitioners (and, for those in ENS and HR, important others) are taught to relate to participants in an empathic, non-judgmental manner even if participants are not ready to stop smoking. Such a caring, accepting, and encouraging style embodies the concept of unconditional positive regard and is consistent with the PHS Guideline. Practitioners reframe failed attempts at smoking cessation as ‘short successes’ and elicit participants’ interpretations of these events [49].
Defining the intervention by time rather than number of visits
The intervention is defined by the period of time that treatment is available, rather than the number of visits made by participants. Offering a time period of available support is expected to enhance relatedness and, to some extent, autonomy because participants differ in the amount of support needed to reach their goals. If the number of visits is fixed, then participants may feel more pressured and less able to develop quality relationships with study providers.
Sample Size Calculations
Power analyses are done using the formula for differences in proportions [50] with a two-tailed alpha of .05 (with Bonferroni adjustment to .025) and a power of 80%. Based on our previous randomized clinical trial testing the intensive SDT-PHS intervention [16] our previous randomized clinical trial of a brief intervention for physicians to motivate cessation [51], the results of several studies providing medications to smokers willing to cut down cigarette use not willing to stop smoking [38–46], and results of the PHS meta-analysis, we estimate 12-month prolonged abstinence rates at 24 months for the three conditions to be 4% in CC, 14% in ENS, and 23% in HR. We planned to randomize 20%of enrollees to CC, and 40% to ENS, and 40% to HR. With an intention-to-treat analysis, the sample size needed to detect a significant difference between ENS and HR is 353 participants per condition. With 353 participants in ENS, a sample size of 112 in CC is more than adequate to detect a significant difference between ENS and CC. The necessary condition sizes to detect the other hypothesized effects are all less than 220. Thus, we plan for a total of 818 participants, with 112 participants randomized to CC and 353 participants randomized to both ENS and HR.
Assessments
Various questionnaire assessments (described below) are performed at the beginning of the intervention (baseline), and at two, four, six, 12, and 18 months post-randomization.
Autonomous self-regulation for tobacco abstinence and medication use
The Treatment Self-Regulation Questionnaire [52–53] presents participants with the following stems: “The reason I would stop smoking permanently or continue not smoking is…” and “The reason I would use medication as recommended is…” Participants rate pre-selected responses assessing external (4 items; e.g., “Because I want others to approve of me”.), introjected (3 items; e.g., “Because I would feel bad about myself if I smoked”.), identified (4 items; e.g., “Because it is very important for being as healthy as possible.”), and integrated (2 items; e.g., “Because using medication to stopping smoking permanently is consistent with my life goals.”) reasons for behavior change. Responses are made on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree).
Perceived competence
The Perceived Competence Scale [15–16, 52] assesses participants’ experience of feeling able to stop smoking successfully (four items; e.g., I feel confident in my ability to stop smoking permanently). Responses are made on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree).
Perceived need support from health care practitioners
The Health Care Climate Questionnaire [15–16, 52] assesses participants’ experience of need support from their health-care provider in consulting with them on their smoking or stopping (15 items; e.g., I feel that my counselor has provided me with choices and options about my smoking). Responses are made on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree).
Perceived need support from important others
The Important Other Questionnaire [15–16, 52] assesses participants’ experience of need support from important others (non-health care professionals) regarding their smoking or stopping (6 items; e.g., I feel that my important other provided me with choices and options about smoking (including quitting or not). Responses are made on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree).
Analytic Overview
Analysis of Variance (ANOVA) is used to test group differences on the primary and secondary outcomes with “intention-to-treat” data, in which all participants are included. Structural Equation Modeling (SEM) is used to test the hypothesized path model (see Figure 1) with “as-reported” data, in which only available responses are included.
Conclusion
Major decision points
It is important to discuss some of the major decisions made in the design of the SHP.
The SHP is a pragmatic comparative effectiveness trial that is characterized by the absence of a run-in period and inclusion of smokers who are typically excluded from efficacy trials. Such a design broadens the applicability of results to real-world smokers. Further, greater variation in motivation among participants at baseline allows for a better test of whether the interventions facilitated internalization and tobacco abstinence.
