Abstract
The arrangement of the early nerve connections in the embryonic vertebrate brain follows a well-conserved pattern, forming the early axon scaffold. The early axon tracts have been described in a number of anamniote species and in mouse, but a detailed analysis in chick is lacking. We have used immunostaining, axon tracing and in situ hybridisation to analyse the development of the early axon scaffold in the embryonic chick brain in relation to the neuromeric organisation of the brain. The first tract to be formed is the medial longitudinal fascicle (MLF), shortly followed by the tract of the postoptic commissure to pioneer the ventral longitudinal tract system. The MLF was found to originate from three different populations of neurones located in the diencephalon. Neurones close to the dorsal midline of the mesencephalon establish the descending tract of the mesencephalic nucleus of the trigeminus. Their axons pioneer the lateral longitudinal tract. At later stages, the tract of the posterior commissure emerges in the caudal pretectum as the first transversal tract. It is formed by dorsally projecting axons from neurones located in the ventral pretectum, and by ventrally projecting axons from neurones located in the dorsal pretectum. The organisation of neurones and axons in the chick brain is similar to that described in the mouse, though tracts form in a different order and appear more clearly distinguished than in the mammalian model.
Keywords: early axon scaffold, medial longitudinal fascicle, prosomeric model, tract of the posterior commisure, tract of the postoptic commissure
Introduction
The complex organisation of the adult vertebrate brain is pioneered during early embryonic development by a small number of neurones and associated axon tracts. The basic array of the early longitudinal tracts, transversal tracts and commissures was first fully described in zebrafish by Chitnis & Kuwada (1990) and Wilson et al. (1990), who termed it the early axon scaffold. Subsequent studies in zebrafish have demonstrated that the neurones of the early axon scaffold have an important function in pioneering the major axon pathways in the brain (e.g. Chitnis & Kuwada, 1991; Bak & Fraser, 2003). The early axon scaffold has since been studied in various anamniotes, like Xenopus (Hartenstein, 1993), turbot (Doldan et al. 2000), medaka (Ishikawa et al. 2004), cat shark (Kuratani & Horigome, 2000) and sea lamprey (Barreiro-Iglesias et al. 2008). In contrast, among amniotes the early tracts have been documented in chick (e.g. Chédotal et al. 1995) and alligator (Pritz, 2010), but only the mouse brain has been studied in detail (Easter et al. 1993; Mastick & Easter, 1996). The basic tract system is remarkably well conserved during evolution. A common feature of all vertebrates analysed is the ventral longitudinal tract (VLT) system, formed by the medial longitudinal fascicle (MLF) and the tract of the postoptic commissure (TPOC). The MLF originates from a cluster of basal neurones located mainly rostral to the diencephalic-mesencephalic boundary (DMB), while the TPOC neurones are located in the rostral end of the basal hypothalamus. Prominent commissures are the postoptic commissure (POC) and anterior commissure (AC) in the rostral prosencephalon, and the posterior commissure (PC) in the caudal diencephalon. The tract of the posterior commissure (TPC) is a well-conserved pretectal transversal tract that runs parallel to the DMB in the alar plate, and whose fibres subsequently adopt longitudinal ascending or descending courses within the basal plate, laterally to the MLF (Diaz et al. 1999). An additional transversal tract, the dorsoventral diencephalic tract (DVDT) is only clearly distinguished in anamniotes (reviewed in Hjorth & Key, 2002), unless it corresponds to an homologue of the retroflex tract (Puelles et al. 1996; Wullimann et al. 1999). On the other hand, in the mouse and sauropsids, neurones in the dorsal mesencephalon form the prominent descending tract of the mesencephalic nucleus of the trigeminus (DTmesV), which has no obvious counterpart in the early anamniote brain. However, anamniotes have a transient population of primary sensory neurones located in the rhombencephalon, the Rohon-Beard cells, which project into the lateral longitudinal tract and may be functionally equivalent to the early DTmesV.
Next to the mouse, the chick is the other main amniote model used in developmental biology. Yet, in contrast to the mouse, the early axon tracts in the chick brain have not been analysed in the same detail as in anamniote species. First descriptions of tracts in the embryonic chick brain date back to the beginning of the 20th century (Mesdag, 1909). Later studies used silver staining (Tello, 1923; Windle & Austin, 1936; Lyser, 1966; Bösel, 1974) and immunohistochemistry (Easter et al. 1994; Chédotal et al. 1995) to analyse the tracts further. Different from the mouse, the first neurones differentiating in the embryonic chick brain were found to be located near the DMB (McConnell & Sechrist, 1980; Puelles et al. 1987); these neurones extend axons to form the MLF (Windle & Austin, 1936; Lyser, 1966; Chédotal et al. 1995). The MLF is a highly conserved tract that in most vertebrates analysed is the first axon tract formed during embryogenesis (reviewed in Ahsan et al. 2007), possibly linked with its function in controlling motor behaviour of free-swimming larval stages (e.g. Gahtan et al. 2002). While previous studies agree on the MLF being the first recognisable tract in the chick brain, the identity and timing of subsequent tracts like DTmesV, TPOC and TPC are less clear.
Previous studies on the early axon tracts have focussed on describing the time of appearance, location and course of the tracts. Yet, only few studies (e.g. Kuratani et al. 1998) have related these tracts to the overall organisation of the rostral brain. While this organisation has long been a matter of debate among neuroanatomists (discussed in Puelles et al. 1987), anatomical and molecular evidence strongly suggests that the rostral neural tube is subdivided into transversal neuromeres and that this subdivision provides the basis for the developmental organisation of the brain (Puelles & Rubenstein, 1993). According to the model, the rostral brain is initially subdivided into mesencephalon and primary prosencephalon, with the latter quickly splitting into diencephalon and secondary prosencephalon. This proneuromeric phase is followed by the early neuromeric phase, during which the diencephalon gets divided into caudal synencephalon (or pretectum, p1) and rostral parencephalon. In the late neuromeric phase the parencephalon separates into posterior parencephalon (or thalamus, p2) and anterior parencephalon (or prethalamus, p3; Puelles et al. 1987; Puelles & Rubenstein, 1993, 2003). Consequently, the neural tube along its whole longitudinal axis displays a similar dorsoventral organisation into roof plate, alar plate, basal plate and floor plate (Shimamura et al. 1995). Prominent transversal boundaries are separating the neuromeres in the rostral brain, including the DMB, the midbrain–hindbrain boundary, and the zona limitans intrathalamica separating p2 and p3. The latter two, along with the anterior neural ridge, are signalling centres that are critical for the correct organisation of the rostral brain (reviewed in Kiecker & Lumsden, 2005).
Although the early axon scaffold is morphologically well defined in most model organisms and is considered to be important for the correct organisation of the mature brain, surprisingly little is known about the molecular mechanisms that control its formation. It is likely that early patterning and transcriptional control are responsible for fate determination of the early differentiating neurones. Yet, to date it has been very difficult to associate gene expression patterns in the brain with specific neurones in the early scaffold, not least because of lack of detail in the anatomical description of the early tracts. In this study, using a combination of immunostaining, axon tracing and in situ hybridisation, we have analysed the early forming tracts in the chick brain in detail. Our study provides the precise location of neurones and axons for the major tracts of the early axon scaffold, and reveals surprising complexity in the organisation of the basal longitudinal tract system and the transversal alar pretectal TPC tract parallel to the DMB. It provides a reference for future studies on patterning and axon guidance molecules, and will be valuable for comparative studies on early brain organisation.
Materials and methods
Fertilised domestic chicken eggs were obtained from Henry Stewart (Peterborough, UK). They were incubated at 38 °C until the required stage. Embryos were prepared in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde/PBS (overnight) or MEMFA (0.1 m MOPS/2 mm EGTA/1 mm MgSO4/3.7% formaldehyde; 30–40 min, and then washed and stored in methanol). Embryos were staged according to Hamburger & Hamilton (1951) criteria.
The early axon scaffold was visualised in chick embryonic brains using pan-neural antibodies. The chick embryos were prepared for immunofluorescence by opening the rhombencephalon and the telencephalic vesicles. Tuj1 mouse (1 : 1000; Abcam ab7751) or rabbit (1 : 1000; Abcam ab18207) and HuC/D (1 : 500; Molecular Probes mAB 16A11) primary antibodies were used. Primary antibodies were visualised with a fluorochrome-conjugated anti-mouse (1 : 500; Invitrogen A11003) or anti-rabbit antibody (1 : 500; Invitrogen A11008). The protocol for immunostaining has been described previously (Lumsden & Keynes, 1989). For combined tract tracing/immunofluorescence staining, 100 μg mL−1 digitonin (Fisher) was used to replace Triton X-100 in the immunostaining protocol to prevent the diffusion of DiI (Matsubayashi et al. 2008). Embryos were transferred into 80% glycerol and the brains were flat-mounted onto microscope slides.
