Abstract
By relating an animal's morphology to its functional role and the behaviours performed, we can further develop our understanding of the selective factors and constraints acting on the adaptations of great apes. Comparison of muscle architecture between different ape species, however, is difficult because only small sample sizes are ever available. Further, such samples are often comprised of different age–sex classes, so studies have to rely on scaling techniques to remove body mass differences. However, the reliability of such scaling techniques has been questioned. As datasets increase in size, more reliable statistical analysis may eventually become possible. Here we employ geometric and allometric scaling techniques, and ancovas (a form of general linear model, GLM) to highlight and explore the different methods available for comparing functional morphology in the non-human great apes. Our results underline the importance of regressing data against a suitable body size variable to ascertain the relationship (geometric or allometric) and of choosing appropriate exponents by which to scale data. ancova models, while likely to be more robust than scaling for species comparisons when sample sizes are high, suffer from reduced power when sample sizes are low. Therefore, until sample sizes are radically increased it is preferable to include scaling analyses along with ancovas in data exploration. Overall, the results obtained from the different methods show little significant variation, whether in muscle belly mass, fascicle length or physiological cross-sectional area between the different species. This may reflect relatively close evolutionary relationships of the non-human great apes; a universal influence on morphology of generalised orthograde locomotor behaviours or, quite likely, both.
Keywords: locomotion, normalisation, positional behaviour, primate
Introduction
Studying the relationship between the functional anatomy of an animal, the behaviours it performs and the habitat it uses is crucial to any investigation of adaptation (Bock & von Wahlert, 1998). Thus, by relating the functional anatomy of extant non-human great apes to the locomotor behaviours they perform in their different habitats, we can expand our knowledge of the influences and constraints upon great-ape adaptations and thereby of locomotor diversification in the hominoids (see Bock & von Wahlert, 1998; Payne et al. 2006; Vereecke, 2006).
Although all the non-human apes can be characterised by their use of generalised orthograde clambering (where the trunk is upright and both fore- and hind-limbs are used to bear weight either under compression or tension; reviewed in Crompton et al. 2008), one might expect anatomical differences between the more terrestrial species (the African non-human apes: chimpanzees, bonobos and gorillas; see Hunt, 1992, 2004), compared to those that are predominantly arboreal (the Asian non-human apes: orangutans and gibbons: see Fleagle, 1999; Thorpe & Crompton, 2005, 2006). Based on these differences, terrestrial species, for example, might be expected to have a reduced need for grasping ability in their feet, compared to more arboreal species, and this might be reflected by reduced mass in their distal limb muscles (see Payne et al. 2006). Furthermore, one might expect an increased need for more arboreal species such as orangutans to produce forces over a greater range of motion than more terrestrial species, due to the need to interact with substrates arranged three-dimensionally around the body (e.g. Thorpe & Crompton, 2005, 2006).
Such potential differences can be established through the measurement of basic anatomical parameters including muscle belly mass and muscle fascicle length, which enable factors such as muscle physiological cross-sectional area (PCSA) to be calculated (e.g. Thorpe et al. 1999; Carlson, 2006; Payne et al. 2006). Fascicle length reflects the number of sarcomeres in series, and the longer the fascicle length, the greater the maximum shortening-velocity of muscle fascicles (see Bodine et al. 1982; Powell et al. 1984; Wickiewicz et al. 1984; Thorpe et al. 1999). PCSA, on the other hand, reflects the number of sarcomeres in parallel, and is calculated from muscle belly mass, muscle density and the length of the muscle fascicles (Alexander & Vernon, 1975). Physiological cross-sectional area provides an indication of the maximum force a muscle can produce when multiplied by the maximum isometric stress of vertebrate muscle (0.3 MPa; Wells, 1965) and therefore a larger PCSA indicates an ability to produce larger forces (Bodine et al. 1982; Sacks & Roy, 1982; Powell et al. 1984; Zajac, 1992; e.g. Thorpe et al. 1999; Carlson, 2006; Payne et al. 2006; Channon et al. 2009; Michilsens et al. 2009). Overall, muscle arrangements range between an optimal ‘in series’ pattern (thus maximising velocity and displacement) to an optimal ‘in parallel’ arrangement (maximising force production) depending on the functions they perform (Wickiewicz et al. 1984; Thorpe et al. 1999).
In recent years, the number of studies reporting such features of hindlimb muscle architecture in non-human apes has increased (e.g. chimpanzee: Thorpe et al. 1999; Carlson, 2006; gibbon and siamang: Vereecke, 2006; Channon et al. 2009; all apes: Payne et al. 2006). However, due to the understandable rarity of non-human ape cadavers, sample sizes are often very small, often consist of varying age–sex classes, and may take many years to collate, all of which complicate inter-specific comparisons of functional capacity. Furthermore, non-human apes range in mass from approximately 5 kg in gibbons (Vereecke, 2006; Channon et al. 2009) to over 200 kg in gorillas (Zihlman & McFarland, 2000). Such a wide range of body mass needs to be taken into careful consideration if we are to be able to interpret inter-specific differences in morphology and the implications of such differences for the dynamic between animals and their habitat.
The calculation of a ‘per-body’ mass ratio – where the variable of interest is divided by a measure of body size, such as body mass – might appear to be an appropriate way to remove the influence of body size (Packard & Boardman, 1987; Nevill et al. 1992). However, it is only suitable in instances where the physiological variable in question varies ‘isometrically’ with body size (Tanner, 1949; Schmidt-Nielson, 1984; Packard & Boardman, 1987; Nevill et al. 1992). Two animals are considered isometrically (or ‘geometrically’) similar if one can be made identical to the other by multiplying all length dimensions by the same factor (Alexander, 2000). In such cases, plotting the variable of interest (e.g. muscle belly mass), against a measure of body size (e.g. body mass) would result in a straight line passing through the origin (see Schmidt-Nielson, 1984; Packard & Boardman, 1987). Where anatomical data does scale geometrically, power function models can be used with masses being proportional to (mass)1, lengths to (mass)1/3 and areas to (mass)2/3 (Alexander et al. 1981; Alexander, 1996). This relationship has been used in numerous previous studies of ape anatomy to normalise data and remove the effects of body mass from the analysis (Thorpe et al. 1999; Payne et al. 2006; Oishi et al. 2008, 2009; Channon et al. 2009; Michilsens et al. 2009). The method may readily be justified for intra-specific comparisons (as in Thorpe et al. 1999) because animals of the same species are more likely to be geometrically similar (although ontogenetic changes should also be taken into account). However, it has been argued that geometric normalization is unlikely to be appropriate for inter-specific comparisons because true geometric scaling rarely occurs in nature, as a consequence of variations during ontogeny or evolution, and adaptations to different lifestyles (Alexander et al. 1981; Nevill et al. 2005). Therefore, the use of geometric scaling to assess species differences may not always be accurate or appropriate. It is recommended that regression analysis should be performed prior to normalisation (even for intra-specific comparisons) to test whether data plot as a straight line through the origin (if plotting raw data), or the exponent equals that predicted by isometry when using log-transformed data (see Tanner, 1949; Alexander et al. 1981; Packard & Boardman, 1987, 1999; Nevill et al. 2005).
More commonly, a plot of the variable of interest against the measure of body size generally results in a linear relationship which does not pass through the origin (when raw data are plotted), or a curvilinear relationship. In such a case the relationship is described as ‘allometric’, and the variable does not alter in direct proportion to body size (see Packard & Boardman, 1987; Brown et al. 2000; Biewener, 2003). The use of power function models, or allometric equations, in these instances, to scale physiological variables is well established (e.g. Kleiber, 1950; Alexander et al. 1981; Schmidt-Nielson, 1984; Pollock & Shadwick, 1994; Nevill and Holder, 1995; Brown et al. 2000) and takes the form:
| (1) |
where a and b are constants and X is body mass. These can be converted into a linear relationship by regressing logarithms of the data to give the equation:
| (2) |
The slope of the line, b, is the exponent of the power function and can be expressed as either a decimal or a fraction (Schmidt-Nielson, 1984; Alexander, 2000). Allometric equations have been established for many biological variables such as rate of oxygen consumption and metabolism (see Schmidt-Nielson, 1984 for examples). However, rather than scaling as third powers, as predicted by isometry, such variables often scale as quarter powers of body mass (M): e.g. mammalian metabolic rate has been found to scale to M3/4 and lifespan to M1/4 (Schmidt-Nielson, 1984; Brown et al. 2000). However, not all studies agree on the values of such exponents. Variations have been identified depending on the range of body sizes studied and the conditions under which measurements were taken, particularly for metabolic scaling exponents (e.g. White & Seymour, 2005; White et al. 2007, 2009; Isaac & Carbone, 2010; Vaca & White, 2010). Furthermore, even if there is agreement over the exponents established, the use of ratios to normalise the data may still introduce bias into the data (Packard & Boardman, 1999). Nevertheless, the use of allometry for scaling anatomical data has remained a key method to compare data between animals of different body mass (e.g. Pollock & Shadwick, 1994; Eng et al. 2008; McGowen et al. 2008).
An alternative approach has been suggested to enable comparison of morphological or physiological data, which is based on statistical models that take account of body size variation but remove the need for scaling. These include general linear model (GLM) or ancova analyses with body mass as a covariate (e.g. Packard & Boardman, 1987, 1999; Green et al. 2005; Halsey et al. 2007; Portugal et al. 2009). Rather than trying to remove the effects of body mass through the use of ratios, these models analyse the amount of variation in the variable of interest due to both body mass and other aspects of interest, e.g. species differences (Packard & Boardman, 1987, 1999; Portugal et al. 2009). They may thus provide a more robust analysis of morphological data where there are differences in body mass between subjects, if an adequate sample size is available.
