Abstract
Desmin is a member of the intermediate filaments, which play crucial roles in the maturation, maintenance and recovery of muscle fibers. Its expression has been examined in human cardiac muscle, rat and chicken, but its spatial distribution in the human fetal heart has not been described. The present study investigated desmin expression in the human fetal heart and associated great vessels in 14 mid-term fetuses from 9 to 18 weeks of gestation. Immunoreactivity for myosin heavy chain (MHC) and alpha smooth muscle actin (α-SMA), as well as neuron-specific enolase (NSE), was also examined. Increased expression of desmin from 9 to 18 weeks was clearly localized in the atrial wall, the proximal portions of the pulmonary vein and vena cava, and around the atrioventricular node. Desmin-positive structures were also positive for MHC. Meanwhile, the great vessels were also positive for α-SMA. The distribution of desmin exhibited a pattern quite different from that described in previous studies of rat and chicken. Thus, desmin in the human fetal heart does not seem to play a general role in myocardial differentiation but rather a specific role closely related to the maturation of the α-isozyme of MHC. Desmin expression in the developing fetal heart also appeared to be induced by mechanical stress due to the involvement of venous walls against the atrium.
Keywords: cardiac atrium, conduction system, desmin, human fetus, myosin heavy chain
Introduction
Desmin is a major component of the muscle intermediate filaments, which play a vital role in the maturation, maintenance and recovery of skeletal and cardiac muscle fibers by forming an interlinking scaffold around myofibrils connected to the sarcolemma and nuclear membrane (Goldfarb et al. 2008; Tidball, 1992). Desmin is mostly observed at myotendinous and neuromuscular junctions in normal adult skeletal muscles, and is crucial for architectural and functional integrity (Carlsson et al. 1999; Goldfarb et al. 2008; Abe et al. 2010).
Desmin has also been reported to have a critical function in cardiac muscle development at the embryonic stage. For instance, expression of desmin has been observed in the outflow tract in rat embryos and fetuses. Moreover, its expression in the ventricle becomes evident on day 10 of gestation, but then shows a decline in intensity on day 19 (Ya et al. 1997). A similar pattern has also been observed in rat fetal atrium, albeit slightly later than that in the ventricle. Localization of desmin has also been reported in the outflow tracts and atria of chick embryos at 4.5–6 days (Sumida et al. 1987).
The distribution pattern of myosin heavy chain (MHC) isozymes in the human fetal heart has already been described in detail. β-MHC is the predominant form in the ventricle and outflow tract, whereas α-MHC predominates in atria (Wessels et al. 1991). In tissue fragments obtained from the left ventricle of the human fetal heart, the relative density of desmin fluorescence progressively increased between 9 and 28 weeks (Kim, 1996). However, a study of cultured cardiac muscles from human fetuses between 8.5 and 14.8 weeks of gestation indicated that there is no difference in desmin content during this period (Torelli et al. 1999). These studies, however, did not describe the spatial distribution of desmin in the human fetal heart. It has been reported that the fetal atrial myocardium contains desmin-positive cells at 5–6 weeks (Hall et al. 2002). In addition, coronary artery, ductus arteriosus, and pulmonary artery and vein in human fetuses also express desmin (Johansson et al. 1999; Hall et al. 2000, 2002).
Difficulty in obtaining human fetal specimens is one of the reasons for the paucity of comprehensive studies of the spatial distribution of desmin in the human fetal heart. Moreover, desmin is probably not a prerequisite for myocardial differentiation, as desmin-deficient mice have been shown not to develop heart anomalies although cardiomyopathy was fostered after birth (Milner et al. 1999; Balogh et al. 2002; Weisleder et al. 2004a,b;). Under these circumstances, interest in the role of desmin in the early-stage human heart has diminished.
Recently, an increase in the expression of intermediate filaments was observed in response to physiological, pathological, mechanical or osmotic stress (Pekny & Lane, 2007). During fetal heart development, dynamic changes occur in the shape, size and topographical relationships of various structures, which might cause mechanical stress. In the present study, therefore, we investigated the spatial distribution of desmin expression in the human fetal heart.
