Abstract
Background:
The purpose of this study was to evaluate the use of intralesional propranolol injection in the management of periocular capillary hemangioma.
Methods:
A prospective study was performed in 22 consecutive patients with periocular hemangioma. Twelve patients underwent intralesional propranolol injection and ten patients underwent intralesional triamcinolone injection. The size of the lesion was measured serially every week during the first month, every 2 weeks for the second month, and then monthly for another 2 months. The refractive error and degree of ptosis if present were measured before injection and at the end of the study.
Results:
There was reduction in the size of hemangioma, astigmatic error, and degree of ptosis in both groups. The difference in outcome between both groups was not statistically significant. Rebound growth occurred in 25% of the propranolol group and 30% of the steroid group but responded to reinjection. No adverse effects were reported during or after intralesional propranolol injection.
Conclusion:
Intralesional propranolol injection is an alternative and effective method for treatment of infantile periocular hemangioma.
Keywords: propranolol, intralesional, periocular capillary hemangioma
Introduction
Hemangiomas are the most common tumors of the eyelids and orbit in children.1 They typically appear at or shortly after birth, with approximately 90% being clinically obvious by 2 months of age.2 Hemangiomas usually exhibit an initial phase of progressive growth followed by spontaneous regression, and 70% regress completely by the age of 7 years.3
Despite the usually benign and self-limiting course of periocular hemangiomas, functional and cosmetic sequelae may persist. Periocular hemangiomas can pose a threat to visual development through anisometropia, amblyopia, or impairment of the visual axis. Hemangiomas can also be complicated by dermatologic problems, such as residual telangiectasias, scarring, and facial disfigurement that can negatively impact emotional development.2
There are different modalities for management of periocular capillary hemangiomas. The most commonly used treatment modalities have been intralesional or systemic corticosteroids.4 Other treatment options include interferon alpha,5 vincristine,6 laser therapy,7 or surgical excision.8
Recently, oral propranolol, a nonselective beta-blocker, has been shown to inhibit vascular proliferation of capillary hemangioma.9–12 However, such therapy requires administration of systemic propranolol for at least 2 months, with possible side effects requiring careful clinical monitoring. In addition, various complications have been reported with systemic propranolol therapy.13–16
A few studies have investigated the use of topical beta-blockers for treatment of capillary hemangioma, and have reported good results with topical application.17,18 Such reports have raised the possibility of using direct intralesional beta-blockers for treatment of capillary hemangioma. Propranolol is available in both oral and parenteral forms. In the present study, we attempted to investigate the effect of direct intralesional propranolol injection in periocular hemangioma. Because corticosteroids have been the gold standard of treatment for capillary hemangioma for a long time, we used intralesional steroid injection in another subset of patients who served as a control group.
Materials and methods
The study protocol was approved by the hospital ethics committee. The study and data collection conformed to all local laws and were compliant with the principles of the Declaration of Helsinki. The parent or guardian of each study patient gave their written informed consent.
This prospective study was performed in patients with periocular hemangiomas requiring intervention. The main indications for intervention included visual-threatening capillary hemangioma, or rapidly progressive or recurrently bleeding hemangioma. Vision-threatening hemangiomas were defined as hemangiomas causing obstruction of the visual axis or inducing astigmatism, strabismus, amblyopia, or anisometropia. In addition, hemangiomas likely to lead to cosmetic deformity in the future or causing psychologic distress to the parents were included.
Any child with cardiovascular problems, bronchial asthma, or diabetes mellitus was excluded. Children who had had previous treatment for hemangioma were also excluded. Prior to inclusion, all patients were subjected to a detailed cardiac evaluation, including history-taking, detailed clinical examination, and a 12-lead electrocardiogram, including calculation of heart rate, voltages, and intervals.
Intralesional injection
The first ten patients received an intralesional steroid injection of triamcinolone 40 mg/mL. The next twelve patients received an intralesional propranolol injection 1 mg/mL. The volume of injected drug depended on the size of the lesion (0.2 mL injected per cm of lesion diameter), with a maximum volume of 1 mL for a lesion of 5 cm diameter. All patients had undiluted injections under general inhalation anesthesia with cardiovascular and respiratory monitoring during and after injection. In addition, a dilated fundus examination was done during injection, with careful examination of the central retinal artery.