The aim of the current study is to explore the use of the different methods reviewed above, in analysing non-human ape hindlimb muscle architecture, and secondarily to add further to the increasing dataset for such data.
Materials and methods
The new material used in this study comprised one chimpanzee (Pan troglodytes; Ptsm), one bonobo (Pan paniscus; Ppam), two gorilla (Gorilla gorilla gorilla; Gam, Gsm) and one orangutan (Pongo abelii; Oaf) cadaver (see Table 1 for subject information). All cadavers were fresh-frozen and positioned in the standard human anatomical position. Only one limb was available for dissection from each cadaver.
Table 1.
Subject information
| Subject code | Ptsm | Ppam | Gam | Gsm | Oaf |
|---|---|---|---|---|---|
| Species | Pan troglodytes | Pan paniscus | Gorilla gorilla gorilla | Gorilla gorilla gorilla | Pongo abelii |
| Obtained from | Zoological Society London | Apenheul Zoo | Twycross Zoo | Twycross Zoo | Paignton Zoo |
| Sex | M | M | M | M | F |
| Body mass (kg) | 50.20 | 41.92 | 175.00 | 152.00 | 54.00 |
| Age at death (years) | ca. 11(sub-adult) | ca. 22(adult) | ca. 30(adult) | ca. 18(sub-adult) | ca. 45(adult) |
| Cause of death | Group violence | Euthanasia | Fibrosing cardiomyopathy | Brain haemorrhage | Euthanasia |
| Hindlimb dissected | Left | Left | Left | Right | Right |
M, male; F, female.
Anatomical measurements and functional groupings
Muscle fascia were removed, and muscles separated and identified before being removed systematically, with their complete tendons attached. Points of origin and insertion were recorded. Muscle–tendon unit lengths were measured, including separate measurements for external tendon lengths at the origin and insertion, and muscle belly length. External tendon length was measured as the distance from either the most proximal (tendon of origin) or distal (tendon of insertion) muscle fibres to the point of tendon attachment to the bone. Any external tendon was then removed and muscle belly mass (including internal tendons) recorded. Finally, the muscle belly was cut either along the line of the internal tendon (pennate muscles) or along the centre of the belly (parallel fibred muscles) to reveal the full length of the muscle fibres, and three measurements of muscle fascicle length were made at different locations throughout the belly (see Thorpe et al. 1999; Payne et al. 2006 for other examples of these methods). Muscle fascicle length assesses the length of a bundle of muscle fibres that is visible to the naked eye. Muscle mass was measured to the nearest 0.1 g and tendon mass to the nearest 0.01 g. All lengths were measured to the nearest millimetre using a metal rule.
Physiological cross-sectional area (PCSA) was calculated using the equation:
where m is muscle belly mass in grams, ρ is the density of fresh muscle (1.06 g cm−3, Mendez & Keys, 1960) and l is muscle fascicle length in cm. In primates the angle of the fascicles to the tendon (pennation angle) is generally < 30o in fore- and hindlimb muscles (Thorpe et al. 1999), thus cosθ, normally present in this equation [PCSA = (cosθ × m)/ρl], is approximately one and can be omitted.
Standard practice in previous studies of ape muscle architecture has been to group muscles for comparison according to their primary function (e.g. Swindler & Wood, 1973; Payne et al. 2006; Channon et al. 2009). Therefore, to enable comparison to previous studies, muscles were grouped following these authorities (see Table 2 for groupings). Eng et al. (2008) highlighted the variability that can occur within functional muscle groups, whereby individual muscles may have different scaling relationships within a single muscle group. Therefore, the scaling of individual muscles was also compared. The intrinsic hip and foot muscles could not be included in the main analysis as measurements could not be recorded for all subjects because of contractures. To increase the size of the dataset to enable more robust comparison of scaling techniques, raw data from previous studies were obtained for analysis of the scaled data and ancova analysis. Data for chimpanzees were taken from Thorpe (1997) (chimp 93 and chimp 94) and Thorpe et al. (1999) (chimp 95) and data for one bonobo (Pp), two gorillas (Gm and Gj) and one orangutan (Ojm) were taken from Payne et al. (2006). Data for Gp and Ojf from Payne et al. (2006) were not included as they were incomplete datasets, and data from Oam were not used because this subject was fixed in alcohol.
Table 2.
Functional muscle groups for the hindlimb
| Muscle group | Muscles |
|---|---|
| Gluteals | Gluteus maximus, gluteus medius, gluteus minimus and scansorius |
| Adductors | Adductor magnus, adductor brevis, adductor longus and pectineus |
| Knee extensors | Rectus femoris and the vasti |
| Knee flexors and hip extensors | Biceps femoris (long and short heads), semimembranosus, semitendinosus |
| Bi-articular knee and hip flexors | Gracilis and sartorius |
| Uni-articular knee flexors | Popliteus |
| Plantarflexors | Gastrocnemius lateralis, gastrocnemius medialis, soleus, plantaris and tibialis posterior |
| Dorsiflexors | Tibialis anterior, extensor hallucis longus, extensor digitorum longus |
| Digital flexors | Flexor hallucis longus, flexor digitorum longus, flexor digitorum fibularis |
| Everters | Peroneus longus and peroneus brevis |
To obtain overall values for each functional muscle group, the individual muscle belly masses were summed to obtain an overall mass value, and the individual PCSA values were also simply summed to provide an estimate of the maximum force-generating capacity of each muscle group. Muscle fascicle length, however, was calculated as a weighted harmonic mean so as to take into account the different sizes of the muscle fibres in a group, using the equation:
| (4) |
where L is the group fascicle length, for a group where the jth member has a mass mj and a fascicle length of lj (Alexander et al. 1981).
Scaling of data
Before scaling using any method, regression analyses should be performed to establish the relationship between the variable of interest and body mass to decide which method of scaling is appropriate, but this has rarely been done (e.g. Thorpe et al. 1999; Carlson, 2006; Oishi et al. 2008, 2009; Channon et al. 2009; Michilsens et al. 2009). Previous studies which have scaled muscle architecture properties in mammals and primates have most often used ordinary least square (OLS) regression to establish scaling exponents (e.g. Alexander et al. 1981; Pollock & Shadwick, 1994; Eng et al. 2008). OLS regression, however, requires a number of assumptions to be met, including independence of data and measurement of the x-variable (e.g. body mass) without error, in addition to being liable to underestimate the scaling exponent when R2 values are low (White, 2011). The alternative use of reduced major axis (RMA) regression has recently been explored in scaling studies (e.g. Smith, 2009; White, 2011). RMA regression may be more appropriate than OLS when there is error in both variables and also symmetry between the two (Smith, 2009). However, as one of the aims of this study was to compare the usability of scaling methods, and so enable comparison with previous studies, OLS regression was employed but the results from RMA regression provided additionally.
OLS regressions of log-transformed data (to ensure linearity) of the three physiological variables of interest (belly mass, fascicle length and PCSA) for functional muscle groups from all studies were plotted against body mass (kg) (using Minitab®; USA). Scaling exponents were then established for muscle belly mass, fascicle length and PCSA for each of those functional muscle groups where a significant relationship with body mass was determined, and overall exponents and a mean exponent (calculated from the muscle group exponents) were provided to enable comparison with previous studies. The same procedure was adopted using RMA regression. Raw data were then scaled using the appropriate exponent established (obtained from OLS regression) for the different functional muscle groups, except in instances where there was no significant relationship with body mass. To further investigate the variation between individual muscles, scaling exponents were established for each individual muscle within a functional group, and a mean scaling exponent for the group calculated, together with standard errors.
For comparative purposes, geometric scaling was applied to all functional muscle groups to explore how conclusions of previous studies of ape muscle architecture based on geometric scaling (see Thorpe et al. 1999; Payne et al. 2006; Channon et al. 2009) compare with those obtainable using the specific exponents calculated from the data.
Statistical analysis
ancovas on log-transformed data, with body mass as a covariate, were used to assess whether differences between individuals reflected species differences and/or variation in body mass, based on the combined dataset from all studies. Species was included in the model as the independent variable, with body mass included as a covariate. ancovas were performed using Minitab® (function GLM) for each physiological variable, for each functional muscle group, across the four species. To achieve a model of best fit, the main effect ‘species’, the covariate ‘body mass’ and the interaction ‘species*body mass’ were first included. The interaction between the variable of interest and the covariate was included to test for homogeneity (Engqvist, 2005). A significant result for the interaction would indicate that the slopes are heterogeneous and it would be inappropriate to continue with an ancova (Engqvist, 2005). However, a significant interaction was not present in any of our models and thus backward elimination could be used to remove each non-significant term (significance taken at the P=0.05 level), one at a time, until the best fitting model remained (see Grafen & Hails, 2002). In cases where species was found to have a significant effect, Tukey's post-hoc tests were performed to establish which species were significantly different (P=0.05).
Results
Descriptive anatomy
Raw data for subjects Ptsm, Ppam, Gam, Gsm and Oaf are provided in Appendix S1. In general, the hindlimb muscle anatomy in this study followed descriptions in previous studies (e.g. Swindler & Wood, 1973; Thorpe et al. 1999; Payne et al. 2006) and origins and insertions did not differ significantly (e.g. chimpanzee: Swindler & Wood, 1973; bonobo: Miller, 1952; gorilla: Preuschoft, 1962; orangutan: Sonntag, 1924; Sigmon, 1974). Variations observed included the absence of scansorius as a clearly separate muscle in all subjects except the adult female orangutan (Oaf), and the presence of a number of muscle belly divisions in different subjects. Adductor magnus was present as two bellies in the bonobo (Ppam) and gorilla (Gam), and adductor brevis was present in two parts in the gorilla (Gsm). Similarly, the bonobo (Ppam) and orangutan (Oaf) also had two parts to their tibialis anterior muscle, whereas the other subjects only had one. It was possible to separate semimembranosus into its two parts, proprius and accessorius, only in the bonobo (Ppam).