Whole-mount in situ hybridisation in combination with immunohistochemistry was performed as described (Schubert & Lumsden, 2005). Probes for Pax6 (Goulding et al. 1993) and En1 (Logan et al. 1992) were employed, while neurones and tracts were detected with the Tuj1 antibody, and visualised with horseradish peroxidase-conjugated anti-mouse antibody (1 : 100; Jackson ImmunoResearch 115-035-044).
Cyanine dyes DiI and DiO (D275; Molecular Probes D282) were used as tracers to label specific axon tracts. The dyes were dissolved in 100% ethanol and injected into formaldehyde-fixed chick brain using fine glass needles. Once injected the embryos were left at room temperature in PBS for 4–5 days for embryos up to HH17, and 1 week for HH18 and older. Embryos were then put into 80% glycerol and the brains were flat-mounted onto microscope slides. To combine tract tracing with antibody staining, chick embryos were injected with DiI and left at room temperature in PBS for 4–5 days.
Low-magnification images of whole chick brains were taken on a Zeiss Stereo Lumar V12 fluorescent microscope, and more detailed images were taken on a Zeiss LSM510 confocal microscope. Images were processed using Axiovision Rel. 4.6, ImageJ and Photoshop CS. ImageJ was used to combine z-stacks taken by the confocal microscope into single images.
Results
Overview of early axon scaffold formation in the chick embryo
To determine when and where the first neurones are differentiating and projecting their initial axons, whole brains of chick embryos between HH10 and HH18 have been analysed by immunofluorescence, using β III-tubulin as a pan-neural marker. The anti-β III-tubulin antibody labels differentiating and mature neurones, but not neuronal precursor cells (Lee et al. 1990). We could not detect any staining of neurones in the brain at HH10 (Figs 1A and 2). The first neurones were labelled near the DMB at HH11 (Figs 1B, 2 and 7a). The number of these neurones had increased at HH12 (Figs 1C and 2), and some had started to grow their axons (Figs 2 and 3A,B). They represent the pioneering MLF neurones. At HH13, some of these neurones projected axons caudally to begin forming the MLF tract (Fig. 1D, filled arrow; Figs 2 and 7b). Also at this stage, the first TPOC neurones appeared at the rostral end of the basal hypothalamus (Fig. 1D, open arrow; Figs 2 and 7b). These neurones started projecting axons at HH14, while the first MLF axons had reached the mesencephalic-rhombencephalic boundary (MRB; Fig. 1E, arrow; Figs 2 and 7c). At this stage, the DTmesV neurones that were first detectable at late HH13 in the dorsal mesencephalon started to project axons ventrally (Figs 1E, 2 and 6C). The number of MLF, TPOC and DTmesV neurones kept increasing during the following stages. By HH15, the TPOC axons had almost reached the DMB (Figs 1F and 2), while neurones were also labelled in the telencephalic area for the first time (Fig. 1F, asterisk; Fig. 2). The latter are likely to originate from the olfactory placode and constitute the terminal nerve ganglion. The VLT made up of TPOC and MLF was well established by HH16 (Figs 1G, 2 and 7d), and their axons are joined by the mamillo-tegmental tract (MTT) originating in the caudal hypothalamus (Figs 2 and 4G). In the mesencephalon, the arciform/circumferential DTmesV axons had started to turn caudally into their longitudinal course, fasciculating within the incipient lateral longitudinal fascicle (LLF) that crosses the MRB at HH17 (Fig. 1H, filled arrow; Fig. 2). In the diencephalon, scattered alar neurones had appeared in the diencephalon, mainly in prospective p3 (Figs 1G and 2). These neurones were projecting their axons a very short distance ventrally, entering the longitudinal TPOC/MLF tract by HH17 (Fig. 1H, open arrow; Fig. 2). The ventral and lateral longitudinal tracts were the most prominent differentiating structures in the HH18 brain (Figs 1I, 2 and 7e), but more postmitotic neurones were emerging in the alar diencephalon, whose transversal axons enter the VLT and course rostrally and caudally along it as they reach the basal plate (Fig. 1I, open arrow; Figs 2 and 7e). The terminal nerve ganglion cells also started to project axons. The TPC became apparent as a transversal tract in the caudal pretectum (Figs 2 and 5B,C). In the alar plate of the mesencephalon, in addition to the DTmesV, an increasing number of neurones were detected that project their axons ventrally to form the tecto-bulbar tract (Figs 1I, 2 and 6F).
Fig. 1.
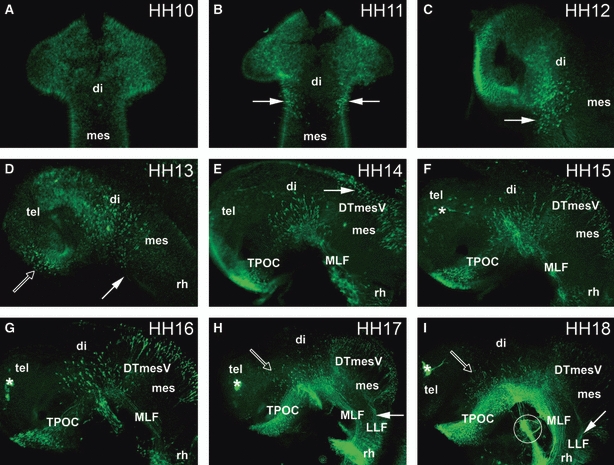
Time series of axon tract development in the chick embryonic brain. (A, B) Dorsal views of the brain; (C–I) lateral views of the brain. (A) HH10: no staining of neurones in the rostral brain. (B) HH11: neurones are located rostral to the DMB, within the diencephalon (arrows). (C) HH12: the number of MLF neurones has increased (arrow). (D) HH13: the first TPOC neurones are labelled in the rostral hypothalamus (open arrow). In the pretectum, MLF neurones are projecting axons caudally (filled arrow). (E) HH14: the MLF axon tract is projecting along the basal mesencephalon, and the TPOC neurones have started to project their axons. DTmesV neurones have started differentiating in the dorsal mesencephalon (arrow). (F) HH15: TPOC axons have reached the pretectum, forming a joint VLT with the MLF. Olfactory placode neurones are labelled in the telencephalic area (asterisk). (G) HH16: the number of MLF, TPOC and DTmesV neurones has increased, and their axons have projected further. (H) HH17: there are neurones located in the alar diencephalon projecting axons ventrally towards the TPOC (open arrow). These neurones are projecting dorsally towards the VLT. In the mesencephalon DTmesV axons are pioneering the LLF (filled arrow). (I) HH18: more alar neurones are present in the diencephalon (open arrow). The LLF is pioneered from the DTmesV (arrow). Circle shows the trigeminal nerve. (F–I) Asterisks mark the olfactory placode. di, diencephalon; DTmesV, descending tract of the mesencephalic nucleus of the trigeminal nerve; LLF, lateral longitudinal fascicle; mes, mesencephalon; MLF, medial longitudinal fascicle; rh; rhombencephalon; tel, telencephalon; TPOC, tract of the postoptic commissure.
Fig. 2.
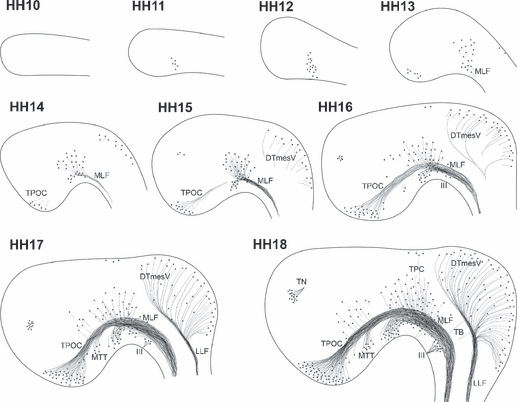
Development of axon tracts in the rostral chick brain. Sketches of the developing neurones and tracts in the rostral brain of chick embryos during the first 72 h of development. Note that the drawings are not representative of the absolute number of neurones present. HH10: no differentiated neurones detectable. HH11: the first MLF neurones differentiate. HH12: increased number of MLF neurones, but no neurones elsewhere yet. HH13: MLF neurones project axons caudally. TPOC neurones are visible in the rostral hypothalamus, and the first DTmesV neurones start appearing at the dorsal midline of the mesencephalon. HH14: MLF neurones are located in three subnuclei. Axons of the TPOC project towards the DMB. HH15: TPOC and MLF form a continuous, VLT. Axons of the DTmesV reach towards the alar-basal boundary in the mesencephalon. The first neurones in the olfactory placode region are detectable. HH16: MTT neurones are detected in the caudal hypothalamus, and their axons join the VLT system. The DTmesV axons turn caudally towards the MHB. HH17: all major tracts increase in complexity. Additional neurones are found scattered in the rostral diencephalon, and neurones also differentiate in the mesencephalic alar plate. HH18: the TPC becomes apparent in the caudal pretectum. VLT and DTmesV dominate the organisation of the rostral brain, but the rising number of additional neurones and axons progressively mask the early axon scaffold. The oculomotor nerve and its neurones are also prominent features of the early basal mesencephalon, but as peripheral nerve are not considered further in this study. III, oculomotor nerve; DTmesV, descending tract of the mesencephalic nucleus of the trigeminal nerve; LLF, lateral longitudinal fascicle; MLF, medial longitudinal fascicle; MTT mamillo-tegmental tract; TB, tecto-bulbar axons; TN, terminal nerve; TPC, tract of the posterior commissure; TPOC, tract of the postoptic commissure.
