Abstract
Some tetrapods hang upside down from tree branches when moving horizontally. The ability to walk in quadrupedal suspension has been acquired independently in at least 14 mammalian lineages. During the stance (supportive) phase of quadrupedal suspension, the elbow joint flexor muscles (not the extensors as in upright vertebrates moving overground) are expected to contract to maintain the flexed limb posture. Therefore muscular control in inverted, suspended quadrupeds may require changes of muscle control, and even morphologies, to conditions opposite to those in upright animals. However, the relationships between musculoskeletal morphologies and elbow joint postures during the stance phase in suspended quadrupeds have not been investigated. Our analysis comparing postures and skeletal morphologies in Choloepus (Pilosa), Pteropus (Chiroptera), Nycticebus (Primates) and Cynocephalus (Dermoptera) revealed that the elbow joints of these animals were kept at flexed angles of 70–100 ° during the stance phase of quadrupedal suspension. At these joint angles the moment arms of the elbow joint flexors were roughly maximized, optimizing that component of antigravity support. Our additional measurements from various mammalian species show that suspended quadrupeds have relatively small extensor/flexor ratios in both muscle masses and maximum moment arms. Thus, in contrast to the pattern in normal terrestrial quadrupeds, suspended quadrupeds emphasize flexor over extensor muscles for body support. This condition has evolved independently multiple times, attendant with a loss or reduction of the ability to move in normal upright postures.
Keywords: elbow, flexor muscle, fruit bat, lorisids, quadrupedal suspension, sloth
Introduction
Some tetrapods can move horizontally below the substrate in an inverted (dorsal-side down) position using all four limbs. This is called quadrupedal suspension (Napier, 1967). The ability to use quadrupedal suspension has evolved at least 14 times in at least eight clades of extant mammals: Diprotodontia (Tarsipes), Pilosa (Bradypus, Choloepus, Cyclopes and Tamandua), Primates (Pongo, Ateles, Potto and Nycticebus), Rodentia (Glirulus, Graphiurus, Petaurista and Pteromys), Scandentia (Tupaia), Chiroptera (Pteropus and Rousettus), Dermoptera (Cynocephalus) and Carnivora (Nasua, Potos and Bassariscus) (Grassé, 1955; Mendel, 1981, 1985; Russell, 1986; Cant, 1987; Jouffroy & Petter, 1990; McClearn, 1992; Trapp, 1972; Sargis, 2001; Youlatos, 2002; Airapetyants & Fokin, 2003; Thorpe & Crompton, 2006; Lim, 2007). Some squamate reptiles such as Gekko (Gekkonidae) and Chamaeleo (Chamaeleonidae) employ quadrupedal suspension as well (Autumn et al. 2002; Losos et al. 1993). Among extinct animals, the locomotion of some extinct primates, such as palaeopropithecids, often is reconstructed in quadrupedal suspension (e.g. Godfrey & Jungers, 2003).
Animals must generate mediolateral torques with their limbs to balance above a thin branch (Lammers & Gauntner, 2008), whereas they can be stable without this torque when in suspension (Napier, 1967). Notably, many suspended quadrupeds maintain their elbow in a flexed pose during the stance (foot in contact with substrate; supportive) phase or in static postures (Grassé, 1955; Mendel, 1981, 1985; Jouffroy & Petter, 1990; Lim, 2007; Thorpe & Crompton, 2006; Nyakatura et al. 2010). This condition differs from brachiation in gibbons, spider monkeys and orangutans, in which the elbows are fully extended in suspension (Jungers & Stern, 1980, 1981; Thorpe & Crompton, 2006), although the forelimb bones still experience considerable tensile strains (Swartz et al. 1989) as likely is the case for suspended quadrupeds. Neither the elbow extensor nor the flexor muscles are active during the stance phase of brachiation (Jungers & Stern, 1980, 1981), whereas the elbow flexors contract during the stance phase of quadrupedal suspension (Jouffroy & Stern, 1990) against an extensor torque incurred by the downward gravitational force (Fig. 1; also Ishida et al. 1990).
Fig. 1.
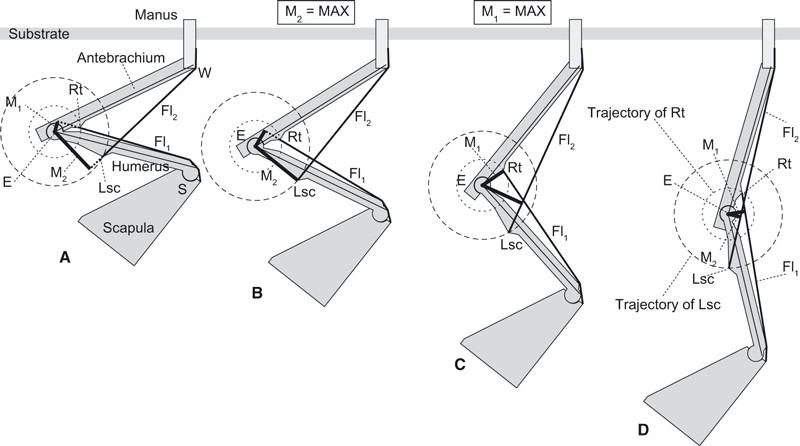
Model of forelimb mechanics during quadrupedal suspension in various elbow joint angles from (A) flexed to (D) extended. The elbow is subject to an extensor torque induced by body weight and resisted by counteracting muscle forces times moment arms (Mn). M2 and M1 are maximized at the specific elbow joint angle shown in (B) and (C), where the lines E-Lsc and E-Rt are perpendicular to Fl2 and Fl1, respectively. E, centre of elbow joint rotation; Fl1 and Fl2, flexor muscle groups along the brachium and antebrachium, respectively; M1 and M2, moment arms of Fl1 and Fl2, respectively; Lsc, lateral supracondylar crest; Rt, radial tuberosity; S and W, shoulder and wrist joints, respectively.
‘Normal’ (dorsal-side up) upright quadrupedal postures are opposite to quadrupedal suspension in both the orientation of the trunk and the activity patterns of elbow joint muscles. In these postures, elbow joint extensor muscles contract to counter moments imposed by the ground reaction force during the stance phase (e.g. Cohen & Gans, 1975; Jenkins & Weijs, 1979; Tuttle et al. 1983; Jouffroy & Stern, 1990; Gregersen et al. 1998; Wickler et al. 2005), and the elbows are kept at an angle where the moment arms of the extensors are nearly maximized (Fujiwara, 2009). This matching of limb postures to moment arm magnitude, irrespective of other critical determinants of support such as muscle force, force-length or force-velocity properties, or moment arms of external forces, concurs with the hypothesis that animals often use nearly optimal muscle moment arms as a control target for effective support (e.g. Lieber, 1997; Hutchinson et al. 2005; Johnson et al. 2008). But does this hypothesis apply to unusually specialized animals, such as suspended quadrupeds?
The unusual mechanics and presumed differences of muscular control in quadrupedal suspension pose other interesting questions about musculoskeletal adaptation, such as how well matched the morphology of elbow flexors is to the demands of quadrupedal suspension. This question could be answered by quantifying the relationship between the elbow angle and the musculoskeletal morphology such as moment arms (leverages of elbow muscles). It is striking that the biomechanics of this unusual locomotor strategy, in which the direction of gravitational pull is inverted relative to the dorsoventral body axis, has hardly been investigated. In contrast, theories about the relation between posture and muscular support have been formulated for many non-inverted animals (e.g. Alexander, 1984; Biewener, 1989, 1990, 2005; Kram & Taylor, 1990; Dickinson et al. 2002; Reilly et al. 2007). Quadrupedal suspension provides a marvellous opportunity to examine how phylogenetic history (i.e. ancestry from animals that did not use quadrupedal suspension) and functional constraints (i.e. conflicting demands for resisting gravitational forces in normal vs. inverted poses) have influenced locomotor form and function. Has evolution resulted in suspended quadrupeds with near-optimal matches between morphology (i.e. elbow flexor muscle moment arms) and behaviour (i.e. elbow postures)? Or have functional constraints or phylogenetic baggage resulted in compromises in which suboptimal moment arms are used, perhaps because muscle force output or external moments constrain the usage of such postures?
To answer these questions we conducted a broad comparative study of elbow joint musculoskeletal form and function during quadrupedal suspension in four taxa that have convergently evolved this locomotor style, and employed comparisons with animals that do not move in inverted, quadrupedally suspended poses. We attached importance especially to limb bone geometry, with an eye to applying our findings to reconstructions of limb postures in extinct animals in the future.
Hypothetical flexor moment arms of the elbow joint
Generally, there are two groups of elbow joint flexor muscles: one runs nearly parallel to the humeral shaft (Fl1), such as M. biceps brachii and M. brachialis, and the other nearly parallel to the antebrachium (Fl2), such as M. extensor carpi radialis and M. brachioradialis (Fig. 1). The former group inserts into the radial tuberosity (Rt) and the latter group originates from the lateral supracondylar crest (Lsc; Fig. 1). The flexor torque (τ) about the elbow joint created by muscle(s) is:
where Fn is the force vector of Fln and Mn is a moment arm of the Fln (perpendicular line from the centre of elbow joint rotation E to the muscle Fln; Fig. 1A–D). A greater elbow flexor torque is created by the muscle (τn) when the moment arm Mn or the scalar quantity of the muscle force Fn gets larger. The value of Mn is measurable from dissection, whereas the muscle force (Fn) is more difficult to determine but can be approximated by muscle mass (see Methods). M1 is maximized at the angle where the Fl1 is perpendicular to the line connecting E and Rt (Fig. 1C), whereas M2 is maximized at the angle where Fl2 is perpendicular to the line connecting E and Lsc (Fig. 1B).
Based on the above mechanical considerations, we hypothesize that the elbow joints during the stance phases of quadrupedal suspension are maintained close to the angle(s) where the moment arms of flexor muscles (M1, M2) are maximized. Our second hypothesis is that, in contrast to the general condition in normal, non-suspended taxa, the elbow flexor muscles in suspended quadrupeds are more developed than the extensors, having both greater moment arms and masses. To test our two hypotheses, we compared the elbow joint angles in vivo during quadrupedal suspension (measured from videos) with those angles at which the flexor moment arms are maximized (estimated from skeletal geometry). We also compared the maximum possible moment arms and actual muscle masses of elbow joint flexors and extensors across a wide diversity of mammals (100 specimens, 67 genera).
