Abstract
Reperfusion therapy is the only approved treatment for acute ischemic stroke. The current approach to patient selection is primarily based on the time from stroke symptom onset. However, this algorithm sharply restricts the eligible patient population, and neglects large variations in collateral circulation that ultimately determine the therapeutic time window in individual patients. Time alone is unlikely to remain the dominant parameter. Alternative approaches to patient selection involve advanced neuroimaging methods including MRI diffusion-weighted imaging, magnetic resonance and computed tomography perfusion imaging and noninvasive angiography that provide potentially valuable information regarding the state of the brain parenchyma and the neurovasculature. These techniques have now been used extensively, and there is emerging evidence on how specific imaging data may result in improved clinical outcomes. This article will review the major studies that have investigated the role of imaging in patient selection for both intravenous and intra-arterial therapies.
Keywords: acute ischemic stroke, CT, intra-arterial, intravenous, MRI, neuroimaging, reperfusion therapy
Acute ischemic stroke (AIS) is the third leading cause of death and the leading cause of severe disability in adults. In total, 795,000 strokes occur annually in the USA, of which 692,000 (87%) are ischemic [1]. Current treatment approaches include intravenous (iv.) tissue plasminogen activator (tPA), thrombolytic and/or mechanical intra-arterial therapies (IATs), and combined iv./intra-arterial (IA) bridging therapy.
There has been over a decade of research into the utility of neuroimaging for the evaluation of AIS. However, the issue of whether advanced imaging can lead to improved clinical outcomes remains open to debate. As a result, stroke duration continues to be the primary determinant of whether to treat.
Despite the lack of clear benefit, there remains a general optimism that advanced imaging can depict an individual patient’s specific cerebro-vascular physiology, and that this information can be used to guide treatment decisions, particularly outside of the current, restrictive time windows [2]. This article will address the current treatment selection paradigm and its limitations, review advanced computed tomography (CT) and MRI stroke imaging techniques, and discuss curent evidence evaluating the role of advanced imaging in patient selection for extended-window reperfusion therapies. This article will focus on MRI-based selection because the higher quality evidence concerns this technique. Where appropriate, we will discuss the analogous CT perfusion approaches.
Current approaches to patient selection: ‘time is brain’ & its limitations
Both iv. and IA treatments are restricted by the time from stroke onset (Table 1). For iv. tPA, the currently approved window is 3 h based on the National Institute of Neurological Disorders and Stroke (NINDS) tPA Stroke Study [3]. Results from the recent European Cooperative Acute Stroke Study (ECASS)-3 trial [4] have led to an American Heart Association guideline recommending iv. tPA treatment between 3–4.5 h in selected patients (class I, LOE B) [5]. For IAT, the time window is 6 h for thrombolysis and up to 8 h for mechanical therapies [6–8]. This time-based approach has been demonstrated to be effective and safe by numerous randomized controlled trials (RCTs) and prospective registries [3,4,9,10], and has helped thousands of patients [11]. In this paradigm, a noncontrast head CT scan is performed to exclude intracranial hemorrhage (ICH) or extensive infarction (e.g., ‘greater than a third of the middle cerebral artery [MCA] territory’).
Table 1.
Clinical trials evaluating the ‘time is brain’ paradigm (noncontrast computed tomography).
| Trial (year) | Type | Patients (n) | Time (min) | Primary outcome | Primary outcome reached | sICH† (%) | Significance | Ref. |
|---|---|---|---|---|---|---|---|---|
| NINDS part 1 | RCT | 291 | 0–180 | 24-h NIHSSS | No | 6.4 vs 0.6‡ | US FDA approval for iv. tPA in <3-h window | [3] |
| NINDS part 2 | RCT | 333 | 0–180 | Global BI/GOS/NIHSSS/mRS | Yes | |||
|
| ||||||||
| ATLANTIS part A | RCT | 142 | 0–360 | 24-h and 30-day NIHSSS | No | 11 vs 0‡ | Failed to extend time window beyond 3 h | [111] |
| ATLANTIS part B | RCT | 547 | 180–300 | 90-day NIHSSS | No | 7.0 vs 1.1‡ | Failed to extend time window beyond 3 h | [112] |
|
| ||||||||
| ECASS§ | RCT | 620 | 0–360 | 90-day BI and mRS | No | 19.8 vs 6.5‡,¶ | Target population (n = 511) demonstrated favorable iv. tPA response | [113]
|
| ECASS-II | RCT | 800 | 0–360 | 90-day mRS | No | 8.8 vs 3.4‡ | Failed to extend time window to 6 h | [114]
|
| ECASS-III | RCT | 821 | 180–270 | 90-day mRS | Yes | 2.4 vs 0.2‡ | AHA recommendation for iv. tPA treatment in a 3–4.5-h window | [4] |
|
| ||||||||
| SITS-MOST | # | 6483 | 0–180 | sICH, 90-day mortality | Yes†† | 7.3 vs 8.6‡‡ | Safety and effectiveness in a large cohort in a 3-h window | [9]
|
| SITS-ISTR | # | 664 vs 11,865§§ | 180–270 | sICH, 90-day mortality and mRS | Yes¶¶ | 2.2 vs 1.6## | Safety and effectiveness between 3 and 4.5 h | [10] |
|
| ||||||||
| Lees et al. (2010) | ††† | 3670 | 0–360 | 90-day mRS | Yes‡‡‡ | 5.2 vs 1.0‡,¶ | Favorable outcome in patients selected with NCCT up to 4.5 h | [15] |
sICH unless otherwise indicated.
Significant at the p < 0.05 level.
iv. tPA dosage of 1.1 mg/kg
Rate of parenchymal hematoma.
Prospective, open, observational patient registry.
No difference in sICH but improved mortality vs pooled RCT data.
Difference to pooled RCT data was not significant.
664 patients treated between 3 and 4.5 h were compared with 11,865 patients treated between 0 and 3 h from symptom onset.
No significant difference between two groups for all end points.
p = 0.24.
Pooled analysis of NINDS, ATLANTIS, ECASS I–III and EPITHET.
Significant favorable outcome in iv. tPA patients compared with placebo was seen until 270 min post-ictus.
AHA: American Heart Association; ATLANTIS: Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke; BI: Barthel index; ECASS: European Cooperative Acute Stroke Study; GOS: Glasgow outcome scale; iv.: Intravenous; mRS: Modified Rankin Scale; NCCT: Noncontrast computed tomography; NIHSSS: NIH Stroke Scale score; NINDS: National Institute of Neurological Disorders and Stroke; RCT: Randomized controlled trial; sICH: Symptomatic intracranial hemorrhage; SITS-ISTR: Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Register; SITS-MOST: Safe Implementation of Thrombolysis in Stroke-Monitoring Study; tPA: Tissue plasminogen activator.
The strict adherence to time windows reflects the idea that ‘time is brain’ [12], and is supported by pooled analyses of multiple RCTs of iv. tPA [13–15], which have demonstrated that the odds for a good outcome decrease with increasing onset to treatment times (OTTs). In a recent analysis [15], data from 3670 patients demonstrated that the adjusted odds of a good 3-month outcome (defined as a modified Rankin scale score [mRS] of 0–1) for iv. tPA versus placebo were 2.55 (95% CI: 1.44–4.52) for 0–90 min, 1.64 (95% CI: 1.12–2.40) for 91–180 min, 1.34 (95% CI: 1.06–1.68) for 181–270 min, and 1.22 (95% CI: 0.92–1.61) for 271–360 min (p = 0.03).
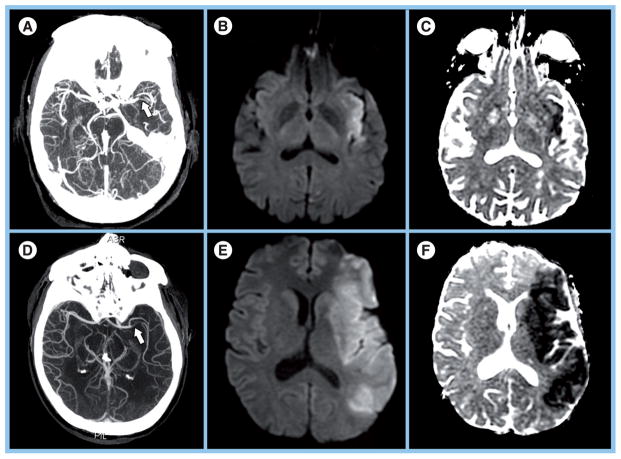
Despite these findings, this time-based approach has been challenged in recent years for two main reasons. First, the time window for iv. tPA is restrictive. Several investigations have shown that only 1–7% of eligible patients receive iv. tPA within the 3-h window [16–18], mainly due to late presentation. Second, ‘time is brain’ paints an incomplete picture of cerebrovascular physiology [19,20]. While a time window may be effective on a population level, it may not hold for individual patients. From clinical experience, we know that there are patients who have large infarcts despite early presentation, and patients with negligible infarcts at later time points (Figure 1). Moreover, studies have demonstrated that there can be substantial volumes of viable penumbral tissue between 12 and 24 h from onset [21–23].
Figure 1. Examples of patients who have large infarcts despite early presentation, and patients with negligible infarcts at later time points.
(A–C) A 66-year-old female with left middle cerebral artery M1 occlusion (arrow) demonstrated on (A) axial maximum intensity projection (MIP) and small infarct (14-ml volume) on (B) diffusion-weighted image (DWI) and (C) apparent diffusion coefficient (ADC) map. MRI was performed 8 h, 58 min post-ictus. The 3-month Modified Rankin Scale (mRS) score was 2. (D–F) A 52-year-old male with left middle cerebral artery M1 occlusion (arrow) demonstrated on (D) axial MIP and extensive infarct (146 ml volume) on (E) DWI and (F) ADC map. MRI was performed 4 h, 16 min post-ictus. The 3-month mRS score was 6.
Reprinted with permission from Massachusetts General Hospital (MA, USA).
Not surprisingly then, recent data exploring the effect of time to reperfusion on outcome in IAT-treated patients is conflicting. In a pooled analysis of the Interventional Management of Stroke (IMS) I and II trials [24], only time to angiographic reperfusion and age independently predicted a good 3-month outcome (mRS: 0–2) in 54 patients with terminal internal carotid artery (ICA) or proximal MCA occlusions who underwent successful reperfusion. On the other hand, in a pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi-MERCI trials [25], neither time to procedure nor time to reperfusion was associated with a good outcome (mRS: 0–2) in 163 patients with anterior circulation proximal artery occlusion (PAO) who were successfully reperfused. These divergent results highlight the central problem with a time-based approach – it neglects the variable degree of cerebral blood flow (CBF) impairment.
In a landmark study, Jones et al. demonstrated that both duration and degree of ischemia determined neuronal progression to cell death using a primate model [26]. With blood flow reduction below a certain threshold, clinical symptoms were observed but the tissue remained viable for several hours. At lower CBF thresholds, infarction ensued more rapidly. These findings have been confirmed in humans using PET [27,28].
For anterior circulation strokes, the pial collateral circulation to the MCA territory, primarily from the anterior cerebral artery, is the main determinant of CBF impairment, and thus the rate of neuronal loss [29]. The strength of the collateral circulation is variable between patients, and has been shown to be a significant predictor of clinical outcome and tissue fate [30–36]. Therefore, a more accurate statement would be ‘time and collaterals are brain’. This idea is further supported by studies of extended window therapies, which have demonstrated two relevant points: there may be a clinical benefit to physiology-based patient selection using advanced imaging beyond 3 h; and in patients selected based on a favorable physiology, OTT is not a predictor of outcome [37–39].
Imaging the acutely ischemic brain
Considerable evidence demonstrates that final infarct size is a critical determinant of clinical outcome [40–43]. In a substudy of the Acute Stroke Accurate Prediction (ASAP) trial involving 169 patients with median NIH Stroke Scale (NIHSS) score of 6 (mild-to-moderate severity), infarct growth (at poststroke day 5) was an independent predictor of an excellent 90-day outcome (mRS: 0–1; p = 0.01) [42]. The adjusted odds ratio for an excellent mRS score was reduced with an increase in infarct volume (OR: 0.57 [95% CI: 0.37–0.88] for every 10-ml increase). In a subgroup analysis of the Echoplanar Imaging Thrombolysis Evaluation Trial (EPITHET) trial, which included 72 patients with a median NIHSS score of 12 (moderate-severe strokes), infarct volume (on poststroke days 3–5) had a strong correlation with the 90-day NIHSS score (Spearman Rho coefficient: 0.81; p < 0.01) [40]. Finally, in a study of 81 IAT patients with anterior circulation PAO and a median NIHSS score of 18 [43], the final infarct volume was the best discriminator of a good 3-month outcome (mRS: ≤2) in ROC analysis (AUC: 0.883).
Reperfusion therapy limits the size of the final infarct by salvaging the ischemic penumbra [27,40,44–48]. Patients with large baseline infarcts have little to no hope of treatment benefit, and should be excluded from the risks of therapy, which include iat-rogenic emboli, ICH and reperfusion edema [20,49]. Therefore, imaging of parenchymal injury at the time of presentation can provide important information for treatment decision making.
Noncontrast CT
As mentioned previously, the major RCTs that guide current clinical practice employed noncontrast CT (NCCT) for assessment of parenchymal injury. Patients were excluded from therapy if they had infarcts that involved greater than a third of the MCA territory or that resulted in severe edema or mass effect (Table 1).
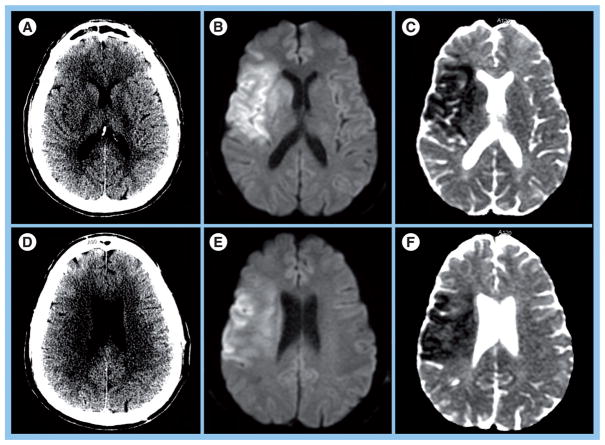
Using NCCT for parenchymal assessment is problematic for two reasons. First, it suffers from a high degree of inter-rater variability for detecting early ischemic changes [50–52], which include parenchymal hypoattenuation, loss of gray-white matter distinction and cortical swelling [52]. Second, NCCT demonstrates poor sensitivity (20–75%) for detecting acute infarction within the 6–8-h window [51–56]. One study reported a sensitivity of 14–43% for identifying infarcts larger than a third of the MCA territory within 7 h of stroke onset (Figure 2) [55]. Hence, the current imaging criterion is highly permissive, and excludes only the largest and most well-established infarcts from reperfusion therapy [57]. For practical purposes, treatment selection is determined primarily by the time window.