The plantaris muscle was present in the chimpanzee (Ptsm) and the bonobo (Ppam), but was not present in the gorillas (Gam and Gsm) or orangutan (Oaf). The digital flexor muscles showed variations in terms of both their presence and their insertions. Most notable was the presence of an additional digital flexor muscle in the orangutan (Oaf), termed ‘flexor digitorum fibularis’ (see Schwartz, 1988), in addition to flexor digitorum longus and flexor hallucis longus. The muscle belly designated flexor digitorum fibularis inserted onto digits three and four, whereas flexor hallucis longus inserted onto digit one. As flexor digitorum longus inserted onto digits two, four and five, the presence of all three muscle bellies resulted in single tendons inserting on all digits except digit four, which had two tendons of insertion. In all other subjects in this study, the muscles flexor digitorum longus and flexor hallucis longus inserted onto all five digits, although the belly which provided a specific tendon of insertion differed between individuals.
Scaled data
Ordinary least-squares regressions of log-transformed data for functional muscle groups are presented in Fig. 1 for all data combined (i.e. including raw data harvested from Thorpe, 1997; Thorpe et al. 1999;Payne et al. 2006). The equation components which were established, including standard errors and 95% confidence intervals, are given in Table 3 for belly mass; Table 4 for fascicle length, and Table 5 for PCSA. Scaling exponents with confidence intervals (CIs) overlapping the exponents that would be predicted by isometry (see Alexander et al. 1981) were identified in some instances and are highlighted in bold in Tables 4–6. Muscle belly mass had a significant relationship with body mass in all instances and nine of the 14 exponents calculated had CIs overlapping the exponent predicted by isometry (M1.0). Fascicle length did not show a significant linear relationship with body mass in any distal muscle group, or in the gluteals, but there was a significant linear relationship with all proximal muscle groups other than the gluteals. Of those muscle groups that scaled significantly to body mass, only two had exponents with CIs overlapping that were predicted by isometry (M0.33). PCSA showed a significant linear relationship with body mass in all functional groups except the everters, and 11 of the 14 exponents had CIs overlapping with that predicted by isometry (M0.67). The results from RMA regression analysis are presented in Table 6 and show a similar trend to OLS regression for the different architectural properties, although the values of the scaling exponents b are generally greater from the RMA regression analysis, as expected.
Fig. 1.
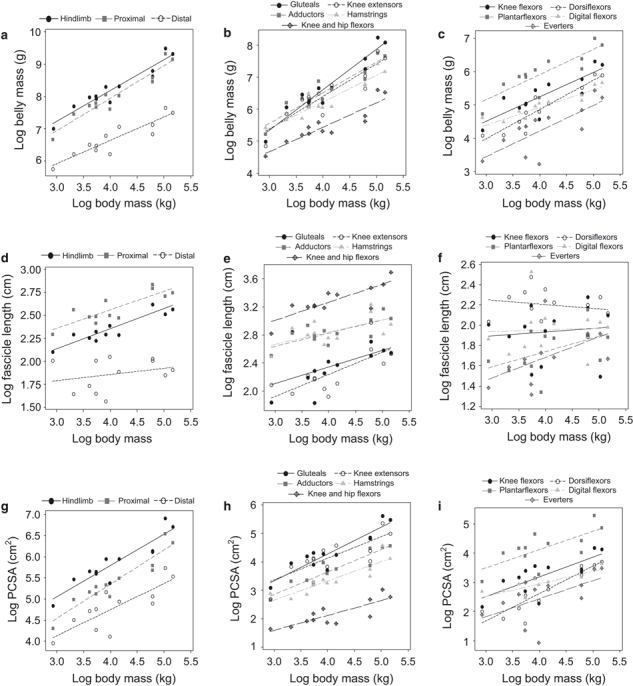
Regression plots of log-transformed data for each of the three variables using data from all subjects. (a–c) Belly mass (g) regressed against body mass (kg) for the overall groupings, proximal muscle groups and distal muscle groups, respectively. (d–f) Fascicle length (cm) regressed against body mass (kg) for the overall groupings, proximal muscle groups and distal muscle groups, respectively. (g–i) PCSA (cm2) regressed against body mass (kg) for the overall groupings, proximal muscle groups and distal muscle groups, respectively.
Table 3.
Allometric equation constants for hindlimb muscle group belly mass (g) ± SE and 95% confidence intervals established using logged data (exponents in bold have CIs overlapping those predicted by isometry)
| Muscle group | a (± SE)* | CI (a)† | b (± SE) | CI (b) | R2 | P |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Total | 0.52 (± 0.53) | ± 0.95 | 0.94 (± 0.13) | ± 0.23 | 0.83 | < 0.001 |
| Proximal | 3.90 (± 0.39) | ± 0.70 | 1.01 (± 0.09) | ± 0.16 | 0.92 | < 0.001 |
| Distal | 3.73 (± 0.43) | ± 0.77 | 0.73 (± 0.10) | ± 0.18 | 0.83 | < 0.001 |
| Proximal | ||||||
| Gluteals | 1.54 (± 0.48) | ± 0.86 | 1.27 (± 0.12) | ± 0.22 | 0.92 | < 0.001 |
| Adductors | 2.69 (± 0.48) | ± 0.86 | 0.96 (± 0.11) | ± 0.20 | 0.87 | < 0.001 |
| Knee extensors | 2.32 (± 0.64) | ± 1.15 | 1.02 (± 0.15) | ± 0.27 | 0.82 | < 0.001 |
| Knee flexors and hip extensors | 2.92 (± 0.41) | ± 0.74 | 0.83 (± 0.10) | ± 0.18 | 0.87 | < 0.001 |
| Bi-articular knee and hip flexors | 2.38 (± 0.45) | ± 0.81 | 0.76 (± 0.11) | ± 0.20 | 0.83 | < 0.001 |
| Distal | ||||||
| Uni-articular knee flexors | 0.19 (± 0.82) | ± 1.47 | 0.78 (± 0.20) | ± 0.36 | 0.64 | 0.003 |
| Plantarflexors | 2.90 (± 0.61) | ± 1.08 | 0.76 (± 0.15) | ± 0.27 | 0.72 | < 0.001 |
| Dorsiflexors | 1.36 (± 0.43) | ± 0.77 | 0.88 (± 0.10) | ± 0.18 | 0.88 | < 0.001 |
| Digital flexors | 2.80 (± 0.36) | ± 0.65 | 0.53 (± 0.09) | ± 0.16 | 0.79 | < 0.001 |
| Everters | 1.15 (± 0.86) | ± 1.54 | 0.77 (± 0.21) | ± 0.38 | 0.58 | 0.004 |
| Mean exp | 0.85 (± 0.06) | ± 0.11 | ||||
Equation takes the form y = aMb, where M is body mass (kg) and a and b are provided above.
Confidence intervals (95%) are provided for the exponents a and b, respectively.
Table 4.
Allometric equation constants for hindlimb muscle fascicle length (cm) ± SE using logged data (exponents in bold have CIs overlapping those predicted by isometry)
| Muscle group | a (± SE)* | CI (a)† | b (± SE) | CI (b) | R2 | P |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Total | 1.48 (± 0.13) | ± 0.23 | 0.22 (± 0.03) | ± 0.05 | 0.84 | < 0.001 |
| Proximal | 1.75 (± 0.17) | ± 0.31 | 0.20 (± 0.04) | ± 0.07 | 0.72 | 0.001 |
| Distal | 1.59 (± 0.31) | ± 0.56 | 0.07 (± 0.07) | ± 0.13 | 0.08 | 0.390 |
| Proximal | ||||||
| Gluteals | 1.41 (± 0.49) | ± 0.88 | 0.24 (± 0.12) | ± 0.22 | 0.22 | 0.073 |
| Adductors | 2.08 (± 0.23) | ± 0.41 | 0.19 (± 0.05) | ± 0.09 | 0.54 | 0.007 |
| Knee extensors | 0.99 (± 0.40) | ± 0.72 | 0.31 (± 0.10) | ± 0.18 | 0.52 | 0.008 |
| Knee flexors and hip extensors | 2.15 (± 0.30) | ± 0.54 | 0.17 (± 0.07) | ± 0.13 | 0.36 | 0.039 |
| Bi-articular knee and hip flexors | 2.24 (± 0.23) | ± 0.41 | 0.26 (± 0.06) | ± 0.11 | 0.67 | 0.001 |
| Distal | ||||||
| Uni-articular knee flexors | 0.48 (± 0.73) | ± 1.32 | 0.28 (± 0.17) | ± 0.31 | 0.22 | 0.141 |
| Plantarflexors | 1.14 (± 0.36) | ± 0.65 | 0.15 (± 0.09) | ± 0.16 | 0.23 | 0.114 |
| Dorsiflexors | 2.38 (± 0.29) | ± 0.52 | −0.04 (± 0.07) | ± 0.13 | 0.04 | 0.562 |
| Digital flexors | 1.89 (± 0.43) | ± 0.77 | 0.02 (± 0.10) | ± 0.18 | 0.00 | 0.875 |
| Everters | 0.88 (± 0.41) | ± 0.74 | 0.20 (± 0.10) | ± 0.18 | 0.29 | 0.071 |
| Mean exponent | 0.15 (± 0.04) | ± 0.07 | ||||
Equation takes the form y = aMb, where M is body mass (kg) and a and b are provided above.