The theoretical framework for the SHP is SDT, which defines maintenance as sustained change over time after the intervention has ended. As such, we expect lower absolute rates of prolonged abstinence from tobacco, relative to trials that require fewer months of health-behavior change and include booster sessions designed to enhance maintenance.
Participants are randomized to one of three intensive interventions without inclusion of a no-treatment control group. This decision comes at the risk of not finding significant group differences in tobacco abstinence, but such risk is attenuated by having data available from our previous trial, which included a no-treatment control group. Indeed, we feel there is much greater risk to the participants in randomizing smokers to a no-treatment control group than from failing to find significant group differences.
There are three differences between the CC and ENS interventions, namely, extending the duration of the intervention from six to 12 months, contact with study providers regardless of whether participants want to stop smoking within 30 days, and training important others to support smokers’ basic psychological needs. We allow for this because the SHP is designed as a pragmatic trial and because we measure perceived need support from health-care providers and important others, which can be used to determine if specific mediators of treatment effects are affected in theoretically consistent ways.
Summary
The SHP is a pragmatic comparative effectiveness trial of three SDT-based intensive tobacco-dependence interventions. Herein, we presented an overview of the SHP and described in detail the need-supportive components of the interventions, which are intended to facilitate long-term tobacco abstinence through internalization of ASR for tobacco abstinence and medication use, enhanced PC, and support for relatedness from both health-care and important others. Comparisons among the intervention groups will determine whether (1) extending the duration of the intervention from six to 12 months and teaching important others to support smokers’ basic psychological needs enhance ASR, PC, medication use, and maintenance of tobacco abstinence (CC vs. ENS), and (2) recommending medication use for participants who do not want to stop smoking completely within 30 days but who are willing to reduce their cigarette use by half enhances these same outcomes (ENS vs. HR).
Supplementary Material
Acknowledgments
Geoffrey C. Williams, Christopher P. Niemiec, Richard M. Ryan, Edward L. Deci, and Holly McGregor Lavigne, Department of Clinical and Social Sciences in Psychology, University of Rochester. Dr. Williams and Dr. McGregor Lavigne are also members of the Department of Medicine, and the Healthy Living Research Center in the Center for Community Health at University of Rochester. Heather Patrick, National Cancer Institute, Division of Cancer Control and Population Sciences, Behavioral Research Program, Health Promotion Research Branch.
This research was supported by grants from the National Cancer Institute [R01-CA106668] awarded to Dr. Geoffrey Williams, MD, PhD; the National Institute of Mental Health and the National Cancer Institute [R01-MH059594] awarded to Dr. Geoffrey Williams, MD, PhD; the National Center for Research Resources [M01-RR00044] awarded to the University of Rochester General Clinical Research Center; and the National Center for Research Resources ARRA Supplement [UL1RR024160] awarded to the University of Rochester’s Clinical and Translational Science Institute.
Footnotes
Trial Registration: ClinicalTrials.gov number NCT00178685
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Geoffrey C. Williams, University of Rochester, Healthy Living Center, Center for Community Health, Rochester, NY 14607 USA
Heather Patrick, National Cancer Institute, Division of Cancer Control and Population Sciences, Bethesda, MD 20892, USA
Christopher P. Niemiec, University of Rochester, Department of Clinical and Social Sciences in Psychology, Rochester, NY 14627 USA