Figure 2. Poor sensitivity of noncontrast computed tomography in acute ischemia.
(A) Noncontrast computed tomography (NCCT) in a 45-year-old male demonstrates subtle hypodensity in the putamen and possible effacement of sulci in the frontal operculum. (B) Diffusion-weighted image (DWI) and (C) apparent diffusion coefficient (ADC) map at the same level show more extensive acute infarct involving the basal ganglia, insula and cortical regions of the frontal and temporal lobe. (D) At a different level in the same patient, NCCT demonstrates subtle hypodensity in the deep white matter. (E) DWI and (F) ADC map reveal a larger region of acute infarction in the gray and white matter of the frontal and parietal lobes that is difficult to identify in the CT scan. Time between computed tomography and MRI was 45 min.
Reprinted with permission from Massachusetts General Hospital (MA, USA).
In order to improve NCCT evaluation of hyper AIS, a more rigorous approach to image analysis is necessary. In current practice, early NCCT changes are poorly defined and may signify varying degrees of ischemia and viability [52,58,59]. For example, two recent studies have shown that isolated cortical swelling may indicate penumbral tissue, while hypoattenuation appears to represent infarct core [58,59]. Further work needs to be carried out, particularly with automated Hounsfield unit analysis.
Advanced imaging of the infarct core
Of the available MRI and CT measurements, abnormalities detected on diffusion-weighted imaging (DWI) are considered the most reliable estimate of the infarct core. The loss of cellular energy metabolism causes a failure of membrane ionic pumps resulting in cytotoxic edema and restricted diffusion of water, which is detectable using diffusion-weighted imaging. DWI is highly sensitive (91–100%) and specific (86–100%) in the identification of early ischemic brain injury (<6 h from onset) [51,60,61], and has a similar accuracy for the prediction of cortical infarction as 11C flumazenil PET [62], a reliable marker of neuronal integrity.
However, certain findings have led some to question the value of DWI for assessing the infarct core [63]. First, the pathophysiologic processes within the DWI lesion are heterogeneous, as evidenced by a high spatial variability in the metabolic rate of oxygen, oxygen extraction fraction and cerebral perfusion within individual lesions [64,65]. Moreover, animal studies demonstrate that the DWI lesion is more closely correlated to areas of pH alteration due to anerobic metabolism [66], suggesting that in the hyperacute phase DWI may depict still viable tissue. Most importantly, there have been several reports of DWI lesion reversibility after deeptreatment, with reported rates ranging in the from 8 to 44% [67–71].
However, the clinical significance of DWI lesion reversal remains unclear. To date, it has not been demonstrated to improve clinical outcomes [70,72]. Tissue reperfusion appears to be a prerequisite for this phenomenon [67,70]. Other predictors include earlier time to imaging [68,70] and smaller reductions in the apparent diffusion coefficient [70], probably reflecting milder reductions in blood flow [73]. Importantly, the mean volume of tissue reversal is relatively small (5.1–16 cm3) [62,67,69,70]. While such small volumes may translate as clinical improvement, it is more likely that any benefit associated with DWI reversal is related to accompanying tissue reperfusion and penumbral salvage. Moreover, delayed regrowth into a previously observed diffusion abnormality frequently occurs [67–68], even when blood flow is restored [74–75]. Finally, the true frequency of DWI reversal may be lower than previously reported when chronic infarct involution is taken into consideration. In a post-hoc analysis of the EPITHET trial [76], true DWI reversal was observed in only 6.4% of patients with a negligible median reversal volume of 2.7 ml (interquartile range: 1.6–6.2 ml).
Taken together, the evidence indicates that DWI is currently the most accurate and well-validated method for assessing acute infarction in the treatment setting, an opinion supported by multiple expert panels (class I, level of evidence A) [57,77]. Advanced CT methods for delineating the infarct core include parenchymal hypoattenuation on CT angiographic source images (CTA-SI) [78–80] and depressed cerebral blood volume (CBV) on processed CT perfusion (CTP) maps [81–83]. However, these methods are less validated (class IIb, level of evidence B) [57], and require further study [84,85]. For example, it has been recently demonstrated that CBV values are dependent on the duration of perfusion-weighted imaging (PWI) scanning, and that the majority of DWI-positive regions may have elevated CBV [86].
Advanced imaging of hypoperfused (penumbral) brain tissue
H215O PET and diffusible-tracer techniques such as 99mTc-HMPAO single-photon emission CT (SPECT) are considered the reference standards for quantifying cerebral perfusion [57], but they are not practical in the clinical setting. On the other hand, MR and CT perfusion imaging are widely available and have been studied extensively in AIS. They are dynamic contrast methods, whereby postprocessing of the source imaging data produces a series of maps that describe the cerebral hemodynamics. Importantly, postprocessing can be performed in several different ways, and may yield different results even for the same patient [87–89]. Indirect parameters, such as bolus arrival time and time-to-peak (TTP), are easy to calculate, but are thought to be less accurate than parameters derived from deconvolution with an arterial input function (AIF). Deconvolution corrects for bolus delay and dispersion up to the level of the AIF [90], and produces maps of tissue-level perfusion parameters such as CBV, cerebral blood flow (CBF) and mean transit time (MTT).
A major limitation of MR and CT perfusion imaging is that they are not reliable for absolute quantification of cerebral perfusion [57]. Numerous studies have demonstrated poor reproducibility and significant variability in these techniques [91–93]. Given these limitations, a semiquantitative approach has been advocated in which a simple volumetric mismatch between the infarct core and the larger region of hypoperfusion constitutes an ‘operational ischemic penumbra’ [94]. The most widely studied method is the MRI diffusion–perfusion mismatch.
Commonly used parameters in MR PWI are TTP, MTT and Tmax, the latter two requiring deconvolution-based processing. TTP is defined as the time to maximum signal intensity loss during contrast transit, and is dependent on multiple factors, including MTT, bolus arrival time and CBF [95]. MTT is the average time for the contrast molecule to traverse the voxel, and has been used in several trials, including Desmoteplase in Acute Ischemic Stroke (DIAS) [39], Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS) [96] and DIAS-2 [97]. It has been quoted as having the most direct physiologic correlation with tissue survival because oxygen extraction is diffusion-limited in brain capillaries and therefore determined by vascular transit time [98,99]. Tmax is the time at which the tissue residue function reaches its maximum, or when the injected contrast arrives in a particular brain voxel. Tmax is abnormal in collaterally perfused tissue even if CBF is normal [100]. Therefore, it does not describe the hemodynamic status of the tissue itself [101] Several studies have used Tmax as the PWI method of choice, such as Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) [102] and EPITHET [103].
Imaging the cerebral vasculature
The initial event in ischemic stroke is vascular occlusion. Angiographically controlled studies have demonstrated that identification of the occluded vessel provides additional prognostic information beyond clinical variables alone [104–106]. Specifically, the presence of a proximal arterial occlusion (e.g., ICA, proximal MCA or basilar artery) is associated with worse clinical outcomes. In a study of 480 patients, patients with PAO or significant parenchymal involvement consistent with PAO accounted for all the deaths, had longer hospital stays, and were more likely to be discharged to a rehabilitation facility (all outcomes; p < 0.0001) [107].
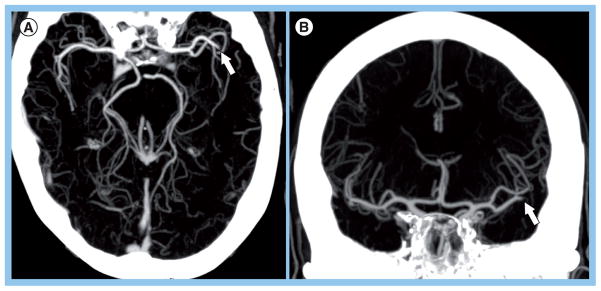
According to recent guidelines, noninvasive vessel imaging should be performed whenever possible in patients with suspected stroke or transient ischemic attack [57]. CT angiography is the best noninvasive test for identifying major intracranial vessel occlusion. In one study of 44 consecutive patients who underwent CTA followed by IAT, CTA demonstrated 98.4% sensitivity, 98.1% specificity and 98.2% accuracy for large vessel occlusions when compared with the gold standard digital subtraction angiography (DSA) [108]. Furthermore, CTA has a high interobserver reliability [108,109]. Identification of PAO, particularly in the second-order M2 (MCA) and A2 (anterior cerebral artery) branches, is facilitated by ‘collapsed’ maximum intensity projection images, which can be constructed immediately at the CT scanner console and along three orthogonal planes (Figure 3). Magnetic resonance angiography (MRA) is another widely used noninvasive test, and is performed with 3D time-of-flight (TOF) technique or after gadolinium administration. Compared to CTA, it is more sensitive to patient motion artifact, as well as to flow artifact. The latter may result in overestimation of vessel stenosis. However, for the question of PAO, 3D TOF MRA performs reasonably well, with a sensitivity of 84–87% and a specificity of 85–98%, compared with DSA [109,110]. The interobserver agreement is fair to moderate (κ = 0.5) [110].
Figure 3. Computed tomography angiography thick slab maximum intensity projection to identify arterial occlusions.
Left middle cerebral artery (MCA) M2 occlusion visualized on (A) axial and (B) coronal maximum intensity projection images in a 72-year-old female with admission NIH Stroke Scale of 9 who was imaged approximately 7 h after stroke onset. The 3-months modified Rankin score was 1. Identification of proximal artery occlusions, particularly in the second-order branches such as the M2 (MCA) branch shown here, is facilitated by thick slab (20–30 mm) maximum intensity projection images, which can be constructed immediately at the CT scanner console and along three orthogonal planes.
Reprinted with permission from Massachusetts General Hospital (MA, USA).
Imaging selection for iv. thrombolysis
Evidence supporting the mismatch hypothesis
Until the ECASS-3 results, previous studies that employed NCCT-based selection failed to extend the time window for iv. tPA beyond 3 h. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS) [111,112], ECASS [113] and ECASS-2 [114] could not demonstrate a clinical benefit that outweighed treatment risk in the 5- to 6-h window. In comparison, multiple studies employing MRI diffusion–perfusion mismatch have demonstrated positive clinical results for extended window treatment with iv. tPA, and provide the strongest evidence for this imaging approach (Table 2).
Table 2.
Clinical trials supporting the mismatch hypothesis (magnetic resonance-based imaging).
| Trial (year) | Type | Patients (n) | Time (min) | Primary outcome | Primary outcome | sICH (%) | Significance | Ref. |
|---|---|---|---|---|---|---|---|---|
| DIAS† | RCT | 104 | 180–540 | Reperfusion‡, 90-day combined NIHSSS/mRS/BI | Yes§ | 2.2 vs 0¶ | Significant desmoteplase effect seen in MRI mismatch patients. Longer OTT not associated with reduced treatment effect | [39] |
| DEDAS† | RCT | 37 | 180–540 | Reperfusion‡, 90-day combined NIHSSS/mRS/BI | Yes# | None | Desmoteplase is safe and effective in mismatch patients up to 9 h | [96] |
| DEFUSE | †† | 74 | 180–360 | 30-day NIHSSS | Yes | 9.5 | Mismatch patients have a more favorable clinical response to early reperfusion than nonmismatch patients‡‡ | [102] |
| Schellinger et al. (2007) | †† | 1210 | 0–1032 | 90-day mRS§§ | Yes¶¶ | 3.4 vs 5.3## | MRI selection for thrombolysis is safer and potentially more effective than NCCT selection Patients with MRI mismatch can be treated up to 6 h with similar or better results vs NCCT selection <3 h | [37] |
| Köhrmann et al. (2006) | †† | 382 | >180 | 90-day mRS§§ | Yes¶¶ | 3 vs 9††† | MRI selection for thrombolysis is safer and potentially more effective than NCCT selection Patients with MRI mismatch can be treated up to 6 h with similar or better results vs NCCT selection <3 h | [38] |
| Thomalla et al. (2006) | †† | 174‡‡‡ | 0–360 | 90-day mRS§§§ | Yes¶¶¶ | 3 vs 8††† | MRI selection for thrombolysis is safer and potentially more effective than NCCT selection Patients with MRI mismatch can be treated up to 6 h with similar or better results vs NCCT selection <3 h | [115] |
Study of intravenous desmoteplase. Patients were selected based on perfusion–diffusion mismatch.
Reperfusion was assessed within 4–8 h after iv. tPA with MRI.
For a 125-μg/kg dose.
One patient who received 90 μg/kg desmoteplase dose.
Significant for clinical outcome but not for reperfusion at 125 μg/kg dose in target population.
Prospective, open, observational study.
Selection of patients for treatment was not based on MRI mismatch. Only 11 patients without a mismatch.
Clinical outcome was compared between patients selected with NCCT, MRI (<3 h) and MRI (>3h).
MRI-based selection >3h led to similar or better clinical outcome than patient selection based on NCCT <3h.
MRI vs NCCT selected patients, respectively (p = 0.05).
MRI vs NCCT selected patients, respectively (significant at the p <0.05 level).
Compared to pooled placebo and tPA patients from the Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke, European Cooperative Acute Stroke Study and National Institute of Neurological Disorders and Stroke trials.
Clinical outcome was compared between placebo patients and iv. tPA patients selected with NCCT vs iv. tPA patients selected with MRI (0–6 h).
iv. tPA patients selected with MRI had significantly better outcomes than pooled placebo patients. MRI based selection >3 h led to similar or better clinical outcome than patient selection based on NCCT <3 h.