Confidence intervals (95%) are provided for the exponents a and b, respectively.
Table 5.
Allometric equation constants for hindlimb muscle group PCSA (cm2) ± SE using logged data (exponents in bold have CIs overlapping those predicted by isometry)
| Muscle group | a (± SE)* | CI (a)† | b (± SE) | CI (b) | R2 | P |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Total | 2.83 (± 0.42) | ± 0.75 | 0.74 (± 0.10) | ± 0.18 | 0.84 | < 0.001 |
| Proximal | 2.11 (± 0.38) | ± 0.68 | 0.81 (± 0.90) | ± 1.62 | 0.89 | < 0.001 |
| Distal | 2.27 (± 0.60) | ± 1.18 | 0.62 (± 0.14) | ± 0.25 | 0.65 | 0.002 |
| Proximal | ||||||
| Gluteals | 0.35 (± 0.65) | ± 1.17 | 0.97 (± 0.16) | ± 0.29 | 0.79 | < 0.001 |
| Adductors | 0.49 (± 0.39) | ± 0.70 | 0.79 (± 0.09) | ± 0.16 | 0.88 | < 0.001 |
| Knee extensors | 1.09 (± 0.72) | ± 1.29 | 0.76 (± 0.17) | ± 0.31 | 0.66 | 0.001 |
| Knee flexors and hip extensors | 0.63 (± 0.46) | ± 0.83 | 0.68 (± 0.11) | ± 0.20 | 0.79 | < 0.001 |
| Bi-articular knee and hip flexors | 0.02 (± 0.46) | ± 0.83 | 0.52 (± 0.11) | ± 0.20 | 0.69 | 0.001 |
| Distal | ||||||
| Uni-articular knee flexors | −0.22 (± 0.81) | ± 1.47 | 0.48 (± 0.19) | ± 0.34 | 0.40 | 0.036 |
| Plantarflexors | 1.63 (± 0.82) | ± 1.47 | 0.62 (± 0.20) | ± 0.36 | 0.50 | 0.010 |
| Dorsiflexors | −1.14 (± 0.64) | ± 1.15 | 0.94 (± 0.15) | ± 0.26 | 0.79 | < 0.001 |
| Digital flexors | 1.22 (± 0.37) | ± 0.66 | 0.43 (± 0.09) | ± 0.16 | 0.70 | 0.001 |
| Everters | −0.06 (± 1.20) | ± 2.16 | 0.62 (± 0.29) | ± 0.52 | 0.32 | 0.056 |
| Mean exp | 0.70 (± 0.05) | ± 0.09 | ||||
Equation takes the form y = aMb, where M is body mass (kg) and a and b are provided above.
Confidence intervals (95%) are provided for the exponents a and b, respectively.
Table 6.
Allometric equation constants for hindlimb muscle group physiological variables from RMA regression of logged data
| Muscle belly mass (g) | Muscle fascicle length (cm) | Muscle PCSA (cm2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle group | a (± SE)* | b (± SE) | R2 | P | a | b | R2 | P | a | b | R2 | P |
| Overall | ||||||||||||
| Total | −1.84 (± 0.41) | 1.01 (± 0.11) | 0.87 | < 0.001 | −2.56 (± 0.57) | 4.21 (± 0.54) | 0.84 | < 0.001 | −1.38 (± 0.40) | 1.24 (± 0.16) | 0.84 | < 0.001 |
| Proximal | −1.54 (± 0.38) | 0.95 (± 0.11) | 0.87 | < 0.001 | −2.93 (± 0.79) | 4.20 (± 0.71) | 0.72 | 0.001 | −0.96 (± 0.29) | 1.16 (± 0.12) | 0.89 | < 0.001 |
| Distal | −1.92 (± 0.49) | 1.27 (± 0.17) | 0.83 | < 0.001 | −1.51 (± 1.01) | 4.07 (± 1.24) | 0.07 | 0.390 | −0.93 (± 0.51) | 1.30 (± 0.24) | 0.65 | 0.002 |
| Proximal | ||||||||||||
| Gluteals | −0.44 (± 0.20) | 0.76 (± 0.07) | 0.92 | < 0.001 | −0.68 (± 0.43) | 2.46 (± 0.42) | 0.73 | 0.073 | 0.05 (± 0.25) | 0.92 (± 0.13) | 0.79 | < 0.001 |
| Adductors | −1.03 (± 0.32) | 0.98 (± 0.11) | 0.87 | < 0.001 | −3.10 (± 1.05) | 3.96 (± 0.85) | 0.54 | 0.007 | −0.14 (± 0.22) | 1.19 (± 0.13) | 0.88 | < 0.001 |
| Knee extensors | −0.72 (± 0.34) | 0.89 (± 0.12) | 0.82 | < 0.001 | −0.49 (± 0.50) | 2.30 (± 0.50) | 0.52 | 0.008 | −0.17 (± 0.37) | 1.07 (± 0.20) | 0.66 | 0.001 |
| K Flx and H Ext† | −1.32 (± 0.35) | 1.13 (± 0.13) | 0.87 | < 0.001 | −2.53 (± 1.09) | 3.47 (± 0.88) | 0.36 | 0.039 | −0.16 (± 0.28) | 1.32 (± 0.19) | 0.79 | < 0.001 |
| KH flexors | −1.08 (± 0.37) | 1.20 (± 0.15) | 0.83 | < 0.001 | −2.80 (± 0.83) | 3.21 (± 0.58) | 0.67 | 0.001 | 0.29 (± 0.27) | 1.59 (± 0.28) | 0.69 | 0.001 |
| Distal | ||||||||||||
| Uni-articular knee flexors | 0.28 (± 0.31) | 1.02 (± 0.21) | 0.64 | < 0.001 | 0.59 (± 0.36) | 1.69 (± 0.50) | 0.22 | 0.752 | 0.79 (± 0.27) | 1.34 (± 0.34) | 0.40 | 0.004 |
| Plantarflexors | −1.15 (± 0.53) | 1.13 (± 0.20) | 0.68 | < 0.001 | −0.65 (± 0.68) | 3.19 (± 0.88) | 0.23 | 0.114 | −0.28 (± 0.47) | 1.13 (± 0.25) | 0.50 | 0.010 |
| Dorsiflexors | −0.52 (± 0.26) | 1.07 (± 0.12) | 0.87 | < 0.001 | 5.96 (± 1.30) | −4.38 (± 1.36) | 0.04 | 0.562 | 0.67 (± 0.17) | 0.95 (± 0.14) | 0.79 | < 0.001 |
| Digital flexors | −1.85 (± 0.55) | 1.68 (± 0.25) | 0.77 | < 0.001 | −0.79 (± 0.82) | 3.02 (± 0.95) | 0.00 | 0.875 | −0.73 (± 0.44) | 1.93 (± 0.33) | 0.70 | 0.001 |
| Everters | −0.07 (± 0.36) | 0.99 (± 0.19) | 0.64 | 0.004 | −0.20 (± 0.53) | 2.67 (± 0.71) | 0.29 | 0.071 | 0.78 (± 0.27) | 0.90 (± 0.23) | 0.32 | 0.056 |
| Mean exp | 0.85 (± 0.06) | 0.15 (± 0.04) | 0.70 (± 0.05) | |||||||||
Equation takes the form y = aMb, where M is body mass (kg) and a and b are provided above.
Muscle group abbreviations: K Flx, knee flexors; H Ext, hip extensors; KH flexors, bi-articular knee and hip flexors.
Figure 2 is an example of the different results obtained for the dorsiflexor muscle group PCSA depending on the scaling exponent used to normalise the data. The geometric scaling exponent was compared to three different allometric exponents obtained from OLS regression (overall mean, distal muscle exponent and the dorsiflexor group exponent), as previous studies (e.g. Alexander et al. 1981; Pollock & Shadwick, 1994) have used different allometric exponents to compare their data, thus highlighting the discrepancies that can occur. From Fig. 2 it can be seen that although the general pattern of variation remains similar, the magnitude of the differences between different individuals varies, and overall PCSA differs substantially, depending on the scaling exponent selected. When a negatively allometric exponent was used (M0.62: total distal hindlimb exponent in the current study) the magnitude of differences between different individuals increased compared to geometrically scaled data (M0.67). However, when a positively allometric exponent was used (M0.92: dorsiflexor muscle group exponent in this study), the magnitude deceased and there was no difference in PSCA between some individuals where variations were previously identified. This discrepancy was also apparent in the other muscle groups, for all physiological variables.
Fig. 2.

Comparison of dorsiflexor PCSA scaled using the scaling exponents, 0.67 (geometric exponent); 0.70 (mean exponent); 0.62 (distal hindlimb exponent); 0.94 (dorsiflexor group exponent).
For overall comparison, all functional muscle group data were additionally scaled geometrically (using the standard exponents; Alexander et al. 1981) and allometrically (using individual group exponents obtained from OLS regression) in instances where there was a significant relationship between body mass and the variable in question. In instances where the variable did not scale significantly with body mass (e.g. fascicle length in the distal muscle groups), raw data are provided alongside geometrically scaled data, as it was not appropriate to scale using body mass if there was no significant correlation to the variable of interest. Comparative data for fascicle length and PCSA are presented in Figs 3 and 4, respectively (belly mass data is not presented as it is functionally less relevant in this study than the other two variables under discussion). There was a large amount of variation between individuals of the same species for all physiological variables, making it very difficult to compare the different species visually, in particular for PCSA (Fig. 4). In general, fascicle length was more uniform within the different species than was PCSA. The orangutans, however, differed in a number of muscle groups with the juvenile orangutan (Ojm), having a larger PCSA than did Oaf in the knee flexors and hip extensors, bi-articular knee and hip flexors, uni-articular knee flexors, plantarflexors, dorsiflexors, digital flexors and everters, indicating a greater potential for force production in these groups. The two bonobos differed in PCSA, particularly in the digital flexors (Pp PCSA greater by 30.9%) and everters (Ppam PCSA greater by 31.5%), and chimp 93 had a smaller PCSA than the other chimpanzees across all distal muscle groups. Within the gorillas, the gluteals, knee extensors, knee flexors and hip extensors and plantarflexors had a larger PCSA in Gsm than the other gorillas, indicating that gorilla Gsm had an enhanced force production capability in these groups. Overall species differences were apparent, in that the orangutans generally had smaller PCSAs but the chimpanzees larger PCSAs, in comparison with the other species. However, in the case of the digital flexors the orangutans appeared to have a greater PCSA than the other species, indicating a greater ability to produce force in this muscle group compared to the other non-human apes.