Patients who underwent intralesional steroid injection were discharged a few hours after injection. Patients who underwent intralesional propranolol injection were kept under observation for the first 24 hours, during which blood pressure and heart rate were measured at hourly intervals. Blood glucose was measured if clinically indicated based on symptoms of clamminess, distress, or irritability.
Follow-up
Patients were followed up on a weekly basis for the first month, then every 2 weeks in the second month, and finally at 4-weekly intervals for a period of 4 months after the injection. The patients were followed up by an investigator blinded to the drug injected. The size of the hemangioma was measured by clinical examination and documented by serial photography. The area was then calculated in mm2 and measured serially. The response to intralesional therapy was graded as excellent, good, fair, or poor, according to the final outcome achieved at the time treatment was stopped (Table 1). Patients with rebound growth after resolution were documented. In addition, cycloplegic refraction was performed for all patients before initiation of therapy and at the end of the study. The degree of ptosis was assessed by comparing the maximum palpebral fissure height on both sides before initiation of therapy and at the end of the follow-up period.
Table 1.
Grading system used in evaluation of intralesional injection for treatment of periocular hemangiomas
| Grade | Response achieved |
|---|---|
| Excellent | Complete resolution achieved |
| Good | Sustained plateau, with ≥50% reduction in size of hemangioma |
| Fair | Sustained plateau, with <50% reduction in size of hemangioma |
| Poor | No response or worsening of hemangioma |
Statistical analysis
Changes in each group were analyzed using repeated-measures analysis of variance. Differences between the groups were analyzed using the t-test for independent samples. Statistical analyses were carried out using StatLab, SPSS for Windows (v 16.0; SPSS, Inc, Chicago, IL).
Results
A total of 22 patients were included in the study. The mean age at treatment was 5.9 months ± 2.7 months in the propranolol group and 6.1 months ± 2.9 months in the steroid group (P = 0.45). The main indication for treatment was vision-threatening hemangioma. Other indications for treatment are listed in Table 2.
Table 2.
Indications for intervention in the present study
| n (%) | |
|---|---|
| Obstruction of visual axis | 10 (45%) |
| Induced astigmatism or anisometropia but no obstruction of visual axis | 4 (18%) |
| Rapidly progressive hemangioma | 4 (18%) |
| Cosmetic disfigurement and/or causing parental distress | 4 (18%) |
Outcome of propranolol treatment
Ten of twelve patients (83%) in the propranolol group showed regression of hemangioma (Table 3). Regression was noted in the first 24 hours in two patients. In the remaining eight patients, onset of regression varied from 1 to 3 weeks after the injection, so that all ten patients showed evidence of regression by the end of the third week. Regression almost always started with a change in the hemangioma color from intense red to lighter purple-blue, together with palpable softening of the lesion. After this initial response, hemangiomas continued to improve as regards regression of their size, flattening of the lesion, and more evident blanching of the color. At the end of the follow-up period, 42% of patients (n = 5) showed an excellent final response (Figure 1), with almost complete resolution of the lesion, 25% (n = 3) showed a good response with more than 50% reduction in the size of the lesion (Figure 2), and 17% (n = 2) showed a fair response with less than 50% reduction in the size of the lesion. Only two patients (17%) were resistant to treatment. In general, patients with smaller hemangiomas tended to respond better to intralesional injection. However, it was difficult to analyze this relationship statistically because of the small number of patients.
Table 3.