BI: Barthel index; DEDAS: Dose Escalation of Desmoteplase for Acute Ischemic Stroke; DEFUSE: Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution; DIAS: Desmoteplase in Acute Ischemic Stroke Trial; iv.: Intravenous; mRS: Modified Rankin Scale score; NCCT: Noncontrast computed tomography; NIHSSS: NIH Stroke Scale score; NINDS: National Institute of Neurological Disorders and Stroke; OTT: Onset to treatment time; RCT: Randomized controlled trial; sICH: Symptomatic intracranial hemorrhage; tPA: Tissue plasminogen activator.
In large multicenter studies, patient selection using the perfusion–diffusion mismatch was demonstrated to safely and effectively extend the treatment window for iv. tPA up to 6 h [37,38,115]. The most frequently employed mismatch definition was a perfusion lesion at least 20% larger than the diffusion lesion. Furthermore, patients were excluded if they had extensive infarcts greater than a third or half of the MCA territory. Notably, patients with a PWI/DWI mismatch treated with iv. tPA beyond 3 h appeared to do just as well as patients treated within the 3-h window using traditional NCCT criteria [37,38,116]. In a multicenter retrospective study of 1210 patients [37], there were no significant differences in a favorable 3-month clinical outcome (mRS: 0–1; 35.4 vs 40.0%), symptomatic ICH (sICH; 5.3 vs 4.4%) and mortality (13.7 vs 13.3%) between patients treated within 3 h using NCCT versus those treated beyond 3 h based on a 20% PWI/DWI mismatch. In multiple logistic regression, use of MRI beyond 3 h significantly increased the odds for a favorable outcome compared with CT-based treatment within 3 h (OR: 1.467; 95% CI: 1.017–2.117). The odds of sICH were reduced to approximately half (OR: 0.520; 95% CI: 0.270–0.999; p < 0.05) with MRI-based selection. Importantly, OTT was not a predictor of safety or efficacy in univariate or multivariate analysis. The perfusion imaging techniques were heterogeneous among the centers and included nonthreshold MTT and TTP with (>4 s delay) and without thresholding. Other studies have found similar results (Table 2) [38,115,116], supporting the idea that a favorable cerebrovascular physiology may be more important than ischemic duration for extended window therapy.
Trials of iv. desmoteplase
Direct RCT evidence supporting the utility of mismatch-based selection was provided by trials of desmoteplase, a thrombolytic agent with high fibrin specificity, low neurotoxicity and a long half-life [117,118].
In the randomized placebo-controlled Phase II DIAS trial [39], patients with OTT within 3–9 h, a perfusion abnormality of 2 cm in diameter involving hemispheric gray matter and a perfusion–diffusion mismatch of ≥20% were included and randomized to 62.5 μg/kg, 90 μg/kg, 125 μg/kg or placebo (Part 2; n = 57 patients). Mismatch volumes were assessed by visual estimation. Compared with placebo (n = 27), the 125 μg/kg desmoteplase dose (n = 15) resulted in a higher reperfusion rate on MRI performed after 4–8 h (71.4 vs 19.2%; p = 0.001) and higher rates of a 90-day favorable clinical outcome (60 vs 22.2%; p = 0.009). As in other studies, OTT interval was not associated with reduced treatment effect. In a similar study design, DEDAS confirmed the clinical benefit of the 125 μg/kg desmoteplase dose relative to placebo in patients who fulfilled all MRI selection criteria, without a difference in sICH rate or mortality [96].
Clinical response of mismatch patients to reperfusion
The beneficial clinical response of mismatch patients to documented reperfusion was demonstrated in DEFUSE [102], a Phase II study examining 74 consecutive patients treated with iv. tPA between 3 and 6 h after stroke onset. Importantly, treatment selection was based on NCCT, not on an MRI mismatch. A total of 1020 patients were screened for 74 patients (7.2%). Patients with a baseline PWI/DWI mismatch (mismatch ratio ≥20%; absolute mismatch ≥10 ml) who underwent early reperfusion (>30% reduction in PWI lesion volume on MRI performed 3–6 h after iv. tPA treatment) had better outcomes than mismatch patients without reperfusion (OR: 5.4; 95% CI: 1.1–25.8). In addition, patients without a PWI/DWI mismatch did not appear to benefit from early reperfusion, although there were only 11 patients without a mismatch in the study. Penumbral imaging utilized Tmax with a 2-second delay.
Computed tomography perfusion-based mismatch (e.g., CBV-MTT mismatch) has been shown to provide similar assessments of treatment eligibility (e.g., infarct core less than a third MCA territory and mismatch ≥20%) to the MRI-based approach [79,119,120]. However, the lack of standardized postprocessing and analysis, including the choice of perfusion parameter, and the absence of well-validated thresholds limit the utility of this approach at this time [85,86,121].
Limitations of the mismatch approach
Despite the favorable data in support of the mismatch hypothesis, there is no conclusive evidence that the mismatch alone identifies patients who will respond to thrombolysis (Table 3) [57,122–124]. This uncertainty was recently highlighted in the results of the Phase III DIAS-2 study [97], which failed to confirm the DIAS and DEDAS findings [39,96]. In this study, stroke patients with a 20% visual mismatch on either MRI or CT perfusion were randomized in a 1:1:1 fashion to 90 μg/kg iv. desmoteplase, 125 μg/kg iv. desmoteplase and placebo. Among the low-dose, high-dose and placebo groups, respectively, the rates of favorable clinical outcome at 90 days were 47, 36 and 46%; the rates of sICH were 3.5, 4.5 and 0%; and the mortality rates were 11, 21 and 6%. A third of enrolled patients were evaluated with CT perfusion.
Table 3.
Clinical trials against the mismatch hypothesis (magnetic resonance and/or computed tomography perfusion imaging).
| Trial | Type | Patients (n) | Time (min) | Primary outcome | Primary outcome reached | sICH (%) | Significance | Ref. |
|---|---|---|---|---|---|---|---|---|
| EPITHET | RCT | 101 | 180–360 | Infarct growth† | No | 7.7 vs 0‡ | Failed to demonstrate difference in reperfusion, infarct growth or clinical outcome between mismatch and non-mismatch patients, for iv. tPA and placebo§ | [103] |
| DIAS-II¶ | RCT | 186 | 180–540 | 90 days combined NIHSSS/mRS/BI | No | 4.1 vs 0 | No clinical benefit demonstrated for mismatch patients treated with desmoteplase between 3 and 9 h# | [97] |
| Mishra et al. (2010) | †† | 502 | 0–540 | 90 days NIHSSS or mRS | No‡‡ | NS§§ | No clinical benefit with iv. thrombolysis in mismatch patients beyond 3 h and up to 9 h | [124] |
Defined as a ratio of geometric means. Comparison is between patients with and without a mismatch treated with iv. tPA.
Treatment vs placebo (significant at p < 0.05 level).
Patients were not selected based on an imaging mismatch.
iv. desmoteplase treatment: 90 and 125 μg/kg doses.
Patients were selected based on 20% imaging mismatch using MRI or CT perfusion. There was a significant increase in mortality with desmoteplase treatment.
Pooled analysis of DEFUSE, EPITHET, DIAS, DIAS II and DEDAS.
iv. thrombolysis was associated with increased reperfusion/recanalization but not with a favorable clinical outcome. There was a nonsignificant increased odds of mortality and sICH with treatment, after excluding data from abandoned desmoteplase doses.
After excluding data from abandoned desmoteplase doses.
BI: Barthel index; EPITHET: Echoplanar Imaging Thrombolytic Evaluation Trial; iv.: Intravenous; mRS Modified Rankin Scale score; NIHSSS: NIH Stroke Scale score; NS: Not significant; RCT: Randomized controlled trial); sICH: Symptomatic intracranial hemorrhage.
In addition, the EPITHET trial [103], a Phase II placebo-controlled randomized trial of iv. tPA administered 3 to 6 h after stroke onset, failed to demonstrate any significant difference in the primary end point of infarct growth, or in reperfusion and clinical outcomes between patients with a PWI/DWI mismatch (PWI/DWI volume >1.2 ml, and absolute mismatch volume ≥10 ml) and those without a mismatch, for both the iv. tPA and placebo groups. As in DEFUSE, MRI findings were not used for patient selection or treatment allocation. These results are consistent with previous studies that have demonstrated that the PWI/DWI mismatch does not predict infarct growth, and that lesion growth is equally common in patients with and without a mismatch [89,125].
A recent meta-analysis of the DEDAS, DIAS, DIAS II, DEFUSE and EPITHET trials analyzed the data from 502 mismatch patients who were treated with iv. thrombolysis or placebo beyond 3 h [124]. While patients with an imaging mismatch demonstrated higher rates of reperfusion with thrombolysis (adjusted OR: 3.0; 95% CI: 1.6–5.8), there was no significant improvement in favorable clinical outcome by thrombolytic therapy (adjusted OR: 1.3; 95% CI: 0.8–2.0). Furthermore, there was a significant increase in mortality and sICH after treatment, but these end points did not remain significant after the exclusion of abandoned desmoteplase doses. These negative results suggest that the clinical and tissue response to reperfusion therapy may not be strictly dependent on the presence of a mismatch as it is currently defined [126].
Several fundamental problems with the mismatch approach exist and may help to explain the conflicting data. First, despite its widespread use, the 20% PWI/DWI mismatch remains an arbitrary value [88,122,127]. Recent studies have sought to optimize this definition. A post-hoc analysis of 45 patients from the DEFUSE study found that a PWI/DWI ratio of 2.6 yielded the highest sensitivity (90%) and specificity (83%) for identifying a favorable clinical response to early reperfusion [128]. Similarly, an unpublished analysis of the EPITHET dataset found that a PWI/DWI ratio of 2 was a better predictor of clinical outcomes than the 20% mismatch [129]. Further studies are required to validate these findings.
In addition, there is no consensus as to which perfusion parameter should be employed in defining the mismatch [122,130]. TTP, Tmax and MTT are commonly used because lesions are easily discernible. However, the choice of parameter affects the perfusion lesion volume and hence the quantitative mismatch [88,89,127]. It has been shown that selection using different parameters would result in 9–63% of patients receiving treatment, a range that is unacceptable for both clinical trials and practice [89].
Another issue is the use of visual estimation for assessing mismatch size. A study by Coutts et al. [131] demonstrated that visual assessment suffers from a high degree of measurement error (standard error of the mean: 21.6%). In the DEDAS study, visual assessment overestimated the true mismatch in 16% of cases [96].
The most significant problem with the mismatch approach is that the commonly used measures of abnormal perfusion (MTT and TTP) overestimate the true territory at risk [132–140]. DEFUSE and EPITHET used a Tmax delay of 2 s, which was subsequently shown to encompass areas of benign oligemia [141]. This poor specificity explains why patients with significant extra- or intracranial vascular stenosis have a larger PWI/DWI mismatch but less infarct growth than patients without stenosis [142,143]. Therefore, the same degree of mismatch may have variable consequences in different patients.
One proposed method to improve tissue prediction using perfusion imaging is the use of threshold techniques [130,144]. Despite the questionable reliability of perfusion measurement, several studies have sought to establish thresholds that distinguish critical hypoperfusion from benign oligemia [81,134,135,138,141,145–149]. Importantly, this analysis must be restricted to patients with persistent occlusions, and only a fraction of studies excluded patients with documented reperfusion or dramatic clinical improvement [81,127,134,135,146]. Unfortunately, these studies utilized different perfusion parameters in their analysis, preventing confirmation of their results. The reported thresholds were an MTT delay of 4–6 s [146], a relative MTT of 145% [81], a Tmax delay of 4–6 s [134] and a first moment prolongation of 3.5 s [127], while one study found that no perfusion parameter distinguished benign oligemia from final infarction (refer to the erratum in [135]). Other studies have compared MR perfusion with H215O PET-derived CBF [92,133,140,150] and have found that a TTP delay between 4–6 s best identifies penumbral flow (e.g., CBF <20 ml/100 g/min). However, this threshold did not reliably correspond to penumbra as defined by PET oxygen extraction fraction >150% [133]. Using MTT prolongation, thresholds >5.3 [150] and 6 s [92] were found to best identify CBF <20 ml/100 g/min. However, these findings disagreed with another study that found an optimal MTT threshold of >10 s that corresponded to xenon CT-derived CBF <20 ml/100 g/min [141].
Based on these results, the use of viability thresholds for clinical decision making cannot be recommended until there is stronger evidence to support it [151]. In order to validate clinically useful perfusion thresholds, not only will it be important to decide on which perfusion parameters to measure, it will also be necessary to use standardized perfusion processing methods and software, as these can introduce variability in perfusion measurement [85,89,93,127]. Even if these issues are addressed, a critical limitation of perfusion imaging is that it measures blood flow at a single moment in time. This is probably insufficient as perfusion may vary over time owing to changes in local perfusion pressure and recruitment or loss of collaterals [29,73].
Alternative methods for penumbral selection
Because a mismatch ratio is a relative measure, the same ratio can equate to different absolute tissue volumes and hence represent varying stroke severities. Despite identical imaging selection criteria for DIAS, DEDAS and DIAS-2, baseline strokes in DIAS-2 were less severe across all study groups (average NIHSS score of 9 vs 12 in DIAS and DEDAS). Similarly, DWI lesion volumes and absolute mismatch volumes were significantly smaller in DIAS-2, despite the higher relative mismatch volume, and the rate of vessel occlusion was lower. The less severe stroke population in DIAS-2 helps to explain the high placebo response rate that contributed to the failure of this study. Likewise, despite similar imaging criteria between DEFUSE and EPITHET (e.g., Tmax threshold of 2 s), the DEFUSE cohort had lower baseline NIHSS score (11.5 vs 13) and smaller baseline DWI (10 vs 21 ml) and PWI (48 vs 192 ml) lesion volumes [152].
To address the mismatch problem, approaches based on absolute lesion volume have been proposed. In support of this concept, a post-hoc analysis of EPITHET recently demonstrated that DWI and PWI lesion volumes influence clinical response to iv. tPA, while mismatch ratios do not [153]. This finding is reasonable when one considers that a small territory at risk will probably result in a favorable clinical outcome even if the perfusion abnormality is 20% larger than the core infarct defined by diffusion imaging. Similarly, a significant neurologic deficit owing to a large core infarct will not be influenced by improved perfusion in the surrounding tissue. It is the size and location of the infarct and the territory at risk that are critical to outcome.