Fig. 3.
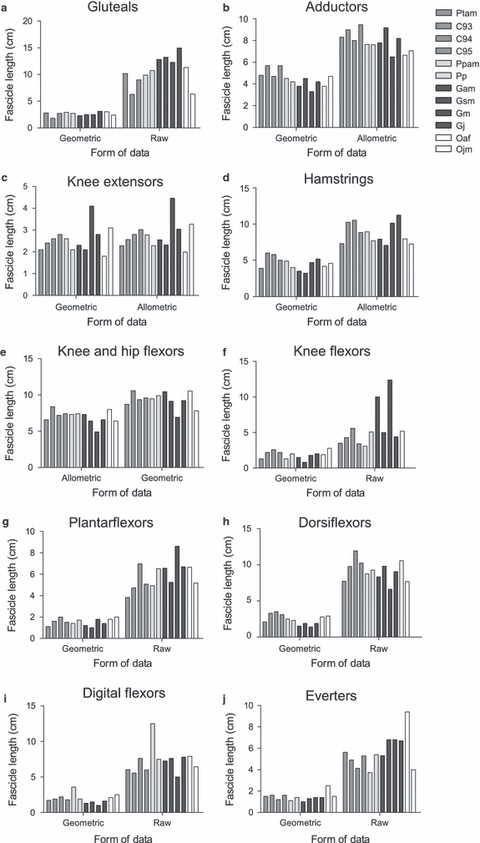
Comparison of raw fascicle length (cm) data scaled using both geometric and individual group allometric exponents. (a) Gluteal muscle group M0.24 (data for chimp 93 not available), (b) adductors M0.19, (c) knee extensors M0.31, (d) knee flexors and hip extensors M0.17, (e) bi-articular knee and hip flexors M0.26, (f) uni-articular knee flexors M0.04, (g) plantarflexors M0.15, (h) dorsiflexors M−0.04, (i) digital flexors M0.02, (j) everters M0.20.
Fig. 4.
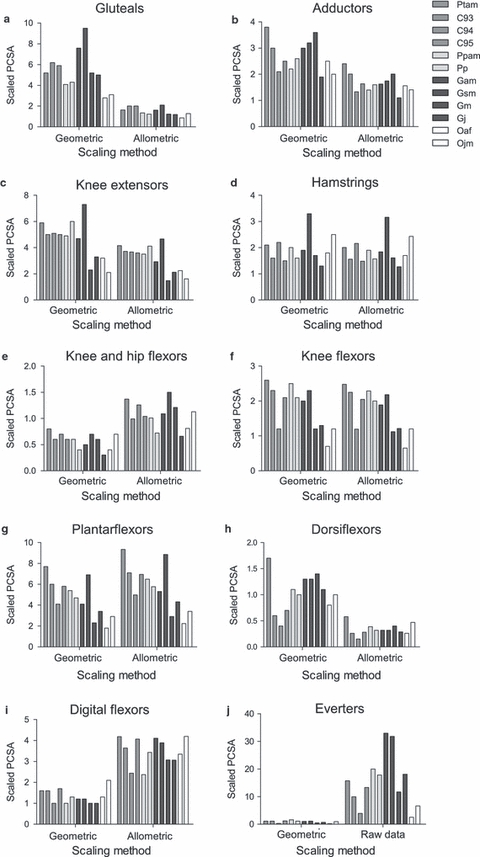
Comparison of raw PCSA (cm2) data scaled using both geometric and individual group allometric exponents. (a) Gluteal muscle group M0.97 (data for chimp 93 not available); (b) adductors M0.79, (c) knee extensors M0.76, (d) knee flexors and hip extensors M0.68, (e) bi-articular knee and hip flexors M0.52, (f) uni-articular knee flexors M0.68, (g) plantarflexors M0.62, (h) dorsiflexors M0.94, (i) digital flexors M0.43, (j) everters M0.62.
The level of variation between individual muscles within a functional muscle group in terms of their scaling relationship was additionally assessed and OLS regression equation components are provided in Appendix S2 (a–c for each physiological variable). The mean scaling exponents b (± SE) for each functional muscle group obtained from these individual muscle exponents are provided in Table 7.
Table 7.
Mean allometric scaling exponent b obtained from individual muscle exponents for muscle belly mass (g), fascicle length (cm) and PCSA (cm2) (± SE)
| Mean scaling exponent b (± SE) | |||
|---|---|---|---|
| Muscle group | Belly mass (g) | Fascicle length (cm) | PCSA (cm2) |
| Proximal | |||
| Gluteals | 1.37 (± 0.13) | 0.29 (± 0.08) | 1.05 (± 0.15) |
| Adductors | 1.00 (± 0.14) | 0.25 (± 0.09) | 0.69 (± 0.19) |
| Knee extensors | 0.96 (± 0.11) | 0.28 (± 0.08) | 0.72 (± 0.05) |
| Knee flexors and hip extensors | 0.86 (± 0.09) | 0.19 (± 0.06) | 0.61 (± 0.07) |
| Bi-articular knee and hip flexors | 0.76 (± 0.03) | 0.28 (± 0.02) | 0.49 (± 0.05) |
| Distal | |||
| Uni-articular knee flexors | 0.78 (± 0.20) | 0.28 (± 0.17) | 0.48 (± 0.19) |
| Plantarflexors | 0.75 (± 0.03) | 0.12 (± 0.03) | 0.59 (± 0.11) |
| Dorsiflexors | 0.86 (± 0.06) | −0.01 (± 0.05) | 0.88 (± 0.11) |
| Digital flexors | 0.52 (± 0.07) | 0.08 (± 0.11) | 0.39 (± 0.02) |
| Everters | 0.80 (± 0.05) | 0.04 (± 0.06) | 0.65 (± 0.05) |
| Mean exponent | 0.89 (± 0.05) | 0.19 (± 0.03) | 0.69 (± 0.05) |
ancova analysis
Results for the most significant ancova models are presented in Table 8. In all ancova models body mass alone explained the largest proportion of the variation in belly mass for all muscle groups, except for the knee extensors and plantarflexors, where both species and body mass had a significant main effect. In the case of the knee extensors, Tukey's post-hoc test identified no significant difference between species pairs, although Fig. 5a shows that the orangutans appear to have a smaller belly mass compared to the other species, in particular the bonobos and chimpanzees. In the plantarflexors, Tukey's post-hoc analysis revealed a significant difference between chimpanzees and orangutans (Tukey, P=0.0288), orangutans possessing a significantly smaller belly mass than chimpanzees (Fig. 5b). From Fig. 5b, the bonobos also appear to have a similar mean to the chimpanzees.
Table 8.
Results from ancova models for hindlimb muscle groups
| Muscle belly mass | Muscle fascicle length | Muscle PCSA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle group | Fdf | R2 | P | Fdf | R2 | P | Fdf | R2 | P |
| Proximal | |||||||||
| Gluteals | 120.21,11 | 0.92 | < 0.001 | 22.861,9 | 0.71 | 0.001 | S: 4.653,11 | 0.92 | 0.043 |
| B: 13.131,11 | 0.92 | 0.008 | |||||||
| Adductors | 69.521,11 | 0.86 | < 0.001 | 11.571,11 | 0.49 | 0.007 | 69.971,11 | 0.86 | < 0.001 |
| Knee extensors | S*: 43.63,11 | 0.90 | 0.050 | 10.81,11 | 0.47 | 0.008 | 19.121,11 | 0.62 | 0.001 |
| B: 18.241,11 | 0.90 | 0.004 | |||||||
| Knee flexors and hip extensors | 69.231,11 | 0.86 | < 0.001 | 5.621,11 | 0.30 | 0.039 | 37.771,11 | 0.77 | < 0.001 |
| Bi-articular knee and hip flexors | 50.391,11 | 0.82 | < 0.001 | 20.621,11 | 0.64 | 0.001 | 22.151,11 | 0.66 | 0.001 |
| Distal | |||||||||
| Uni-articular knee flexors | 25.251,11 | 0.69 | 0.001 | 0.111,11 | 0.00 | 0.752 | 13.321,11 | 0.53 | 0.004 |
| Plantarflexors | S: 5.503,11 | 0.87 | 0.029 | 2.991,11 | 0.15 | 0.114 | 9.961,11 | 0.45 | 0.010 |
| B: 9.941,11 | 0.87 | 0.016 | |||||||
| Dorsiflexors | 71.291,11 | 0.86 | < 0.001 | 0.361,11 | 0.00 | 0.562 | 37.431,11 | 0.77 | < 0.001 |
| Digital flexors | 37.791,11 | 0.77 | < 0.001 | 0.031,11 | 0.00 | 0.875 | 23.361,11 | 0.67 | 0.001 |
| Everters | 13.721,11 | 0.54 | 0.004 | 4.071,11 | 0.22 | 0.071 | 4.661,11 | 0.25 | 0.056 |
Best model included both species (S) and body mass (B) as significant effects.