Materials and methods
The present study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). Paraffin-embedded sections of 14 mid-term fetuses (10 males and four females) were used. The specimens were classified according to week of gestation based on craniorump length (CRL). Four specimens were classified as 9 weeks (with a CRL of 40 mm, 41 mm, 45 mm and 50 mm), four were classified as 12 weeks (with a CRL of 71 mm, 75 mm, 78 mm and 85 mm), four were classified as 15 weeks (with a CRL of 107 mm, 115 mm, 120 mm and 125 mm), and two were classified as 18 weeks (CRL 150 mm and 155 mm). All specimens were part of the archive collection at the Embryology Institute of Universidad Complutense, Madrid, and were the products of miscarriages and ectopic pregnancies managed at the Department of Obstetrics of the university. The university ethics committee granted approval for the study. Specimens were thoroughly examined to confirm that there was no pathological condition in the developing umbilical vessels, liver, intestine, adrenal or kidney. Gender was identified on the basis of the urogenital fold and the presence of the primitive uterus. The specimens had been fixed and stocked in neutralized formalin solution (10% v/v in aqua) for 3 months to 1 year.
After routine histological procedures for paraffin-embedding, horizontal or sagittal serial sections 5 μm thick were prepared. Some sections were stained with hematoxylin and eosin, and others were used for immunohistochemistry. The primary antibodies used were (i) mouse monoclonal anti-human desmin (1 : 50; Dako, Glostrup, Denmark), (ii) mouse monoclonal anti-human striated muscle MHC (1 : 100; Dako), (iii) mouse monoclonal anti-human alpha smooth muscle actin (α-SMA) (1 : 10; Dako) and (iv) rabbit polyclonal anti-human neuron-specific enolase (NSE) (1 : 100; Abcam, Cambridge, UK). Autoclave pretreatment was not conducted because of the loose nature of the fetal tissues. The secondary antibody (Dako Chem Mate Envision Kit; Dako) was labeled with horseradish peroxidase (HRP), and antigen-antibody reactions were detected via an HRP-catalyzed reaction with diaminobenzidine (DAB), followed by counterstaining with hematoxylin.
Results
Slight variations in the results of immunostaining were observed among specimens even at the same stage. Desmin immunoreactivity was restricted to the atrial walls, proximal parts of the pulmonary vein and superior vena cava, cavernous sinus, and the atrioventricular node or close by. All the figures presented here show the strongest case of the stage-group of immunoreactivity. Larger specimens appeared to have stronger immunoreactivity. In contrast to the atrium, with a smooth inner surface (parts derived from the venous wall), the auricle of the atrium was almost negative for desmin at 9 weeks (Fig. 1), weakly positive at 12 weeks (Fig. 2), and strongly positive at 15 and 18 weeks (Fig. 3). A valve-like structure between the right and left atria was also positive for desmin (Fig. 2B). The atrioventricular node, which was positive for desmin, was also positive for NSE (Fig. 3B insert). Moreover, part of the pulmonary vein located outside the pulmonary hilum was also desmin-positive (Figs 1A, 2C and 3A). The proximal part of the inferior vena cava, at a much higher level than the hepatic vein confluence, was also positive for desmin, as were the muscle layers of the esophagus (figure not shown). In contrast, the ventricle and great arteries such as the aorta, ductus arteriosus and pulmonary artery were negative for desmin. Slight and irregular staining in the ventricle appeared to be non-specific (Fig. 3A).
Fig. 1.