Changes in size of hemangioma after injection
| Propranolol group | Steroid group | |
|---|---|---|
| Size of hemangioma before injection (cm2) | 8.1 ± 3.4, range 3.6–14.3 | 7.9 ± 3.6, range 3.5–14.1 |
| Mean ± standard deviation, range | ||
| Final size of hemangioma after 4 months (cm2) | 3.6 ± 2.6, range 0– 7.1 | 3.7 ± 2.5, range 0–7.5 |
| Mean ± standard deviation, range |
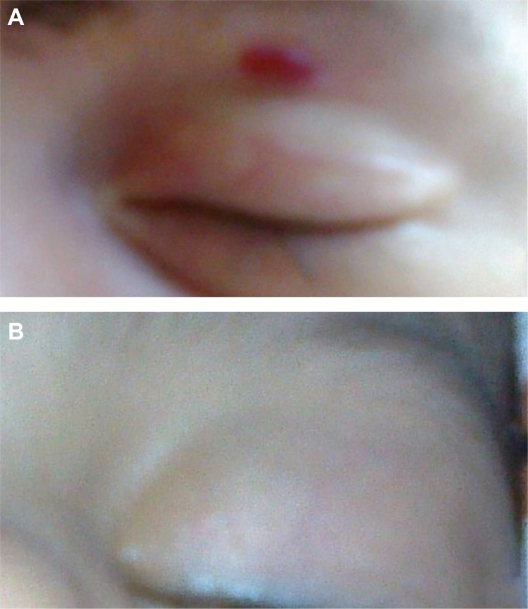
Figure 1.
Clinical photographs of a representative case showing a 4-month-old patient with periocular hemangioma. (A) Initial photographs before injection and (B) 4 months after propranolol injection showing excellent response with almost complete resolution of the lesion.
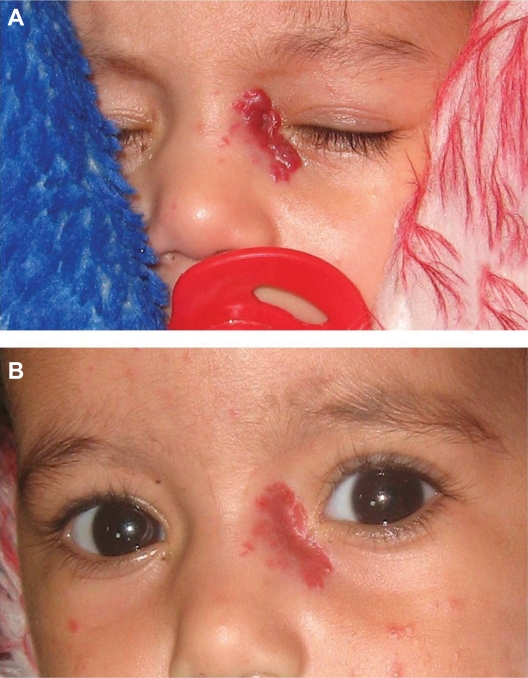
Figure 2.
Clinical photographs of a representative case showing a 5-month-old patient with periocular hemangioma. (A) Initial photographs before injection and (B) 4 months after propranolol injection showing good response with marked reduction in size of the lesion.
Outcome of steroid treatment
Eight of ten patients in the intralesional steroid group showed regression of hemangioma (Table 3). Onset of regression was slightly more delayed than in the propranolol group. Regression started to appear in the first week in one patient. In the remaining seven patients, the onset of regression varied from 2 to 3 weeks after injection. The clinical course of regression was similar to that in the propranolol group as regards change in color and regression in size and depth of the lesion. At the end of the follow-up period, 40% of patients (n = 4) showed an excellent final response with almost complete resolution of the lesion, 20% (n = 2) showed a good response, and 20% (n = 2) showed a fair response. Two patients (20%) previously documented to be resistant to treatment showed a poor final response.
Change in refractive error
Of the 22 patients, twelve patients had significant astigmatism more than 1 D (mean = 2.1 ± 1.5 D). Four months after injection, there was a reduction in astigmatic error (mean = 1.3 ± 1.1 D). The reduction in astigmatic error was statistically significant in both the propranolol group (P = 0.02) and the steroid group (P = 0.03). There was no statistically significant difference in the final astigmatic error between both groups (P = 0.34).