Materials and methods
Comparison between observed and estimated elbow joint angles
The elbow joint angles observed in vivo for quadrupedal suspension and the angles estimated to maximize muscle moment arms (from skeletons) were compared to each other in four taxa of different mammalian clades: the two-toed sloth (Choloepus; Pilosa), fruit bat (Pteropus, Chiroptera), colugo (Cynocephalus, Dermoptera) and slow loris (Nycticebus, Primates). The measurements of both observed and estimated elbow joint angles were taken from one to two species for each genus. Here we assume that interspecific variation within a genus (whether caused by inherited or environmental factors, including captivity) is relatively small, which our qualitative observations from external and skeletal anatomy as well as dissections support. Although we lack sufficient sample size to test this assumption statistically, it should not influence our general, qualitative results and conclusions.
The changes of elbow joint angle during the stance phases of quadrupedal suspension for 16 step cycles (strides) in total were collected for Choloepus hoffmanni (n = 6), Pteropus dasymallus (n = 6) and Nycticebus coucang (n = 4) at the Ueno Zoo (Tokyo, Japan). The angles were measured from lateral view video clips (30 Hz video; FVM300, Canon, Japan). Orientations of forelimb skeletal elements in vivo are generally difficult to observe through the surrounding soft tissues. However, our dissections and radiographs of Choloepus and Nycticebus demonstrated that the cranial (flexor) margin of the upper arm and the line connecting the olecranon and the ulnar edge of the wrist joint are nearly parallel to the shaft of the humerus and antebrachium, respectively, and thus these boundaries can be used as a proxy for the orientations of forelimb elements (Fig. 2). In Pteropus and Cynocephalus, the shoulder, the elbow and the wrist positions are quite recognizable through the membrane that covers the forelimb, so the elbow joint angle was measured between the lines from the shoulder to the elbow and wrist joint centres.
Fig. 2.
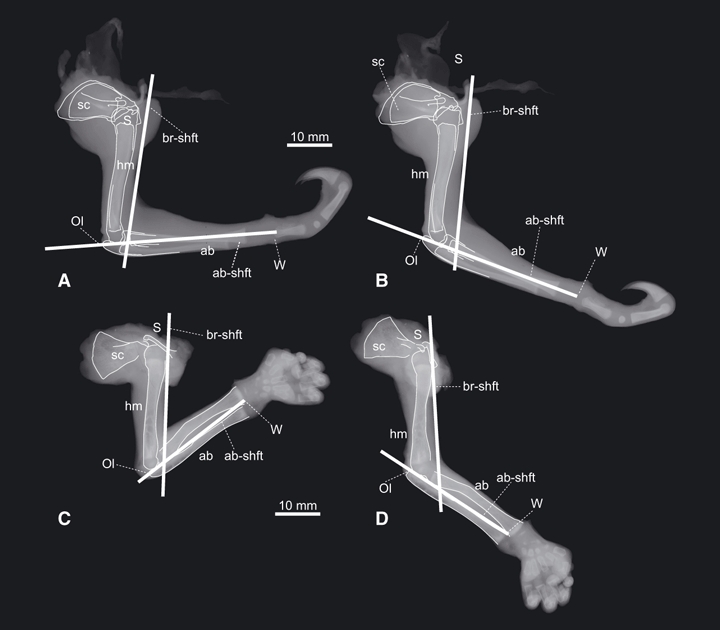
Radiographs of (A,B) Choloepus (UMUT unnumbered) and (C,D) Nycticebus (NSM M 35960) forelimbs in (A,C) flexed and (B,D) extended elbow joint angles. The cranial margin of the humerus is assumed to be nearly parallel to the shaft of the humerus (br-shft), and the line connecting the olecranon (Ol) and the wrist joint (W) is assumed to be nearly parallel to the shaft of the antebrachium (ab-shft). ab, antebrachium; hm, humerus; sc, scapula; S and W, shoulder and wrist joints, respectively.
Animals were moving at steady, normal walking speeds. Speeds were not measured for this study due to lack of consistent scale objects in the field of view to calibrate distances, but qualitatively were very consistent and speeds did not vary obviously among trials.
An additional problem our analysis encountered was that the actual elbow joint angles could not be very accurately determined from lateral view video footage when the humerus was abducted (Fig. S1). Humeral abduction and antebrachial supination occur during the first half of the stance phase. These out-of-sagittal plane motions obscure the flexion/extension angle of the elbow. They are followed by humeral adduction and pronation during the latter half in Choloepus and Nycticebus (S.-I. Fujiwara, personal observation), which facilitated more reliable quantification of joint angles. Therefore in our analysis we separated the abducted portion of the stance phase from the adducted portion, and emphasize the latter here.
The static elbow joint angles during rest were also measured from lateral view photographs of C. hoffmanni (n = 5), P. dasymallus (n = 6), Nycticebus [N. coucang (n = 2), N. pygmaeus (n = 3)], and Cynocephalus variegatus (n = 4). We used photographs taken at Ueno Zoo as well as photographs from the literature (Lim, 2007).
The range of elbow joint motion permitted by the musculoskeletal system was measured from fresh carcasses that we used for this study's dissections: two Choloepus, three Pteropus, two Cynocephalus, and two Nycticebus (Table 1). Carcasses that had been deeply frozen were not used for these measurements, nor were specimens that had been fixed or otherwise dehydrated. The original flexibility of the elbow joints was assumed to be roughly maintained in these specimens. The purpose of this measurement was not to determine precisely the actual range of motion in vivo, but to determine where the optimal elbow joint angle estimated from the geometries of bones (below) lay within the range of possible elbow joint motion.
Table 1.
Elbow joint angle estimated from skeletons. Elbow joint angles where the moment arms of Fl1 and 2 (elbow flexors along the brachium and antebrachium, respectively) are maximized, and the range of elbow joint motion (ROM) measured from fresh carcasses is indicated. The mean value (ave.) of each measurement is also indicated for each genus, followed by number of measurements in parentheses
| Genus | Specimen | Fl1 | Fl2 | ROM |
|---|---|---|---|---|
| Choloepus | ave. 70 ° (n = 5) | ave. 72.2 ° (n = 5) | ||
| C. hoffmanni | NSM M 10137 | 72 ° | 71 ° | – |
| UMUT unnumbered (juvenile)* | 72 ° | 71 ° | 53–133 ° | |
| UMUT unnumbered* | 70 ° | 74 ° | 50–120 ° | |
| C. didactylus | NSM PO 134 | 66 ° | 72 ° | – |
| IC | 70 ° | 73 ° | – | |
| Pteropus | ave. 69.75 ° (n = 4) | ave. 67.75 ° (n = 4) | ||
| P. dasymallus | NSM PO 127 | 60 ° | 69 ° | – |
| P. pselaphon | NSM M 34798 | 76 ° | 71 ° | – |
| NSM M 35961* | 69 ° | 65 ° | 29–118 ° | |
| P. sp. | UMUT unnumbered* | 74 ° | 66 ° | 11–145 ° |
| UMUT unnumbered* | – | – | 14–140 ° | |
| Nycticebus | ave. 85.25 ° (n = 4) | ave. 72.25 ° (n = 4) | ||
| N. coucang | NSM M 335 | 81 ° | 71 ° | – |
| NSM M 35960 (juvenile)* | 87 ° | 76 ° | 59–131 ° | |
| NSM M 36100 | 87 ° | 73 ° | – | |
| KPM 3674* | 86 ° | 69 ° | 59–139 ° | |
| Cynocephalus | ave. 68 ° (n = 3) | ave. 66.67 ° (n = 3) | ||
| C. variegatus | ZRC 4.8183* | – | – | 28–104 ° |
| ZRC 4.9464 (juvenile)* | – | – | 35–137 ° | |
| ZRC 4.8187 | 67 ° | 66 ° | – | |
| ZRC 4.8119 | 68 ° | 65 ° | – | |
| ZRC 4.8112 | 69 ° | 69 ° | – |
Institution abbreviations: IC, personal collection of N. Inuzuka, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan; KPM, Kanagawa Prefectural Museum, Odawara, Japan; NSM, National Science Museum, Tokyo, Japan; UMUT, The University Museum, the University of Tokyo, Japan; ZRC, Zoological Collection, Raffles Museum of Biodiversity Research, Singapore.
Fresh specimens used.
In the next step, we estimated the elbow joint angle where the flexor moment arms are maximized from 18 forelimb skeletons for our study genera (Table 1). The humerus and the antebrachium were photographed in the plane of elbow extension/flexion. The centres of elbow joint rotation (E) was determined from the curvature of the articular surfaces of the trochlea (humerus) and the arc formed by the trochlear notch (ulna) and sagittal crest (radius) of the antebrachium (Figs 1 and 3). To simplify our model, the radius and the ulna were held in semi-supinated positions in Choloepus and Nycticebus, in accordance with the limb posture during the second half of stance phase (when the humerus is adducted). In Pteropus and Cynocephalus, the antebrachium has no pronation/supination mobility and is fixed into a semi-supinated position. The Supporting Information contains images that document these non-parasagittal locomotor postures for three of our study genera (Choloepus, Pteropus and Nycticebus: Fig. S1); Cynocephalus was only photographed in static poses (see Fig. 4E).
Fig. 3.
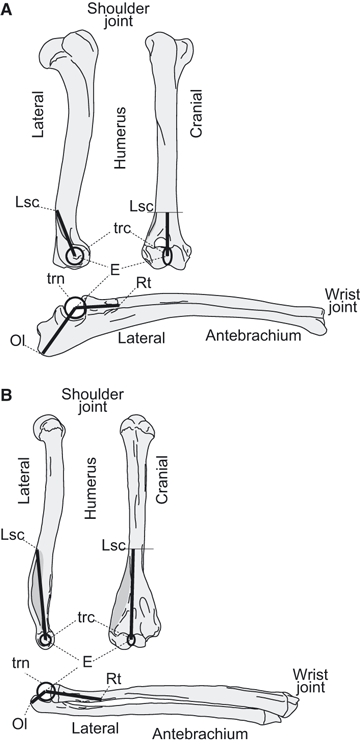
Measurements of distances E-Ol, E-Rt and E-Lsc used to calculate the maximum possible moment arms of elbow joint extensors and flexors, respectively, along the brachium and antebrachium. (A) Non-scansorial upright quadruped example (Canis; Type A). (B) Non-upright suspended quadruped example (Choloepus; Type F). See Materials and methods and Fig. 5 for the categories of locomotor ability. E, centre of elbow joint rotation; Lsc, lateral supracondylar crest; Ol, olecranon; Rt, radial tuberosity; trc, trochlea; trn, trochlear notch.
Fig. 4.