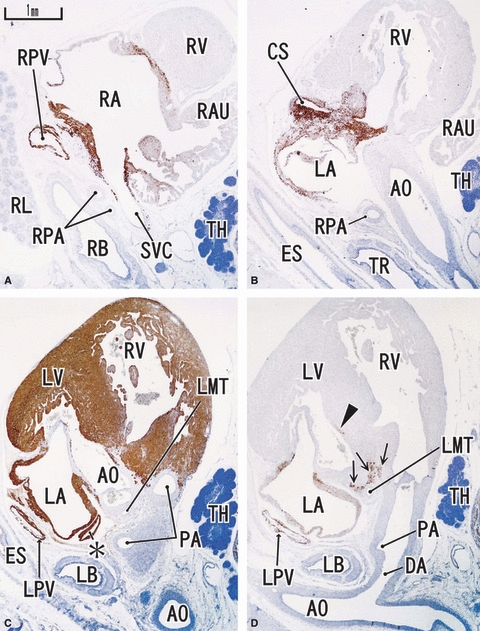
Sagittal sections of a 9-week human fetus. Panel A (D) is the most rightward (leftward) in the figure. Right side corresponds to the ventral side of the body. Intervals between panels are 0.6 mm (A–B), 0.5 mm (B–C) and 0.2 mm (C–D), respectively. Panels A, B and D represent desmin expression; panel C represents MHC. Desmin reactivity is seen along the atrium (LA, RA), proximal parts of the pulmonary vein (LPV, RPV), superior vena cava (SVC) and cavernous sinus (CS) at and around the atrioventricular node (arrows in panel D). The auricle is almost negative. MHC is positive in the ventricle as well as in the atrium. Asterisk in panel C indicates a protrusion of the left atrium. DA, ductus arteriosus (Botallo's duct). All panels were prepared at the same magnification (scale bar in panel A). TH, thymus.
Fig. 2.
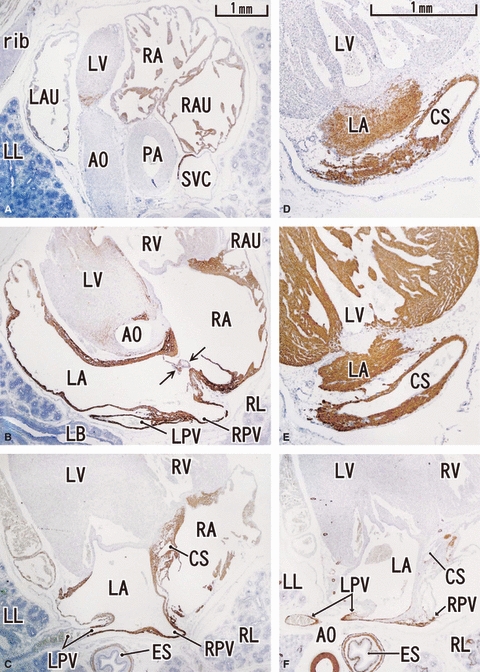
Horizontal sections of a 12-week human fetus. Panel A (F) is the most cranial (caudal) in the figure. Upper part corresponds to the ventral side of the body. Intervals between panels are 1.2 mm (A–B), 1.0 mm (B–C) and 1.2 mm (C–F), respectively. Panels D and E are magnified sections in the intermediate levels between panels C and F. Panels A–D represent desmin, panel E is MHC and panel F is α-SMA. Desmin expression is seen along the atrium (LA, RA), proximal parts of the pulmonary vein (LPV, RPV), superior vena cava (SVC) and the cavernous sinus (CS). The auricle is weakly positive. MHC (panel E) is positive in the ventricle as well as in the atrium. α-SMA (panel F) is positive along the vascular wall. Arrows in panel B indicate a valve-like structure between the atria. Panels A–C and F (D and E) were prepared at the same magnification (scale bars in panels A and D).
Fig. 3.
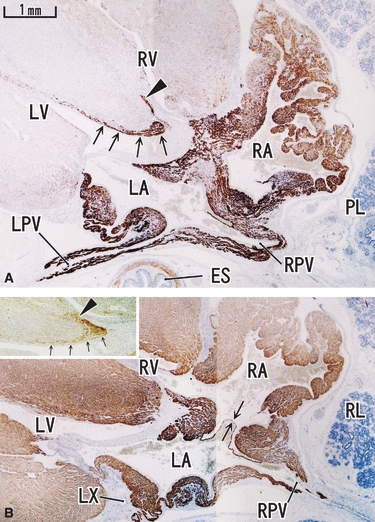
Horizontal section of an 18-week human fetus. Panel A (desmin) is located 5 mm superior to panel B (MHC). Upper part corresponds to the ventral side of the body. Proximal parts of the pulmonary vein (LPV, RPV) are positive for desmin, but the reactivity disappears suddenly in the distal parts (panel A). The auricle around the right atrium (RA) is also strongly positive for desmin. Arrows and arrowhead in panel A indicate the desmin-positive conduction system. These structures with labels are also seen in the insert in panel B (section near panel A is stained for NSE). Arrows in panel B indicate a valve-like structure between the atria. All panels and inserts were prepared at the same magnification (scale bar in panel A). LX, left circumferential branch of the coronary artery.