Fig. 5.
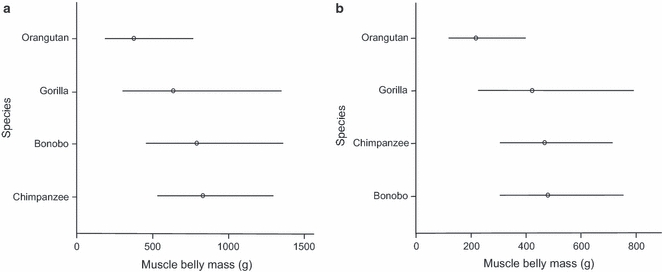
Adjusted mean values and 95% confidence intervals for the muscle belly mass (g) for the different species. Values presented are back-transformed from logged data. (a) Knee extensor belly mass. (b) Plantarflexor belly mass.
For fascicle length, significant models were found for all proximal muscle groups but for none of the distal muscle groups. For the proximal muscles, species was not found to be significantly related to fascicle length. Instead, the majority of variation was accounted for by body mass (Table 8), although the model fit (R2) was rather low for the knee flexors and hip extensors, knee extensors and adductors. The lack of significance in the distal muscle group models indicates that there is no significant linear relationship between muscle fascicle length and body mass for these muscle groups. All PCSA models were significant except that for the everters. Body mass explained the largest proportion of the variation in all cases, except for the gluteals, where both species and body mass had a significant effect. Tukey's post-hoc test again revealed a significant difference between chimpanzees and orangutans (Tukey, P=0.0372), orangutans having a significantly smaller PCSA and thus less ability to produce force compared to chimpanzees (Fig. 6). From Fig. 6 it can be seen that the difference between the gorillas and orangutans was also close to significance, the mean being close to that of the chimpanzees (although the confidence interval range was larger). In this instance the bonobos were more similar to the orangutans than to the other species (Fig. 6).
Fig. 6.
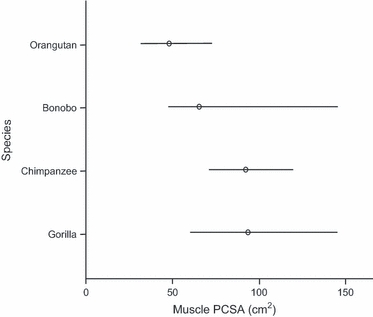
Adjusted mean values and 95% confidence intervals for gluteal PCSA for the different species. Values presented are back-transformed from logged data.
Discussion
Morphological variations
Variations in morphology may be expected between individuals and between species, even if their general body patterns are similar. In this study, scansorius was only present in the adult orangutan (Oaf). Scansorius is generally more common in orangutans than in other apes (Sigmon, 1969; Payne et al. 2006) and this is probably related to higher levels of arboreality in orangutans, as scansorius may provide an increased ability to rotate the thigh (Sigmon, 1974). The presence of the plantaris muscle in the chimpanzee (Ptsm) and bonobo (Ppam), but not in the gorillas (Gam and Gsm) or the orangutan (Oaf), also agrees with other studies, as it has been found to be absent in ∼ 10% of humans, ∼ 39% of chimpanzees, ∼ 95% of orangutans and ∼ 99% of gorillas (Langdon, 1990). Variations in the structure of the muscle bellies and points of insertion of the digital flexor muscles are also relatively common. In particular, the presence of an additional digital flexor muscle belly (termed flexor digitorum fibularis accessory belly in this study), in addition to flexor digitorum fibularis and flexor digitorum tibialis, in the orangutan has been described before by Sonntag (1924) and Schwartz (1988), where the flexor digitorum fibularis muscle was described as consisting of two distinct bellies, inserting onto digits three and four, one of the which could be the additional belly described here.
Scaling exponents and muscle architecture
The concept of geometric similarity between species has been used in previous comparative studies of ape muscle architecture (e.g. Thorpe et al. 1999; Payne et al. 2006; Oishi et al. 2008, 2009; Channon et al. 2009; Michilsens et al. 2009). While this may be appropriate in instances where comparisons are between individuals of the same species and age–sex class, some authors have argued that it is unlikely to be the most appropriate method to compare different species (Alexander et al. 1981; Schmidt-Nielson, 1984). In general, our results support the latter argument, as the regression analyses showed that the relationships between the physiological variables and body mass in great apes were variable; in some instances the exponents obtained overlapped those predicted by isometry, but this was not the case in many of functional muscle groups that were better described by allometric scaling. Furthermore, in some instances the physiological variables, particularly fascicle length, did not scale to body mass at all, either when functional groups or when individual muscle data were regressed against body mass.
Previous studies have examined the allometric scaling of hindlimb muscle architecture in quadrupedal mammals (Pollock & Shadwick, 1994), including primates (Alexander et al. 1981: Galago, Cercopithecus, Colobus, Papio and Homo) using OLS regression. The mean allometric scaling exponents obtained for hindlimb muscle belly mass in the present study (M0.85 from overall mean functional muscle group data and M0.89 mean from individual muscle exponents) were lower than the means obtained in previous primate studies: M1.05 (Alexander et al. 1981; Payne et al. 2006) and, in other mammals, M0.98 (Pollock & Shadwick, 1994). The mean allometric scaling exponent obtained for hindlimb PCSA in this study (M0.70 from functional groups, M0.69 mean from individual muscle exponents) was also lower than the value obtained elsewhere, both in primates (M0.80; Alexander et al. 1981) and in quadrupedal mammals (M0.88; Pollock & Shadwick, 1994). However, our values still indicate that both scale with positive allometry, which reflects the ability of muscles of the larger animals to exert disproportionally greater forces. In particular, the gluteals and dorsiflexors had the largest PCSA scaling exponents, indicating their ability to produce greater forces than the other muscles for any given body mass (Pollock & Shadwick, 1994). From the individual muscle exponents it can be seen that within the gluteals, gluteus minimus has the largest influence on this difference, while within the dorsiflexors, tibialis anterior is the muscle with the largest exponent. This may reflect the importance of vertical climbing in the non-human great ape locomotor repertoire: where powerful gluteal muscles provide propulsion, particularly that muscle which is the most redundant gluteal muscle in humans for force production: gluteus minimus (Sigmon, 1974; Stern and Susman, 1981); and as the ankle is usually dorsiflexed during push off, this likely requires a large amount of force production in the dorsiflexors, e.g. tibialis anterior (J. P. Myatt; personal observation; chimpanzees and orangutans).
Previous studies have found wide variation in the scaling exponents for fascicle length: e.g. M0.05 and M0.24 in Pollock & Shadwick (1994) for quadrupedal mammals; M0.30 and M0.17 in Alexander et al. (1981) for primates; and M0.30 and M0.34 in Payne et al. (2006) for non-human apes, for the proximal and distal hindlimbs, respectively. The exponents for fascicle length from our study (OLS regression) overlapped with these values (mean from overall functional group: M0.15; proximal: M0.20; distal: M0.07; overall mean from individual muscle exponents: M0.19). The scaling of fascicle length with negative allometry, and the lack of a significant relationship with body mass in some instances, in particular for the distal muscle groups, indicates that these muscles will likely have disproportionally shorter fascicle lengths in larger animals. This likely reflects the increased amount of external tendon length in the distal limb (Ker et al. 1988).
From this, it can be seen that although the exponents from other studies (Alexander et al. 1981; Pollock & Shadwick, 1994), and within this study, may be similar in range and indeed agree with isometry in some cases, there is a large amount of variation between them, and even a small difference in exponent can affect results substantially (Schmidt-Nielson, 1984 and see Fig. 2). Using an alternative form of regression, e.g. RMA regression as opposed to OLS regression (as in traditional studies; e.g. Alexander et al. 1981), also results in further discrepancies in scaling exponents and the use of these different methods may not always be appropriate, depending on the specific dataset, as has recently been found for the scaling of basal metabolic rate (White, 2011). Therefore, care is required when deciding the form of regression to employ, particularly if the wish is to compare data directly with that in previous studies.
In addition, when discussing the best regression method for analysis of muscle architecture data (Smith, 2009; White, 2011), the grouping of the data will affect the scaling exponents obtained, although not to the same degree. For example, in the present study, scaling exponents were calculated for overall functional muscle groups in addition to individual muscles. The use of functional muscle groups provides a useful indicator of where the key differences occur when relating data to locomotor patterns. However, retention of individual muscle data allows the specific muscles which contribute to observed differences to be established and compared at a finer level. We therefore suggest that future studies should take this variation into account, although there is not scope within the present study fully to investigate this. Overall, differences in method and the data obtained can result in different scaling exponents being obtained. The magnitude of the exponents used for fascicle length and PCSA, in particular, influences interpretations of the maximum speed of shortening and of the muscle forces which different individuals and/or species can exert, and thus in turn influences conclusions which can be drawn about the interactions between animals and their habitat. Therefore, methodological discrepancies between studies could lead to very different conclusions being drawn.