Fig. 7.
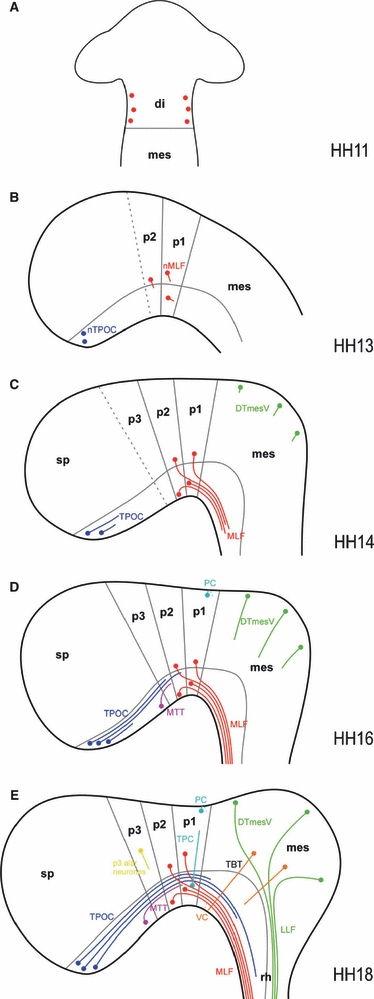
Developmental series of the chick early axon scaffold. Schematic representation of axon tract formation in the early embryonic chick brain. Neuromere boundaries (based on e.g. Larsen et al. 2001; Puelles & Rubenstein, 2003; Ferran et al. 2007) and the alar-basal plate border (reflecting the dorsal margin of Nkx2.2 expression and ventral margin of Pax3 expression) are shown in grey, while neurones and axons of the various early tracts are colour-coded (DTmesV, green; MLF, red; MTT, purple; PC/TPC, turquoise; TPOC, blue; VC, orange; dispersed alar p3 neurones, yellow). (A) HH11: the first MLF neurones appear within the diencephalon. (B) HH13: while the MLF neurones in the alar and basal pretectum begin to project axons, the TPOC neurones arise in the rostral basal hypothalamus. (C) HH14: in the pretectum, three populations of neurones contributing axons to the MLF in alar p1/p2, basal p1 and basal p2 are apparent. The MLF consists of a tight bundle of basal axons, close to the ventral midline. The TPOC axons have now started to project axons caudally through the secondary prosencephalon. The DTmesV neurones have appeared along the dorsal midline of the mesencephalon and start to project axons ventrally. (D) HH16: the TPOC axons have reached the MLF in p1. Located in between the TPOC and MLF neurones, the MTT neurones have arisen in the basal plate of the caudal secondary prosencephalon. These axons initially follow the TPOC axons to join the VLT. Neurones at the dorsal midline of p1 project axons contralaterally to pioneer the PC. (E) HH18: the principal tracts of the chick early axon scaffold have formed. In the pretectum, TPC neurones have appeared in ventral p1 and project axons dorsally along the DMB, while in the mesencephalon tectobulbar neurones project their axons ventrally. di, diencephalon; DTmesV, descending tract of the mesencephalic nucleus of the trigeminal nerve; LLF, lateral longitudinal fascicle; mes, mesencephalon; MLF, medial longitudinal fascicle; MTT, mamillo-tegmental tract; p1, prosomere 1/pretectum; p2, prosomere 2/thalamus; p3, prosomere 3/prethalamus; PC, posterior commissure; rh, rhombencephalon; sp, secondary prosencephalon; TBT, tectobulbar tract; TPC, tract of the posterior commissure; TPOC, tract of the postoptic commissure; VC, ventral commissure.
Fig. 3.
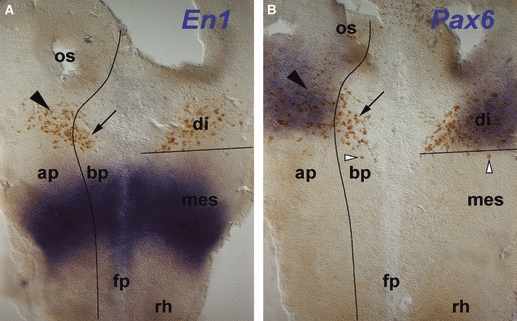
Patterning and MLF neurone location. In situ hybridisation (blue) for patterning genes En1 (A) and Pax6 (B) combined with immunohistochemistry (brown) for neurones detected with the Tuj1 antibody in HH12 flat-mounted chick brains. Black lines indicate the DMB as defined by the caudal limit of the Pax6 expression domain, and the alar plate-basal plate boundary as defined by the ventral limit of the Pax6 expression domain. (A) En1 is expressed in the mesencephalon. All of the neurones are located rostral to the En1 expression domain in alar (arrowhead) and basal plate (arrow). (B) Pax6 is expressed in the alar plate of the diencephalon. Neurones are located both in the Pax6-positive alar plate (filled arrowhead) and in the corresponding basal plate (arrow). Some neurones are just caudal to the Pax6 expression domain (open arrowheads). ap; alar plate; bp, basal plate; di, diencephalon; fp, floor plate; mes, mesencephalon; os, optic stalk; rh, rhombencephalon.
Fig. 6.
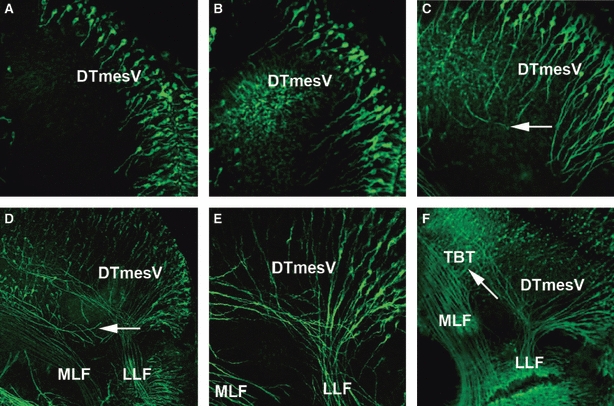
Formation of the DTmesV in the dorsal mesencephalon. (A) HH14: neurones have differentiated along the dorsal midline of the mesencephalon and have begun projecting their axons ventrally into the mesencephalon. (B) HH15: the axons of the DTmesV have projected further into the alar plate of the mesencephalon. (C) HH16: DTmesV axons have started turning caudally as indicated with arrow. (D) HH17: the caudally projecting DTmesV axons have formed a dense bundle (LLF) in the alar plate. Some MLF axons project from the MLF towards the DTmesV (arrow). (E) HH17: high magnification of the MRB, showing the LLF projecting as a compact bundle into the rhombencephalon. (F) HH18: LLF and MLF are well separated, and no axons crossing between them are detected. In the mesencephalon, tecto-bulbar axons project perpendicular to LLF and MLF towards the ventral midline (arrow). DTmesV, descending tract of the mesencephalic nucleus of the trigeminal nerve; LLF, lateral longitudinal fascicle; MLF, medial longitudinal fascicle; TBT, tectobulbar tract.
Fig. 4.