One alternative based on volume considerations is the clinical-DWI mismatch (CDM) [154]. Dávalos et al. defined CDM as a baseline NIHSS score ≥8 and admission DWI lesion volume ≤25 ml. In their study, 87 out of 166 hemispheric stroke patients (52%) presenting within 12 h were found to have CDM. CDM patients were more likely to have early neurological deterioration (NIHSS score worsening ≥4 at 72 h) than those with NIHSS score <8 (OR: 9.0; 95% CI: 1.9–42) and those with DWI volume >25 ml (OR: 2.0; 95% CI: 0.8–4.9). Furthermore, CDM was associated with the greatest infarct growth at days 3 and 30 (p < 0.001). The association of CDM with greater infarct growth was also reported by Prosser et al. [155], who also found that CDM was highly specific (93%) but not sensitive (53%) for identifying a PWI/DWI mismatch >20%. However, other studies were not able to demonstrate that CDM was associated with an improved clinical response to iv. tPA or reperfusion [156,157]. Similarly, an analogous NCCT-based approach failed to predict response to iv. tPA in the CLOTBUST dataset [158].
Another recently described approach utilizes DWI and PWI lesion volumes in conjunction with NIHSS thresholds [159]. In a study of 54 AIS patients imaged using MRI within 9 h of onset, baseline DWI and MTT volumes predicted independent outcome (mRS: 0–2) at 3 months better than mismatch volume or percent mismatch. Thresholds identifying poor outcome (mRS: 3–6) with 100% specificity were DWI lesion volume >72 ml and NIHSS score >20. Thresholds identifying good outcome with 100% specificity were MTT lesion volume <47 ml and NIHSS score <8. Using these combined thresholds, dichotomized outcome was predicted in 70% of cases, higher than for clinical (43%) or imaging (54%) thresholds alone (p = 0.01). Using these criteria, a target population for treatment would be patients with DWI lesion volume ≤72 ml, MTT volume ≥47 ml and NIHSS score ≥8 and ≤20. However, this approach awaits clinical validation in an independent cohort.
A third approach combines vessel imaging and baseline DWI lesion volume. In a post-hoc analysis of DEFUSE, the MRA-DWI mismatch was defined as:
A proximal artery occlusion with DWI lesion volume <25 ml;
Proximal artery stenosis or abnormal distal vessel with DWI lesion volume <15 ml [160].
Proximal arteries were the terminal ICA and MCA M1 segment. Distal arteries were the MCA M2 segments and the anterior and posterior cerebral arteries. In total, 27 out of 62 patients (44%) had an MRA-DWI mismatch. The agreement between the MRA-DWI and PWI-DWI mismatch models was 63% (95% CI: 50–74%). Early reperfusion (3–6 h after tPA administration) was associated with increased odds of favorable outcome in patients with MRA-DWI mismatch (OR: 12.3; 95% CI: 1.8–84.0). There was a decrease in favorable outcomes with early reperfusion among patients without MRA-DWI mismatch (OR: 0.2; 95% CI: 0.0–0.8), although this was not significant after adjusting for baseline characteristics.
Ongoing imaging studies of iv. thrombolysis
Despite numerous studies, there is conflicting evidence that perfusion imaging improves clinical outcomes [161]. The mismatch approach, in particular, awaits further testing. Multiple ongoing trials are exploring MRI- and CT-based imaging signatures for identifying favorable candidates for iv. reperfusion therapies (Table 4).
Table 4.
Ongoing imaging trials of intravenous thrombolysis.
| Trial | Type | Patients (n) | Time (min) | Imaging criteria | Drug | Primary outcome |
|---|---|---|---|---|---|---|
| DIAS-iv. | RCT | 400 | 180–540 | MRA/CTA† | Desmoteplase | 90-day mRS |
| START-EXTEND‡ | RCT | 400 | 180–540 | PWI-DWI mismatch§ | Alteplase | 90-day mRS |
| 1000Plus | ¶ | 1200 | 0–1440 | MTT-DWI mismatch | None required | Infarct growth at day 5–7 |
| ITAIS-II | # | 200 | 180–540 | CTP-CTASI mismatch†† | Alteplase | 90-day mRS |
| ITAIS-III | # | 200 | 180–540 | CTP-CTASI mismatch†† | Alteplase | 90-day mRS |
Proximal occlusion seen on MRA or CTA. Patients with ICA occlusion or extensive early infarcts will be excluded.
A pilot study (START-EXTEND) will include 100 patients in selected centers. After successful completion of the pilot, EXTEND will be initiated.
Mismatch defined as >20% and >10-ml difference between Tmax thresholded to 6-s delay and DWI lesion volume.
Single-center, prospective, observational study.
Prospective, single-arm, multicenter trial.
Mismatch defined as >20% difference between CTP and CTASI lesion volume and a CTP lesion >2 cm.
CTA: Computed tomography angiogram; CTASI: CTA source image; CTP: Computed tomography perfusion; DIAS: Desmoteplase in Acute Ischemic Stroke; DWI: Diffusion-weighted imaging; ITAIS: Imaging-Based Thrombolysis Trial in Acute Ischemic Stroke; MRA: Magnetic resonance angiogram; mRS: Modified Rankin score; MTT: Mean transit time; PWI: Perfusion-weighted imaging; RCT: Randomized controlled trial; START-EXTEND: Stroke Imaging Prevention and Treatment-Extending the Time for Thrombolysis in Emergency Neurological Deficits.
The DIAS-4 is a Phase III, placebo-controlled RCT that aims to confirm the efficacy and safety of iv. desmoteplase (90 μg/kg dose) for AIS patients in the 3–9-h window [201,202]. Unlike previous desmoteplase trials, the use of a mismatch criterion based on MR or CT perfusion imaging has been abandoned. Major inclusion criteria include NIHSS score between 4 and 24, and proximal cerebral artery occlusion or high-grade stenosis on MRA or CTA. Major exclusion criteria include ICA occlusion and evidence of extensive early infarction on MRI or CT scan. The primary outcome will be a 90-day mRS score. The planned enrollment is 400 patients, and is expected to be completed in mid-2012.
The Stroke Imaging Prevention and Treatment-Extending the Time for Thrombolysis in Emergency Neurological Deficits (START-EXTEND) trial is a pilot study of the planned Phase III Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND) trial [201,202]. In total, 100 patients will be enrolled in the pilot study to receive either iv. tPA (0.9 mg/kg) or placebo in a randomized fashion, after which more centers will accrue a final sample size of 400 patients. Eligibility criteria include NIHSS score between 4 and 26, treatment with iv. tPA between 3 (or 4.5 h per local protocol) to 9 h after onset, and a significant penumbral pattern (PWI-DWI mismatch of >20% and ≥10 ml mismatch volume using Tmax thresholded to >6-s delay). Patients with a DWI lesion volume >70 ml will be excluded. The primary outcome will be a 90-day mRS of 0–1.
The 1000Plus study is a single-center, prospective observational study to assess whether the prognostic value of the mismatch is dependent on time to imaging and vessel status [201,162]. The study will enroll patients with AIS or transient ischemic attack within 24 h of onset. Patients will undergo MRI/MRA with neurologic evaluation at days 1, 2 and 5–7. MRI will be performed on a 3 Tesla MR scanner, and the PWI-DWI mismatch will be assessed using MTT maps and semi-automated software. Thrombolytic treatment is permissible but not required. The primary outcome will be infarct growth between baseline and days 5–7. Planned enrollment is 1200 patients, and the estimated completion date is October 2011.
Finally, Imaging-Based Thrombolysis Trial in Acute Ischemic Stroke (ITAIS)-II and ITAIS-III are ongoing trials that will each recruit 200 patients in the 3–9-h time window [163,203]. Patients with a mismatch between CTP (penumbra) and CTA source image (core) lesion volume of ≥20% and a CTP lesion >2 cm will be enrolled and treated with iv. tPA (0.9 mg/kg). The primary end points will be a 90-day mRS of 0–1, ICH at 24–36 h, and reperfusion at 24–48 h. Comparisons will be made with published trial data using standard CT-based criteria in the 0–3 and 3–6-h windows.
Imaging selection for intra-arterial therapies
There has been a marked growth in the use of endovascular revascularization therapies in recent years [164]. However, there remains little evidence for improved outcomes with this approach. The only RCT to demonstrate such a benefit was the Prolyse in Acute Cerebral Thromboembolism (PROACT) II study [165]. The MERCI [7], Multi-MERCI [166] and Penumbra Pivotal [8] trials were prospective, single-arm studies of mechanical devices that used reperfusion, rather than clinical outcome, as their primary end points.
According to the Stroke Therapy Academic Industry Roundtable (STAIR), equipoise exists between catheter-based intervention and supportive medical care, and IA therapy should be further tested in the setting of RCTs [2]. Moreover, STAIR supports the use of imaging-based penumbral selection for such extended window trials. The problem is that there are few data on the effectiveness of advanced imaging for selecting patients for IAT. To date, the major trials of IA therapy have utilized NCCT to exclude patients with extensive infarctions (e.g., greater than a third of the MCA territory) or with significant edema and mass effect (Table 5).
Table 5.
Patient selection in the major trials of intra-arterial therapy.
| Trial | Time (min) | NIHSSS | NCCT | Angiography | Ref. |
|---|---|---|---|---|---|
| IMS I and II† | 0–180 | ≥10 | Infarct ≤1/3 of the MCA territory ‡; no ICH or midline shift | Occlusion of ACA, MCA, PCA or BA | [65] |
| PROACT II | 0–360 | ≥4 and ≤30 | Infarct ≤1/3 of the MCA territory §; no ICH, midline shift or tumor | TIMI grade 0–1 of MCA M1 or M2 | [165] |
| MERCI | 180–480¶ | ≥8 | Infarct ≤1/3 of the MCA territory ‡; no ICH or midline shift | Occlusion of VA, BA, ICA, MCA M1 or M2 | [7] |
| Multi-MERCI | 180–480¶ | ≥8 | No midline shift | Occlusion of VA, BA, ICA, MCA M1 or M2 | [166] |
| Penumbra Pivotal | 180–480¶ | ≥8 | Infarct ≤1/3 of the MCA territory; no severe edema or ICH | Treatable large intracranial vessel occlusion | [8] |
Combined intravenous and IA tPA bridging therapy.
Hypodensity (sulcal effacement and loss of gray-white matter differentiation allowed).
Hypodensity or sulcal effacement.
0–180 min if ineligible or refractory to iv. tPA.
ACA: Anterior cerebral artery; BA: Basilar artery; ICA: Internal carotid artery; ICH: Intracranial hemorrhage; IMS: Interventional Management of stroke; M1: MCA stem; M2: MCA post-bifurcation segment; MCA: Middle cerebral artery; NCCT: Noncontrast computed tomography; NIHSSS: NIH Stroke Scale score; PCA: Posterior cerebral artery; PROACT II: Prolyse in Acute Cerebral Thromboembolism II; TIMI: Thrombolysis in Myocardial Infarction grading system; tPA: Tissue palsminogen activator; VA: Vertebral artery.
Can the perfusion–diffusion mismatch identify favorable IAT candidates?
The primary requirement for IAT is the presence of a proximal artery occlusion, such as the ICA or proximal MCA. Given the large parenchymal territory supplied by these vessels, the mismatch approach is a poor discriminator for identifying IAT candidates with a favorable collateral physiology. One reason is that perfusion imaging methods are highly sensitive for identifying hypoperfused tissue, but not specific for delineating true territory at risk. In a recent analysis of 116 patients with ICA or proximal MCA occlusions, 90 out of 93 patients (96.8%) with baseline DWI lesion volume ≤100 ml had at least 100% mismatch [167]. The results were similar when using nonthresholded MTT or TTP with a 4-s delay.
The importance of pretreatment core infarct volume
While the true extent of the penumbra cannot be known with certainty in the clinical setting, it can be reasonably assumed that this territory is quite large in patients with PAO who present with significant neurological deficit (e.g., NIHSS score >10). On average, the MCA territory and the ICA territory (anterior cerebral artery + MCA) constitute approximately 250–300 and 400–450 cm3 of brain tissue, respectively [168]. Therefore, substantial tissue at risk is probably present even when the core infarct exceeds 100 cm3 (~a-third of the MCA territory) [167]. In this setting, the benefit of penumbral salvage will be outweighed by the extensive deficit and procedural risk related to the large infarct core. This suggests that treatment response in anterior circulation PAO patients may be better predicted by baseline infarct size, rather than the size of the penumbra. This idea is supported by a study in which 36 patients with MCA M1 occlusions were evaluated with xenon-enhanced CT within 6 h of symptom onset [169]. The authors found that the core infarct volume (percent MCA territory with CBF <8 ml/100 g/min) was an independent predictor of favorable outcome (mRS: 0–1; OR: 0.85; 95% CI: 0.74–0.98), while the penumbral volume (percent MCA territory with CBF: 8–20 ml/100 g/min) was not associated with outcome in univariate or multivariate analysis (p = 0.5). In this study, the treatments were variable: 26 patients received iv. and/or IA therapy, and ten patients were not treated.
The utility of core infarct size for treatment selection in PAO is supported by multiple studies demonstrating that:
Patients with large pretreatment DWI lesions do poorly despite successful reperfusion;
Patients with small DWI lesions will do well depending largely on timely reperfusion.
Several studies have demonstrated that PAO patients who have early extensive DWI lesions have a worse clinical course with a significantly higher risk of malignant brain swelling [71,170,171]. Among the studies where MRI was performed within 6 h of onset, DWI lesion volume thresholds of >82 cm3 (sensitivity 87/specificity 91%) [170] and >89 cm3 (85.7/95.7%) [71] optimally predicted malignant cerebral edema. Furthermore, the DEFUSE study demonstrated that acute stroke patients with early (3–6 h after onset) and extensive infarcts generally had poor outcomes despite early reperfusion [102]. In this study, only one out of six patients with a ‘malignant profile’ (DWI lesion ≥100 cm3 and/or PWI lesion ≥100 cm3 with ≥8 s of Tmax delay) had a favorable outcome despite early reperfusion in three patients.