Richard M. Ryan, University of Rochester, Department of Clinical and Social Sciences in Psychology, Rochester, NY 14627 USA
Edward L. Deci, University of Rochester, Department of Clinical and Social Sciences in Psychology, Rochester, NY 14627 USA
Holly McGregor Lavigne, University of Rochester, Healthy Living Center, Center for Community Health, Rochester, NY 14607 USA
References
- 1.Ryan RM, Deci EL. Self-Determination Theory and the Facilitation of Intrinsic Motivation, Social Development, and Well-Being. Am Psychol. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 2.Deci EL, Ryan RM. The “What” and “Why” of Goal Pursuits: Human Needs and the Self-Determination of Behavior. Psychol Inquiry. 2000;11:227–68. [Google Scholar]
- 3.Niemiec CP, Ryan RM, Deci EL. Self-Determination Theory and the Relation of Autonomy to Self-Regulatory Processes and Personality Development. In: Hoyle RH, editor. Handbook of Personality and Self-Regulation. Malden, MA: Blackwell Publishing Ltd; 2010. [Google Scholar]
- 4.Vansteenkiste M, Niemiec CP, Soenens B. The Development of the Five Mini-Theories of Self-Determination Theory: An Historical Overview, Emerging Trends, and Future Directions. In: Urdan TC, Karabenick SA, editors. Advances in Motivation and Achievement. London: Emerald Group Publishing Limited; 2010. pp. 105–65. [Google Scholar]
- 5.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual Causes of Death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 6.Doll R, Peto R, Boreham J, Sutherland I. Mortality from Cancer in Relation to Smoking: 50 Years Observations on British Doctors. Br J Cancer. 2005;92:426–9. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strandberg AY, Strandberg TE, Pitkala K, Salomaa VV, Tilvis RS, Miettinen TA. The Effect of Smoking in Midlife on Health-Related Quality of Life in Old Age: A 26-Year Prospective Study. Arch Intern Med. 2008;168:1968–74. doi: 10.1001/archinte.168.18.1968. [DOI] [PubMed] [Google Scholar]
- 8.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating Tobacco Use and Dependence. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 2000. [Google Scholar]
- 9.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Currey SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 2008. [Google Scholar]
- 10.Maciosek MV, Edwards NM, Coffield AB, Flottemesch TJ, Nelson WW, Goodman MJ, et al. Priorities Among Effective Clinical Preventive Services: Methods. Am J Prev Med. 2006;31:90–6. doi: 10.1016/j.amepre.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The Effects of a Smoking Cessation Intervention on 14.5-Year Mortality. Ann Intern Med. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. Intensive Smoking Cessation Intervention Reduces Mortality in High-Risk Smokers With Cardiovascular Disease. Chest. 2007;131:446–52. doi: 10.1378/chest.06-1587. [DOI] [PubMed] [Google Scholar]
- 13.Rothman AJ. Toward a Theory-Based Analysis of Behavioral Maintenance. Health Psychol. 2000;19:64–9. doi: 10.1037/0278-6133.19.suppl1.64. [DOI] [PubMed] [Google Scholar]
- 14.Ryan RM, Patrick H, Deci EL, Williams GC. Facilitating Health Behavior Change and its Maintenance: Interventions Based on Self-Determination Theory. Euro Health Psychol. 2008;20:2–5. [Google Scholar]
- 15.Williams GC, McGregor HA, Sharp D, Levesque C, Kouides RW, Ryan RM, et al. Testing a Self-Determination Theory Intervention for Motivating Tobacco Cessation: Supporting Autonomy and Competence in a Clinical Trial. Health Psychol. 2006;25:91–101. doi: 10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Williams GC, McGregor HA, Sharp D, Kouides RW, Levesque C, Ryan RM, et al. A Self-Determination Multiple Risk Intervention Trial to Improve Smokers’ Health. J Gen Intern Med. 2006;21:1288–94. doi: 10.1111/j.1525-1497.2006.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams GC, Minicucci DS, Kouides RW, Levesque CS, Chirkov VI, Ryan RM, et al. Self-Determination, Smoking, Diet and Health. Health Educ Res. 2002;17:512–21. doi: 10.1093/her/17.5.512. [DOI] [PubMed] [Google Scholar]