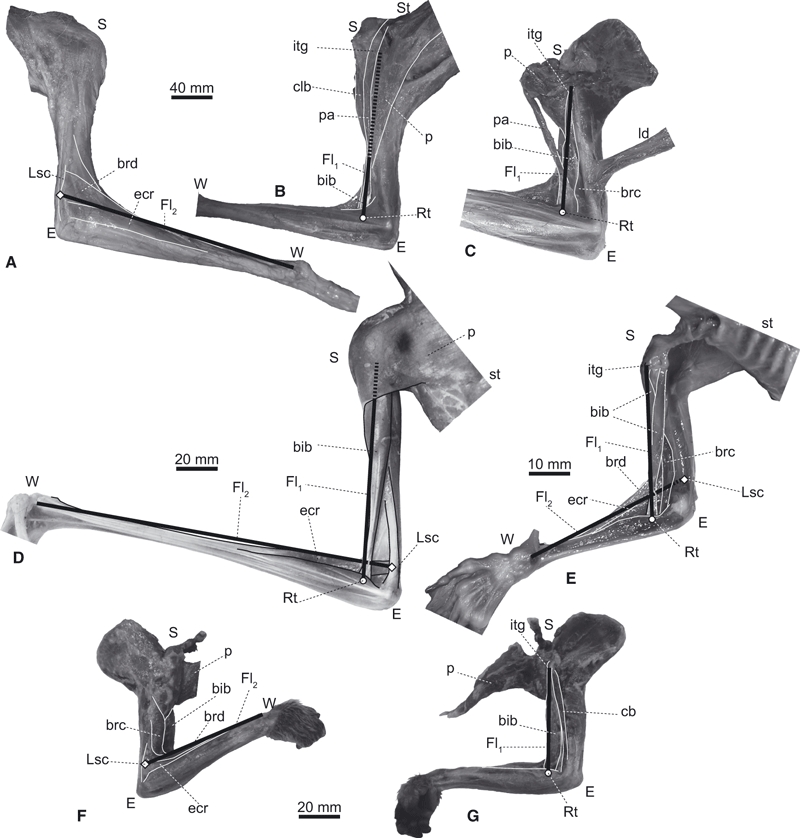
Elbow joint flexors of (A–C) Choloepus (UMUT unnumbered), (D) Pteropus (UMUT unnumbered), (E) Cynocephalus (ZRC 4.9464) and (F,G) Nycticebus (KPM 3684), in lateral (A,F) and medial views (B–E,G). Pectoral muscles are reflected or removed in C, E and G. A flexor muscle along the brachium (Fl1) is represented as a line connecting the inter-turbecular groove (itg) and the radial shaft (Rt). A flexor muscle along the antebrachium (Fl2) is represented as a line connecting the mid-portion of the lateral supracondylar crest (Lsc) and the wrist joint (W). bib, M. biceps brachii; brd, M. brachioradialis; cb, M. coracobrachialis; clb, M. cleidobrachialis; ecr, M. extensor carpi radialis; ld, M. latissimus dorsi; p, M. pectoralis; pa, part of M. pectoralis which inserts onto the antebrachium; st, sternum; E, S, and W, elbow, shoulder, and wrist joints, respectively. Note that our Fl1 and Fl2 groups best represent the paths of M. biceps brachii and M. extensor carpi radialis, respectively, but nonetheless are reasonable approximations of the paths, and thus moment arms, of the two major groups of elbow flexor muscles.
We estimated the optimal elbow joint angle for the moment arms of M. biceps brachii (Fl1; Fig. 1C) and M. extensor carpi radialis (Fl2; Fig. 1B) from the skeletal geometry. The path of Fl1 was assumed to be the line connecting the surface of the cranial side of the intertubercular groove of the humerus and the radial tuberosity (Rt; Fig. 3A,B). The path of Fl2 was assumed to be the line connecting the midpoint of the lateral supracondylar crest (Lsc) and the distalmost portion of the radius (Fig. 4A,B). Measurements of moment arms based on the bone geometry are useful when the joint has a single degree of freedom constrained by its pulley action, and the lines of the muscle actions are nearly straight from the origin and the insertion (An et al. 1984). In the elbow joints of mammals, the trochlear notch moves along the arc of the closely fitting trochlea: therefore, the joint axis is not expected to deviate much from point E (Fig. 1). We also confirmed by dissection that, at least in all four study genera, both the distal portion of M. biceps brachii (Fl1) and the proximal portion of M. extensor carpi radialis (Fl2) do not wrap around the elbow joint even when the elbows are fully extended (to their limits of ∼ 150 °). We validated our assumption that the paths of the flexor muscles approximate straight lines using radiographs (SOFTEX CMB-80; Softex Co., Ltd, National Museum of Nature and Science, Tokyo, Japan) or dissections of fresh carcasses for some specimens (Figs 2 and 4).
Measurements were made using calipers (0–200 mm; Mitutoyo Mfg. Co., Ltd.) and a Martin-type anthropometer (200–1950 mm; Takei Scientific Instruments Co., Ltd.). In the final step of our analysis we compared the observed and the estimated elbow joint angles to test our first hypothesis.
Ratios of flexor/extensor muscle moment arm and muscle mass
For the second part of our analysis we categorized our study specimens into six qualitative groups of forelimb-based locomotor abilities based on the presence or absence of terrestrial quadrupedal abilities [upright (sagittal)/non-upright (crawling or sprawling)] and arboreal abilities [non-scansorial/scansorial (climbing)/quadrupedal suspension]. These categories were: Type A, upright animals with no scansorial abilities; Type B, upright animals with scansorial abilities but with no quadrupedal suspension abilities; Type C, upright animals with quadrupedal suspension abilities; Type D, non-upright animals with no scansorial abilities; Type E, non-upright animals with scansorial abilities but with no quadrupedal suspension abilities; and Type F, non-upright animals with quadrupedal suspension abilities (Fig. 5). We did not distinguish habitual bipeds (e.g. Macropus), amphibious (e.g. Enhydra), flying (e.g. Pteropus), gliding (e.g. Cynocephalus and Petaurista) or fossorial animals (e.g. Mogera and Dasypus) from the other mammals, simply emphasizing quadrupedal abilities (Fig. 5). An animal was categorized as a scansorial or suspended quadruped if these behaviours were reported in the literature, but the levels of those abilities were not taken into account because such fine categorization was deemed too arbitrary (Fig. 5; Table 2).
Fig. 5.
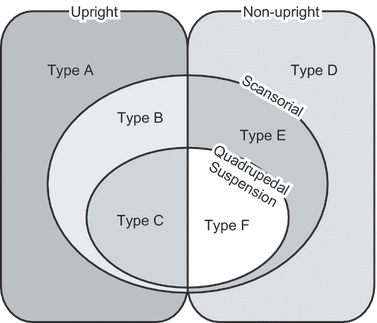
Locomotor abilities of mammals categorized into six types (see Materials and methods).
Table 2.
Lists of maximum possible moment arms (in millimetres) of elbow joint flexor muscles along the brachium (Fl1) and antebrachium (Fl2), and of the elbow joint extensors (Ex), the extensor/flexor moment arm ratio (MAR), and types of locomotor abilities (LA) in the mammals studied. The Reference column indicates where information on locomotor abilities (LA column) was checked
| Taxonomy | Moment arms (mm) | MAR | ||||||
|---|---|---|---|---|---|---|---|---|
| Order/family | Taxa | Specimen | Fl1 | Fl2 | Ex | Ex/Fl | LA | References |
| Monotremata | ||||||||
| Tachyglossidae | Tachyglossus aculeatus | NSM M 28691 | 11.3 | 25.4 | 24.2 | 0.953 | D | Nowak (1999) |
| Diprotodontia | ||||||||
| Phalangeridae | Trichosurus vulpecuis | NSM M 34964 | 18.9 | 14.7 | 14.4 | 0.761 | B | Weisbecker & Warton (2006) |
| Phascolarctidae | Phascolarctos cinereus | NSM M 821 | 35.2 | 30.2 | 23.3 | 0.662 | B | Weisbecker & Warton (2006) |
| Macropodidae | Macropus giganteus | NSM M 35838 | 56.9 | 42.4 | 37.3 | 0.655 | A | Weisbecker & Warton (2006) |
| M. giganteus | UMUT unnumbered* | 48.6 | 49.6 | 38.4 | 0.774 | A | Weisbecker & Warton (2006) | |
| M. giganteus | UMUT 0140 | 29.2 | 21.9 | 20.8 | 0.714 | A | Weisbecker & Warton (2006) | |
| M. agilis | UMUT 0047 | 40.3 | 27.6 | 26.1 | 0.648 | A | Weisbecker & Warton (2006) | |
| Afrosoricida | ||||||||
| Tenrecidae | Tenrec ecaudatus | UMZC E.5431.H | 11.9 | 12.4 | 12.3 | 0.988 | A | Salton & Sargis (2009) |
| Setifer setosus | UMZC E.5450.B | 7.4 | 6.9 | 6.2 | 0.844 | A | Salton & Sargis (2009) | |
| Hemicentetes nigriceps | UMZC E.5445.B | 5.7 | 6.9 | 7.5 | 1.088 | A | Salton & Sargis (2009) | |
| Chrysochloridae | Amblysomus hottentotus | UMZC 2010.15.A | 3.7 | 2.3 | 7.5 | 2.011 | D | Nowak (1999) |
| Tublidentata | ||||||||
| Orycteropodidae | Orycteropus afer | NSM M 34334 | 69.3 | 27.6 | 56.3 | 0.813 | A | Nowak (1999) |
| O. afer | UMZC E.1326 | 54.3 | 33.0 | 59.4 | 1.094 | A | Nowak (1999) | |
| Hyracoidea | ||||||||
| Procaviidae | Procavia capensis | NSM M 34896 | 11.2 | 15.1 | 16.3 | 1.079 | B | Nowak (1999) |
| P. sp. | UMZC E.4980.K | 11.4 | 17.2 | 15.1 | 0.878 | B | Nowak (1999) | |
| Dendrohyrax arboreus | UMZC H.5281 | 7.7 | 13.0 | 12.1 | 0.931 | B | Nowak (1999) | |
| Proboscidea | ||||||||
| Elephantidae | Elephas maximus | NSM M 33109 | 314.0 | 109.5 | 262.8 | 0.837 | A | Nowak (1999) |
| E. maximus | UMUT 0701 | 221.7 | 207.3 | 168.1 | 0.758 | A | Nowak (1999) | |
| E. maximus | UMZC H.4611 | 249.8 | 293.4 | 208.4 | 0.710 | A | Nowak (1999) | |
| Cingulata | ||||||||
| Dasypodidae | Dasypus novemcinctus | IC | 20.1 | 8.6 | 27.7 | 1.379 | A | Nowak (1999) |
| Chaetopractus villosus | UMZC E.1062 | 27.5 | 11.1 | 19.2 | 0.698 | A | Nowak (1999) | |
| Tolypeutes muriei | UMZC E. 1182 | 11.2 | 8.5 | 18.8 | 1.674 | A | Nowak (1999) | |
| Chlamydophorus truncates | UMZC E.1201 | 8.9 | 2.8 | 10.5 | 1.186 | A | Nowak (1999) | |
| Pilosa | ||||||||
| Bradypodidae | Bradypus tridactylus | UMZC E.21 | 44.9 | 31.8 | 11.0 | 0.245 | F | Mendel (1985) |
| B. tridactylus | UMZC E.23 | 45.8 | 32.9 | 11.2 | 0.245 | F | Mendel (1985) | |
| Megalonychidae | Choloepus hoffmanni | NSM M 10137 | 66.5 | 31.3 | 15.9 | 0.239 | F | Mendel (1981) |