Degree of ptosis
Fifteen patients had significant ptosis of more than 1 mm difference between the eyes (mean = 1.5 ± 1.4 mm). Four months after injection, there was a reduction in the degree of ptosis (mean = 0.9 ± 1.1 mm). The reduction in degree of ptosis was statistically significant in both the propranolol group (P = 0.02) and the steroid group (P = 0.02). There was no statistically significant difference in the degree of ptosis between the groups (P = 0.46).
Rebound growth
Of the patients who showed evidence of regression with therapy, seven later showed evidence of rebound growth. This included four patients in the propranolol group and three patients in the steroid group (Table 4). Rebound growth occurred in the form of a sudden increase in size and worsening of color. All patients received reinjection and responded to treatment in a shorter duration than for the first time. Reinjection was done using the same initial therapeutic agent given.
Table 4.
Summary of data on patients who developed rebound growth after cessation of therapy
| Patient number | Gender | Age at start (months) | Treatment given | Response | Onset of relapse after injection (weeks) |
|---|---|---|---|---|---|
| 1 | F | 3 | Steroids | Excellent | 12 |
| 2 | F | 4 | Steroids | Good | 10 |
| 3 | M | 4 | Steroids | Fair | 10 |
| 4 | M | 5 | Propranolol | Excellent | 10 |
| 5 | M | 3 | Propranolol | Good | 12 |
| 6 | F | 3 | Propranolol | Fair | 12 |
| 7 | F | 3 | Propranolol | Fair | 12 |
Side effects
None of the patients developed local side effects during or after injection. Patients in the propranolol group had no significant change in heart rate or blood pressure during or after injection. However, we did not investigate and assess for possible systemic side effects of intralesional steroids, such as adrenal suppression.
Discussion
Capillary hemangioma occurs in about 10% of infants, with a predominance in females, premature infants and Caucasians.19 Capillary hemangiomas are characterized by rapid proliferation during the first year (proliferative phase), followed by slow involution over the next 5 years (involuting phase), during which cellular elements are replaced by fibro-fatty deposition.20 However, functional or cosmetic sequelae may occur and require intervention.21
The use of intralesional corticosteroids to induce involution of capillary hemangiomas was first introduced in 1982.22 Since then, steroids have remained the first-line treatment for infantile hemangioma in the vast majority of cases. However, adverse effects have been reported with intralesional steroids, in particular, skin changes and adrenal-related changes. Reported skin-related complications have included skin atrophy and/or necrosis, skin depigmentation, and fat atrophy. Adrenal-related changes are even more serious, and include reversible cushingoid facies, as well as adrenal suppression that may require replacement therapy.4 A serious complication, ie, central retinal artery occlusion, has also been reported with intralesional steroid injection.4
In 2008, Leatue-Labreze et al reported a dramatic effect of systemic propranolol in inducing accelerated involution of infantile hemangioma.9 There is as yet no generally accepted consensus on the ideal treatment regime for propranolol. The main differences between the different published reports were the dosage of propranolol used and the duration of treatment.10–12 The mechanism of action of propranolol in hemangiomas is unknown. It is speculated that it may act through increased apoptosis and downregulation of vascular endothelial growth factor as a result of vasoconstriction.23,24
Despite the initial promise that propranolol may replace oral corticosteroids as the treatment of choice for infantile hemangioma, there are many potential side effects of propranolol, including bradycardia, hypotension, hypoglycemia, rash, gastrointestinal discomfort/reflux, fatigue, and bronchospasm.13–16 Lethargy and hypothermia have also been reported, and required termination of therapy.13 Additionally, infants with PHACE syndrome with tortuous, aneurysmal, or stenotic intracranial arteries would potentially be at increased risk of stroke if treated with propranolol.24 Holmes et al10 and Leboulanger et al11 recommended that a full cardiovascular workup be performed prior to initiation of propranolol therapy, to identify patients in whom commencement of propranolol therapy could be dangerous. In addition, infants with large facial hemangioma should be evaluated for a PHACE association before initiation of oral propranolol.25
In this prospective clinical study, conducted on 22 consecutive patients with infantile hemangioma, intralesional propranolol was shown to be almost as effective as intralesional steroids. Intralesional propranolol was not associated with significant cardiovascular changes or local adverse effects during or after injection. One may speculate, although not be able to prove it, that the systemic effects of direct intralesional injection may possibly be reduced by intralesional injection. Because hemangiomas are very vascular lesions, accidental systemic absorption may occur. The injection is done under general anesthesia with careful cardiovascular monitoring for immediate management if significant bradycardia or hypotension occurs. Atropine would be given in the event of such a complication. However, we did not encounter such complications during injection in our study. However, it is worth mentioning that we did not study the possible systemic side effects of intralesional steroids. The intralesional steroid group only served as a control group to allow evaluation of our results, given that intralesional steroids are considered close to a gold standard therapy.