Recent evidence suggests that the core infarct volume threshold for poor outcomes may be even lower than a third of the MCA territory. Three studies have shown that baseline DWI lesion volume >70 cm3 is highly specific for poor outcomes with or without therapy [159,172,173]. In a retrospective study of 34 patients who underwent pretreatment MRI followed by IAT [174], there were six patients with baseline DWI lesion size >70 cm3 (‘Futile group’), all of whom had a poor 3-month outcome (mRS: 3–6) despite reperfusion in three patients. Within the study population, the Futile group patients had the largest infarct growth.
For PAO patients who do not have extensive infarcts on hyper-acute imaging (<6 h), clinical and tissue outcome is strongly dependent on early reperfusion. As previously mentioned, a post-hoc analysis of DEFUSE [160] demonstrated that PAO patients with DWI lesion volume <25 ml had marked improvement in outcomes if they underwent early reperfusion (OR: 12.5; 95% CI: 1.8–83.9). Similarly, among anterior circulation PAO patients treated with IAT [174], those with a pretreatment DWI lesion volume <70 cm3 had higher rates of good outcomes (3-month mRS of ≤2) with early reperfusion (64%) compared with late (17%) or no (0%) reperfusion (p < 0.016). Furthermore, there was greater infarct growth in patients who underwent delayed or no reperfusion. Despite differences in the actual threshold, these studies illustrate the importance of core infarct size in shaping the clinical response to reperfusion, and suggest that PAO patients with small core infarcts may represent a target population for IAT. Further studies are needed to better define the relationship between core infarct size, early reperfusion and clinical outcome in this patient population.
The importance of baseline core infarct size in IAT-treated patients has also been demonstrated in studies using CT-based methods for infarct delineation, including NCCT (using the Alberta Stroke Program Early CT Score [ASPECTS] classification) [175,176], CTA source images [177] and CTP-CBV maps [178].
Ongoing imaging studies of IAT
Please refer to Table 6. MR and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) is an ongoing Phase II RCT comparing mechanical revascularization therapy using the Merci Retriever or the Penumbra Stroke System plus standard medical care versus standard medical care alone [201,202]. All patients will undergo pretreatment MRI with PWI or CT perfusion imaging. The aim is to determine whether pretreatment imaging findings influence the response to IAT. Eligibility criteria include anterior circulation PAO (ICA, MCA M1 or M2 segment), NIHSS score ≥6 and procedure initiation within 8 h from onset. The primary outcome measure is a 90-day mRS score. Approximately 70 out of the planned 120 patients have been accrued.
Table 6.
Ongoing imaging trials of intra-arterial therapy.
| Trial | Type | Patients (n) | Time (min) | Imaging criteria | Device | Primary outcome |
|---|---|---|---|---|---|---|
| MR RESCUE | RCT | 120 | 0–480 | PWI-DWI or CTP mismatch | Merci Retriever and Penumbra Stroke System | 90-day mRS |
| START | † | 200 | 0–480 | NCCT plus CTASI, CTP or DWI | Penumbra Stroke System | 90-day mRS |
| DEFUSE II | † | 100 | 0–720 | PWI-DWI ratio >1.8‡ | IA thrombolytic and/or mechanical therapy | 30-day NIHSSS |
Prospective, multicenter, single-arm trial.
Tmax with a threshold of 6 s will be used. DWI must be <70 ml and Tmax must be <100 ml.
CTASI: Computed tomography angiographic source image; CTP: Computed tomography perfusion; DEFUSE: Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution; DWI: Diffusion-weighted imaging; IA: Intra-arterial; MR RESCUE: MR and Recanalization of Stroke Clots Using Embolectomy; mRS: Modified Rankin score; NCCT: Noncontrast computed tomography; NIHSSS: NIH Stroke Scale score; PWI: Perfusion-weighted imaging; RCT: Randomized controlled trial; START: Stroke Treatment And Revascularization Therapy.
The START trial is a Phase IV, prospective single-arm study that will test whether pretreatment core infarct volume is correlated with functional outcome in patients treated with the Penumbra Stroke System [201,202]. All patients will undergo NCCT as well as one advanced imaging modality for delineation of core infarct size: CTA source images, CT perfusion and MRI DWI. Inclusion criteria include anterior circulation PAO, NIHSS score >10 and presentation within 8 h of onset. The primary outcome measures are a 90-day mRS of 0–2, angiographic assessment of reperfusion (TIMI/TICI scores) and procedural serious adverse events. Approximately 70 of the planned 200 patients have been accrued.
DEFUSE 2 is a prospective single-arm study which will test whether a specified PWI/DWI mismatch can predict which PAO patients will benefit from early reperfusion via IAT. Eligibility criteria include hemispheric stroke with NIHSS score ≥5 and baseline MRI including DWI, PWI and MRA. The MRI findings may be used for clinical decision making. Automated software will create research maps for calculating the mismatch. A target mismatch will be defined as a PWI volume (Tmax >6 s) that is at least 1.8 times as large as the DWI volume. Furthermore, the DWI lesion must be <70 ml and the PWI lesion <100 ml. The primary end point will be an NIHSS improvement of at least 8 points or score of 0–1 at 30 days. Thus far, 50 of the planned 100 patients have been enrolled [Albers G, Pers. Comm.].
Using imaging to assess treatment-related risk: predictors of hemorrhagic transformation
The benefits of reperfusion therapy must be balanced against its risks, particularly post-treatment ICH. Evidence from both iv. and IA studies suggests that pretreatment neuroimaging may be able to identify patients at high risk for hemorrhagic transformation. Early ischemic changes on NCCT (e.g., loss of gray-white matter differentiation, effacement of sulci) have been associated with sICH and parenchymal hematomas [179–181], although other studies were not able to confirm these findings [182,183]. MRI appears to provide a better assessment of clinically significant hemorrhage risk than NCCT [37,184]. In a retrospective multicenter study of 645 patients with anterior circulation ischemic stroke treated with iv. or IA thrombolysis [185], DWI lesion size was an independent risk factor for sICH (OR 1.080; 95%CI 1.012–1.153 per 10 ml increase). The rates of symptomatic ICH were 2.8, 7.8 and 16.1% for small (≤10 ml), moderate (10–100 ml) and large (>100 ml) DWI lesions, respectively (p < 0.05). Of the study cohort, 109 patients (16.9%) underwent intra-arterial therapy. Numerous studies [184,186,187] have confirmed this finding. Moreover, the elevated risk in patients with large DWI lesions appears to be further increased when there is subsequent reperfusion. In a post-hoc analysis of the DEFUSE study [184], only the interaction between DWI lesion volume and reperfusion status was an independent predictor of sICH (OR: 1.77; 95% CI: 1.25–2.50 per 10 ml increase in DWI volume) when early reperfusion status was included in the model. Therefore, an acute infarct volume threshold may not only identify patients who will respond to reperfusion, but may also be used to exclude patients with an increased risk of harm from treatment.
Feasibility & practicality of advanced neuroimaging in the treatment setting
If advanced neuroimaging selection is validated for extended window therapies, there will be challenges to the implementation of these imaging protocols, particularly for MRI. While MRI has been demonstrated to be feasible in the treatment setting [188–190], multiple patient-related factors prevent imaging of all stroke patients, including contraindications to MRI, agitation, diminished consciousness and unstable medical condition [191,192]. Fast imaging protocols (under 15 min) and head immobilization may alleviate the issues related to patient agitation and medical instability [188,189,193]. In addition, there are organizational hurdles such as scanner availability, presence of trained personnel and time delays to imaging [191]. However, increased experience has been shown to facilitate logistics and decrease imaging-related delays [189]. In one study, there was no significant difference in door-to-needle times between MRI and CT [190].
Keeping in mind that no treatment is offered beyond 3 or 4.5 h in the majority of medical centers, stroke MR imaging for extended window therapy would produce an increase in the number of treatable patients, despite the aforementioned challenges. In fact, the majority of late-presenting patients (up to 85%) should be able to undergo such imaging [188,190–192]. It should be acknowledged that such an approach will probably increase health care costs, although this could be justified if there is a proven clinical benefit [189].
Expert commentary & five-year view
The use of time from stroke onset and noncontrast CT scans for reperfusion therapy decisions in AIS is likely to be supplemented by advanced neuroimaging that provides critical information on relevant brain physiology in the extended time window. However, despite the extensive number of investigations, there is no clear-cut evidence that the most widely employed advanced neuroimaging approach improves clinical outcomes (Table 7). Specifically, studies of the MRI diffusion–perfusion mismatch have yielded conflicting results. It is apparent that alternative imaging criteria are needed to better identify which patients will respond to early reperfusion.
Table 7.
Rationale for various imaging-based triage algorithms for acute ischemic stroke reperfusion therapy.
| Imaging approach | Advantages | Disadvantages |
|---|---|---|
| Noncontrast CT (and time from stroke onset) | Has been validated in large RCTs of iv. tPA and in PROACT II for IA thrombolysis Widely available and well established in the clinical setting | Low number of eligible patients No clinical benefit for iv. tPA beyond 4.5 h Poor sensitivity for detecting ischemic change, especially in large early infarcts Poor interobserver reliability |
| Mismatch approach (advanced imaging) | Has been demonstrated in multiple large studies to extend the iv. tPA window up to 6 h Benefit for iv. desmoteplase up to 9 h in mismatch patients (DIAS, DEDAS) Mismatch patients demonstrate favorable clinical response to early reperfusion (DEFUSE) |
Failure of DIAS-2 to validate clinical benefit of iv. desmoteplase in a 3–9-h window in mismatch patients No difference between mismatch and nonmismatch patients for reperfusion, infarct growth and clinical outcome after iv. tPA treatment (EPITHET) 20% mismatch arbitrary, and not optimal in post-hoc analyses Same mismatch ratio may correspond to varying stroke severities MR and CT perfusion not standardized and not true territory at risk |
| Absolute lesion volumes (advanced imaging): Clinical-diffusion mismatch Combined DWI/MTT/NIHSS thresholds MRA-DWI mismatch |
DWI and PWI lesion volumes correlate better with outcome after iv. tPA than mismatch ratio in post-hoc analysis of EPITHET Multiple studies have demonstrated the utility of core infarct volume in selecting patients for iv. tPA and for IAT Baseline DWI lesion size shown to influence risk of intracerebral hemorrhage following treatment |
Requires further validation MRI is not widely available in the clinical setting CT perfusion requires proof of reliability and standardization of technique |
CT: Computed tomography; DEDAS: Dose Escalation of Desmoteplase for Acute Ischemic Stroke; DEFUSE: Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution; DIAS: Desmoteplase in Acute Ischemic Stroke; DWI: Diffusion-weighted imaging; EPITHET: Echoplanar Imaging Thrombolysis Evaluation Trial; IA: Intra-arterial; iv.: Intravenous; MR: Magnetic resonance; MRA: Magnetic resonance angiography; MTT: Mean transit time; NIHSS: NIH Stroke Scale; PROACT II: Prolyse in Acute Cerebral Thromboembolism II; PWI: Perfusion-weighted imaging; RCT: Randomized controlled trial; tPA: Tissue plasminogen activator.
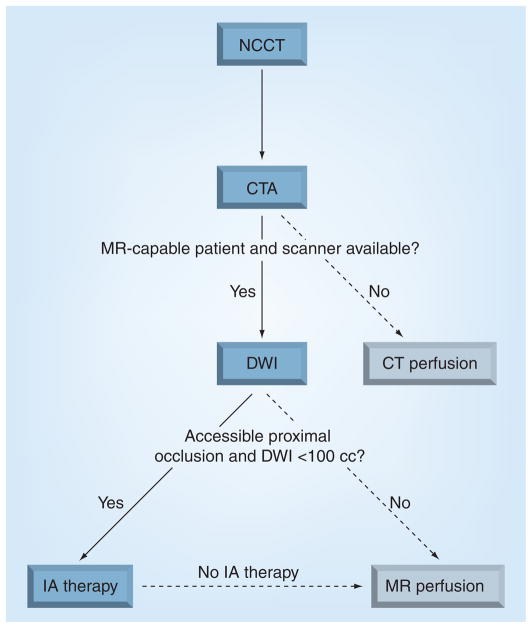
In the next 5 years, we believe that the mismatch hypothesis will be invalidated. Inherent flaws of the mismatch approach include its relative nature that results in a nonspecific representation of ischemic stroke physiology. We believe that approaches based on the severity of the clinical deficit, site of arterial occlusion and absolute core infarct volume will prove to be more accurate for identifying patients who will respond to treatment by excluding those patients who cannot be rescued (e.g., very large core infarcts) or in whom treatment will provide no further benefit (e.g., mild deficits and no visible occlusion). It is probable that such criteria will also consider the effectiveness of the treatment approach (iv. vs IA therapy). We have already incorporated these principles in our current algorithm for triaging patients to endovascular therapy (Figure 4).
Figure 4. Massachusetts General Hospital imaging algorithm for deciding which patients to treat with endovascular stroke therapy.
CT: Computed tomography; CTA: Computed tomography angiography; DWI: Diffusion-weighted imaging; IA: Intra-arterial; MR: Magnetic resonance; NCCT: Noncontrast computed tomography.
Reprinted with permission from Massachusetts General Hospital (MA, USA).
Key issues.
The current approach to patient selection for stroke reperfusion therapies is based on the time from stroke symptom onset. This approach is reasonable in the first 3–4.5 h after stroke when substantial salvageable tissue probably exists in the majority of patients. However, it neglects the variable collateral physiology that exists between individual patients and probably plays a critical role beyond this time window.
Advanced neuroimaging can provide important information on the state of the brain parenchyma and of the neurovasculature, which may guide treatment outside of current time windows.
Despite extensive studies in the setting of intravenous thrombolysis, there is no clear evidence that the MRI diffusion–perfusion mismatch can improve clinical outcomes.
Based on recent data, alternative approaches employing absolute lesion volumes of the core infarct and of the surrounding region of hypoperfusion appear promising, but require validation.
Limited data in the setting of intra-arterial stroke therapies suggest that the baseline core infarct volume in the presence of a major artery occlusion and severe neurological deficit (NIH stroke scale score ≥10) may provide sufficient information for predicting treatment response.