The extensive intra-specific variation observed in the present study may have been a consequence of the multiple age–sex classes represented for each species, as there were males and females, juveniles and adults within the dataset. Studies both in the field and in captivity suggest that juveniles may be more active than adults, or may perform different behaviours to adults (e.g. Hunt, 1992; Thorpe et al. 2009), and these behavioural distinctions may result in differences in morphology, as were observed between the adult and juvenile orangutan in this study. The greater ability of the juvenile to produce force (as indicated by larger PCSAs) across a number of its muscle groups possibly reflects an increased use of arboreal-type supports and behaviours in the captive environment, and generally increased amount of activity, compared to adults (e.g. Doran, 1992; J. P. Myatt, personal observation). Depending on the exponents used (e.g. mean, functional muscle group, individual muscle), there was either an increase in the magnitude of the difference between individuals when scaled allometrically, or a decrease (depending on whether the exponent was positively or negatively isometric) This further highlights the fact that the interpretation of species differences and of the relationship between morphology, behaviour and habitat may be influenced by the choice of scaling exponent. However, although some individuals stood out in comparison with the others, there were relatively few major differences apparent between the different species. This recalls the finding of Payne et al. (2006) that the non-human apes were generally characterised by longer fascicle length and smaller PCSAs when compared to humans, reflecting their need to produce moderate forces over a range of joint motions during arboreal locomotion (Thorpe et al. 1999).
Species comparisons using statistical analyses
ancovas on log-transformed data were found in this study to identify species differences more clearly than was possible through visual analysis of scaled data. The results suggest that the variation in muscle belly mass observed between different non-human great ape species is the result of differences in body mass more than species per se. No firm conclusion in that respect can be drawn without a much larger sample size, as our present small sample size is reflected in wide confidence intervals and may reduce the power of the statistical model (Grafen & Hails, 2002). Furthermore, the large amount of variation between individuals makes these data difficult to analyse robustly. Therefore, we do not propose that either the use of ancovas or of scaling are ideally suited to species comparisons at the present time. Rather, data should be explored using both methods until such a time when an adequate sample size becomes available.
Tukey's post-hoc test for the plantarflexors highlighted a significant difference between the orangutans and the panins: the orangutans have muscles with the smallest mass of all species, and the chimpanzees and bonobos the heaviest. However, there was no significant difference between the PCSAs, and thus the force production capacity, of these muscles. Species did not have a significant effect on fascicle length, and non-significant models resulted for all distal muscle groups, indicating that neither species nor body mass explained the variation observed in these distal muscles. PCSA differed significantly between species only in the case of the gluteals, where orangutans, with the smallest PCSA of all species, differed significantly from the chimpanzees, the gorillas being more similar to the chimpanzees and the bonobos more similar to the orangutans. The smaller PCSAs in the orangutan gluteals probably reflects their increased need for mobility around the hip joint compared with the more terrestrial chimpanzees, during orangutans’ more frequent use of arboreal behaviours (since a reduced PCSA often reflects a longer fascicle length, although this was not found to be a significant relationship). As bonobos are also more arboreal than chimpanzees or gorillas (Doran, 1993; Remis, 1995), this case in bonobos further supports the above interpretation. Kinematic differences between the species during equivalent behaviours may also be influential, for example in vertical climbing, where orangutans use a greater range of motion at the hip (Isler, 2005) than panins or gorillines.
Concluding remarks
Overall, from this study, the differences in hindlimb muscle architecture between the different species appear to be small, and non-significant in most cases, both from the scaled data and the ancova models. This seems to suggest that even though the non-human great apes live in different habitats and perform given locomotor behaviours at different frequencies, their basic functional morphology remains very similar. This likely reflects a close evolutionary history, their ability to use a wide variety of locomotor modes and substrates, and the fact that their locomotion is characterised overall by orthograde positional behaviours (Thorpe & Crompton, 2006; Crompton et al. 2008). However, macro-architecture may not provide the whole picture, as further variation may be found to lie in the micro-architecture (i.e. the proportions of different muscle fibre types), which may also modulate the functional capability of muscles (e.g. Acosta & Roy, 1987; Myatt et al. 2011).
The different methods of analysis in this study each have both benefits and disadvantages for comparing the data. The use of allometric exponents to normalise data enables a visual comparison of the different individuals: however, it is difficult to compare species accurately using this method due to the large amount of intra-specific variation in the present study. The use of ancovas (GLMs), on the other hand, enables significant differences between species to be established, but is more appropriate for larger sample sizes.
It should be emphasized that although the differences between the species may appear small from an analytical perspective, the magnitude of the difference may not need to be that large to have a functional impact. Finally, although the present dataset is one of the largest compiled for non-human ape anatomy, it still suffers from a small sample size, which would be expected to impact on the reliability of the statistical analysis performed. Therefore, we advocate caution when interpreting these results.
As sample sizes of primate cadaveric material increase, there will be exciting opportunities to move beyond broad-based comparisons of maximum musculo-skeletal ability, which rather inevitably tend to show that animals are designed for the behaviours we already know they exhibit, towards a more refined analysis of the subtleties of the relationship between form and function. In the case of primate data, it may take many years to collate a sufficient amount of data to allow robust statistical analyses. Until such a time, we recommend exploration of the data using multiple methods to provide a more comprehensive comparison of data.
Acknowledgments
Thanks go to Dr Andrew Kitchener for supplying the cadavers and to Russ Savage, Dr Evie Vereecke, Dr Robert Ker, Dr Anthony Channon, Dr Sam Coward and Katy Wareing for their assistance with the dissections. Additional thanks go to Dr Steven Portugal for his assistance with the statistical analysis. Thanks also go to three anonymous reviewers for their comments on this manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Raw data from subjects dissected for present study.
Appendix S2. (a) Allometric equation constants for individual muscle belly mass (g) ± SE (logged data). (b) Allometric equation constants for individual muscle fascicle length (cm) ± SE (logged data). (c) Allometric equation constants for individual muscle PCSA (cm2) ± SE (logged data).
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Acosta L, Roy RR. Fiber-type composition of selected hindlimb muscles of a primate (Cynomolgus monkey) Anat Rec. 1987;218:136–141. doi: 10.1002/ar.1092180207. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Principles of Animal Locomotion. Princeton: Princeton University Press; 1996. [Google Scholar]
- Alexander RM. Hovering and jumping: contrasting problems in scaling. In: Brown JH, West GB, editors. Scaling in Biology. Oxford: Oxford University Press; 2000. pp. 37–50. [Google Scholar]
- Alexander RM, Jayes AA, Maloiy GMO, et al. Allometry of the leg muscles of mammals. J Zool (Lond) 1981;194:539–552. [Google Scholar]
- Alexander RM, Vernon A. The dimensions of the knee and ankle muscles and the forces they exert. J Hum Mov Stud. 1975;1:115–123. [Google Scholar]
- Biewener AA. Animal Locomotion. New York: Oxford University Press; 2003. [Google Scholar]
- Bock WJ, von Wahlert G. Adaptation and the form-function complex. In: Allen C, Bekoff M, Lauder G, editors. Nature's Purposes: Analyses of Function and Design in Biology. Cambridge, MA: The MIT Press; 1998. pp. 117–167. [Google Scholar]
- Bodine SC, Roy RR, Meadows DA, et al. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J Neurophysiol. 1982;48:192–201. doi: 10.1152/jn.1982.48.1.192. [DOI] [PubMed] [Google Scholar]
- Brown JH, West GB, Enquist BJ. Scaling in biology: patterns and processes, causes and consequences. In: Brown JH, West GB, editors. Scaling in Biology. New York: Oxford University Press; 2000. pp. 1–24. [Google Scholar]
- Carlson KJ. Muscle architecture of the common chimpanzee (Pan troglodytes): perspectives for investigating chimpanzee behavior. Primates. 2006;47:218–229. doi: 10.1007/s10329-005-0166-4. [DOI] [PubMed] [Google Scholar]
- Channon AJ, Gunther MM, Crompton RH, et al. Mechanical constraints on the functional morphology of the gibbon hind limb. J Anat. 2009;215:383–400. doi: 10.1111/j.1469-7580.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton RH, Vereecke EE, Thorpe SKS. Locomotion and posture from the common hominoid ancestor to fully modern hominins, with special reference to the last common panin/hominin ancestor. J Anat. 2008;212:501–543. doi: 10.1111/j.1469-7580.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran DM. The ontogeny of chimpanzee and pygmy chimpanzee locomotor behavior: a case study of paedomorphism and its behavioral correlates. J Hum Evol. 1992;23:139–157. [Google Scholar]
- Doran DM. Comparative locomotor behaviour of chimpanzees and bonobos – the influence of morphology on locomotion. Am J Phys Anthropol. 1993;91:83–98. doi: 10.1002/ajpa.1330910106. [DOI] [PubMed] [Google Scholar]