In contrast to the distribution of desmin, MHC was expressed in both the ventricle and atrium, but not in the walls of the great vessels (Figs 1C, 2E and 3B). The walls of the great vessels and the coronary artery were apparently negative for both desmin and MHC (Figs 1C and 3B). Otherwise, α-SMA was expressed in the great vessels and esophagus, but not in the myocardium (Fig. 2F). Thin vessels including the coronary artery, their endothelia, and smooth muscles were also positive for α-SMA.
Discussion
The present study has demonstrated the chronological changes in the spatial distribution of desmin in the human fetal heart. Desmin was previously considered to play a critical role in cardiac muscle development. The increased expression of desmin between 9 and 18 weeks suggested that it is probably involved in development of the human fetal heart, even though it was localized only in the atrium.
The chronological changes and distributions of both desmin and α-SMA during stages E10–20 in hearts of rat embryos and fetuses have been characterized previously. α-SMA was shown to be expressed in all parts of the heart and showed a peak at E13–14, whereas desmin expression appeared at E13–19 (Ya et al. 1997). However, the results of the present study of human fetal hearts showed that desmin was restricted to the atrium, and that α-SMA was absent. The present results also differed from those obtained in chick embryos. It is possible that desmin and α-SMA might have been expressed in the ventricle before 9 weeks, but materials at this stage were not included in the present study. However, not even rudimentary expression of desmin or α-SMA was detected in the ventricle at 9 weeks. The expression of these molecules has been shown not to disappear suddenly in rats (Ya et al. 1997). Also, Hall et al. (2000, 2002) mentioned that, in humans, the pulmonary artery and vein expressed desmin at 14 and 8 weeks of gestation, respectively. Likewise, Nanaev et al. (1991) observed strong expression of desmin in the walls of the femoral and brachial veins at 18–20 weeks of gestation, in contrast to the lack of concomitant reactivity in the walls of arteries. Thus, the expression of desmin appears to differ between the arterial and venous systems. In fact, in contrast to α-SMA and MHC, desmin expression was not observed during the initial stages of heart development in rats (Ya et al. 1997). Therefore, desmin is probably not essential during the early stage of human ventricular development, as no heart anomalies were found in desmin-deficient mutant mice (Milner et al. 1999; Balogh et al. 2002; Weisleder et al. 2004a,b;). Furthermore, in the human ventricle, desmin expression most likely commences after 18 weeks of gestation. These data indicate that the spatial distribution of desmin in the fetal heart differs considerably among animal species.
Cardiac muscles express the α and β isoforms of MHC. The relative distribution of the two isoforms in cardiac muscles is regulated physiologically and pathologically. α-MHC is responsible for a faster rate of heart activity, and β-MHC accounts for a slower rate. In the human fetal heart, α-MHC expression was restricted to the atrial walls and sinoatrial and atrioventricular nodes, whereas β-MHC was expressed in the ventricular wall (Bouvagnet et al. 1987; Wessels et al. 1991, 1992). At birth, an increased level of thyroid hormone seems to be associated with the induction of a substantial increase of β-MHC in the atrium and α-MHC in the ventricle (Chizzonite & Zak, 1984). In pathological conditions, hypertrophy of the human atrium is associated with α to β transition, suggesting that fiber stretching could trigger an isomyosin switch (Tsuchimochi et al. 1984). Moreover, both the α- and β-MHC isoforms are acetylated, although the β-isoform has been shown to be acetylated to a greater degree than the α-isoform when rat myocytes are subjected to stress (Samant et al. 2010). Although the present study obtained no information on changes in desmin expression in relation to myosin isozyme switching, in large mammals the MHC isoform shift is not a major event regulating myosin ATPase activity. It is likely that MHC acetylation contributes to a spatial or focused increase in the motor activity of myosin (Samant et al. 2010). In this regard, desmin expression in the atrium is not related to MHC isozyme switching attributable to stress, but rather is a feature of α-MHC isozyme maturation.