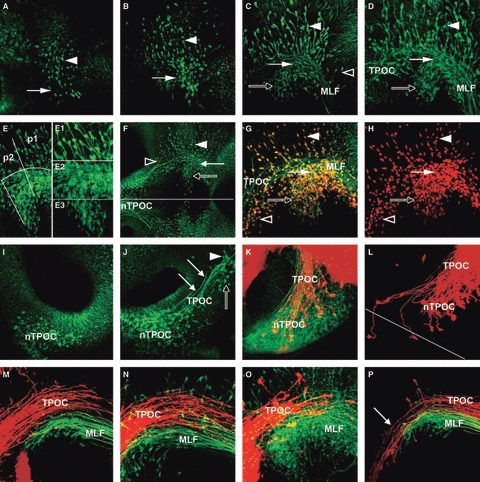
Development of the VLT. (A–D) Formation of the MLF axon tract. (A) HH11: first neurones are detectable in the alar plate (arrowhead) and basal plate (arrow) of the caudal diencephalon. (B) HH13: the number of neurones in the diencephalon has increased and some basal neurones have begun projecting axons caudally (arrow). (C) HH14: neurones in alar plate (arrowhead) and basal plate (arrows) of the diencephalon project axons caudally into the MLF, which runs along the floor of the mesencephalon. Some scattered neurones in the basal mesencephalon also project into the VLT (open arrowhead). (D) HH15: the MLF neurones are organised into three distinct populations in the alar plate (dorsal population, arrowhead), in line with the MLF (central, filled arrow) and ventral to the MLF (rostroventral, open arrow). (E) Detail of the three MLF neurone populations in a HH15 chick embryo. The alar population is spread across p1 and p2. In the basal plate, the central population is mainly located in p1, while the rostroventral population is in p2. E1, E2 and E3 are higher magnifications of each neurone population. (E1) The dorsal neurones have large cell bodies and are dispersed across the alar plate. They initially project their axons ventrally before turning caudally. (E2) The central population of neurones is very dense and intermingled with the MLF axons. (E3) Neurones in the rostroventral population have smaller cell bodies. They project axons first at an angle dorsal-caudally before joining the MLF. (F) Overview of the longitudinal tract in a HH15 chick embryo. TPOC (open arrowhead) and MLF form a seemingly continuous tract system running along the basal plate of the prosomeres and the mesencephalon. The three populations of MLF neurones are indicated (filled arrowhead: dorsal population; filled arrow: central population; open arrow: rostroventral population). Line indicates ventral midline. (G,H) The developing MTT in HH16 chick embryo. Double-labelling of the early axon scaffold with Tuj1 (green) and HuC/D (red). MTT neurones (open arrowhead) are located rostral to the MLF neurones project their axons caudally with the TPOC axon tract. The three populations of MLF neurones are indicated (filled arrowhead: dorsal population; filled arrow: central population; open arrow: rostroventral population). (H) Only the red HuC/D staining labelling only the neuronal cell bodies. A neurone-free gap separates the MTT neurones (open arrowhead) from the most rostral MLF neurones in p2 (open arrowhead). (I–P) Formation of the TPOC axon tract. (I) HH13: the first TPOC neurones differentiate in the basal plate of the rostral secondary prosencephalon, ventral to the optic stalk. (J) HH15: the TPOC neurones have started to project caudally towards the DMB (arrows). The TPOC neurones are clearly separated from the MTT neurones in the caudal secondary prosencephalon (open arrow). There are also neurones located rostral to the nMLF, which are projecting axons back towards the nTPOC (arrowhead). (K) HH17: retrograde labelling of the TPOC (DiI, red) combined with Tuj1 staining (green). The nTPOC is now a very dense population of neurones. Retrograde labelled neurones are scattered across the whole population of neurones. (L) HH18: retrograde labelling of TPOC axons from the mesencephalon. Most of the labelled neurones are densely packed in the ipsilateral nTPOC, but some contralateral neurones are also labelled. Line indicates anterior midline. (M–P) Relative location of MLF and TPOC. (M) HH17; (N) HH18. Retrograde labelling of the MLF (DiO, green) and anterograde labelling of the TPOC (DiI, red). The two axon tracts are running close together, but the TPOC axons are projecting just dorsal to the MLF. (O) HH17: DiI labelling of the TPOC (DiI, red) combined with Tuj1 (green) staining. The TPOC axons project well dorsal to the rostroventral population of MLF neurones and also dorsal to most of the central population. (P) HH19: retrograde labelling of TPOC (DiI, red) and MLF (DiO, green) from the rostral rhombencephalon. Both dyes were injected into the basal plate, but DiO was injected slightly more ventrally. The DiI-labelled axons can be traced back to the rostral hypothalamus (arrow), while DiO labelling depicts the three populations of MLF neurones, indicating that TPOC (dorsal) and MLF (ventral) axons remain mostly separated also in their caudal projection. MLF, medial longitudinal fascicle; nTPOC, nucleus of the TPOC; p1, prosomere 1; p2, prosomere 2; TPOC, tract of the postoptic commissure.
Fig. 5.
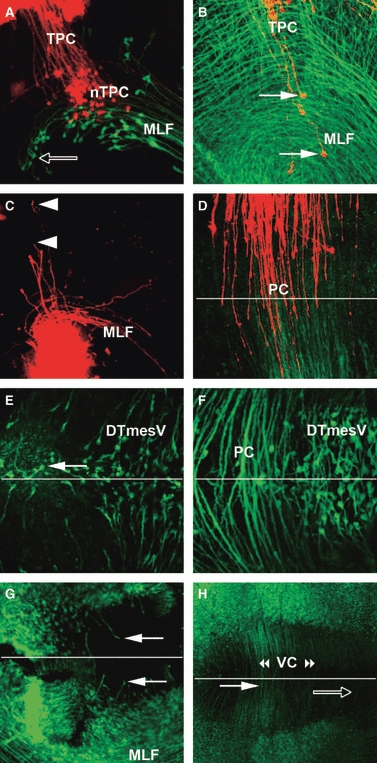
Development of TPC and VC. (A) HH19: retrograde labelling of the TPC (DiI, red) and the MLF (DiO, green). The TPC neurones are located ventrally within the MLF axon tract, interspersed with the dorsal and central populations of MLF neurones in p1. They project their axons dorsally towards the dorsal midline. Arrow labels the rostroventral population of MLF neurones in basal p2. (B) HH18: retrograde labelling of TPC neurones (DiI, red) combined with Tuj1 staining (green). Arrows point to TPC neurones that are located within the MLF axon tract and project axons dorsally. (C) HH18: anterograde labelling of the TPC from the ventral p1. Growth cones can clearly be seen projecting axons dorsally towards the dorsal midline (arrowheads). Some MLF axons have also been labelled as the nTPC and nMLF are in very close association with each other. (D–F) Dorsal view of the dorsal midline of p1. (D) HH22: TPC axons from right (DiO, green) and left side (DiI, red) were anterograde labelled from ventral p1. The TPC axons have reached the midline (indicated with line) and indeed crossed to the other side of the brain. (E) HH16: Tuj1 staining shows a small number of neurones at the dorsal midline projecting axons across the dorsal midline (arrow). (F) HH21: the PC is well formed and many axons have crossed the dorsal midline. Only few neurones are located at the dorsal midline of p1, in contrast to the dense population of DTmesV neurones in the neighbouring mesencephalon. (G, H) Open book, flat-mount preparations of ventral brains stained with Tuj1. (G) HH17: the first axons projecting into the floor plate of the mesencephalon are visible (arrows), pioneering the VC. (H) HH21: many axons have crossed the ventral midline of diencephalon (filled arrow) and mesencephalon (open arrow), forming the VC. DTmesV, descending tract of the mesencephalic nucleus of the trigeminal nerve; MLF, medial longitudinal fascicle; nTPC, nucleus of the tract of the posterior commissure; PC, posterior commissure; TPC, tract of the posterior commissure; VC, ventral commissure.
Development of the MLF
The MLF has been recognised in previous studies as the earliest tract forming in the embryonic chick brain (Mesdag, 1909; Tello, 1923; Windle & Austin, 1936; Lyser, 1966; Bösel, 1974; Chédotal et al. 1995), but there is some discrepancy about when the first neurones appear (reviewed in Puelles et al. 1987). The neurones contributing to the MLF, classically known as forming the ‘interstitial nucleus of Cajal’ (Ramón y Cajal, 1911), are poorly characterised. Also, an additional group of neurones located just rostral to the retroflex tract in p2 has been described as a pre-oculomotor centre in adult mammals, and was termed the ‘rostral interstitial nucleus of the MLF’ (Büttner-Ennever & Büttner, 1978), but its origin during development is unknown. We therefore analysed the formation of the MLF in detail. The first MLF neurones were detected at HH11 (Figs 1B and 4A). To determine their position with respect to the neuromeres, we combined immunohistochemistry for the neurones and in situ hybridisation for the mesencephalic marker En1 (Araki & Nakamura, 1999) and the diencephalic marker Pax6 (Matsunaga et al. 2000). The neurones were located rostral to the mesencephalic En1 expression domain (Fig. 3A), within the Pax6-positive alar plate (Fig. 3B, black arrowhead) and the basal plate (Fig. 3B, arrow) of the caudal diencephalon. At this stage, the DMB is already molecularly defined, while the prosomere boundaries in the diencephalon are not yet apparent (Ferran et al. 2007).