- 18.Williams GC, Niemiec CP, Patrick H, Ryan RM, Deci EL. The Importance of Supporting Autonomy and Perceived Competence in Facilitating Long-Term Tobacco Abstinence. Ann Behav Med. 2009;37:315–24. doi: 10.1007/s12160-009-9090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niemiec CP, Ryan RM, Deci EL, Williams GC. Aspiring to Physical Health: The Role of Aspirations for Physical Health in Facilitating Long-Term Tobacco Abstinence. Patient Ed Couns. 2009;74:250–7. doi: 10.1016/j.pec.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan RM. Agency and Organization: Instrinsic Motivation, Autonomy and the Self in Psychological Development. In: Jacobs J, editor. Nebraska Symposium on Motivation: Developmental Perspectives on Motivation. Lincoln, NE: University of Nebraska Press; 1993. pp. 1–56. [PubMed] [Google Scholar]
- 21.Ryan RM, Connell JP. Perceived Locus of Causality and Internalization: Examining Reasons for Acting in Two Domains. J Pers Soc Psychol. 1989;57:749–61. doi: 10.1037//0022-3514.57.5.749. [DOI] [PubMed] [Google Scholar]
- 22.Fortier MS, Sweet S, O’Sullivan TL, Williams GC. A Self-Determination Process Model of Physical Activity Adoption in the Context of a Randomized Controlled Trial. Psychol Sport Exercise. 2007;8:741–57. [Google Scholar]
- 23.Silva M, Markland D, Minderico C, Vieira P, Castro M, Coutinho S, et al. A Randomized Controlled Trial to Evaluate Self-Determination Theory for Exercise Adherence and Weight Control: Rationale and Intervention Description. BMC Public Health. 2008;8:234. doi: 10.1186/1471-2458-8-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halvari A, Halvari H. Motivational Predictors of Change in Oral Health: An Experimental Test of Self-Determination Theory. Motivation and Emotion. 2006;30:294–305. [Google Scholar]
- 25.Silva MN, Vieira PN, Coutinho SR, Matos MG, Sardinha LB, Teixeira PJ. Using Self-Determination Theory to Promote Physical Activity and Weight Control: A Randomized Controlled Trial in Women. J Behav Med. 2010;33:110–22. doi: 10.1007/s10865-009-9239-y. [DOI] [PubMed] [Google Scholar]
- 26.Williams GC, Lynch MF, McGregor HA, Ryan RM, Sharp D, Deci EL. Validation of the “Important Other” Climate Questionnaire: Assessing Autonomy Support for Health-Related Change. Families, Systems & Health. 2006;24:179–94. [Google Scholar]
- 27.ABIM, ACP-ASIM, Medicine EFoI. Medical Professionalism in the New Millennium: A Physician Charter. Ann Intern Med. 2002;136:243–6. doi: 10.7326/0003-4819-136-3-200202050-00012. [DOI] [PubMed] [Google Scholar]
- 28.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 5. New York: Oxford University Press, Inc; 2001. [Google Scholar]
- 29.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 6. New York: Oxford University Press, Inc; 2009. [Google Scholar]
- 30.U.S. Department of Health and Human Services. The Benefits of Smoking Cessation: A Report of the Surgeon General (DHHS Publication No. CDC 90-8416) Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; [Google Scholar]
- 31.Godwin M, Ruhland L, Casson I, MacDonald S, Delva D, Birtwhistle R, et al. Pragmatic Controlled Clinical Trials in Primary Care: The Struggle Between External and Internal Validity. BMC Med Res Methodol. 2003;3:28. doi: 10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pringle M, Churchill R. Randomised Controlled Trials in General Practice. BMJ. 1995;311:1382–3. doi: 10.1136/bmj.311.7017.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes JR, Keely J, Niaura R, Ossip-Klein D, Richmond R, Swan G. Measures of Abstinence in Clinical Trials: Issues and Recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 34.Pierce JP, Gilpin E. A Minimum 6-Month Prolonged Abstinence Should be Required for Evaluating Smoking Cessation Trials. Nicotine Tob Res. 2003;5:151–3. doi: 10.1080/0955300031000083427. [DOI] [PubMed] [Google Scholar]
- 35.Pojer R, Whitfield JB, Poulos V, Eckhard IF, Richmond R, Hensley WJ. Carboxyhemoglobin, Cotinine, and Thiocyanate Assay Compared for Distinguishing Smokers from Non-Smokers. Clin Chemistry. 1984;30:1377–80. [PubMed] [Google Scholar]