Major trials investigating imaging selection are ongoing and aim to validate these refined approaches.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Albert J Yoo has received research support from Penumbra, Inc. R Gilberto González has received research support from the National Institutes for Health and from Penumbra, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40(7):2594–2600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 5.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 7.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36(7):1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 8.Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40(8):2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 9.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 10.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372(9646):1303–1309. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- 11.Saver JL, Smith EE, Fonarow GC, et al. The ‘golden hour’ and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41(7):1431–1439. doi: 10.1161/STROKEAHA.110.583815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saver JL. Time is brain – quantified. Stroke. 2006;37(1):263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 13.Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. 2000;55(11):1649–1655. doi: 10.1212/wnl.55.11.1649. [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 15••.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–1703. doi: 10.1016/S0140-6736(10)60491-6. Pooled analysis of the major randomized controlled trials for intravenous tissue plasminogen activator, which provides the strongest evidence for the ‘time is brain’ paradigm. [DOI] [PubMed] [Google Scholar]
- 16.Cocho D, Belvis R, Marti-Fabregas J, et al. Reasons for exclusion from thrombolytic therapy following acute ischemic stroke. Neurology. 2005;64(4):719–720. doi: 10.1212/01.WNL.0000152041.20486.2F. [DOI] [PubMed] [Google Scholar]
- 17.Katzan IL, Furlan AJ, Lloyd LE, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA. 2000;283(9):1151–1158. doi: 10.1001/jama.283.9.1151. [DOI] [PubMed] [Google Scholar]
- 18.Smith MA, Doliszny KM, Shahar E, McGovern PG, Arnett DK, Luepker RV. Delayed hospital arrival for acute stroke: the Minnesota Stroke Survey. Ann Intern Med. 1998;129(3):190–196. doi: 10.7326/0003-4819-129-3-199808010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez RG. Imaging-guided acute ischemic stroke therapy: From ‘time is brain’ to ‘physiology is brain’. AJNR Am J Neuroradiol. 2006;27(4):728–735. [PMC free article] [PubMed] [Google Scholar]
- 20.Caplan LR. Stroke thrombolysis: slow progress. Circulation. 2006;114(3):187–190. doi: 10.1161/CIRCULATIONAHA.106.638973. [DOI] [PubMed] [Google Scholar]
- 21.Marchal G, Beaudouin V, Rioux P, et al. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET-CT study with voxel-based data analysis. Stroke. 1996;27(4):599–606. doi: 10.1161/01.str.27.4.599. [DOI] [PubMed] [Google Scholar]
- 22.Baird AE, Benfield A, Schlaug G, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41(5):581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 23.Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40(2):216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- 24.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73(13):1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of clinical outcomes in acute ischemic stroke patients undergoing thrombectomy: pooled analysis of the MERCI and Multi MERCI trials. Stroke. 2009;40(4):26. doi: 10.1161/STROKEAHA.109.561431. [DOI] [PubMed] [Google Scholar]
- 26.Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54(6):773–782. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- 27.Baron JC. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis. 2001;11(Suppl 1):2–8. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- 28.Heiss WD, Kracht LW, Thiel A, Grond M, Pawlik G. Penumbral probability thresholds of cortical flumazenil binding and blood flow predicting tissue outcome in patients with cerebral ischaemia. Brain. 2001;124(Pt 1):20–29. doi: 10.1093/brain/124.1.20. [DOI] [PubMed] [Google Scholar]
- 29.Liebeskind DS. Collateral circulation. Stroke. 2003;34(9):2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 30••.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26(7):1789–1797. Angiographically controlled study clearly illustrates the importance of the pial collateral circulation in determining tissue fate and clinical outcome after acute ischemic stroke. [PMC free article] [PubMed] [Google Scholar]
- 31.Kucinski T, Koch C, Eckert B, et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology. 2003;45(1):11–18. doi: 10.1007/s00234-002-0881-0. [DOI] [PubMed] [Google Scholar]
- 32.Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30(3):525–531. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bang OY, Saver JL, Buck BH, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79(6):625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ringelstein EB, Biniek R, Weiller C, Ammeling B, Nolte PN, Thron A. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology. 1992;42(2):289–298. doi: 10.1212/wnl.42.2.289. [DOI] [PubMed] [Google Scholar]
- 35.Takada T, Yasaka M, Minematsu K, Naritomi H, Yamaguchi T. Predictors of clinical outcome in patients receiving local intra-arterial thrombolysis without subsequent symptomatic intracranial hemorrhage against acute middle cerebral artery occlusion. AJNR Am J Neuroradiol. 2004;25(10):1796–1801. [PMC free article] [PubMed] [Google Scholar]
- 36.von Kummer R, Holle R, Rosin L, Forsting M, Hacke W. Does arterial recanalization improve outcome in carotid territory stroke? Stroke. 1995;26(4):581–587. doi: 10.1161/01.str.26.4.581. [DOI] [PubMed] [Google Scholar]
- 37••.Schellinger PD, Thomalla G, Fiehler J, et al. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows: an analysis of 1210 patients. Stroke. 2007;38(10):2640–2645. doi: 10.1161/STROKEAHA.107.483255. This large, multicenter study demonstrates that imaging evidence of favorable collateral physiology may be more important than stroke duration for determining clinical outcomes following extended window therapy. [DOI] [PubMed] [Google Scholar]
- 38.Kohrmann M, Juttler E, Fiebach JB, et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: a cohort study. Lancet Neurol. 2006;5(8):661–667. doi: 10.1016/S1474-4422(06)70499-9. [DOI] [PubMed] [Google Scholar]
- 39.Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a Phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36(1):66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 40.Ebinger M, Christensen S, De Silva DA, et al. Expediting MRI-based proof-of-concept stroke trials using an earlier imaging end point. Stroke. 2009;40(4):1353–1358. doi: 10.1161/STROKEAHA.108.532622. [DOI] [PubMed] [Google Scholar]
- 41.Warach S, Pettigrew LC, Dashe JF, et al. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Ann Neurol. 2000;48(5):713–722. [PubMed] [Google Scholar]
- 42.Barrett KM, Ding YH, Wagner DP, Kallmes DF, Johnston KC. Change in diffusion-weighted imaging infarct volume predicts neurologic outcome at 90 days: results of the Acute Stroke Accurate Prediction (ASAP) trial serial imaging substudy. Stroke. 2009;40(7):2422–2427. doi: 10.1161/STROKEAHA.109.548933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hakimelahi R, Romero J, Nogueira RG, et al. American Society of Neuroradiology. Vancouver, BC, Canada: 2009. Final infarct volume is the best predictor of outcome in large vessel occlusion patients treated with IAT. [Google Scholar]
- 44.Ginsberg MD, Pulsinelli WA. The ischemic penumbra, injury thresholds, and the therapeutic window for acute stroke. Ann Neurol. 1994;36(4):553–554. doi: 10.1002/ana.410360402. [DOI] [PubMed] [Google Scholar]
- 45.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36(4):557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 46.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke. 1981;12(6):723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 47.Soares BP, Tong E, Hom J, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization. A proof of concept using CT in acute ischemic stroke patients. Stroke. 2009;41(1):e34–e40. doi: 10.1161/STROKEAHA.109.568766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermier M, Nighoghossian N, Adeleine P, et al. Early magnetic resonance imaging prediction of arterial recanalization and late infarct volume in acute carotid artery stroke. J Cereb Blood Flow Metab. 2003;23(2):240–248. doi: 10.1097/01.WCB.0000043340.09081.7E. [DOI] [PubMed] [Google Scholar]
- 49.King S, Khatri P, Carrozella J, et al. Anterior cerebral artery emboli in combined intravenous and intra-arterial rtPA treatment of acute ischemic stroke in the IMS I and II trials. AJNR Am J Neuroradiol. 2007;28(10):1890–1894. doi: 10.3174/ajnr.A0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiebach J, Jansen O, Schellinger P, et al. Comparison of CT with diffusion-weighted MRI in patients with hyperacute stroke. Neuroradiology. 2001;43(8):628–632. doi: 10.1007/s002340100542. [DOI] [PubMed] [Google Scholar]
- 51.Fiebach JB, Schellinger PD, Jansen O, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke. 2002;33(9):2206–2210. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 52.Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment – systematic review. Radiology. 2005;235(2):444–453. doi: 10.1148/radiol.2352040262. [DOI] [PubMed] [Google Scholar]
- 53.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barber PA, Darby DG, Desmond PM, et al. Identification of major ischemic change. Diffusion-weighted imaging versus computed tomography. Stroke. 1999;30(10):2059–2065. doi: 10.1161/01.str.30.10.2059. [DOI] [PubMed] [Google Scholar]
- 55.Lansberg MG, Albers GW, Beaulieu C, Marks MP. Comparison of diffusion-weighted MRI and CT in acute stroke. Neurology. 2000;54(8):1557–1561. doi: 10.1212/wnl.54.8.1557. [DOI] [PubMed] [Google Scholar]
- 56.Camargo EC, Furie KL, Singhal AB, et al. Acute brain infarct: detection and delineation with CT angiographic source images versus nonenhanced CT scans. Radiology. 2007;244(2):541–548. doi: 10.1148/radiol.2442061028. [DOI] [PubMed] [Google Scholar]
- 57••.Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute ischemic stroke. A scientific statement from the American Heart Association. Stroke. 2009;40(11):3646–3678. doi: 10.1161/STROKEAHA.108.192616. Expert panel recommendation that provides a comprehensive review of the available neuroimaging techniques and their utility in acute ischemic stroke management. [DOI] [PubMed] [Google Scholar]
- 58.Butcher KS, Lee SB, Parsons MW, et al. Differential prognosis of isolated cortical swelling and hypoattenuation on CT in acute stroke. Stroke. 2007;38(3):941–947. doi: 10.1161/01.STR.0000258099.69995.b6. [DOI] [PubMed] [Google Scholar]
- 59.Parsons MW, Pepper EM, Bateman GA, Wang Y, Levi CR. Identification of the penumbra and infarct core on hyperacute noncontrast and perfusion CT. Neurology. 2007;68(10):730–736. doi: 10.1212/01.wnl.0000256366.86353.ff. [DOI] [PubMed] [Google Scholar]
- 60.Mullins ME, Schaefer PW, Sorensen AG, et al. CT and conventional and diffusion-weighted MR imaging in acute stroke: study in 691 patients at presentation to the emergency department. Radiology. 2002;224(2):353–360. doi: 10.1148/radiol.2242010873. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez RG, Schaefer PW, Buonanno FS, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology. 1999;210(1):155–162. doi: 10.1148/radiology.210.1.r99ja02155. [DOI] [PubMed] [Google Scholar]
- 62.Heiss WD, Sobesky J, Smekal U, et al. Probability of cortical infarction predicted by flumazenil binding and diffusion-weighted imaging signal intensity: a comparative positron emission tomography/magnetic resonance imaging study in early ischemic stroke. Stroke. 2004;35(8):1892–1898. doi: 10.1161/01.STR.0000134746.93535.9b. [DOI] [PubMed] [Google Scholar]
- 63.Kranz PG, Eastwood JD. Does diffusion-weighted imaging represent the ischemic core? An evidence-based syst ematic review. AJNR Am J Neuroradiol. 2009;30(6):1206–1212. doi: 10.3174/ajnr.A1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guadagno JV, Warburton EA, Jones PS, et al. How affected is oxygen metabolism in DWI lesions?: a combined acute stroke PET-MR study. Neurology. 2006;67(5):824–829. doi: 10.1212/01.wnl.0000233984.66907.db. [DOI] [PubMed] [Google Scholar]
- 65.Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35(4):904–911. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 66.Kohno K, Hoehn-Berlage M, Mies G, Back T, Hossmann KA. Relationship between diffusion-weighted MR images, cerebral blood flow, and energy state in experimental brain infarction. Magn Reson Imaging. 1995;13(1):73–80. doi: 10.1016/0730-725x(94)00080-m. [DOI] [PubMed] [Google Scholar]
- 67.Kidwell CS, Saver JL, Starkman S, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52(6):698–703. doi: 10.1002/ana.10380. [DOI] [PubMed] [Google Scholar]
- 68.Schaefer PW, Hassankhani A, Putman C, et al. Characterization and evolution of diffusion MR imaging abnormalities in stroke patients undergoing intra-arterial thrombolysis. AJNR Am J Neuroradiol. 2004;25(6):951–957. [PMC free article] [PubMed] [Google Scholar]
- 69.Kidwell CS, Saver JL, Mattiello J, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47(4):462–469. [PubMed] [Google Scholar]
- 70.Fiehler J, Knudsen K, Kucinski T, et al. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke. 2004;35(2):514–519. doi: 10.1161/01.STR.0000114873.28023.C2. [DOI] [PubMed] [Google Scholar]
- 71.Arenillas JF, Rovira A, Molina CA, Grive E, Montaner J, Alvarez-Sabin J. Prediction of early neurological deterioration using diffusion- and perfusion-weighted imaging in hyperacute middle cerebral artery ischemic stroke. Stroke. 2002;33(9):2197–2203. doi: 10.1161/01.str.0000027861.75884.df. [DOI] [PubMed] [Google Scholar]
- 72.Chalela JA, Kang DW, Luby M, et al. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: Insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol. 2004;55(1):105–112. doi: 10.1002/ana.10781. [DOI] [PubMed] [Google Scholar]