The first neurones were just scattered across the diencephalon (Fig. 4A), but shortly thereafter the MLF neurones appeared organised into three separate populations that occupy different positions (topographically central, dorsal and rostroventral), have different morphologies and different projection patterns. At HH13, the central cell group is aligned with the MLF within the basal plate of p1 (Fig. 4B, arrow), whereas the dorsal group lies in the overlying alar plate (Fig. 4B, arrowhead). The more densely arranged rostroventral population became distinctly visible at HH14 and lies within the basal plate of p2 (Fig. 4C, open arrow). The distinction between the two basal cell groups appears after the definition of the p1/p2 boundary (Ferran et al. 2007), though it is noteworthy that the boundary between the cell groups is diffuse at this early stage. Apart from the diencephalic neurones, a few scattered neurones in the basal mesencephalon also projected axons into the path of the MLF (Fig. 4C, open arrowhead).
By HH15 the MLF axons formed a tight bundle that extended close to the midline (Fig. 4D), projecting caudally into the rhombencephalon. At this stage, the three populations of MLF neurones were well defined (Fig. 4E). The population of neurones dorsal to the MLF axon tract (Fig. 4E1) were dispersed around the DMB and first projected their axons ventrally, before turning to project caudally into the MLF axon tract. The centrally located population of neurones (Fig. 4E2) sent their axons directly caudal into the MLF axon tract. The population of neurones ventral to the MLF (Fig. 4E3) first projected their axons dorsally, before turning and projecting caudally in the MLF axon tract with the rest of the MLF axons. At HH18, the MLF axon tract had projected far into the rhombencephalon, and the number of neurones continued to increase (Fig. 1I). By then the number of neurones and axons in the basal pretectum made it difficult to distinguish individual cell groups.
Development of the TPOC
The TPOC is the most prominent tract in the early rostral brain and is a major contributor to the VLT, yet in amniotes it is surprisingly little characterised. The first TPOC neurones were present in the rostral basal hypothalamus (retrochiasmatic area) at HH13 (Figs 1D and 4I). The TPOC axons began projecting caudally at HH14 (Fig. 1E), and by HH15 had grown through the prosencephalon (Fig. 4J, arrows). Interestingly, at this stage there were also alar p3 neurones visible that projected their axons rostrally using the TPOC axon tract (Fig. 4J, arrowhead). By HH17, the nucleus of the TPOC consisted of a very dense population of neurones, but only few of these neurones were retrogradely labelled from the pretectum (Fig. 4K). When the TPOC axons were labelled with DiI at HH18 (Fig. 4L), two contralateral neurones were labelled in addition to the large population of ipsilateral neurones. Despite these isolated axons crossing the midline before following the contralateral TPOC, there was no indication of a well-defined commissure at this stage.
The TPOC reached the DMB at HH16, but its course thereafter was obscured due to the large number of MLF and TPOC axons coursing the ventral mesencephalon at later stages. Hence, we applied retrograde and anterograde labelling to characterise the projection pattern of the TPOC and MLF in more detail. When the TPOC was anterograde labelled from the hypothalamus, and the MLF retrograde labelled from the ventral rhombencephalon, the TPOC axons were located dorsal to the MLF axons in the mesencephalon at HH17 (Fig. 4M) and HH18 (Fig. 4N). This preferential course of TPOC axons dorsal to the MLF was confirmed by retrograde labelling of both tracts from the rhombencephalon. In this case, DiI was applied slightly more dorsal than DiO. As a result, the dorsal, DiI-labelled axons could be traced back into the hypothalamus, while the ventral, DiO-labelled axons originated from neurones located in the caudal prosomeres (Fig. 4P). However, it is noteworthy that MLF and TPOC axons together form the VLT, which is well separated from the LLF.
Development of the MTT
At HH15, the VLT that runs along the prosencephalon and mesencephalon basal plate and extends caudally into the spinal cord was already visible (Fig. 4F). While its main contributors are the MLF and the TPOC, a separate group of neurones located in the caudal hypothalamus became apparent at HH16. These neurones projected their axons first dorsally, then caudally to join the VLT (Fig. 4G, open arrowhead). Location and course of the axons suggest this is the incipient MTT. While the axons take a course that seems continuous with the MLF, immunostaining of the neuronal cell bodies with anti-HuC/D demonstrated that the MTT neurones are located rostral to the MLF neurones in p1 and p2, and are well separated from them by a gap corresponding to basal p3 (Fig. 4G,H).
Development of the TPC
While the longitudinal tracts are prominent features of the early chick brain, the transversal TPC was not clearly visible in the overview images (Fig. 1), although it could be detected at higher magnification from HH18 (data not shown). However, the density of tracts and neurones at this stage made it difficult to identify the TPC neurones. Therefore, DiI and DiO were used to specifically label the TPC and MLF. When DiI was applied at the alar plate of the caudal pretectum, labelled TPC axons could be traced back to ipsilateral neurones in a ventral area rostral to the DMB, largely within p1 (pretectum). These neurones were located within the MLF axon tract (Fig. 5A,B), intermingled with the central and dorsal populations of MLF neurones. Conversely, when DiI was injected at the basal plate of the caudal pretectum, anterograde labelled axons projected dorsally into the alar plate (Fig. 5C), while notably no retrograde labelled neurones were detected in the alar plate at this stage. In contrast, immunolabelling with Tuj1 revealed neurones at the dorsal midline of the pretectum already at HH16 (Fig. 5E). While no crossing axons could be detected at this stage, the PC was formed across the whole pretectal roof plate by HH21. At this stage, anterograde labelling of TPC axons from the basal plate of the pretectum using DiI and DiO on the opposite sides of the brain showed the axons crossing at the dorsal midline (Fig. 5D). The axons crossing the pretectal midline stopped caudally precisely at the DMB (Fig. 3D,F; see also Ferran et al. 2007).
Axons crossing the ventral midline
In anamniotes like zebrafish, axons crossing the ventral midline in the neighbourhood of the DMB have been described to form a distinct ventral commissure, the VC (e.g. Chitnis & Kuwada, 1990). Using immunostaining, in the rostral chick brain the first axons projecting towards the ventral midline were detected at HH17, located in the mesencephalon tegmentum (Fig. 5G). At later stages, many axons crossed the ventral midline of the pretectum and the mesencephalon (Fig. 5H). Some of these, presumably those located more caudally (Fig. 5H, open arrow), are likely to be the crossed tecto-bulbar axons (incipient dorsal tegmental decussation), though another possibility is represented by the crossing axons of the rubrospinal tract, coming from the adjacent magnocellular red nucleus (these subsequently form the ventral tegmental decussation).
Development of the mesencephalic tract of the trigeminus
Unlike most anamniotes studied so far, in amniotes a significant feature of the early dorsal mesencephalon is the DTmesV (Easter et al. 1993). In the chick, the first DTmesV neurones appeared towards the end of HH13. The neurones lined up along the dorsal midline of the mesencephalon and began projecting arciform/circumferential axons ventrally at HH14 (Fig. 6A). They continued growing transversally through the alar plate until HH16, when they started turning caudally and fasciculating together at a specific alar plate location (Fig. 6C, arrow), thus pioneering the LLF (Fig. 6D,E). The DTmesV fibres projected into the rhombencephalon, where they eventually bifurcate into a dendritic branch that grows peripherally through the trigeminal nerve, targeting muscular propioceptive sensors, and the axon, which supposedly connects with diverse brainstem motor nuclei, including the trigeminal motor nucleus. At later stages the DTmesV axons were joined in the alar plate by the tecto-bulbar axons that projected contralaterally across the ventral midline (Fig. 6F, arrow).
Because for mouse it has been reported that the TPOC joined the LLF in the mesencephalon (Mastick & Easter, 1996), we investigated the spatial relation of the ventral and lateral longitudinal tracts in more detail. In some HH16 and HH17 embryos there were stray axons projecting out of the VLT towards the LLF (Fig. 6D, arrow), but by HH18 the two tracts were well separated (Fig. 6E).
Discussion
This paper has described the formation of the first neurones and axon tracts in the early embryonic chick brain during the first 4 days of incubation. At the end of this period, the early axon scaffold is fully established. In line with previous studies (Windle & Austin, 1936; Lyser, 1966; Chédotal et al. 1995), three tract systems are clearly distinguishable in the chick: in the basal plate a VLT comprising the TPOC, MTT and MLF; in the alar plate the DTmesV forming the lateral longitudinal tract; and rostral to the DMB, the pretectal transversal TPC, whose fibres also contribute to the longitudinal pathway as they reach the basal plate (Diaz et al. 1999). The basic organisation of the initial tracts is similar to that described for the mouse (Easter et al. 1993; Mastick & Easter, 1996), though the tracts are more clearly distinguished in the chick brain. Similar to anamniotes (Hjorth & Key, 2002), the axon tracts originate at distinct clusters of basal neurones (as first concluded by Puelles et al. 1987). Our findings are consistent with the neuromeric model, in particular characterising MLF, MTT and TPOC as part of the VLT system traversing the basal plate from the rostral prosencephalon into rhombencephalon and spinal cord.