- Eng CM, Smallwood LH, Rainiero MP, et al. Scaling of muscle architecture and fiber types in the rat hindlimb. J Exp Biol. 2008;211:2336–2345. doi: 10.1242/jeb.017640. [DOI] [PubMed] [Google Scholar]
- Engqvist L. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim Behav. 2005;70:967–971. [Google Scholar]
- Fleagle JR. Primate Adaptation and Evolution. San Diego: Academic Press; 1999. [Google Scholar]
- Grafen A, Hails R. Modern Statistics for the Life Sciences. Oxford: Oxford University Press; 2002. [Google Scholar]
- Green JA, White CR, Butler PJ. Allometric estimation of metabolic rate from heart rate in penguins. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:478–484. doi: 10.1016/j.cbpa.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Halsey LG, Fahlman A, Handrich Y, et al. How accurately can we estimate energetic costs in a marine top predator, the king penguin? Zoology. 2007;110:81–92. doi: 10.1016/j.zool.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Hunt KD. Positional behaviour of Pan troglodytes in the Mahale Mountains and Gombe Stream National Parks, Tanzania. Am J Phys Anthropol. 1992;87:83–105. doi: 10.1002/ajpa.1330870108. [DOI] [PubMed] [Google Scholar]
- Hunt KD. The special demands of great ape locomotion and posture. In: Russon AE, Begun DR, editors. The Evolution of Thought: Evolutionary Origins of Great Ape Intelligence. Cambridge: Cambridge University Press; 2004. pp. 172–189. [Google Scholar]
- Isaac NJB, Carbone C. Why are metabolic scaling exponents so controversial? Quantifying variance and testing hypotheses. Ecol Lett. 2010;13:728–735. doi: 10.1111/j.1461-0248.2010.01461.x. [DOI] [PubMed] [Google Scholar]
- Isler K. 3D-kinematics of vertical climbing in hominoids. Am J Phys Anthropol. 2005;126:66–81. doi: 10.1002/ajpa.10419. [DOI] [PubMed] [Google Scholar]
- Ker RF, Alexander RM, Bennett MB. Why are mammalian tendons so thick? J Zool. 1988;216:309–324. [Google Scholar]
- Kleiber M. Physiological meaning of regression equations. J App Physiol. 1950;2:417–423. [Google Scholar]
- Langdon JH. Variations in cruropedal musculature. Int J Primatol. 1990;11:575–606. [Google Scholar]
- McGowen CP, Skinner J, Biewener AA. Hind limb scaling of kangaroos and wallabies (superfamily Macropodoidea): implications for hopping performance, safety factor and elastic savings. J Anat. 2008;212:153–163. doi: 10.1111/j.1469-7580.2007.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Michilsens F, Vereecke EE, D'Août K, et al. Functional anatomy of the gibbon forelimb: adaptations to a brachiating lifestyle. J Anat. 2009;215:335–354. doi: 10.1111/j.1469-7580.2009.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. The musculature of Pan paniscus. Am J Anat. 1952;91:183–232. doi: 10.1002/aja.1000910202. [DOI] [PubMed] [Google Scholar]
- Myatt JP, Schilling N, Thorpe SKS. Distribution patterns of fibre types in the triceps surae muscle group of chimpanzees and orangutans. J Anat. 2011;218:402–412. doi: 10.1111/j.1469-7580.2010.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevill AM, Ramsbottom R, Williams C. Scaling physiological measurements for individuals of different body size. Eur J Appl Physiol Occup Physiol. 1992;65:110–117. doi: 10.1007/BF00705066. [DOI] [PubMed] [Google Scholar]
- Nevill AM, Bate S, Holder RL. Modeling physiological and anthropometric variables known to vary with body size and other confounding variables. Yearb Phys Anthropol. 2005;48:141–153. doi: 10.1002/ajpa.20356. [DOI] [PubMed] [Google Scholar]
- Nevill AM, Holder RL. Normalizing and ‘per-ratio’ standards, an allometric modeling approach. J Appl Physiol. 1995;79:1027–1031. doi: 10.1152/jappl.1995.79.3.1027. [DOI] [PubMed] [Google Scholar]
- Oishi M, Ogihara N, Endo H, et al. Muscle architecture of the upper limb in the orangutan. Primates. 2008;49:204–209. doi: 10.1007/s10329-008-0082-5. [DOI] [PubMed] [Google Scholar]
- Oishi M, Ogihara N, Endo H, et al. Dimensions of forelimb muscles in orangutans and chimpanzees. J Anat. 2009;215:373–382. doi: 10.1111/j.1469-7580.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard GC, Boardman TJ. The misuse of ratios to scale physiological data that vary allometrically with body size. In: Feder ME, Bennett AF, Burggren WW, Huey RB, editors. New Directions in Ecological Physiology. Cambridge, MA: Cambridge University Press; 1987. pp. 216–239. [Google Scholar]
- Packard GC, Boardman TJ. The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comp Biochem Physiol A Mol Integr Physiol. 1999;122:37–44. [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. Morphological analysis of the hindlimb in apes and humans. I. Muscle architecture. J Anat. 2006;208:709–724. doi: 10.1111/j.1469-7580.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CM, Shadwick RE. Allometry of muscle, tendon, and elastic energy storage capacity in mammals. Am J Physiol. 1994;266:1022–1031. doi: 10.1152/ajpregu.1994.266.3.R1022. [DOI] [PubMed] [Google Scholar]
- Portugal SJ, Thorpe SKS, Green JA, et al. Testing the use/disuse hypothesis: pectoral and leg muscle changes in captive barnacle geese Branta leucopsis during wing moult. J Exp Biol. 2009;212:2403–2410. doi: 10.1242/jeb.021774. [DOI] [PubMed] [Google Scholar]
- Powell PL, Roy RR, Kanim P, et al. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Preuschoft H. Muskeln und Gelenke der Hinterextremitat des Gorilla (Gorilla gorilla) Morph Jahrbuch. 1962;101:432–540. [PubMed] [Google Scholar]
- Remis M. Effects of body size and social context on the arboreal activities of lowland gorillas in the in the Central African Republic. Am J Phys Anthropol. 1995;97:413–433. doi: 10.1002/ajpa.1330970408. [DOI] [PubMed] [Google Scholar]
- Sacks RD, Roy RR. Architecture of the hindlimb muscles of cats – functional significance. J Morphol. 1982;173:185–195. doi: 10.1002/jmor.1051730206. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielson K. Scaling, Why is Animal Size so Important? Cambridge: Cambridge University Press; 1984. [Google Scholar]
- Schwartz JH. Orang-utan Biology. New York: Oxford University Press; 1988. [Google Scholar]
- Sigmon BA. The scansorius muscles in pongids. Primates. 1969;10:247–261. [Google Scholar]
- Sigmon BA. A functional-analysis of pongid hip and thigh musculature. J Hum Evol. 1974;3:161–185. [Google Scholar]
- Smith RJ. Use and misuse of the reduced major axis for line-fitting. Am J Phys Anthropol. 2009;140:476–486. doi: 10.1002/ajpa.21090. [DOI] [PubMed] [Google Scholar]
- Sonntag CF. On the anatomy, physiology, and pathology of the orang-outan. Proc Zool Soc Lond. 1924;24:349–390. [Google Scholar]
- Stern JT, Susman RL. Electromyography of the gluteal muscles in Hylobates, Pongo and Pan: implications for the evolution of hominid bipedality. Am J Phys Anthropol. 1981;55:153–166. [Google Scholar]
- Swindler DR, Wood CD. An Atlas of Primate Gross Anatomy. Seattle: University of Washington Press; 1973. [Google Scholar]
- Tanner JM. Fallacy of per-weight and per-surface area standards and their relation to spurious correlation. J Appl Physiol. 1949;2:1–15. doi: 10.1152/jappl.1949.2.1.1. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS. University of Leeds; 1997. Bipedal locomotion in humans and chimpanzees: biomechanics and implications for hominid evolution. Thesis (PhD) [Google Scholar]
- Thorpe SKS, Crompton RH. Locomotor ecology of wild orangutans (Pongo abelii) in the Gunung Leuser ecosystem, Sumatra, Indonesia: a multivariate analysis using log-linear modelling. Am J Phys Anthropol. 2005;127:58–78. doi: 10.1002/ajpa.20151. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH. Orangutan positional behavior and the nature of arboreal locomotion in Hominoidea. Am J Phys Anthropol. 2006;131:384–401. doi: 10.1002/ajpa.20422. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH, Günther MM, et al. Dimensions and moment arms of the hind- and forelimb muscles of common chimpanzees (Pan troglodytes) Am J Phys Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Holder RL, Crompton RH. Orangutans employ unique strategies to control branch flexibility. Proc Natl Acad Sci USA. 2009;212:2403–2410. doi: 10.1073/pnas.0811537106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca HF, White CR. Environmental modulation of metabolic allometry in ornate rainbowfish Rhadinocentrus ornatus. Biol Lett. 2010;6:136–138. doi: 10.1098/rsbl.2009.0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke EE. University of Antwerp; 2006. The functional morphology and bipedal locomotion of Hylobates lar and its implications for the evolution of bipedalism in humans. Thesis (PhD) [Google Scholar]
- Wells JB. Comparison of mechanical properties between slow and fast mammalian muscles. J Physiol Lond. 1965;178:252–269. doi: 10.1113/jphysiol.1965.sp007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR. Allometric estimation of metabolic rates in animals. Comp Biochem Physiol A Mol Integr Physiol. 2011;158:346–357. doi: 10.1016/j.cbpa.2010.10.004. [DOI] [PubMed] [Google Scholar]
- White CR, Seymour RS. Sample size and mass range effects on the allometric exponent of basal metabolic rate. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:74–78. doi: 10.1016/j.cbpa.2005.07.013. [DOI] [PubMed] [Google Scholar]
- White CR, Cassey P, Blackburn TM. Allometric exponents do not support a universal metabolic allometry. Ecology. 2007;88:315–323. doi: 10.1890/05-1883. [DOI] [PubMed] [Google Scholar]
- White CR, Blackburn TM, Seymour RS. Phylogenetically informed analysis of the allometry of mammalian basal metabolic rate supports neither geometric nor quarter-power scaling. Evolution. 2009;63:2658–2667. doi: 10.1111/j.1558-5646.2009.00747.x. [DOI] [PubMed] [Google Scholar]
- Wickiewicz TL, Roy RR, Powell PL, et al. Muscle-architecture and force-velocity relationships in humans. J Appl Physiol. 1984;57:435–443. doi: 10.1152/jappl.1984.57.2.435. [DOI] [PubMed] [Google Scholar]
- Zajac FE. How musculotendon architecture and joint geometry affect the capacity of muscles to move and exert force on objects – a review with application to arm and forearm tendon transfer design. J Hand Surg Am. 1992;17A:799–804. doi: 10.1016/0363-5023(92)90445-u. [DOI] [PubMed] [Google Scholar]
- Zihlman AL, McFarland RK. Body mass in lowland gorillas: a quantitative analysis. Am J Phys Anthropol. 2000;113:61–78. doi: 10.1002/1096-8644(200009)113:1<61::AID-AJPA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