- 73.Olivot JM, Mlynash M, Thijs VN, et al. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke. 2009;40(5):1692–1697. doi: 10.1161/STROKEAHA.108.538082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neumann-Haefelin T, Kastrup A, de Crespigny A, et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood–brain barrier damage, and edema formation. Stroke. 2000;31(8):1965–1972. doi: 10.1161/01.str.31.8.1965. discussion 1972–1963. [DOI] [PubMed] [Google Scholar]
- 75.Li F, Liu KF, Silva MD, et al. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats: correlation with histopathology. Stroke. 2000;31(4):946–954. doi: 10.1161/01.str.31.4.946. [DOI] [PubMed] [Google Scholar]
- 76.Chemmanam T, Campbell BC, Christensen S, et al. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion–diffusion mismatch. Neurology. 2010;75(12):1040–1047. doi: 10.1212/WNL.0b013e3181f39ab6. [DOI] [PubMed] [Google Scholar]
- 77.Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75(2):177–185. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schramm P, Schellinger PD, Fiebach JB, et al. Comparison of CT and CT angiography source images with diffusion-weighted imaging in patients with acute stroke within 6 hours after onset. Stroke. 2002;33(10):2426–2432. doi: 10.1161/01.str.0000032244.03134.37. [DOI] [PubMed] [Google Scholar]
- 79.Schramm P, Schellinger PD, Klotz E, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours’ duration. Stroke. 2004;35(7):1652–1658. doi: 10.1161/01.STR.0000131271.54098.22. [DOI] [PubMed] [Google Scholar]
- 80.Hunter GJ, Silvennoinen HM, Hamberg LM, et al. Whole-brain CT perfusion measurement of perfused cerebral blood volume in acute ischemic stroke: probability curve for regional infarction. Radiology. 2003;227(3):725–730. doi: 10.1148/radiol.2273012169. [DOI] [PubMed] [Google Scholar]
- 81.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37(4):979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 82.Schaefer PW, Roccatagliata L, Ledezma C, et al. First-pass quantitative CT perfusion identifies thresholds for salvageable penumbra in acute stroke patients treated with intra-arterial therapy. AJNR Am J Neuroradiol. 2006;27(1):20–25. [PMC free article] [PubMed] [Google Scholar]
- 83.Muir KW, Halbert HM, Baird TA, McCormick M, Teasdale E. Visual evaluation of perfusion computed tomography in acute stroke accurately estimates infarct volume and tissue viability. J Neurol Neurosurg Psychiatry. 2006;77(3):334–339. doi: 10.1136/jnnp.2005.074179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu R, Yoo AJ, Hakimelahi R, et al. CTA source images in the era of fast, volume CT scanning: are they still ‘DWI’ weighted?. Presented at: International Stroke Conference; San Antonio, TX, USA. 14–26 February 2010. [Google Scholar]
- 85.Wintermark M, Albers GW, Alexandrov AV, et al. Acute stroke imaging research roadmap. AJNR Am J Neuroradiol. 2008;29(5):e23–e30. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Ipolyi AR, Wu O, Schaefer PW, et al. Cerebral blood volume measurements in acute ischemic stroke are technique-dependent and cannot substitute for DW imaging. Presented at: American Society of Neuroradiology Annual Meeting; Boston, MA, USA. 15–20 May 2010. [Google Scholar]
- 87.Konstas AA, Goldmakher GV, Lee TY, Lev MH. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: theoretic basis. AJNR Am J Neuroradiol. 2009;30(4):662–668. doi: 10.3174/ajnr.A1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Butcher KS, Parsons M, MacGregor L, et al. Refining the perfusion–diffusion mi smatch hypothesis. Stroke. 2005;36(6):1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- 89.Kane I, Carpenter T, Chappell F, et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke. 2007;38(12):3158–3164. doi: 10.1161/STROKEAHA.107.483842. [DOI] [PubMed] [Google Scholar]
- 90.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 91.Carroll TJ, Teneggi V, Jobin M, et al. Absolute quantification of cerebral blood flow with magnetic resonance, reproducibility of the method, and comparison with H2(15)O positron emission tomography. J Cereb Blood Flow Metab. 2002;22(9):1149–1156. doi: 10.1097/00004647-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 92.Takasawa M, Jones PS, Guadagno JV, et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke. 2008;39(3):870–877. doi: 10.1161/STROKEAHA.107.500090. [DOI] [PubMed] [Google Scholar]
- 93.Fiorella D, Heiserman J, Prenger E, Partovi S. Assessment of the reproducibility of postprocessing dynamic CT perfusion data. AJNR Am J Neuroradiol. 2004;25(1):97–107. [PMC free article] [PubMed] [Google Scholar]
- 94.Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53(7):1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- 95.Perthen JE, Calamante F, Gadian DG, Connelly A. Is quantification of bolus tracking MRI reliable without deconvolution? Magn Reson Med. 2002;47(1):61–67. doi: 10.1002/mrm.10020. [DOI] [PubMed] [Google Scholar]
- 96.Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37(5):1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 97.Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion–diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8(2):141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sette G, Baron JC, Mazoyer B, Levasseur M, Pappata S, Crouzel C. Local brain haemodynamics and oxygen metabolism in cerebrovascular disease. Positron emission tomography. Brain. 1989;112(Pt 4):931–951. doi: 10.1093/brain/112.4.931. [DOI] [PubMed] [Google Scholar]
- 99.Mihara F, Kuwabara Y, Tanaka A, et al. Reliability of mean transit time obtained using perfusion-weighted MR imaging; comparison with positron emission tomography. Magn Reson Imaging. 2003;21(1):33–39. doi: 10.1016/s0730-725x(02)00629-x. [DOI] [PubMed] [Google Scholar]
- 100.Zaharchuk G. Theoretical basis of hemodynamic MR imaging techniques to measure cerebral blood volume, cerebral blood flow, and permeability. AJNR Am J Neuroradiol. 2007;28(10):1850–1858. doi: 10.3174/ajnr.A0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zaharchuk G, Yamada M, Sasamata M, Jenkins BG, Moskowitz MA, Rosen BR. Is all perfusion-weighted magnetic resonance imaging for stroke equal? The temporal evolution of multiple hemodynamic parameters after focal ischemia in rats correlated with evidence of infarction. J Cereb Blood Flow Metab. 2000;20(9):1341–1351. doi: 10.1097/00004647-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 102.Albers GW, Thijs VN, Wechsler L, et al. Ma gnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 103.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 104.Smith WS, Lev MH, English JD, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40(12):3834–3840. doi: 10.1161/STROKEAHA.109.561787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith WS, Tsao JW, Billings ME, et al. Prognostic significance of angiographically confirmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocrit Care. 2006;4(1):14–17. doi: 10.1385/ncc:4:1:014. [DOI] [PubMed] [Google Scholar]
- 106.Sims JR, Rordorf G, Smith EE, et al. Arterial occlusion revealed by CT angiography predicts NIH stroke score and acute outcomes after IV tPA treatment. AJNR Am J Neuroradiol. 2005;26(2):246–251. [PMC free article] [PubMed] [Google Scholar]
- 107.Cipriano LE, Steinberg ML, Gazelle GS, Gonzalez RG. Comparing and predicting the costs and outcomes of patients with major and minor stroke using the Boston Acute Stroke Imaging Scale Neuroimaging Classification System. AJNR Am J Neuroradiol. 2009;30(4):703–709. doi: 10.3174/ajnr.A1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr. 2001;25(4):520–528. doi: 10.1097/00004728-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 109.Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26(5):1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 110.Tomanek AI, Coutts SB, Demchuk AM, et al. MR angiography compared to conventional selective angiography in acute stroke. Can J Neurol Sci. 2006;33(1):58–62. doi: 10.1017/s0317167100004704. [DOI] [PubMed] [Google Scholar]
- 111.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Thromblytic Therapy in Acute Ischemic Stroke Study Investigators. Stroke. 2000;31(4):811–816. doi: 10.1161/01.str.31.4.811. [DOI] [PubMed] [Google Scholar]
- 112.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282(21):2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 113.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274(13):1017–1025. [PubMed] [Google Scholar]
- 114.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European–Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 115.Thomalla G, Schwark C, Sobesky J, et al. Outcome and symptomatic bleeding complications of intravenous thrombolysis within 6 hours in MRI-selected stroke patients: comparison of a German multicenter study with the pooled data of ATLANTIS, ECASS, and NINDS tPA trials. Stroke. 2006;37(3):852–858. doi: 10.1161/01.STR.0000204120.79399.72. [DOI] [PubMed] [Google Scholar]
- 116.Ribo M, Molina CA, Rovira A, et al. Safety and efficacy of intravenous tissue plasminogen activator stroke treatment in the 3- to 6-hour window using multimodal transcranial Doppler/MRI selection protocol. Stroke. 2005;36(3):602–606. doi: 10.1161/01.STR.0000155737.43566.ad. [DOI] [PubMed] [Google Scholar]
- 117.Reddrop C, Moldrich RX, Beart PM, et al. Vampire bat salivary plasminogen activator (desmoteplase) inhibits tissue-type plasminogen activator-induced potentiation of excitotoxic injury. Stroke. 2005;36(6):1241–1246. doi: 10.1161/01.STR.0000166050.84056.48. [DOI] [PubMed] [Google Scholar]
- 118.Liberatore GT, Samson A, Bladin C, Schleuning WD, Medcalf RL. Vampire bat salivary plasminogen activator (desmoteplase): a unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke. 2003;34(2):537–543. doi: 10.1161/01.str.0000049764.49162.76. [DOI] [PubMed] [Google Scholar]
- 119.Wintermark M, Meuli R, Browaeys P, et al. Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment. Neurology. 2007;68(9):694–697. doi: 10.1212/01.wnl.0000255959.30107.08. [DOI] [PubMed] [Google Scholar]
- 120.Schaefer PW, Barak ER, Kamalian S, et al. Quantitative assessment of core/penumbra mismatch in acute stroke: CT and MR perfusion imaging are strongly correlated when sufficient brain volume is imaged. Stroke. 2008;39(11):2986–2992. doi: 10.1161/STROKEAHA.107.513358. [DOI] [PubMed] [Google Scholar]
- 121.Konstas AA, Lev MH. CT perfusion imaging of acute stroke: the need for arrival time, delay insensitive, and standardized postprocessing algorithms? Radiology. 2010;254(1):22–25. doi: 10.1148/radiol.09091610. [DOI] [PubMed] [Google Scholar]
- 122.Kane I, Sandercock P, Wardlaw J. Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date. J Neurol Neurosurg Psychiatry. 2007;78(5):485–491. doi: 10.1136/jnnp.2006.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schabitz WR. MR mismatch is useful for patient selection for thrombolysis: no. Stroke. 2009;40(8):2908–2909. doi: 10.1161/STROKEAHA.109.552885. [DOI] [PubMed] [Google Scholar]
- 124••.Mishra NK, Albers GW, Davis SM, et al. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke. 2010;41(1):e25–e33. doi: 10.1161/STROKEAHA.109.566869. Meta-analysis of trials investigating the mismatch hypothesis (ratio ≥1.2) failed to demonstrate a clinical benefit of delayed (>3 h) thrombolysis in mismatch patients. [DOI] [PubMed] [Google Scholar]
- 125.Rivers CS, Wardlaw JM, Armitage PA, et al. Do acute diffusion- and perfusion-weighted MRI lesions identify final infarct volume in ischemic stroke? Stroke. 2006;37(1):98–104. doi: 10.1161/01.STR.0000195197.66606.bb. [DOI] [PubMed] [Google Scholar]
- 126.Davis SM, Donnan GA. MR mismatch and thrombolysis: appealing but validation required. Stroke. 2009;40(8):2910. doi: 10.1161/STROKEAHA.109.552893. [DOI] [PubMed] [Google Scholar]
- 127.Christensen S, Mouridsen K, Wu O, et al. Comparison of 10 perfusion MRI parameters in 97 sub-6-hour stroke patients using voxel-based receiver operating characteristics analysis. Stroke. 2009;40(6):2055–2061. doi: 10.1161/STROKEAHA.108.546069. [DOI] [PubMed] [Google Scholar]
- 128.Kakuda W, Lansberg MG, Thijs VN, et al. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab. 2008;28(5):887–891. doi: 10.1038/sj.jcbfm.9600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Donnan GA, Baron JC, Ma H, Davis SM. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 2009;8(3):261–269. doi: 10.1016/S1474-4422(09)70041-9. [DOI] [PubMed] [Google Scholar]
- 130.Butcher KS, Parsons MW, Davis S, Donnan G. PWI/DWI mismatch: better definition required. Stroke. 2003;34(11):e215–e216. doi: 10.1161/01.STR.0000099066.23627.24. author reply e215–e216. [DOI] [PubMed] [Google Scholar]
- 131.Coutts SB, Simon JE, Tomanek AI, et al. Reliability of assessing percentage of diffusion-perfusion mismatch. Stroke. 2003;34(7):1681–1683. doi: 10.1161/01.STR.0000078840.96473.20. [DOI] [PubMed] [Google Scholar]
- 132.Sobesky J, Zaro Weber O, Lehnhardt FG, et al. Does the mismatch match the penumbra? Magnetic resonance imaging and positron emission tomography in early ischemic stroke. Stroke. 2005;36(5):980–985. doi: 10.1161/01.STR.0000160751.79241.a3. [DOI] [PubMed] [Google Scholar]
- 133.Heiss WD, Sobesky J, Hesselmann V. Identifying thresholds for penumbra and irreversible tissue damage. Stroke. 2004;35(11 Suppl 1):2671–2674. doi: 10.1161/01.STR.0000143329.81997.8a. [DOI] [PubMed] [Google Scholar]
- 134.Olivot JM, Mlynash M, Thijs VN, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40(2):469–475. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kucinski T, Naumann D, Knab R, et al. Tissue at risk is overestimated in perfusion-weighted imaging: MR imaging in acute stroke patients without vessel recanalization. AJNR Am J Neuroradiol. 2005;26(4):815–819. [PMC free article] [PubMed] [Google Scholar]
- 136.Kidwell CS, Alger JR, Saver JL. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke. 2004;35(11 Suppl 1):2662–2665. doi: 10.1161/01.STR.0000143222.13069.70. [DOI] [PubMed] [Google Scholar]