| C. hoffmanni | UMUT unnumbered* | 23.6 | 12.5 | 7.9 | 0.249 | F | Mendel (1981) | |
| C. hoffmanni | UMUT unnumbered* | 65.2 | 36.7 | 16.3 | 0.335 | F | Mendel (1981) | |
| C. didactylus | NSM PO 134 | 45.7 | 55.8 | 15.5 | 0.278 | F | Mendel (1981) | |
| C. didactylus | IC | 60.7 | 30.9 | 14.9 | 0.245 | F | Mendel (1981) | |
| Cyclopedidae | Cyclopes didactylus | UMZC E.621 | 15.1 | 9.0 | 7.7 | 0.510 | C | Nowak (1999) |
| Myrmecophagidae | Myrmecophaga tridactylus | NSM M 34333 | 79.7 | 31.7 | 52.3 | 0.657 | B | Young et al. (2003) |
| Tamandua tetradactyla | NSM M unnumbered* | 57.8 | 19.2 | 29.8 | 0.515 | C | S.-I. Fujiwara, personal observation | |
| T. tetradactyla | UMZC E.581 | 49.1 | 16.5 | 25.1 | 0.515 | C | S.-I. Fujiwara, personal observation | |
| T. sp. | IC | 47.9 | 13.7 | 24.0 | 0.501 | C | S.-I. Fujiwara, personal observation | |
| Dermoptera | ||||||||
| Cynocephalidae | Cynocephalus variegatus | ZRC 4.8187* | 42.4 | 21.4 | 6.8 | 0.161 | F | Grassé (1955) and Lim (2007) |
| C. variegatus | ZRC 4.9464* | 19.4 | 10.6 | 3.7 | 0.191 | F | Grassé (1955) and Lim (2007) | |
| C. variegatus | ZRC 4.8119* | 44.9 | 21.5 | 6.9 | 0.154 | F | Grassé (1955) and Lim (2007) | |
| C. variegatus | ZRC 4.8112* | 31.4 | 15.4 | 6.1 | 0.194 | F | Grassé (1955) and Lim (2007) | |
| Primates | ||||||||
| Lemuridae | Varecia sp. | NSM M 33114 | 39.7 | 19.6 | 15.9 | 0.401 | B | Nowak (1999) |
| Loridae | Nycticebus coucang | NSM M 35960* | 11.2 | 5.4 | 3.8 | 0.336 | C | Jouffroy & Petter (1990) |
| N. coucang | NSM M 36100* | 31.7 | 10.8 | 6.6 | 0.207 | C | Jouffroy & Petter (1990) | |
| N. coucang | KPM 3674* | 27.6 | 12.8 | 6.7 | 0.243 | C | Jouffroy & Petter (1990) | |
| Cercopithecidae | Macaca fuscata | KPM 4191* | 50.6 | 33.3 | 24.1 | 0.477 | B | Nowak (1999) |
| Pongidae | Pongo pygmaeus | NSM M 31996 | 120.6 | 61.8 | 27.4 | 0.227 | C | Thorpe & Crompton (2006) |
| P. pygmaeus | NSM M 4226 | 110.9 | 58.2 | 24.4 | 0.220 | C | Thorpe & Crompton (2006) | |
| Hominidae | Pan troglodytes | NSM M 33042 | 91.8 | 61.8 | 33.0 | 0.360 | B | Nowak (1999) |
| P. troglodytes | NSM M 32559 | 88.8 | 58.6 | 31.8 | 0.358 | B | Nowak (1999) | |
| Lagomorpha | ||||||||
| Leporidae | Oryctolagus cuniculus | NSM M 35751 | 9.9 | 10.8 | 10.7 | 0.991 | A | Nowak (1999) |
| Lepus brachyurus | NSM unnumbered | 9.9 | 9.3 | 12.6 | 1.279 | A | Nowak (1999) | |
| Rodentia | ||||||||
| Sciuridae | Petaurista leucogenys | NSM PO 94 | 23.5 | 13.9 | 8.2 | 0.348 | C | S.-I. Fujiwara, personal observation |
| P. leucogenys | KPM unnumbered* | 16.5 | 12.3 | 5.8 | 0.350 | C | S.-I. Fujiwara, personal observation | |
| Gliridae | Glirulus japonicas | NSM unnumbered* | 3.7 | 3.8 | 2.6 | 0.680 | C | Minato 2009 pers. comm. |
| Graphiurus murinus | UMUT unnumbered* | 3.8 | 3.4 | 2.5 | 0.647 | C | S.-I. Fujiwara, personal observation | |
| Caviidae | Cavia porcellus | NSM M 35862 | 8.3 | 7.4 | 8.8 | 1.060 | A | Weisbecker & Schmid (2007) |
| Dolichotis patagonum | NSM M 35831 | 23.1 | 22.1 | 26.7 | 1.154 | A | Weisbecker & Schmid (2007) | |
| Hystricidae | Erethizon dorsatum | NSM M 34319 | 26.6 | 21.1 | 13.7 | 0.514 | B | Weisbecker & Schmid (2007) |
| Soricomorpha | ||||||||
| Talpidae | Mogera kobeae | NSM PO 123 | 5.3 | 3.6 | 9.5 | 1.792 | D | Nowak (1999) |
| Cetartiodactyla | ||||||||
| Suidae | Sus scrofa | KPM 3681* | 36.5 | 22.6 | 53.9 | 1.477 | A | Nowak (1999) |
| S. scrofa domesticus | UMUT unnumbered* | 39.0 | 40.2 | 53.8 | 1.339 | A | Nowak (1999) | |
| Tayassuidae | Tayassu tajacu | UMUT 0279 | 28.4 | 21.1 | 46.8 | 1.646 | A | Nowak (1999) |
| Giraffidae | Giraffa camelopardalis | KPM 3928* | 81.0 | 71.6 | 64.8 | 0.800 | A | Nowak (1999) |
| G. camelopardalis | UMUT unnumbered | 65.5 | 119.0 | 147.3 | 1.238 | A | Nowak (1999) | |
| Cervidae | Rangifer tarandus | UMUT 0036 | 51.7 | 46.3 | 65.8 | 1.273 | A | Nowak (1999) |
| Bovidae | Bos gaurus | KPM 3937* | 113.0 | 65.8 | 132.2 | 1.170 | A | Nowak (1999) |
| Bubalus bubalis | UMUT unnumbered | 89.7 | 108.1 | 131.9 | 1.215 | A | Nowak (1999) | |
| Carnivora | ||||||||
| Mustelidae | Gulo gulo | NSM M 35843 | 42.8 | 30.4 | 27.3 | 0.637 | B | Van Vankenburgh (1987) |
| Lutra lutra | NSM M 33858 | 20.2 | 14.4 | 16.5 | 0.817 | B | Leblanc (2003) | |
| Enhydra lutris | UMUT unnumbered* | 39.1 | 25.9 | 21.0 | 0.538 | A | Iwaniuk (2000) | |
| Ailuridae | Ailurus fulgens | NSM M 34320 | 29.9 | 19.8 | 16.9 | 0.565 | B | Iwaniuk (2000) |
| Ursidae | Ailuropoda melanoleuca | NSM M 32901 | 111.1 | 54.2 | 56.2 | 0.506 | B | Iwaniuk (2000) |
| Tremarctos ornatus | NSM M 22995 | 87.5 | 48.8 | 56.3 | 0.643 | B | Van Vankenburgh (1987) | |
| Melursus ursinus | NSM M 25234 | 101.1 | 49.5 | 60.1 | 0.595 | B | Van Vankenburgh (1987) | |
| Ursus maritimus | NSM M 31634 | 112.0 | 61.8 | 70.1 | 0.626 | A | Iwaniuk (2000) | |
| U. maritimus | UMUT unnumbered* | 149.0 | 102.0 | 99.1 | 0.665 | A | Iwaniuk (2000) | |
| Canidae | Nyctereutes procyonoides | NSM M 35491 | 20.0 | 18.9 | 19.0 | 0.944 | B | Kauhala & Saeki (2004) |
| Chrysocyon brachyrus | NSM M 16003 | 48.0 | 40.4 | 41.1 | 0.856 | A | Iwaniuk (2000) | |
| C. brachyrus | NSM M 36655* | 55.4 | 38.5 | 43.3 | 0.781 | A | Iwaniuk (2000) | |
| Canis familiaris | UMUT unnumbered* | 56.1 | 41.0 | 53.3 | 0.950 | A | Van Vankenburgh (1987) | |
| Herpestidae | Mungos mungo | NSM M 35866 | 21.8 | 11.4 | 12.3 | 0.566 | A | Gittleman (1986) |
| Helogale parvula | NSM M 35753 | 12.2 | 6.1 | 6.7 | 0.549 | B | Iwaniuk (2000) | |
| Felidae | Felis catus | NSM M 35590 | 23.5 | 17.9 | 17.5 | 0.744 | B | Nowak (1999) |
| Pronailurus bengaliensis | NSM M 14329 | 12.0 | 12.9 | 12.2 | 0.946 | B | Iwaniuk (2000) | |
| Leptailurus serval | NSM M 27664 | 46.7 | 25.7 | 26.8 | 0.574 | B | Nowak (1999) | |
| Caracara caracal | NSM M 2609 | 30.8 | 21.0 | 26.3 | 0.855 | B | Van Vankenburgh (1987) | |
| Acinonyx jubatus | NSM M 31465 | 60.7 | 29.7 | 47.4 | 0.780 | B | Van Vankenburgh (1987) | |
| A. jubatus | NSM M 31466 | 49.2 | 29.6 | 45.2 | 0.918 | B | Van Vankenburgh (1987) | |
| A. jubatus | NSM M 36698 * | 56.9 | 67.8 | 47.1 | 0.694 | B | Van Vankenburgh (1987) | |
| Neofelis nebulosa | NSM M 31826 | 43.7 | 30.5 | 30.9 | 0.706 | B | Van Vankenburgh (1987) | |
| Uncia uncia | NSM M 33876 | 54.2 | 33.9 | 44.2 | 0.816 | B | Iwaniuk (2000) | |
| Panthera pardalis | KPM 3653* | 99.3 | 48.4 | 54.2 | 0.528 | B | Van Vankenburgh (1987) | |
| P. tigris | NSM M 33189 | 87.6 | 62.7 | 75.3 | 0.859 | B | Van Vankenburgh (1987) | |
| P. leo | NSM M 33055 | 83.7 | 59.9 | 66.7 | 0.797 | B | Van Vankenburgh (1987) | |
| Perissodactyla | ||||||||
| Equidae | Equus caballus | NSM PO 131 | 72.0 | 105.0 | 109.5 | 1.043 | A | Nowak (1999) |
| E. caballus | NSM M 36001* | 65.6 | 67.6 | 103.0 | 1.524 | A | Nowak (1999) | |
| E. asinus | KPM 3932* | 69.4 | 83.9 | 86.4 | 1.030 | A | Nowak (1999) | |
| Tapiridae | Tapirus indicus | KPM 3936* | 64.3 | 61.6 | 88.5 | 1.376 | A | Nowak (1999) |
| Chiroptera | ||||||||
| Pteropodidae | Pteropus pselaphon | NSM M 35961* | 15.3 | 10.2 | 5.8 | 0.376 | F | S.-I. Fujiwara, personal observation |
| P. sp. | UMUT unnumbered* | 16.6 | 4.1 | 7.7 | 0.462 | F | S.-I. Fujiwara, personal observation | |
| Rousettus aegyptiacus | NSM M 34803 | 9.8 | 8.0 | 3.4 | 0.347 | F | S.-I. Fujiwara, personal observation | |
Institution abbreviations: IC, personal collection of N. Inuzuka, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan; KPM, Kanagawa Prefectural Museum, Odawara, Japan; NSM, National Science Museum, Tokyo, Japan; UMUT, The University Museum, the University of Tokyo, Japan; UMZC, University Museum of Zoology, Cambridge, UK; ZRC, Zoological Collection, Raffles Museum of Biodiversity Research, Singapore.