Monreal et al. (2010) reported that, in ventricular septal defect, desmin upregulation in the adult myocardium is related to right ventricular remodeling. In the context of myocardial overload and remodeling, it was speculated that in fetuses, involvement of the venous wall in atrial development caused mechanical stress, thus inducing desmin expression. Notably, desmin expression in the atrium with a smooth inner surface (derived from the venous wall) was stronger and appeared earlier than that in the atrial auricle. It can thus be inferred that desmin is expressed in the atrium in response to mechanical stress.
An increase in the expression of intermediate filaments has recently been recognized as a physiological or pathological response to mechanical or osmotic stress (Pekny & Lane, 2007). At the muscle-tendon interface, intermediate filaments seem to develop in response to mechanical stress resulting from early muscle contraction (Abe et al. 2010). In addition, the absence of α-SMA in human fetal myocardium seems to be related to the human-specific isozyme distribution of MHC.
Conclusions
The spatial distribution of desmin in the human fetal heart does not seem to play a general role in myocardial differentiation, and may instead be a result of α-myosin isozyme maturation. Moreover, desmin expression may also be found in areas subjected to mechanical stress during human fetal development.
Acknowledgments
This research was supported by Oral Health Science Center Grant hrc8 from Tokyo Dental College, and by a Project for Private Universities matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology), Japan, 2010–2012.
References
- Abe S, Rhee SK, Osonoi M, et al. Expression of intermediate filaments at muscle insertions of human fetuses. J Anat. 2010;217:167–173. doi: 10.1111/j.1469-7580.2010.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh J, Merisckay M, Li Z, et al. Hearts from mice lacking desmin have a myopathy with impaired active force generation and unaltered wall compliance. Cardiovasc Res. 2002;53:439–450. doi: 10.1016/s0008-6363(01)00500-4. [DOI] [PubMed] [Google Scholar]
- Bouvagnet P, Neveu S, Montoya M, et al. Developmental changes in the human cardiac isomyosin distribution: an immunohistochemical study using monoclonal antibodies. Circ Res. 1987;61:329–336. doi: 10.1161/01.res.61.3.329. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Li Z, Paulin D, et al. Nestin is expressed during development and in myotendinous and neuromuscular junctions in wild type and desmin knock-out mice. Exp Cell Res. 1999;251:213–223. doi: 10.1006/excr.1999.4569. [DOI] [PubMed] [Google Scholar]
- Chizzonite RA, Zak R. Regulation of myosin isozyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J Biol Chem. 1984;259:12628–12632. [PubMed] [Google Scholar]
- Goldfarb LG, Olivé M, Vicart P, et al. Intermediate filament diseases: desminopathy. Adv Exp Med Biol. 2008;642:131–164. doi: 10.1007/978-0-387-84847-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Hislop AA, Pierce CM, et al. Prenatal origins of human intrapulmonary arteries. Formation and smooth muscle maturation. Am J Respir Cell Mol Biol. 2000;23:194–203. doi: 10.1165/ajrcmb.23.2.3975. [DOI] [PubMed] [Google Scholar]
- Hall SM, Alison A, Hislop AA, et al. Origin, differentiation, and maturation of human pulmonary veins. Am J Respir Cell Mol Biol. 2002;26:333–340. doi: 10.1165/ajrcmb.26.3.4698. [DOI] [PubMed] [Google Scholar]
- Johansson B, Eriksson A, Thrnell LE. Intermediate filament proteins in developing human arteries. Anat Embryol. 1999;199:225–231. doi: 10.1007/s004290050223. [DOI] [PubMed] [Google Scholar]