Despite the good efficacy of propranolol, relapses may occur. In this study, four patients (25%) showed evidence of rebound growth after cessation of therapy. All of these patients were younger than 5 months at the time of injection. This may be explained by the fact that, in the early months of life, capillary hemangioma is in its active proliferative phase when there are high levels of proangiogenic factors. Later on, there may be a shift of balance towards proapoptotic factors, reducing the chance of rebound growth.20
Qin et al12 performed one of the largest studies to date using propranolol in 58 infants with infantile hemangioma at a dose of 1–1.5 mg/kg/day. They reported a response rate of “good to excellent” in 67% of patients. In our study, we achieved a similar response with intralesional propranolol injection. This suggests that direct intralesional injection of propranolol can achieve similar results to systemic propranolol therapy. However, this would require adequate controlled double-blind studies to allow accurate comparison of the results.
Furthermore, we noted a significant reduction in astigmatic error and degree of ptosis after injection. This is consistent with previous studies reporting improvement in refractive error after systemic propranolol therapy and after intralesional steroid therapy.26,27
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Haik B, Karcioglu Z, Gordon RA, Pechous BP. Capillary hemangioma (infantile periocular hemangioma) Surv Ophthalmol. 1994;38:399–426. doi: 10.1016/0039-6257(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 2.Garza G, Fay A, Rubin PA. Treatment of pediatric vascular lesions of the eyelid and orbit. Int Ophthalmol Clin. 2001;41:43–55. doi: 10.1097/00004397-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667–680. [Google Scholar]
- 4.Wasserman BN, Medow NB, Homa-Palladino M, Hoehn ME. Treatment of periocular capillary hemangiomas. J AAPOS. 2004;8:175–181. doi: 10.1016/j.jaapos.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Ezekowitz RAB, Phil CBD, Muliken JB, Fokman J. Interferon alfa-2a therapy for life threatening hemangiomas of infancy. N Engl J Med. 1992;326:1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 6.Fawcett SL, Grant I, Hall PN, et al. Vincristine as a treatment for a large haemangioma threatening vital functions. Br J Plast Surg. 2004;57:168–171. doi: 10.1016/j.bjps.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Garden JM, Bakus AD, Paller AS. Treatment of cutaneous hemangiomas by the flashlamp-pumped pulsed dye laser: prospective analysis. J Pediatr. 1992;120:555–560. doi: 10.1016/s0022-3476(05)82481-3. [DOI] [PubMed] [Google Scholar]
- 8.Walker RS, Custer PL, Nerad JA. Surgical excision of periorbital capillary hemangiomas. Ophthalmology. 1994;101:1333–1340. doi: 10.1016/s0161-6420(94)31165-1. [DOI] [PubMed] [Google Scholar]
- 9.Leatue-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:649–651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 10.Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for infantile hemangiomas. Plast Reconstr Surg. 2010;125:420–421. doi: 10.1097/PRS.0b013e3181c2a731. [DOI] [PubMed] [Google Scholar]
- 11.Leboulanger N, Fayoux P, Teissier N, et al. Propranolol in the therapeutic strategy of infantile laryngotracheal hemangioma: a preliminary retrospective study of French experience. Int J Pediatr Otorhinolaryngol. 2010;74:1254–1257. doi: 10.1016/j.ijporl.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Qin ZP, Liu XJ, Li KL, et al. Treatment of infantile hemangiomas with low dose propranolol: evaluation of short term efficacy and safety. Zhonghua Yi Xue Za Zhi. 2009;89:3130–3134. Chinese. [PubMed] [Google Scholar]