Measurements taken from carcasses. MAR is calculated as (Ex/Fl1) or (Ex/Fl2), whichever is smaller.
The maximum possible moment arms of elbow joint extensor and flexor muscles were measured in dried skeletons and carcasses of 100 mammal specimens representing 67 genera, 40 families and 17 orders (Table 2). We used specimens from KPM (Kanagawa Prefectural Museum, Odawara, Japan), IC (personal collections of N. Inuzuka, Graduate School of Medicine, The University of Tokyo, Japan), NSM (National Science Museum, Tokyo, Japan), UMUT (The University Museum, The University of Tokyo, Japan), UMZC (University Museum of Zoology, Cambridge, UK), and ZRC (Zoological Reference Collection, Raffles Museum of Biodiversity Research, Singapore).
The distance between the centre of elbow joint rotation (E) and the most distant (i.e. caudal) point from E on the olecranon (Ol) was assumed to be a reasonable approximation of the maximum moment arm of the extensors, such as M. triceps brachii and M. dorsiepitrochlealis (Fig. 3A,B). Similarly, the distances between E and the distalmost point of the Rt or the proximal edge of Lsc were respectively used to estimate the maximum moment arm of Fl1 (M. biceps brachii, M. brachialis, M. brachioradialis and M. pectoantebrachialis) and Fl2 (M. extensor carpi radialis and M. brachioradialis: Fig. 3A,B). Because there were no landmarks on the antebrachium and humerus for determining the centre of elbow joint rotation (E), we first calculated the radii of the trochlear notch and the trochlea from measurements of their diameters (as above). Next, the distances between E and the most distant point from E on the olecranon (Ol) and the distalmost point of the radial tuberosity (Rt) were calculated by adding the measurement of the minimum length from the margin of the trochlear notch to Ol and Rt, and the radius of the trochlear notch, respectively (Fig. 3A,B). Likewise, the distance between E and the proximal edge of the lateral supracondylar crest (Lsc) was calculated by subtracting the radius of the trochlea from the distance between the distalmost portion of the trochlea and Lsc (Fig. 3A,B). The length ‘E-Ol’ divided by the length ‘E-Rt or E-Lsc, whichever is larger’ is defined here as the index of elbow extensor/flexor moment arm ratio.
The masses of the elbow joint extensor and flexor muscles were also measured from fresh carcasses in 37 specimens representing 26 genera (Fig. 6). We used specimens from KPM, NSM, UMUT, ZRC (above), and personal collections of J. R. Hutchinson. These mass measurements were made using an electronic balance (0.001 g of accuracy: Shimadzu Co., Ltd.). The flexor/extensor function of each muscle was determined from dissections by pulling the muscle along its line of action because the functions of homologous muscles are not always the same among taxa [e.g. M. triceps brachii caput mediale does not extend the elbow in Tamandua (Taylor, 1978)], and also because unusual muscles function as elbow extensors/flexors in some taxa [e.g. M. pectoantebrachialis, a pectoral muscle which inserts onto the antebrachium as an elbow flexor in Choloepus (Fig. 4B,C; Lucae, 1884)].
Fig. 6.
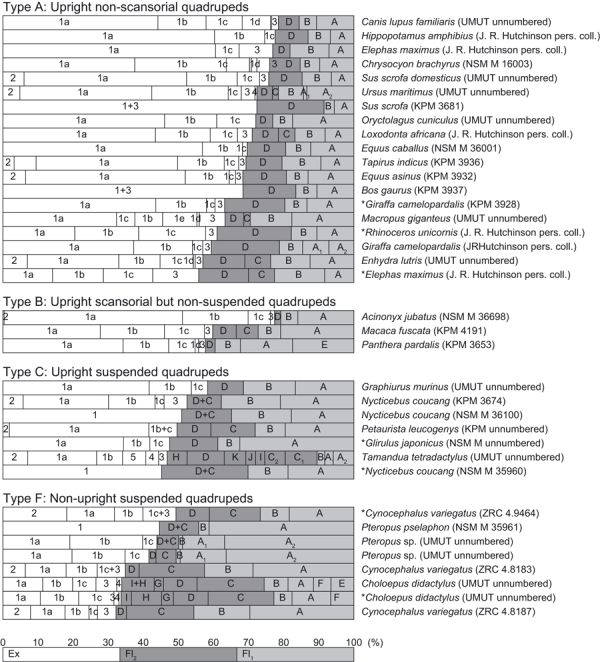
Muscle mass ratios of elbow joint extensors (Ex) and flexors along the brachium (Fl1) and antebrachium (Fl2). The length of each bar represents the relative mass of each muscle, when the total mass of the extensors and flexors of an individual is 100% (see bottom bar for abstract example). No data were obtainable for taxa in types D and E. Asterisks indicate juvenile specimens. (1) M. triceps brachii (1a, long head; 1b, lateral head; 1c, medial head; 1d, accessory head; 1e, intermediate head); (2) M. dorsiepitrochlearis; (3) M. anconeus; (4) M. epitrochleoanconeus; (5) M. flexor carpi ulnaris. A, M. biceps brachii (A1, short head; A2, long head); B, M. brachialis; C, M. brachioradialis (C1, short head; C2, long head); D, M. extensor carpi radialis; E, M. cleidobrachialis; F, M. ectoantebrachialis; G, M. supinator; H, M. pronator teres; I, M. flexor carpi radii; J, M. flexor digiti profundus; K, M. flexor digiti sublimis. See institution abbreviations in Tables 1 and 2.
Limitations of the analyses
Our methods have several technical limitations yet we contend these should not greatly influence our results. Because the videos were taken only from lateral views, we could not measure the elbow angles throughout the stance phase (Fig. 7) or three-dimensionally (but see Fig. S1). However, our estimates of the elbow joint angle during the latter half of the stance phase in Choloepus (Fig. 6A) match the measurements by Nyakatura et al.(2010) based on three-dimensional cineradiographs. Specifically, the elbow joint angle is around 60 ° in mid-stance, and increases to around 110 ° toward the end of the stance phase (Nyakatura et al. 2010).
Fig. 7.
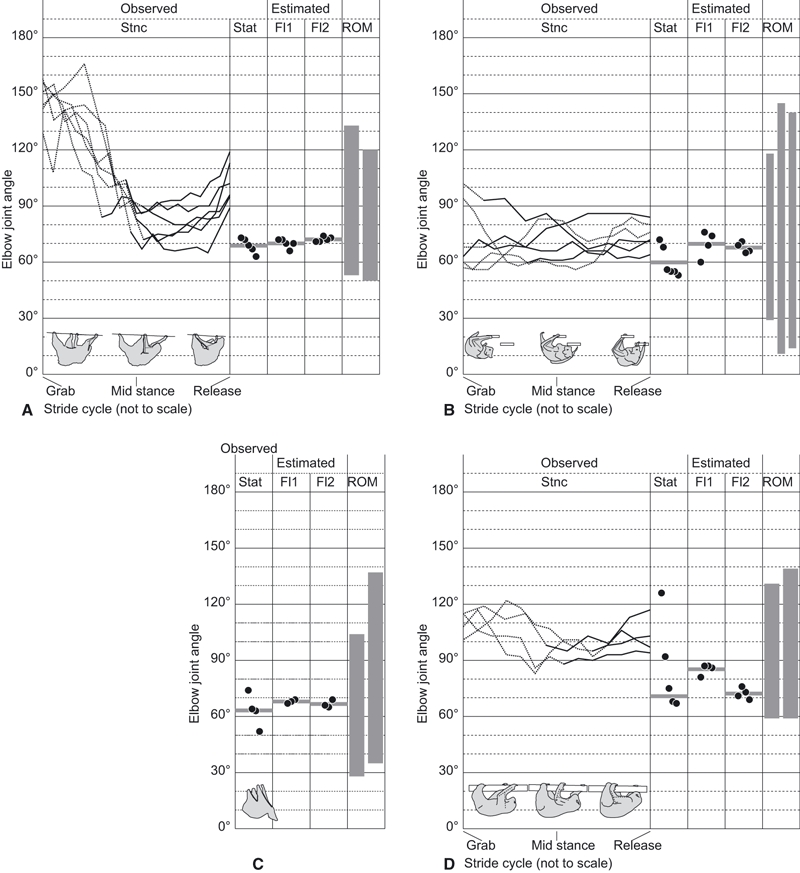
(A–D) Observed elbow joint angles, including the changes of the joint angle during stance phase (Stnc) and the angle in static postures (Stat), estimated elbow joint angles where the moment arms of the flexors along the brachium (Fl1) and the antebrachium (Fl2) are maximized, and the ranges of elbow joint motion (ROM), are compared for our study taxa of four suspended quadrupeds: (A) Choloepus, (B) Pteropus, (C) Cynocephalus (no Stnc data recorded) and (D) Nycticebus. Solid and dotted lines of the transitions in Stnc indicate, respectively, the portions of the stance phase where the humerus is more abducted (first half) or adducted (second half). The horizontal bar in each section of Stat, Fl1 and Fl2 is a mean value of the measurements.