Ventral longitudinal tracts
The most prominent longitudinal fibre system in the early vertebrate brain is the VLT that eventually runs from the prosencephalon to the spinal cord, and assembles a wide range of ascending and descending fibres. This is consistently pioneered by two tracts of the early axon scaffold, MLF and TPOC. Here, we demonstrate that in the chick the first MLF neurones emerge even earlier than described in some previous studies (Windle & Austin, 1936; Lyser, 1966; Chédotal et al. 1995): neurones in p1/p2 are detectable using the Tuj1 antibody already at HH11 (Fig. 7A), consistent with the identification of early neurones by acetylcholinesterase activity (Puelles et al. 1987). The MLF neurones are likely to correspond to the neurones unprecisely reported as lying ‘at the di-mesencephalic boundary’ that supposedly exit the cell cycle already at HH5 (McConnell & Sechrist, 1980; see critical comments about this analysis in Puelles et al. 1987, who concluded that birthdates occur closer to the moment in which the neurones become stained with early differentiation markers). These neurones are the first neurones differentiating in the rostral chick brain (Easter et al. 1994; Chédotal et al. 1995). This is in contrast to the mouse, where the DTmesV is the first tract to appear (Easter et al. 1993), but is in line with findings in anamniote species (e.g. Chitnis & Kuwada, 1990; Wilson et al. 1990). Our immunolabelling revealed two distinct basal populations of p1 and p2 neurones all contributing axons to the MLF, plus associated alar plate cells at both loci that apparently send their axons also into the MLF as well (Fig. 7C–E; alar p3 neurones tend to project rostralwards along the TPOC). The distribution of MLF neurones across p1 and p2 was also observed by Larsen et al. (2001), and their distinction into separate groups has already been noted by Puelles et al. (1987), who associated neurones in the pretectal tegmentum (p1) with the area nucleus fasciculi longitudinalis medialis (aflm), the basal thalamic tegmentum (p2) with the area tuberculi posterioris (atp), and cells in the p1 alar plate as the area commissuralis (acom). Based on the position of these areas, the rostroventral MLF neurones described in our study correspond to the atp (p2 tegmentum), possibly equivalent to the so-called ‘rostral interstitial MLF nucleus’ (Büttner-Ennever & Büttner, 1978), and the central neurones correspond to the aflm (p1 tegmentum), or classic interstitial MLF nucleus of Cajal (Ramón y Cajal, 1911). The dorsal population lies in the acom, which also contains neurones contributing to the TPC (Fig. 7E, and see below). Chédotal et al. (1995) indeed described the central population as the interstitial nucleus of Cajal, and referred to the dorsal population as the neurones of the thalamo-tegmental tract (adopting the nomenclature used by Windle & Austin, 1936). Interestingly, distinct nuclei contributing to the MLF have also been described by Keene & Hewer (1933) in the human embryos. They distinguished four different nuclei, of which their ‘nucleus 2’ appears to correspond to our central population, ‘nucleus 3’ to the dorsal group and ‘nucleus 4’ to the rostroventral neurones. While the majority of axons in the MLF originate from neurones in the diencephalon, some scattered neurones in the basal mesencephalon, presumably are part of the reticular formation, also send their axons ipsilaterally into the VLT (see also Schubert & Lumsden, 2005; Ahsan et al. 2007).
The MLF neurones predate the differentiation of TPOC neurones in the rostral basal hypothalamus. Our results show the first TPOC neurones appearing at HH13 (Fig. 7B), consistent with the previous description of neurones in the area retrochiasmatica at this stage (Puelles et al. 1987). The differentiation of neurones in the juxta-optic region, likely to be the TPOC, was mentioned by Lyser (1966) at the 21-somite stage (HH14). Similar to the MLF, the TPOC axons project caudad, along the dorsal part of the VLT (Fig. 7C–E). There has been some debate in the literature whether MLF and TPOC form a continuous VLT system, particularly as it was not clear how far into the mesencephalon the TPOC axons project, and whether caudally they join the MLF or rather the LLF pioneered by the DTmesV axons. Our results indicate that by stage 19 at the latest TPOC axons project beyond the DMB to reach the MRB. Across the ventral mesencephalon, MLF axons favour a ventral path close to the floor plate, while the TPOC axons follow a slightly more dorsal, but adjacent path (Fig. 7E). Thus, in the chick the TPOC axons are well separated from the DTmesV or the LLF.
In mouse a further contribution to the ventral longitudinal fibre system is made by neurones in the caudal hypothalamus that form the MTT (Easter et al. 1993; Mastick & Easter, 1996). The MTT was also identified in the chick by Windle & Austin (1936). They observed the tract first at HH19, while our study demonstrates MTT neurones already at HH16 (Fig. 7D), consistent with the appearance of the acetylcholinesterase-positive neurones in the area mammillaris lateralis roughly at this stage (Puelles et al. 1987).
Decussations and commissures
Commissures are a main feature of the early axon scaffold in anamniotes. In zebrafish, four early-formed commissures have been described: AC and POC in the rostral prosencephalon; PC in the pretectum, in front of the DMB; and ventral tegmental commissure at the floor of pretectum (p1) and mesencephalon (Chitnis & Kuwada, 1990; Wilson et al. 1990). The appearance of the major brain commissures during embryonic development has not been studied in much detail in the chick. In anamniotes, axons from the rostral basal hypothalamus project contralaterally to form the POC (Chitnis & Kuwada, 1990; Wilson et al. 1990). Similar to mouse (Easter et al. 1993), we did not observe a commissure in the rostral prosencephalon of the early chick embryos, though consistent with Windle & Austin’ observations (1936) axon tracing in this study revealed some axons tentatively crossing the midline. Crossing fibres of AC and supraoptic decussation (the avian counterpart to the POC) have been reported in E4 chick embryos (Windle & Austin, 1936), indicating that their development appears to be just delayed in chick compared with anamniotes.
In contrast to the rostral commissures, the PC forms part of the early axon scaffold also in mouse (Easter et al. 1993) and chick (Windle & Austin, 1936; Chédotal et al. 1995; this study). We found the first axons crossing the dorsal midline of the pretectum in chick at HH16 (Fig. 7D). These axons originate from neurones located dorsally, close to the midline. However, axon tracing revealed that a major contribution to the later TPC originates from neurones in the ventral pretectum (Fig. 7E). These neurones are interspersed with the dorsal population of MLF neurones, but project their axons dorsally to cross the midline. Their location corresponds to the acom, where neurones have been found to differentiate at HH16 (Puelles et al. 1987). The close proximity of MLF and TPC neurones has also been reported in human embryos (Keene & Hewer, 1933; ‘nucleus 1’). Projections into the TPC, but also the MLF, have been attributed to the nucleus of Darkschewitsch, which lies across pretectum and rostral mesencephalon (Luis Puelles, personal communication). TPC neurones have also been described to form separate parvocellular and magnocellular interstitial nuclei of the PC at later stages (Ferran et al. 2009). The exact relationship of the early differentiating neurones in the ventral alar plate to the adult alar plate nuclei is unclear.
In zebrafish (Chitnis & Kuwada, 1990; Wilson et al. 1990) and other anamniotes, as well as in mammals (Meynert's decussation and Forel's decussation formed by tectobulbar/tectospinal and rubrospinal axons, respectively), distinct ventral tegmental decussations are formed across the caudal diencephalic and mesencephalon floor plate. In the chick we identified axons projecting towards the ventral midline of the mesencephalon at HH17, and by HH21 a large number of axons have crossed. Some of these axons diverge from the VLT system, or originate presumptively from the tegmental rubrospinal magnocellular neurones, while others originate in the tectum. The former represent the ventral tegmental decussation of the mesencephalon, whereas the latter represent the dorsal tegmental decussation of the crossed tectobulbar and tectospinal axons (see below). There is no information about the axonal contingents crossing in the pretectal ventral decussation, though presumably part of them arises in the alar plate, like those in the mesencephalon. The axons crossing the ventral midline in this general area are numerous but, in contrast to anamniotes and mammals, they do not form a compact commissure and instead are spread over the floor of pretectum and mesencephalon.
Dorsal axons in the mesencephalon
In zebrafish (Chitnis & Kuwada, 1990; Wilson et al. 1990) and other anamniotes, the dorsal mesencephalon is initially devoid of neurones. In contrast, neurones in the mesencephalic trigeminal nucleus are the first neurones to differentiate in the rostral mouse brain (Easter et al. 1993). Lyser (1966) described neurones beginning to differentiate along the dorsal midline of the mesencephalon in HH13-chick embryos. We could detect neurones in the dorsal mesencephalon first at HH14 (Fig. 7C), similar to other studies (Puelles et al. 1987; Chédotal et al. 1995). Windle & Austin (1936) observed mesencephalic axons at about HH15. They referred to these axons as part of the tectobulbar and tectospinal tracts, but the position of the neurones along the dorsal midline and their axon projection pattern rather identify them as cells of the mesencephalic trigeminal nucleus (Hunter et al. 2001). The development of the DTmesV at early embryonic stages interestingly has also been described in some anamniotes, including medaka (Ishikawa et al. 2004) and cat shark (Kuratani & Horigome, 2000), but not in zebrafish (Chitnis & Kuwada, 1990; Wilson et al. 1990), turbot (Doldan et al. 2000) or Xenopus (Hartenstein, 1993). However, at adult stages the DTmesV has long been recognised as a common feature of all gnathostomes (Weinberg, 1928). In Amblystoma the DTmesV is absent at early stages but could be seen in early swimming stages (Herrick, 1937), indicating heterochrony of DTmesV formation in different species.