- 137.Parsons MW, Yang Q, Barber PA, et al. Perfusion magnetic resonance imaging maps in hyperacute stroke: relative cerebral blood flow most accurately identifies tissue destined to infarct. Stroke. 2001;32(7):1581–1587. doi: 10.1161/01.str.32.7.1581. [DOI] [PubMed] [Google Scholar]
- 138.Neumann-Haefelin T, Wittsack HJ, Wenserski F, et al. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30(8):1591–1597. doi: 10.1161/01.str.30.8.1591. [DOI] [PubMed] [Google Scholar]
- 139.Shih LC, Saver JL, Alger JR, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003;34(6):1425–1430. doi: 10.1161/01.STR.0000072998.70087.E9. [DOI] [PubMed] [Google Scholar]
- 140.Sobesky J, Zaro Weber O, Lehnhardt FG, et al. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004;35(12):2843–2847. doi: 10.1161/01.STR.0000147043.29399.f6. [DOI] [PubMed] [Google Scholar]
- 141.Olivot JM, Mlynash M, Zaharchuk G, et al. Perfusion MRI (Tmax and MTT) correlation with xenon CT cerebral blood flow in stroke patients. Neurology. 2009;72(13):1140–1145. doi: 10.1212/01.wnl.0000345372.49233.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neumann-Haefelin T, Wittsack HJ, Fink GR, et al. Diffusion- and perfusion-weighted MRI: influence of severe carotid artery stenosis on the DWI/PWI mismatch in acute stroke. Stroke. 2000;31(6):1311–1317. doi: 10.1161/01.str.31.6.1311. [DOI] [PubMed] [Google Scholar]
- 143.Kim SJ, Seok JM, Bang OY, et al. MR mismatch profiles in patients with intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. J Cereb Blood Flow Metab. 2009;29(6):1138–1145. doi: 10.1038/jcbfm.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hirano T, Read SJ, Abbott DF, et al. Prediction of the final infarct volume within 6 h of stroke using single photon emission computed tomography with technetium-99m hexamethylpropylene amine oxime. Cerebrovasc Dis. 2001;11(2):119–127. doi: 10.1159/000047623. [DOI] [PubMed] [Google Scholar]
- 145.Rohl L, Ostergaard L, Simonsen CZ, et al. Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted MRI and apparent diffusion coefficient. Stroke. 2001;32(5):1140–1146. doi: 10.1161/01.str.32.5.1140. [DOI] [PubMed] [Google Scholar]
- 146.Thijs VN, Adami A, Neumann-Haefelin T, Moseley ME, Marks MP, Albers GW. Relationship between severity of MR perfusion deficit and DWI lesion evolution. Neurology. 2001;57(7):1205–1211. doi: 10.1212/wnl.57.7.1205. [DOI] [PubMed] [Google Scholar]
- 147.Wittsack HJ, Ritzl A, Fink GR, et al. MR imaging in acute stroke: diffusion-weighted and perfusion imaging parameters for predicting infarct size. Radiology. 2002;222(2):397–403. doi: 10.1148/radiol.2222001731. [DOI] [PubMed] [Google Scholar]
- 148.Grandin CB, Duprez TP, Smith AM, et al. Usefulness of magnetic resonance-derived quantitative measurements of cerebral blood flow and volume in prediction of infarct growth in hyperacute stroke. Stroke. 2001;32(5):1147–1153. doi: 10.1161/01.str.32.5.1147. [DOI] [PubMed] [Google Scholar]
- 149.Grandin CB, Duprez TP, Smith AM, et al. Which MR-derived perfusion parameters are the best predictors of infarct growth in hyperacute stroke? Comparative study between relative and quantitative measurements. Radiology. 2002;223(2):361–370. doi: 10.1148/radiol.2232010673. [DOI] [PubMed] [Google Scholar]
- 150.Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. MRI perfusion maps in acute stroke validated with 15O-water positron emission tomography. Stroke. 2010;41(3):443–449. doi: 10.1161/STROKEAHA.109.569889. [DOI] [PubMed] [Google Scholar]
- 151.Bandera E, Botteri M, Minelli C, Sutton A, Abrams KR, Latronico N. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006;37(5):1334–1339. doi: 10.1161/01.STR.0000217418.29609.22. [DOI] [PubMed] [Google Scholar]
- 152.Toth G, Albers GW. Use of MRI to estimate the therapeutic window in acute stroke: is perfusion-weighted imaging/diffusion-weighted imaging mismatch an EPITHET for salvageable ischemic brain tissue? Stroke. 2009;40(1):333–335. doi: 10.1161/STROKEAHA.108.525683. [DOI] [PubMed] [Google Scholar]
- 153•.Parsons MW, Christensen S, McElduff P, et al. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010;30(6):1214–1225. doi: 10.1038/jcbfm.2010.3. Post-hoc analysis of Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) demonstrated that absolute diffusion-weighted imaging and perfusion-weighted imaging lesion volumes were more important than the mismatch ratio for determining the clinical response to intravenous tissue plasminogen activator. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davalos A, Blanco M, Pedraza S, et al. The clinical-DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology. 2004;62(12):2187–2192. doi: 10.1212/01.wnl.0000130570.41127.ea. [DOI] [PubMed] [Google Scholar]
- 155.Prosser J, Butcher K, Allport L, et al. Clinical-diffusion mismatch predicts the putative penumbra with high specificity. Stroke. 2005;36(8):1700–1704. doi: 10.1161/01.STR.0000173407.40773.17. [DOI] [PubMed] [Google Scholar]
- 156.Lansberg MG, Thijs VN, Hamilton S, et al. Evaluation of the clinical-diffusion and perfusion–diffusion mismatch models in DEFUSE. Stroke. 2007;38(6):1826–1830. doi: 10.1161/STROKEAHA.106.480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ebinger M, Iwanaga T, Prosser JF, et al. Clinical-diffusion mismatch and benefit from thrombolysis 3 to 6 hours after acute stroke. Stroke. 2009;40(7):2572–2574. doi: 10.1161/STROKEAHA.109.548073. [DOI] [PubMed] [Google Scholar]
- 158.Choi JY, Pary JK, Alexandrov AV, et al. Does clinical-CT ‘mismatch’ predict early response to treatment with recombinant tissue plasminogen activator? Cerebrovasc Dis. 2006;22(5–6):384–388. doi: 10.1159/000094856. [DOI] [PubMed] [Google Scholar]
- 159.Yoo AJ, Barak ER, Copen WA, et al. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke. 2010;41(8):1728–1735. doi: 10.1161/STROKEAHA.110.582874. [DOI] [PubMed] [Google Scholar]
- 160.Lansberg MG, Thijs VN, Bammer R, et al. The MRA-DWI mismatch identifies patients with stroke who are likely to benefit from reperfusion. Stroke. 2008;39(9):2491–2496. doi: 10.1161/STROKEAHA.107.508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Provenzale JM, Shah K, Patel U, McCrory DC. Systematic review of CT and MR perfusion imaging for assessment of acute cerebrovascular disease. AJNR Am J Neuroradiol. 2008;29(8):1476–1482. doi: 10.3174/ajnr.A1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hotter B, Pittl S, Ebinger M, et al. Prospective study on the mismatch concept in acute stroke patients within the first 24 h after symptom onset – 1000Plus study. BMC Neurol. 2009;9:60. doi: 10.1186/1471-2377-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Y, Liao X, Zhao X, et al. Imaging-based thrombolysis trial in acute ischemic stroke-II (ITAIS-II) Int J Stroke. 2009;4(1):49–53. doi: 10.1111/j.1747-4949.2009.00245.x. [DOI] [PubMed] [Google Scholar]
- 164.Hirsch J, Yoo AJ, Nogueira RG, et al. Case volumes of intra-arterial and intravenous treatment of ischemic stroke in the U.S. J NeuroInterv Surg. 2009;1(1):27–31. doi: 10.1136/jnis.2009.000166. [DOI] [PubMed] [Google Scholar]
- 165.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282(21):2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 166.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39(4):1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 167.Hakimelahi R, Copen WA, Schaefer PW, et al. A small DWI lesion in the setting of an anterior circulation proximal artery occlusion predicts the presence of a large diffusion-perfusion mismatch. Presented at: American Society of Neuroradiology Annual Meeting; Vancouver, BC, USA. 16–21 May 2009. [Google Scholar]
- 168.van der Zwan A, Hillen B, Tulleken CA, Dujovny M. A quantitative investigation of the variability of the major cerebral arterial territories. Stroke. 1993;24(12):1951–1959. doi: 10.1161/01.str.24.12.1951. [DOI] [PubMed] [Google Scholar]
- 169.Jovin TG, Yonas H, Gebel JM, et al. The cortical ischemic core and not the consistently present penumbra is a determinant of clinical outcome in acute middle cerebral artery occlusion. Stroke. 2003;34(10):2426–2433. doi: 10.1161/01.STR.0000091232.81947.C9. [DOI] [PubMed] [Google Scholar]
- 170.Thomalla GJ, Kucinski T, Schoder V, et al. Prediction of malignant middle cerebral artery infarction by early perfusion- and diffusion-weighted magnetic resonance imaging. Stroke. 2003;34(8):1892–1899. doi: 10.1161/01.STR.0000081985.44625.B6. [DOI] [PubMed] [Google Scholar]
- 171.Oppenheim C, Samson Y, Manai R, et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000;31(9):2175–2181. doi: 10.1161/01.str.31.9.2175. [DOI] [PubMed] [Google Scholar]
- 172.Arsava E, Ay H, Singhal AB, Ona Wu, Furie KL, Sorenson AG. An Infarct Volume Threshold on Early DWI to Predict Unfavorable Clinical Outcome. Presented at: International Stroke Conference; New Orleans, LA, USA. 20–22 February 2008. [Google Scholar]
- 173.Sanak D, Nosal V, Horak D, et al. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48(9):632–639. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 174•.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40(6):2046–2054. doi: 10.1161/STROKEAHA.108.541656. Demonstrates that baseline core infarct size is an important determinant of the clinical response to intra-arterial reperfusion in patients with proximal artery occlusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hill MD, Rowley HA, Adler F, et al. Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-urokinase by using ASPECTS. Stroke. 2003;34(8):1925–1931. doi: 10.1161/01.STR.0000082483.37127.D0. [DOI] [PubMed] [Google Scholar]
- 176.Hill MD, Demchuk AM, Tomsick TA, Palesch YY, Broderick JP. Using the baseline CT scan to select acute stroke patients for IV-IA therapy. AJNR Am J Neuroradiol. 2006;27(8):1612–1616. [PMC free article] [PubMed] [Google Scholar]
- 177.Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke. 2001;32(9):2021–2028. doi: 10.1161/hs0901.095680. [DOI] [PubMed] [Google Scholar]
- 178.Gasparotti R, Grassi M, Mardighian D, et al. Perfusion CT in patients with acute ischemic stroke treated with intra-arterial thrombolysis: predictive value of infarct core size on clinical outcome. AJNR Am J Neuroradiol. 2009;30(4):722–727. doi: 10.3174/ajnr.A1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brekenfeld C, Remonda L, Nedeltchev K, et al. Symptomatic intracranial haemorrhage after intra-arterial thrombolysis in acute ischaemic stroke: assessment of 294 patients treated with urokinase. J Neurol Neurosurg Psychiatry. 2007;78(3):280–285. doi: 10.1136/jnnp.2005.078840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European–Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32(2):438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 181.Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke. 1997;28(11):2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 182.Patel SC, Levine SR, Tilley BC, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA. 2001;286(22):2830–2838. doi: 10.1001/jama.286.22.2830. [DOI] [PubMed] [Google Scholar]
- 183.Demchuk AM, Hill MD, Barber PA, Silver B, Patel SC, Levine SR. Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA Stroke Study. Stroke. 2005;36(10):2110–2115. doi: 10.1161/01.STR.0000181116.15426.58. [DOI] [PubMed] [Google Scholar]
- 184.Lansberg MG, Thijs VN, Bammer R, et al. Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007;38(8):2275–2278. doi: 10.1161/STROKEAHA.106.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185••.Singer OC, Humpich MC, Fiehler J, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008;63(1):52–60. doi: 10.1002/ana.21222. This large, multicenter study demonstrates that pretreatment diffusion-weighted imaging lesion size is an important predictor of symptomatic intracerebral hemorrhage following intravenous or intra-arterial reperfusion therapy. [DOI] [PubMed] [Google Scholar]
- 186.Singer OC, Kurre W, Humpich MC, et al. Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke. 2009;40(8):2743–2748. doi: 10.1161/STROKEAHA.109.550111. [DOI] [PubMed] [Google Scholar]
- 187.Selim M, Fink JN, Kumar S, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002;33(8):2047–2052. doi: 10.1161/01.str.0000023577.65990.4e. [DOI] [PubMed] [Google Scholar]
- 188.Sunshine JL, Tarr RW, Lanzieri CF, Landis DM, Selman WR, Lewin JS. Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology. 1999;212(2):325–332. doi: 10.1148/radiology.212.2.r99au52325. [DOI] [PubMed] [Google Scholar]
- 189.Schellinger PD, Jansen O, Fiebach JB, et al. Feasibility and practicality of MR imaging of stroke in the management of hyperacute cerebral ischemia. AJNR Am J Neuroradiol. 2000;21(7):1184–1189. [PMC free article] [PubMed] [Google Scholar]
- 190.Solling C, Ashkanian M, Hjort N, Gyldensted C, Andersen G, Ostergaard L. Feasibility and logistics of MRI before thrombolytic treatment. Acta Neurol Scand. 2009;120(3):143–149. doi: 10.1111/j.1600-0404.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 191.Hand PJ, Wardlaw JM, Rowat AM, Haisma JA, Lindley RI, Dennis MS. Magnetic resonance brain imaging in patients with acute stroke: feasibility and patient related difficulties. J Neurol Neurosurg Psychiatry. 2005;76(11):1525–1527. doi: 10.1136/jnnp.2005.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Singer OC, Sitzer M, du Mesnil de Rochemont R, Neumann-Haefelin T. Practical limitations of acute stroke MRI due to patient-related problems. Neurology. 2004;62(10):1848–1849. doi: 10.1212/01.wnl.0000125320.53244.fa. [DOI] [PubMed] [Google Scholar]
- 193.Graves MJ, U-King-Im J, Howarth S, Gillard JH. Ultrafast magnetic resonance imaging protocols in stroke. Expert Rev Neurother. 2006;6(6):921–930. doi: 10.1586/14737175.6.6.921. [DOI] [PubMed] [Google Scholar]
Websites
- 201.US National Institutes of Health. http://ClinicalTrials.gov.
- 202.Internet Stroke Center, Washington University School of Medicine. Stroke Trials Registry. www.strokecenter.org/trials.
- 203.BioMed Central. Current Controlled Trials. www.controlled-trials.com.