We have only sampled four main genera as representatives from four clades that include highly specialized suspended quadrupeds, of at least eight extant clades that use these behaviours. We predict that future studies of clades/genera we have not yet sampled would bolster our results and allow more robust phylogenetic hypotheses to be tested (e.g. the evolutionary sequences that have produced/enabled quadrupedal suspension). However, access to these rare, often endangered species for measurement and dissection will remain an obstacle. Nonetheless, our study is the first broadly comparative analysis of suspended quadrupeds — all previous studies have focused on one species or genus in isolation. Furthermore as noted above, our sample sizes were too small to characterize fully individual variation within species (see Kikuchi, 2010 for an approach that could be conducted with larger samples of our study taxa).
We used a geometric method instead of the tendon travel method (An et al. 1984; Spoor & Van Leeuwen, 1992) or other approaches (e.g. MRI, cineradiography) to quantify the moment arms of muscles, although the latter may provide more accurate data. However, moment arm analyses have previously been conducted using the tendon travel method for the elbow joints in taxa which have similar musculoskeletal geometries to our study taxa. These similarities include that the origins and insertions of the elbow flexor muscles are located near the shaft of the humerus and the antebrachium, respectively. These studies show that the elbow flexor muscle moment arms are maximized at flexed angles of around 90 ° (Homo, Pan, Symphalangus and Macaca: Murray et al. 1995; Thorpe et al. 1999; Graham & Scott, 2003; Michilsens et al. 2010) as in our results (see below). This trend, however, is not observed when the moment arm-joint angle relationships are approximated by straight lines and quadratic equations (e.g. studies of hares and greyhounds by Williams et al. 2007, 2008) but we suspect that this discrepancy may be an artefact of the methods of the latter studies, particularly the quadratic equations, which reduce accuracy for estimating moment arms at some joint angles (Channon et al. 2010). Overall, these published data validate our simple geometric model.
Furthermore, our model focuses on the largest and presumably most important elbow flexors, grouped as Fl1 (especially M. biceps brachii) and Fl2 (especially M. extensor carpi radialis), for our study taxa. Our qualitative observations support our assumption that this focus is justified, because other elbow flexors in groups Fl1 (e.g. M. brachialis, M. brachiocephalicus) and Fl2 (e.g. M. brachioradialis) follow roughly parallel lines of action that should give them similar moment arms and moment arm-angle trajectories, and in most cases have nearly identical origins and/or insertions (Fig. 4).
Our dataset of muscle masses alone is insufficient to quantify muscle force outputs fully because it is not the muscle mass but the physiological cross-section area (PCSA) of the muscle that indicates its force-producing capability. However, maximal force output should still correlate strongly with muscle mass. This is because PCSA is proportional to the product of the muscle mass and the cosine of the pennation angle, and is inversely proportional to the product of the density of muscle and the fibre length (Gans & Bock, 1965; Payne et al. 2005; Williams et al. 2007, 2008). Thus our qualitative conclusions about the differences of extensor : flexor muscle masses in suspended vs. upright taxa should be sufficiently reliable.
Results
Do suspended quadrupeds match their elbow joint angles to maximize flexor muscle moment arms?
Our motion analyses revealed that the elbow joints of Choloepus, Pteropus and Nycticebus are well flexed, below 120 °, during the adducted portion of the stance phase (Fig. 7A,B,D). The elbow joint angle at mid-stance, where the ground reaction force is expected to be maximal (e.g. Biewener, 1989, 1990; Ishida et al. 1990), was flexed to a 60–100 ° angle at least during the periods when the humeri were more adducted at mid-stance (Fig. 7A,B,D). The elbow joint in the abducted portion was relatively extended, but the actual angles in the abducted portion are expected to be less than the measured angles (dotted lines in Fig. 7A,B,D).
The elbows of Choloepus, Pteropus, Nycticebus and Cynocephalus were flexed mostly below 80 ° (similar to mid-stance) in static postures when they were resting or sleeping, except for a few cases in Nycticebus, where the elbows were extended up to 130 ° when the animal was alert and actively inspecting its surroundings (Fig. 7).
The elbows in other suspended quadrupeds, such as Bradypus and Pongo, can be fully extended to about 180 ° (e.g. Nowak, 1999; Thorpe & Crompton, 2006) but according to our manipulations of carcasses the elbows of our four study genera can only extend to 110–150 ° (Fig. 7). The ranges of elbow joint motions are restricted not only by the muscles and tendons, but also by the geometry of the joint surfaces.
The relative position of Lsc (the origin of Fl2) on the humerus does not vary between our study taxa; its position remains on the distal third of the humeral shaft in lateral views (Fig. 8A–D). Therefore, the estimated elbow joint angles where the Fl2 moment arms are maximized do not vary appreciably between our study taxa. On the other hand, the relative position of Rt (the insertion of Fl1) varies more widely between the four genera. In the antebrachia of Pteropus, Cynocephalus (fixed antebrachium) and Choloepus (even in pronated or semi-supinated positions) the M. biceps brachii (Fl1) insertion (Rt) spanned the space between the radius and the ulna (Fig. 8A–D). However, in Nycticebus the relative position of Rt is located cranially in accordance with supination of the antebrachia (Fig. 7D). Consequently, the estimated elbow joint angle where the Fl1 moment arm is maximized showed interspecific variation: the optimal angles are at about 70 ° in Choloepus, Pteropus and Cynocephalus, and at about 90 ° in Nycticebus (Figs 7 and 8).
Fig. 8.
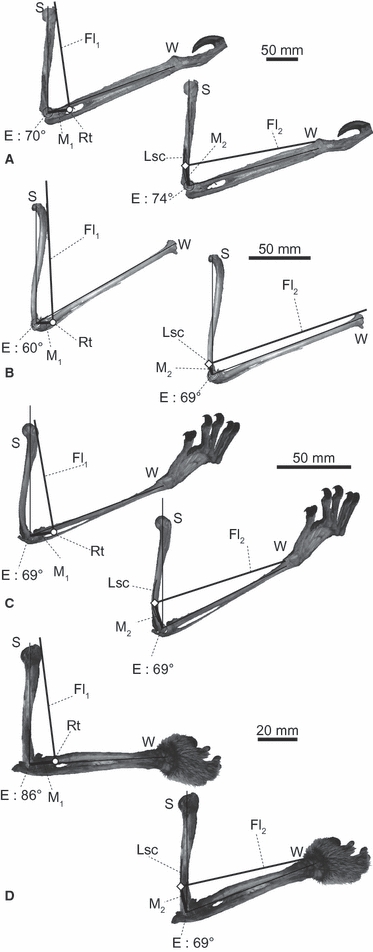
Estimated elbow joint angles (maximizing flexor moment arms) for the Fl1 and Fl2 muscles of selected specimens. ROM was measured after the flight membrane was removed in Pteropus specimens: (A) Choloepus hoffmanni (UMUT unnumbered), (B) Pteropus dasymallus (NSM PO 127), (C) Cynocephalus variegatus (ZRC 4.8112) and (D) Nycticebus coucang (KPM 3674). The antebrachia of Choloepus and Nycticebus specimens are held in semi-supinated position. E, centre of elbow joint rotation; Lsc, lateral supracondylar crest; Rt, radial tuberosity; S and W, shoulder and wrist joints, respectively.
Considering the observed angles in vivo and the ranges of motion allowed at the elbow, the elbow joints of all four study taxa were neither maximally extended nor flexed, at least during the adducted portion of the stance phase. Most importantly, the estimated moment arm-maximizing angles for both Fl1 and Fl2 and the observed angles were close to each other, especially in Choloepus, Pteropus and Cynocephalus (Fig. 7A–C). In Nycticebus, the optimal elbow joint angle estimated for the Fl2 moment arm was close to the observed angles in static postures, whereas the optimal angle estimated for the Fl1 moment arm was close (< 20 ° difference) to the angles observed at mid-stance of locomotion (Fig. 7D). Overall, our first hypothesis is well supported, although elbow joint function in Nycticebus deserves more investigation.
Do suspended quadrupeds have larger flexor vs. extensor muscle masses and moment arms?
The elbow extensor/flexor muscle moment arm ratios of quadrupeds varied between the different categories of locomotor abilities, descending (i.e. more strongly emphasizing flexors) in order from type A, to B, to C, to F (using median values; Figs 9 and 10A). There were only three data points for taxa in type D, so clear conclusions about this group cannot be drawn, although they were most similar to type A (Fig. 10A). No animals categorized in type E could be obtained for measurement. The ranges of the elbow joint extensor/flexor moment arm ratio do not overlap between types A (upright non-scansorial taxa) and F (non-upright suspended quadrupeds). The median extensor/flexor muscle moment arm ratios were four times smaller for our study taxa (type F) vs. type A.
Fig. 9.
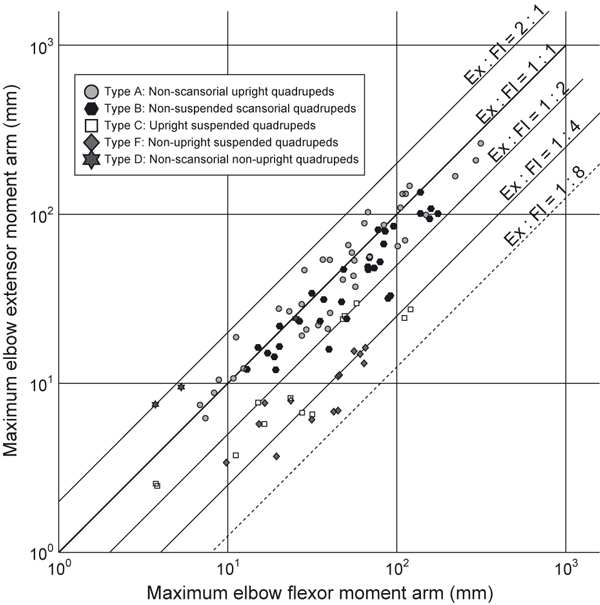
Comparison between maximum possible moment arms of the elbow joint extensor (Ex: vertical axis) and flexor (Fl1 or Fl2, whichever is larger: horizontal axis) muscles. See Figs 3 and 5 and main text for the details of the measurements and the locomotor ability categories. Animals plotted on the upper left possess relatively large extensor moment arms (e.g. upright quadrupeds), and the one on lower right possesses relatively large flexor moment arms (e.g. suspended quadrupeds).
Fig. 10.