We could identify tectobulbar axons at HH18 (Fig. 7E). These axons, while originating in the dorsal mesencephalon and growing ventrally, take a different path compared with the DTmesV axons: the tectobulbar axons grow circumferentially to the ventral midline, while the DTmesV axons grow dorsoventrally only until they reach the LLF. Moreover, many of the tectobulbar axons cross the midline before joining the DTmesV axons in the contralateral LLF (Kröger & Schwarz, 1990).
The early axon scaffold in chick and other vertebrates
Our description of the early neurones and tracts in the embryonic chick brain complements previous work on axon tract development (Tello, 1923; Windle & Austin, 1936; Lyser, 1966; Bösel, 1974; Chédotal et al. 1995) and early neuronal differentiation (McConnell & Sechrist, 1980; Puelles et al. 1987) in the chick, and investigations into the early axon scaffold of other vertebrates (Herrick, 1937; Chitnis & Kuwada, 1990; Wilson et al. 1990; Easter et al. 1993; Hartenstein, 1993; Mastick & Easter, 1996; Doldan et al. 2000; Kuratani & Horigome, 2000; Ishikawa et al. 2004; Barreiro-Iglesias et al. 2008). Similar to mouse, the first neurones in the chick brain are located in three distinct locations: ventral pretectum, rostral hypothalamus and dorsal mesencephalon. Clustering of early neurones has also been found in zebrafish (reviewed in Hjorth & Key, 2002). Two of the zebrafish clusters have counterparts in the chick: the ventrocaudal cluster (vcc) corresponds to the ventral pretectal group and gives rise to MLF and TPC, while the ventrorostral cluster (vrc) is the equivalent of the TPOC neurones in the rostral hypothalamus. No match for the zebrafish dorsorostral cluster (drc) could be found in the early chick brain. Equally, the early zebrafish brain is lacking the dorsal mesencephalic neurones that are prominent in the chick.
We have identified five distinct tracts (MLF, TPOC, DTmesV/LLF, MTT, TPC) and the diffuse VC in the early chick brain. This arrangement is similar to mouse, but different from zebrafish where eight tracts form the early axon scaffold (reviewed in Hjorth & Key, 2002). However, only four of these tracts (MLF, TPOC, TPC and VC) are also found in the early amniote brain. Among the remaining four tracts, AC and POC will form later in amniotes. The amniote equivalent of the zebrafish supraoptic tract (or telencephalic tract) is the cerebral peduncle, or its earliest component, possibly forming part of the medial prosencephalon bundle. It is unclear if a tract corresponding to the zebrafish DVDT exists in amniotes, though scattered neurones in similar position are present in mouse and chick within dorsocaudal parts of the p2 alar plate, representing the origin of the fasciculus retroflexus (habenulo-interpeduncular tract). This tract is known to course dispersedly – analogously to DTmesV – within the frog thalamus (Puelles et al. 1996) and likewise in the chick (Huber & Crosby, 1929), maybe giving rise to the myth that it is not present in these species; however, the main target, the interpeduncular nucleus, is constant in all vertebrates. The DTmesV, prominent in the early amniote brain, is missing in most anamniotes at early stages but will develop later. Thus, despite differences in the developmental timing of early tract formation, the basic organisation of the initial tracts is remarkably well conserved among vertebrates. In particular, the most basic elements of the early axon scaffold, the two basal longitudinal tracts (MLF and TPOC), the TPC and the VC, are a common feature of the early brain in all vertebrates analysed to date.
Although the early axon scaffold is a basic feature of vertebrate brain development, we know remarkably little about the genetic programme governing its formation. Most molecular studies have focussed on the guidance of the early axons (e.g. Anderson & Key, 1999; Hjorth & Key, 2001; Molle et al. 2004; Devine & Key, 2008; Farmer et al. 2008; Riley et al. 2010), rather than the specification and differentiation of the neurones. Signalling from the prechordal plate influences the size of the vcc in zebrafish (Tallafuss et al. 2003), while in chick the homeobox gene Sax1 has been implicated with the development of the MLF (Schubert & Lumsden, 2005). Moreover, several homeobox genes have been found to be expressed in specific longitudinal domains in the basal mesencephalon and diencephalon at later stages (e.g. Sanders et al. 2002; Ahsan et al. 2007). However, in contrast to intermediate and advanced stages of brain development (e.g. Ferran et al. 2007, 2009), a detailed map of gene expression patterns correlated with neurone identity is missing for the early brain. The characterisation of the chick early axon scaffold presented here provides a framework to map and interpret gene expression data in the future.
Acknowledgments
The authors gratefully acknowledge the funding of this study by a studentship to M.W. by the Anatomical Society of Great Britain and Ireland. In addition, the study benefited from funding by the EU through the Interreg IV programme ‘Advanced Microscopy Network’ (AdMiN). The authors are indebted to Luis Puelles for his expert advice on vertebrate brain anatomy and the neuromeric model, and for his highly valuable comments on the manuscript.
References
- Ahsan M, Riley KL, Schubert FR. Molecular mechanisms in the formation of the medial longitudinal fascicle. J Anat. 2007;211:177–187. doi: 10.1111/j.1469-7580.2007.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RB, Key B. Novel guidance cues during neuronal pathfinding in the early scaffold of axon tracts in the rostral brain. Development. 1999;126:1859–1868. doi: 10.1242/dev.126.9.1859. [DOI] [PubMed] [Google Scholar]
- Araki I, Nakamura H. Engrailed defines the position of dorsal di-mesencephalic boundary by repressing diencephalic fate. Development. 1999;126:5127–5135. doi: 10.1242/dev.126.22.5127. [DOI] [PubMed] [Google Scholar]
- Bak M, Fraser SE. Axon fasciculation and differences in midline kinetics between pioneer and follower axons within commissural fascicles. Development. 2003;130:4999–5008. doi: 10.1242/dev.00713. [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Villar-Cheda B, Abalo XM, et al. The early scaffold of axon tracts in the brain of a primitive vertebrate, the sea lamprey. Brain Res Bull. 2008;75:42–52. doi: 10.1016/j.brainresbull.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Bösel R. Das erste Nervenfaserwachstum im Gehirn von Hühnerembryonen bis zum Stadium HH 17. J Hirnforsch. 1974;15:23–48. [PubMed] [Google Scholar]
- Büttner-Ennever JA, Büttner U. A cell group associated with vertical eye movements in the rostral mesencephalic reticular formation of the monkey. Brain Res. 1978;151:31–47. doi: 10.1016/0006-8993(78)90948-4. [DOI] [PubMed] [Google Scholar]
- Chédotal A, Pourquié O, Sotelo C. Initial tract formation in the brain of the chick embryo: selective expression of the BEN/SC1/DM-GRASP cell adhesion molecule. Eur J Neurosci. 1995;7:198–212. doi: 10.1111/j.1460-9568.1995.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Chitnis AB, Kuwada JY. Axonogenesis in the brain of zebrafish embryos. J Neurosci. 1990;10:1892–1905. doi: 10.1523/JNEUROSCI.10-06-01892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis AB, Kuwada JY. Elimination of a brain tract increases errors in pathfinding by follower growth cones in the zebrafish embryo. Neuron. 1991;7:277–285. doi: 10.1016/0896-6273(91)90266-3. [DOI] [PubMed] [Google Scholar]
- Devine CA, Key B. Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev Biol. 2008;313:371–383. doi: 10.1016/j.ydbio.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Diaz C, Perez-Santana L, Martinez-de-la-Torre M, et al. Diencephalic neuronal populations projecting axons into the basal plate in a lizard (Gallotia galloti) Eur J Morphol. 1999;37:130–133. doi: 10.1076/ejom.37.2.130.4734. [DOI] [PubMed] [Google Scholar]
- Doldan MJ, Prego B, Holmqvist B, et al. Emergence of axonal tracts in the developing brain of the turbot (Psetta maxima) Brain Behav Evol. 2000;56:300–309. doi: 10.1159/000047214. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Ross LS, Frankfurter A. Initial tract formation in the mouse brain. J Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter SS, Jr, Burrill J, Marcus RC, et al. Initial tract formation in the vertebrate brain. Prog Brain Res. 1994;102:79–93. doi: 10.1016/S0079-6123(08)60533-6. [DOI] [PubMed] [Google Scholar]