- 36.Riggs RL, Hughes JR, Pillitteri JL. Two Behavioral Treatments for Smoking Reduction: A Pilot Study. Nicotine Tob Res. 2001;3:71–6. doi: 10.1080/14622200020032114. [DOI] [PubMed] [Google Scholar]
- 37.Williams GC, Quill TE, Deci EL, Ryan RM. The Facts Concerning the Recent Carnival of Smoking in Connecticut and Elsewhere. Ann Intern Med. 1991;115:59–63. doi: 10.7326/0003-4819-115-1-59. [DOI] [PubMed] [Google Scholar]
- 38.Bollinger CT, Zellweger JP, Danielsson T, van Biljon X, Robidou A, Westin A, et al. Smoking Reduction with Oral Nicotine Inhalers: Double Blind, Randomised Clinical Trial of Efficiacy and Safey. BMJ. 2000;321:329–33. doi: 10.1136/bmj.321.7257.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Croghan GA, Hurt RD, Croghan IT, Wolter TD, Offord KP. A Pilot Study to Demonstrate that Smoking Reduction Occurs with the Nicorette Inhaler and Results in Harm Reduction. J Addict Dis. 1999 [Google Scholar]
- 40.Daughton DM, Rennard SI, Thompson AB, Floreani AA, Romberger DJ, Millatmal T. The Effects of Nicotine Replacement Therapy on Cigarette Smoking Reduction. Pamphlet from the American Thoracic Association; Boston, MA: 1994. [Google Scholar]
- 41.Etter JF, Laszlo E, Zellweger JP, Perrot C, Perneger TV. Nicotine Replacement to Reduce Cigarette Consumption in Smokers Who Are Unwilling to Quit: A Randomized Trial. J Clin Psychopharmacol. 2002;22:487–95. doi: 10.1097/00004714-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Fagerstrom KO, Tejding R, Ake W, Lunell E. Aiding Reduction of Smoking with Nicotine Replacement Medications: Hope for the Recalcitrant Smoker? Tob Control. 1997;3:311–6. doi: 10.1136/tc.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hughes JR. Reduced Smoking: An Introduction and Review of Evidence. Addiction. 2000;95:S3–S7. doi: 10.1080/09652140032008. [DOI] [PubMed] [Google Scholar]
- 44.Hughes JR, Cummings KM, Hyland A. Ability of Smokers to Reduce Their Smoking and its Association with Future Smoking Cessation. Addiction. 1999;94:109–14. doi: 10.1046/j.1360-0443.1999.9411097.x. [DOI] [PubMed] [Google Scholar]
- 45.Hurt RD, Croghan GA, Wolter TD, Croghan IT, Offord KP, Williams GM, et al. Does Smoking Reduction Result in Reduction of Biomarkers Associated with Harm? A Pilot Study Using a Nicotine Inhaler. Nicotine Tob Res. 2000;2:327–36. doi: 10.1080/713688154. [DOI] [PubMed] [Google Scholar]
- 46.Rennard SI, Glover E, Leischow S, Daughton DM, Glover P, Muramoto M, et al. Nicotine Tob Res. PA6. 2002. Efficacy of the Nicotine Inhaler in Smoking Reduction; p. 27. [DOI] [PubMed] [Google Scholar]
- 47.Kasser T, Ryan RM. Further Examining the American Dream: Differential Correlates of Intrinsic and Extrinsic Goals. Pers Soc Psychol Bull. 1996;22:280–7. [Google Scholar]
- 48.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of Cardiovascular Risk by Use of Multiple-Risk-Factor Assessment Equations: A Statement for Healthcare Professionals From the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–92. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 49.Rogers CRR. The Process Equation of Psychotherapy. Am J Psychother. 1961;15:27–45. doi: 10.1176/appi.psychotherapy.1961.15.1.27. [DOI] [PubMed] [Google Scholar]
- 50.Fleiss JL. Statistical Methods for Rates and Proportions. 2. New York, NY: Wiley; 1981. [Google Scholar]
- 51.Williams GC, Gagne M, Ryan RM, Deci EL. Facilitating Autonomous Motivation for Smoking Cessation. Health Psychol. 2002;21:40–50. [PubMed] [Google Scholar]
- 52.Williams GC, McGregor HA, Zeldman A, Freedman ZR, Deci EL. Testing a Self-Determination Theory Process Model for Promoting Glycemic Control Through Diabetes Self-Management. Health Psychol. 2004;23:58–66. doi: 10.1037/0278-6133.23.1.58. [DOI] [PubMed] [Google Scholar]
- 53.Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the Theoretical Structure of the Treatment Self-Regulation Questionnaire (TSRQ) Across Three Different Health Behaviors. Health Educ Res. 2007;22:691–702. doi: 10.1093/her/cyl148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.