- 13.Lawley LP, Siegfried MD, Todd JL. Propranolol treatment for haemangioma of infancy: risks and recommendations. Pediatr Dermatol. 2009;26:610–614. doi: 10.1111/j.1525-1470.2009.00975.x. [DOI] [PubMed] [Google Scholar]
- 14.Abbott J, Parulekar M, Shahidullah H, Taibjee S, Moss C. Diarrhea associated with propranolol treatment for hemangioma of infancy (HOI) Pediatr Dermatol. 2010;27:558. doi: 10.1111/j.1525-1470.2010.01221.x. [DOI] [PubMed] [Google Scholar]
- 15.Pavlakovic H, Kietz S, Lauerer P, Zutt M, Lakomek M. Hyperkalemia complicating propranolol treatment of an infantile hemangioma. Pediatrics. 2010;126:e1589–e1593. doi: 10.1542/peds.2010-0077. [DOI] [PubMed] [Google Scholar]
- 16.Holland KE, Frieden IJ, Frommelt PC, Mancini AJ, Wyattt D, Drolet BA. Hypoglycemia in children taking propranolol for the treatment of infantile hemangioma. Arch Dermatol. 2010;146:775–778. doi: 10.1001/archdermatol.2010.158. [DOI] [PubMed] [Google Scholar]
- 17.Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564–565. doi: 10.1001/archdermatol.2010.67. [DOI] [PubMed] [Google Scholar]
- 18.Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta blocker solution. Arch Ophthalmol. 2010;128:255–256. doi: 10.1001/archophthalmol.2009.370. [DOI] [PubMed] [Google Scholar]
- 19.Mueller BU, Mulliken JB. The infant with a vascular tumour. Semin Perinatol. 1999;23:332–340. doi: 10.1016/s0146-0005(99)80041-x. [DOI] [PubMed] [Google Scholar]
- 20.Tan ST, Velickovic M, Ruger BM, et al. Cellular and extracellular markers of hemangioma. Plast Reconstr Surg. 2000;106:529–537. doi: 10.1097/00006534-200009030-00001. [DOI] [PubMed] [Google Scholar]
- 21.Stigmar G, Crawford JS, Ward CM, Thomson HG. Ophthalmic sequelae of infantile hemangiomas of the eyelids and orbit. Am J Ophthalmol. 1978;85:806–813. doi: 10.1016/s0002-9394(14)78109-7. [DOI] [PubMed] [Google Scholar]
- 22.Kushner BJ. Intralesional corticosteroid injection for infantile adnexal hemangioma. Am J Ophthalmol. 1982;93:496–506. doi: 10.1016/0002-9394(82)90140-4. [DOI] [PubMed] [Google Scholar]
- 23.Léauté-Labrèze C, Taïeb A. Efficacy of beta-blockers in infantile capillary haemangiomas: the physiopathological significance and therapeutic consequences. Ann Dermatol Venereol. 2008;135:860–862. doi: 10.1016/j.annder.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Frieden IJ, Drolet BA. Propranolol for infantile hemangiomas: promise, peril, pathogenesis. Pediatr Dermatol. 2009;26:642–644. doi: 10.1111/j.1525-1470.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 25.Maguiness SM, Frieden IJ. Current management of infantile hemangiomas. Semin Cutan Med Surg. 2010;29:106–114. doi: 10.1016/j.sder.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Fabian ID, Ben-Zion I, Samuel C, Spierer A. Reduction in astigmatism using propranolol as first-line therapy for periocular capillary hemangioma. Am J Ophthalmol. 2011;151:53–58. doi: 10.1016/j.ajo.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Weiss AH, Kelly JP. Reappraisal of astigmatism induced by periocular capillary hemangioma and treatment with intralesional corticosteroid injection. Ophthalmology. 2008;115:390–397. doi: 10.1016/j.ophtha.2007.03.077. [DOI] [PubMed] [Google Scholar]