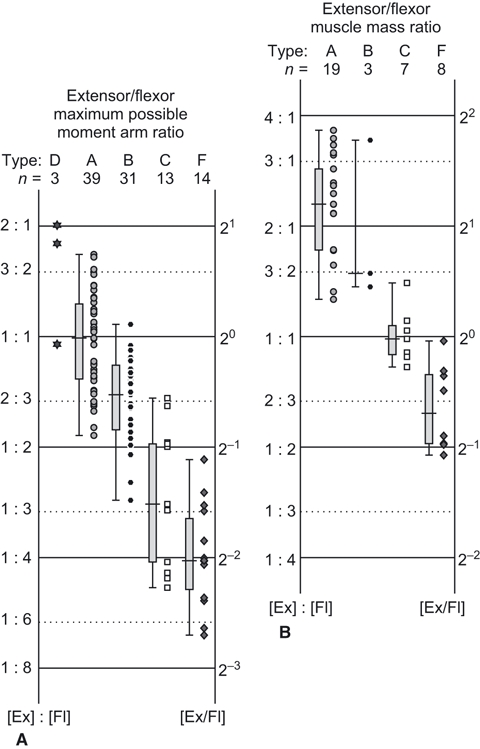
Elbow joint extensor/flexor ratios for (A) maximum possible moment arm and (B) total muscle masses. Extensor (Ex) and flexor (Fl) ratios are plotted along the vertical axis, and quartiles for each type of locomotor ability are shown in box plots.
Similarly, the median value of extensor/flexor muscle mass ratio decreased (i.e. more strongly emphasized flexors) in order from type A, to C, to F (Fig. 10B). There were only three samples for type B taxa but the muscle mass ratio was larger than in types C and F (Fig. 10B). Unfortunately, no samples were available for types D or E. However, for type A taxa, the flexor masses were relatively large in some species with additional locomotor abilities, such as in Macropus (habitual bipeds), Enhydra (amphibious) and Giraffa (‘normal’ upright quadruped, but with relatively long distal elements that may require large flexor torques for limb protraction), and in a juvenile (but not adult) Elephas and Ceratotherium. Overall, our second hypothesis remains well supported by our results: much like the moment arm ratios, the median values for extensor/flexor muscle mass ratios in suspended, non-upright quadrupeds were approximately four times smaller than those for upright non-scansorial taxa (type A). We did not find conclusively significant correlations between body mass and the elbow extensor/flexor muscle moment arm or muscle mass ratio within each type (see Supporting Information Data S1, Tables S1–S4, Figs S2 and S3).
Discussion and Conclusions
We find that suspended quadrupeds use poses that are approximately optimal for supporting their elbow joints during locomotion (i.e. maximizing flexor muscle moment arms) and that their morphology has evolved to match these demands, increasing the relative leverages and masses of flexor vs. extensor muscles. Therefore, in both upright (Fujiwara, 2009) and suspended (this study) postures, the moment arms of antigravity muscles are a key factor in the selection of elbow joint poses in extant quadrupeds as in some other taxa (Lieber, 1997; Hutchinson et al. 2005; Johnson et al. 2008).
We might expect then that the application of biomechanical theory proposed for normal, non-inverted taxa (see Introduction) would still hold for the response of the musculoskeletal system to gravitational constraints in suspended quadrupeds, but with one major change. Flexor, rather than extensor, muscles dominate the antigravity support role in these taxa, and thus changes predicted for flexor (i.e. leg-swinging) muscles in normal taxa should apply to extensor muscles in suspended quadrupeds (e.g. Cohen & Gans, 1975; Tuttle et al. 1983; Jouffroy & Stern, 1990; Payne et al. 2005; Williams et al. 2007). This condition has evolved convergently in at least our four study taxa, and we predict that this will hold for all other extant suspended quadrupeds.
As far as upright, scansorial and suspended quadrupedal abilities are concerned, our analyses support the notion that there are mechanical trade-offs between locomotor abilities. Our locomotor types A and F are drastically different in these abilities and their specializations in extensor vs. flexor muscles of the elbow may be integral to these differences. However, as we acknowledged in the Introduction, biomechanical factors other than the muscle moment arm (e.g. muscle properties and gravitational or ground reaction force moment arms) are important determinants of the elbow and other joint angles that animals use. We find it exciting and a bit surprising that even suspended quadrupeds tend to adopt postures in which their elbow flexor muscle moment arms are near maximal and hence that component of their antigravity support is optimized. Yet the interplay of this component and others remains to be fully determined for any species or behaviour.
Regardless, our study shows how the inverted lifestyle of suspended quadrupeds has inverted the ‘normal’ biomechanical functions of their antigravity vs. leg-swinging muscles. Their muscular changes have made them adroit at this upside-down lifestyle, but at the repeatedly evolved cost of reducing or even losing the ability to support themselves in the upright posture of their distant common mammalian ancestors.
Acknowledgments
This paper was supported by the Japan Society of Promotion for Science (Grant number: 22-4730). We also thank S. Kawada (NSM), H. Taru (KPM), K. K. P. Lim (ZRC), N. Lim (University of California, Davis), and M. Lowe (UMZC) for providing the specimens for dissection and measurements, M. Ohga (Ueno Zoo) for giving us the opportunity to film at Ueno Zoo, A. Hayashida (Kobe Oji Zoo), D. Koyabu, Y. Nakajima, K. Mori, N. Miwa and M. Hosojima (The University of Tokyo) for assisting with the dissections, R. Matsumoto (University College London), N. Egi (Kyoto University) and G. Suwa (The University of Tokyo) for providing critical literature, T. Kubo (Fukui Prefectural Dinosaur Museum) for assisting with filming at zoos, A. Spence (Royal Veterinary College), G. Byrnes (University of California, Berkeley), N. Inuzuka (The University of Tokyo), M. Takai (Primate Research Institute, Kyoto University) and S. Minato (Yamane Museum) for their helpful advice, and three anonymous reviewers for improving this paper. J.R.H. thanks the BBSRC for funding via grants BB/C516844/1, BB/F001169/1 and BB/H002782/1.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. The scaling of body mass and elbow extensor . flexor ratios of maximum muscle moment arms and masses in each locomotor type (A–F).
Fig. S1. Images from video clips of stance phases (from top to bottom) of (A) Choloepus and (B) Pteropus in near-frontal views, and (C) Nycticebus in lateral view. Bars on the right side of each image sequence indicate the phases of humeral abduction. adduction during the stance . swing phases of right (R) and left (L) forelimbs. The image sequences are not in equal intervals. See Fig. 4E for a representative image of Cynocephalus
Fig. S2. Reduced major axis scaling plots for body mass and elbow extensor . flexor muscle moment arm ratios in locomotor types A–D and F. See Table S1 for the original data and Table S3 for the regression statistics.
Fig. S3. Reduced major axis scaling plots for body mass and elbow extensor . flexor muscle mass ratios in locomotor types A–C and F. See Table S2 for the original data and Table S4 for the regression statistics.
Table S1. Mean values of log extensor . flexor ratio of elbow joint muscle moment arms (Log Ex/ Fl), sample size (n), body mass (BM), mean value of the range of log body masses (Log BM), and locomotor ability (LA) in each study taxon. See main text and Fig. 5).
Table S2. Mean values of the log extensor/flexor ratio of elbow joint muscle masses (Log Ex . Fl), sample size (n), body mass (BM), mean value of the range of log body masses (Log BM), and locomotor ability (LA) in each study taxon. See main text and Fig. 5).
Table S3. Sample size (n), correlation coefficient (r), coefficient of determination (r2) and significance probability (P) calculated by reduced major axis regression analysis of the relationship between body mass and elbow extensor . flexor muscle moment arm ratio in each locomotor type (A.D, and F: Fig. S2). See main text and Fig. 5.
Table S4. Sample size (n), correlation coefficient (r), coefficient of determination (r2), and significance probability (P) calculated by reduced major axis regression analysis of the relationship between body mass and elbow extensor . flexor muscle mass ratio in each locomotor type (A–C, and F: Fig. S3). See main text and Fig. 5.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Airapetyants AE, Fokin IM. Biology of European flying squirrel Pteromys volans L. (Rodentia: Pteromyidae) in the North-West of Russia. Russ J Theriol. 2003;2:105–113. [Google Scholar]
- Alexander RM. The gaits of bipedal and quadrupedal animals. Int J Rob Res. 1984;3:49–59. [Google Scholar]
- An KN, Takahashi K, Harrigan TP, et al. Determination of muscle orientations and moment arms. J Biomech Eng ASME. 1984;106:280–282. doi: 10.1115/1.3138494. [DOI] [PubMed] [Google Scholar]
- Autumn K, Sitti M, Liang YA, et al. Evidence for van der Waals adhesion in gecko setae. PNAS. 2002;99:12252–12256. doi: 10.1073/pnas.192252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener AA. Scaling body support in mammals: limb posture and muscle mechanics. Science. 1989;245:45–48. doi: 10.1126/science.2740914. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Biomechanics of mammalian locomotion. Science. 1990;250:1097–1103. doi: 10.1126/science.2251499. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Biomechanical consequences of scaling. J Exp Biol. 2005;208:1665–1676. doi: 10.1242/jeb.01520. [DOI] [PubMed] [Google Scholar]
- Cant JGH. Positional behaviour of female Bornean orangutans (Pongo pygmaeus) Am J Primatol. 1987;12:71–90. doi: 10.1002/ajp.1350120104. [DOI] [PubMed] [Google Scholar]
- Channon AJ, Crompton RH, Gunther MM, et al. Muscle moment arms of the gibbon hind limb: implications for hylobatid locomotion. J Anat. 2010;216:446–462. doi: 10.1111/j.1469-7580.2009.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AH, Gans C. Muscle activity in rat locomotion: movement analysis and electromyography of the flexors and extensors of the elbow. J Morphol. 1975;146:177–196. doi: 10.1002/jmor.1051460202. [DOI] [PubMed] [Google Scholar]
- Dickinson MH, Farley CT, Full RJ, et al. How animals move: an integrative view. Science. 2002;288:100–106. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- Fujiwara S-I. Olecranon orientation as an indicator of elbow joint angle in the stance phase, and estimation of forelimb posture in extinct quadruped animals. J Morphol. 2009;270:1107–1121. doi: 10.1002/jmor.10748. [DOI] [PubMed] [Google Scholar]