- Farmer WT, Altick AL, Nural HF, et al. Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. Development. 2008;135:3643–3653. doi: 10.1242/dev.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferran JL, Sanchez-Arrones L, Sandoval JE, et al. A model of early molecular regionalization in the chicken embryonic pretectum. J Comp Neurol. 2007;505:379–403. doi: 10.1002/cne.21493. [DOI] [PubMed] [Google Scholar]
- Ferran JL, de Oliveira ED, Merchan P, et al. Genoarchitectonic profile of developing nuclear groups in the chicken pretectum. J Comp Neurol. 2009;517:405–451. doi: 10.1002/cne.22115. [DOI] [PubMed] [Google Scholar]
- Gahtan E, Sankrithi N, Campos JB, et al. Evidence for a widespread brain stem escape network in larval zebrafish. J Neurophysiol. 2002;87:608–614. doi: 10.1152/jn.00596.2001. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Lumsden A, Gruss P. Signals from the notochord and floor plate regulate the region-specific expression of two Pax genes in the developing spinal cord. Development. 1993;117:1001–1016. doi: 10.1242/dev.117.3.1001. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hartenstein V. Early pattern of neuronal differentiation in the Xenopus embryonic brainstem and spinal cord. J Comp Neurol. 1993;328:213–231. doi: 10.1002/cne.903280205. [DOI] [PubMed] [Google Scholar]
- Herrick CJ. Development of the brain of Amblystoma in early functional stages. J Comp Neurol. 1937;67:381–422. [Google Scholar]
- Hjorth JT, Key B. Are pioneer axons guided by regulatory gene expression domains in the zebrafish forebrain? High-resolution analysis of the patterning of the zebrafish brain during axon tract formation. Dev Biol. 2001;229:271–286. doi: 10.1006/dbio.2000.9980. [DOI] [PubMed] [Google Scholar]
- Hjorth J, Key B. Development of axon pathways in the zebrafish central nervous system. Int J Dev Biol. 2002;46:609–619. [PubMed] [Google Scholar]
- Huber GC, Crosby EC. The nuclei and fiber paths of the avian diencephalon, with consideration of telencephalic and certain mesencephalic centers and connections. J Comp Neurol. 1929;48:1–225. [Google Scholar]
- Hunter E, Begbie J, Mason I, et al. Early development of the mesencephalic trigeminal nucleus. Dev Dyn. 2001;222:484–493. doi: 10.1002/dvdy.1197. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Kage T, Yamamoto N, et al. Axonogenesis in the medaka embryonic brain. J Comp Neurol. 2004;476:240–253. doi: 10.1002/cne.20220. [DOI] [PubMed] [Google Scholar]
- Keene MF, Hewer EE. The development and myelination of the posterior longitudinal bundle in the Human. J Anat. 1933;67:522–535. [PMC free article] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat. Rev. Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- Kröger S, Schwarz U. The avian tectobulbar tract: development, explant culture, and effects of antibodies on the pattern of neurite outgrowth. J Neurosci. 1990;10:3118–3134. doi: 10.1523/JNEUROSCI.10-09-03118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani S, Horigome N. Developmental morphology of branchiomeric nerves in a cat shark, Scyliorhinus torazame, with special reference to rhombomeres, cephalic mesoderm, and distribution patterns of cephalic crest cells. Zool Sci. 2000;17:893–909. [Google Scholar]
- Kuratani S, Horigome N, Ueki T, et al. Stereotyped axonal bundle formation and neuromeric patterns in embryos of a cyclostome, Lampetra japonica. J Comp Neurol. 1998;391:99–114. [PubMed] [Google Scholar]
- Larsen CW, Zeltser LM, Lumsden A. Boundary formation and compartition in the avian diencephalon. J Neurosci. 2001;21:4699–4711. doi: 10.1523/JNEUROSCI.21-13-04699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, et al. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Logan C, Hanks MC, Noble-Topham S, et al. Cloning and sequence comparison of the mouse, human, and chicken engrailed genes reveal potential functional domains and regulatory regions. Dev Genet. 1992;13:345–358. doi: 10.1002/dvg.1020130505. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Lyser KM. The development of the chick embryo diencephalon and mesencephalon during the initial phases of neuroblast differentiation. J Embryol Exp Morphol. 1966;16:497–517. [PubMed] [Google Scholar]
- Mastick GS, Easter SS., Jr Initial organization of neurons and tracts in the embryonic mouse fore- and midbrain. Dev Biol. 1996;173:79–94. doi: 10.1006/dbio.1996.0008. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Iwai L, Kawasaki H. Fluorescent double-labeling with carbocyanine neuronal tracing and immunohistochemistry using a cholesterol-specific detergent digitonin. J Neurosci Meth. 2008;174:71–81. doi: 10.1016/j.jneumeth.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Araki I, Nakamura H. Pax6 defines the di-mesencephalic boundary by repressing En1 and Pax2. Development. 2000;127:2357–2365. doi: 10.1242/dev.127.11.2357. [DOI] [PubMed] [Google Scholar]
- McConnell JA, Sechrist JW. Identification of early neurons in the brainstem and spinal cord: I. An autoradiographic study in the chick. J Comp Neurol. 1980;192:769–783. doi: 10.1002/cne.901920410. [DOI] [PubMed] [Google Scholar]
- Mesdag TM. Bijdrage tot de ontwikkelings-geschiedenis van de structuur der hersenen bij het kipembryo.) 1909. Groeningen.
- Molle KD, Chedotal A, Rao Y, et al. Local inhibition guides the trajectory of early longitudinal tracts in the developing chick brain. Mech Dev. 2004;121:143–156. doi: 10.1016/j.mod.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Pritz MB. Forebrain and midbrain fiber tract formation during early development in Alligator embryos. Brain Res. 2010;1313:34–44. doi: 10.1016/j.brainres.2009.11.081. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Puelles L, Amat JA, Martinez-de-la-Torre M. Segment-related, mosaic neurogenetic pattern in the forebrain and mesencephalon of early chick embryos: I. Topography of AChE-positive neuroblasts up to stage HH18. J Comp Neurol. 1987;266:247–268. doi: 10.1002/cne.902660210. [DOI] [PubMed] [Google Scholar]
- Puelles L, Javier Milan F, Martinez-de-la-Torre M. A segmental map of architectonic subdivisions in the diencephalon of the frog Rana perezi: acetylcholinesterase-histochemical observations. Brain Behav Evol. 1996;47:279–310. doi: 10.1159/000113247. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du Système Nerveux de l'Homme et des Vertébrés. 1911. A. Maloine, Paris.
- Riley KL, Gledhill S, Schubert FR. Early expression of axon guidance molecules in the embryonic chick mesencephalon and pretectum. Int J Dev Biol. 2010;54:743–753. doi: 10.1387/ijdb.082803kr. [DOI] [PubMed] [Google Scholar]
- Sanders TA, Lumsden A, Ragsdale CW. Arcuate plan of chick midbrain development. J Neurosci. 2002;22:10 742–10 750. doi: 10.1523/JNEUROSCI.22-24-10742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert FR, Lumsden A. Transcriptional control of early tract formation in the embryonic chick midbrain. Development. 2005;132:1785–1793. doi: 10.1242/dev.01731. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, et al. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Tallafuss A, Adolf B, Bally-Cuif L. Selective control of neuronal cluster size at the forebrain/midbrain boundary by signaling from the prechordal plate. Dev Dyn. 2003;227:524–535. doi: 10.1002/dvdy.10329. [DOI] [PubMed] [Google Scholar]
- Tello JF. Les différenciations neuronales dans l'embryon du poulet, pendant les premiers jours de l'incubation. Trab Lab Invest Biol Univ Madr. 1923;21:1–93. [Google Scholar]
- Weinberg E. The mesencephalic root of the fifth nerve. A comparative anatomical study. J Comp Neurol. 1928;46:249–405. [Google Scholar]
- Wilson SW, Ross LS, Parrett T, et al. The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development. 1990;108:121–145. doi: 10.1242/dev.108.1.121. [DOI] [PubMed] [Google Scholar]
- Windle WF, Austin MF. Neurofibrillar development in the central nervous system of chick embryos up to 5 days incubation. J Comp Neurol. 1936;63:431–463. [Google Scholar]
- Wullimann MF, Puelles L, Wicht H. Early postembryonic neural development in the zebrafish: a 3-D reconstruction of forebrain proliferation zones shows their relation to prosomeres. Eur J Morphol. 1999;37:117–121. doi: 10.1076/ejom.37.2.117.4739. [DOI] [PubMed] [Google Scholar]