- Kim HD. Expression of intermediate filament desmin and vimentin in the human fetal heart. Anat Rec. 1996;246:271–278. doi: 10.1002/(SICI)1097-0185(199610)246:2<271::AID-AR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Taffet GE, Wang X, et al. The absence of desmin leads to cardiomyocyte hypertrophy and cardiac dilation with compromised systolic function. J Mol Cell Cardiol. 1999;31:2063–2076. doi: 10.1006/jmcc.1999.1037. [DOI] [PubMed] [Google Scholar]
- Monreal G, Youtz DJ, Phillips AB, et al. Right ventricular remodeling in restrictive ventricular septal defect. J Mol Cell Cardiol. 2010;49:699–706. doi: 10.1016/j.yjmcc.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanaev AK, Shirinsky VP, Birukov KG. Immunofluorescent study of heterogeneity in smooth muscle cells of human fetal vessels using antibodies to myosin, desmin, and vimentin. Cell Tissue Res. 1991;266:535–540. doi: 10.1007/BF00318595. [DOI] [PubMed] [Google Scholar]
- Pekny M, Lane EB. Intermediate filaments and stress. Exp Cell Res. 2007;313:2244–2254. doi: 10.1016/j.yexcr.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Samant SA, Courson DS, Sundaresan NR, et al. HDAC-3 dependent reversible lysine acetylation of cardiac myosin heavy chain isoforms modulates their enzymatic and motor activity. J Biol Chem. 2010 doi: 10.1074/jbc.A110.163865. doi: http://www.jbc.org/cgi/doi/10.1074/jbc.M110.163865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida H, Nakamura H, Akimoto N, et al. Desmin distribution in the cardiac outflow tract of the chick embryo during aortic-pulmonary septation. Arch Histol Jpn. 1987;50:525–531. doi: 10.1679/aohc.50.525. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Desmin at myotendinous junctions. Exp Cell Res. 1992;199:206–212. doi: 10.1016/0014-4827(92)90425-8. [DOI] [PubMed] [Google Scholar]
- Torelli S, Ferlini A, Obici L, et al. Expression, regulation and localization of dystrophin isoform in human foetal skeletal and cardiac muscle. Neuromuscul Disord. 1999;9:541–551. doi: 10.1016/s0960-8966(99)00048-6. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, Sugi M, Kuro-o M, et al. Isozymic changes in myosin of human atrial myocardium induced by overload: immunohistochemical study using monoclonal antibodies. J Clin Invest. 1984;74:662–665. doi: 10.1172/JCI111466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder N, Soumake E, Abbasi S, et al. Cardiomyocyte-specific desmin rescue of desmin null cardiomyopathy excludes vascular involvement. J Mol Cell Cardiol. 2004a;36:121–128. doi: 10.1016/j.yjmcc.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Weisleder N, Taffet GE, Capetanaki Y. Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc Natl Acad Sci U S A. 2004b;101:769–774. doi: 10.1073/pnas.0303202101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A, Vermeulen JLM, Virágh SZ, et al. Spatial distribution of ‘tissue-specific’ antigens in the developing human heart and skeletal muscle. II. An immunohistochemical analysis of myosin heavy chain isoform expression patterns in the embryonic heart. Anat Rec. 1991;229:355–368. doi: 10.1002/ar.1092290309. [DOI] [PubMed] [Google Scholar]
- Wessels A, Vermeulen JLM, Verbeek FJ, et al. Spatial distribution of ‘tissue-specific’ antigens in the developing human heart and skeletal muscle. III. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat Rec. 1992;232:97–111. doi: 10.1002/ar.1092320111. [DOI] [PubMed] [Google Scholar]
- Ya J, Markman MW, Wagenaar GT, et al. Expression of the smooth-muscle proteins alpha-smooth-muscle actin and calponin, and of the intermediate filament protein desmin are parameters of cardiomyocyte maturation in the prenatal rat heart. Anat Rec. 1997;249:495–505. doi: 10.1002/(SICI)1097-0185(199712)249:4<495::AID-AR9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]