- Gans C, Bock WJ. The functional significance of muscle architecture — a theoretical analysis. Ergeb Anat Entwicklungsgesch. 1965;38:115–142. [PubMed] [Google Scholar]
- Gittleman JL. Carnivore brain size, behavioral ecology, and phylogeny. J Mamm. 1986;67:23–36. [Google Scholar]
- Godfrey LR, Jungers WL. The extinct sloth lemurs of Madagascar. Evol Anthropol. 2003;12:252–263. [Google Scholar]
- Graham KM, Scott SH. Morphometry of Macaca mulatto forelimb. III. Moment arm of shoulder and elbow muscles. J Morphol. 2003;255:301–314. doi: 10.1002/jmor.10064. [DOI] [PubMed] [Google Scholar]
- Grassé PP. Ordre des dermoptères. In: Grassé PP, editor. Traité de Zoologie, Anatomie, Systématique, Biologie, Tome XVII Mammifères: Systématique et Éthologie. Paris: Libraires de l'Académie de Médecine; 1955. pp. 1713–1728. [Google Scholar]
- Gregersen CS, Silverton NA, Carrier DR. External work and potential for elastic storage at the limb joints of running dog. J Exp Biol. 1998;201:3197–3210. doi: 10.1242/jeb.201.23.3197. [DOI] [PubMed] [Google Scholar]
- Hutchinson JR, Anderson FC, Blemker S, et al. Analysis of hindlimb muscle moment arms in Tyrannosaurus rex using a three-dimensional musculoskeletal computer model. Paleobiol. 2005;31:676–701. [Google Scholar]
- Ishida H, Jouffroy FK, Nakano Y. Comparative dynamics of pronograde and upside down horizontal quadrupedalism in the slow loris (Nycticebus coucang) In: Jouffroy FK, Stack MH, Niemitz C, editors. Gravity, Posture and Locomotion in Primates. Florence: Il Sedicesimo; 1990. pp. 209–220. [Google Scholar]
- Iwaniuk AN. The Evolution of Skilled Forelimb Movements in Carnivores. Canada: University of Lethbridge; 2000. Msc Thesis. [Google Scholar]
- Jenkins FA, Weijs WA. The functional anatomy of the shoulder in the Virginia opossum (Didelphis virgninana) J Zool Lond. 1979;188:379–410. [Google Scholar]
- Johnson WL, Jindrich DL, Roy RR, et al. A three-dimensional model of the rat hindlimb: musculoskeletal geometry and muscle moment arms. J Biomech. 2008;41:610–619. doi: 10.1016/j.jbiomech.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouffroy FK, Petter A. Gravity-related kinematic changes in lorisine horizontal locomotion in relation to position of body. In: Jouffroy FK, Stack MH, Niemitz C, editors. Gravity, Posture and Locomotion in Primates. Florence: Il Sedicesimo; 1990. pp. 199–208. [Google Scholar]
- Jouffroy FK, Stern JT. Telemetred EMG study of the antigravity versus propulsive actions of knee and elbow muscles in the slow loris (Nycticebus coucang) In: Jouffroy FK, Stack MH, Niemitz C, editors. Gravity, Posture and Locomotion in Primates. Florence: Il Sedicesimo; 1990. pp. 221–236. [Google Scholar]
- Jungers WL, Stern JT. Telemetered electromyography of forelimb muscle chains in gibbons (Hylobates lar) Science. 1980;208:617–619. doi: 10.1126/science.7367886. [DOI] [PubMed] [Google Scholar]
- Jungers WL, Stern JT. Preliminary electromyographical analysis of brachiation in gibbon and spider monkey. Int J Primatology. 1981;2:19–33. [Google Scholar]
- Kauhala K, Saeki M. Finnish and Japanese raccoon dogs — on the road to speciation? In: Macdonald DW, Sillero-Zubiri C, editors. Biology and Conservation of Wild Canids. Oxford: Oxford University Press; 2004. pp. 215–226. [Google Scholar]
- Kikuchi Y. Quantitative analysis of variation in muscle internal parameters in crab-eating macaques (Macaca fascicularis) Anthropol Sci. 2010;118:9–21. [Google Scholar]
- Kram R, Taylor CR. Energetics of running: a new perspective. Nature. 1990;346:265–267. doi: 10.1038/346265a0. [DOI] [PubMed] [Google Scholar]
- Lammers AR, Gauntner T. Mechanics of torque generation during quadrupedal arboreal locomotion. J Biomech. 2008;41:2388–2395. doi: 10.1016/j.jbiomech.2008.05.038. [DOI] [PubMed] [Google Scholar]
- Leblanc F. Protecting fish farms from predation by the Eurasian otter (Lutra lutra) in the Limousin region of central France: first results. IUCN Otter Spec Group Bull. 2003;20:31–34. [Google Scholar]
- Lieber RL. Muscle fiber length and moment arm coordination during dorsi- and plantarflexion in the mouse hindlimb. Acta Anat. 1997;159:84–89. doi: 10.1159/000147970. [DOI] [PubMed] [Google Scholar]
- Lim N. Colugo: The Flying Lemur of South-East Asia. Singapore: Draco Publishing, National University of Singapore; 2007. [Google Scholar]
- Losos JB, Walton BM, Bennett AF. Trade-offs between sprinting and clinging ability in Kenyan chameleons. Funct Ecol. 1993;7:281–286. [Google Scholar]
- Lucae JCG. Statik und Mechanik der Quadrupeden an dem Muskeln des Lemur und Choloepus. Abhandl Senkenberg Naturforsch Gesellsch Frankfurt. 1884;13:1–92. [Google Scholar]
- McClearn D. Locomotion, posture, and feeding behavior of kinkajous, coatis, and raccoons. J Mamm. 1992;73:245–261. [Google Scholar]
- Mendel FC. Use of hands and feet of two-toed sloths (Choloepus hoffmanni) during climbing and terrestrial locomotion. J Mamm. 1981;62:413–421. [Google Scholar]
- Mendel FC. Use of hands and feet of three-toed sloths (Bradypus variegatus) during climbing and terrestrial locomotion. J Mamm. 1985;66:359–366. [Google Scholar]
- Michilsens F, Vereecke EE, D'Août K, et al. Muscle moment arms and function of the siamang forelimb during brachiation. J Anat. 2010;217:521–535. doi: 10.1111/j.1469-7580.2010.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray WM, Delp SL, Buchanan TS. Variation of muscle moment arms with elbow and forearm position. J Biomech. 1995;28:513–525. doi: 10.1016/0021-9290(94)00114-j. [DOI] [PubMed] [Google Scholar]
- Napier JR. Evolutionary aspects of primate locomotion. Am J Phys Anthropol. 1967;27:333–341. doi: 10.1002/ajpa.1330270306. [DOI] [PubMed] [Google Scholar]
- Nowak RM. Walker's Mammals of the World. 6th edn. Baltimore: The Johns Hopkins University Press; 1999. [Google Scholar]
- Nyakatura JA, Petrovitch A, Fischer MS. Limb kinematics during locomotion in the two-toed sloth (Choloepus didactylus, Xenarthra) and its implications for the evolution of the sloth locomotor apparatus. Zoology. 2010;113:221–234. doi: 10.1016/j.zool.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, et al. Functional specialization of pelvic limb anatomy in horses (Equus caballus) J Anat. 2005;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SM, McElroy EJ, Biknevicius AR. Posture, gait and the relevance of locomotor costs and energy-saving mechanisms in tetrapods. Zoology. 2007;110:271–289. doi: 10.1016/j.zool.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Russell EM. Observations on the behaviour of the honey possum, Tarsipes rostratus (Marsupialia: Tarsipedidae) in captivity. Aust J Zool Suppl Ser. 1986;34:1–63. [Google Scholar]
- Salton JA, Sargis EJ. Evolutionary morphology of the Tenrecoidea (Mammalia) hindlimb skeleton. J Morphol. 2009;270:367–387. doi: 10.1002/jmor.10697. [DOI] [PubMed] [Google Scholar]
- Sargis EJ. The grasping behaviour, locomotion and substrate use of the tree shrews Tupaia minor and T. tana (Mammalia, Scandentia) J Zool Lond. 2001;253:485–490. [Google Scholar]
- Spoor CW, Van Leeuwen JL. Knee muscle moment arms from MRI and from tendon travel. J Biomech. 1992;25:201–206. doi: 10.1016/0021-9290(92)90276-7. [DOI] [PubMed] [Google Scholar]
- Swartz SM, Bertram JEA, Biewener AA. Telemetered in vivo strain analysis of locomotor mechanics of brachiating gibbons. Nature. 1989;342:270–272. doi: 10.1038/342270a0. [DOI] [PubMed] [Google Scholar]
- Taylor BK. The anatomy of the forelimb in the anteater (Tamandua) and its functional implications. J Morphol. 1978;157:347–368. doi: 10.1002/jmor.1051570307. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH. Orangutan positional behavior and the nature of arboreal locomotion in hominoidea. Am J Phys Anthropol. 2006;131:384–401. doi: 10.1002/ajpa.20422. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH, Gunther MM, et al. Dimensions and moment arms of the hind- and forelimb muscles of common chimpanzees (Pan troglodytes) Am J Phys Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Trapp GR. Some anatomical and behavioural adaptations of ringtails, Bassariscus astutus. J Mamm. 1972;53:549–557. [Google Scholar]
- Tuttle R, Velte MJ, Basmajian JV. Electromyography of brachial muscles in Pan troglodytes and Pongo pygmaeus. Am J Phys Anthropol. 1983;61:75–83. doi: 10.1002/ajpa.1330610108. [DOI] [PubMed] [Google Scholar]
- Van Vankenburgh B. Skeletal indicators of locomotor behavior in living and extinct carnivores. J Vert Paleont. 1987;7:162–182. [Google Scholar]
- Weisbecker V, Schmid S. Autopodial skeletal diversity in hystricognath rodents: functional and phylogenetic aspects. Mamm Biol. 2007;72:27–44. [Google Scholar]
- Weisbecker V, Warton DI. Evidence at hand: diversity, functional implications, and locomotor prediction in intrinsic hand proportions of diprotodontian marsupials. J Morphol. 2006;267:1469–1485. doi: 10.1002/jmor.10495. [DOI] [PubMed] [Google Scholar]
- Wickler SJ, Hoyt DF, Biewener AA, et al. In vivo muscle function vs speed. II. Muscle function trotting up an incline. J Exp Biol. 2005;208:1191–1200. doi: 10.1242/jeb.01485. [DOI] [PubMed] [Google Scholar]
- Williams SB, Wilson AM, Payne RC. Functional specialization of the thoracic limb of the hare (Lepus europeus) J Anat. 2007;210:491–505. doi: 10.1111/j.1469-7580.2007.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Wilson AM, Daynes J, et al. Functional anatomy and muscle moment arms of the thoracic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris) J Anat. 2008;213:373–382. doi: 10.1111/j.1469-7580.2008.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youlatos D. Positional behaviour of black spider monkeys (Ateles paniscus) in French Guiana. Int J Primatol. 2002;23:1071–1093. [Google Scholar]
- Young RJ, Coelho CM, Wieloch DR. A note on the climbing abilities of giant anteaters, Myrmecophaga tridactyla (Xenarthra, Myrmecophagidae) Bol Mus Biol Mello Leitão. 2003;15:41–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


