Abstract
With the dissemination of optical coherence tomography over the past two decades, the role of persistent vitreomacular adhesion (VMA) in the development of numerous macular pathologies – including idiopathic macular hole, vitreomacular traction syndrome, cystoid and diabetic macular edema, neovascularization in diabetic retinopathy and retinal vein occlusion, exudative age-related macular degeneration, and myopic traction maculopathy – has been established. While invasive vitreoretinal procedures have long been utilized to address complications related to these disorders, such an approach is hampered by incomplete vitreoretinal separation and vitreous removal, surgical complications, and high costs. In light of such limitations, investigators have increasingly looked to nonsurgical means for the treatment of persistent pathologic VMA. Chief among these alternative measures is the intravitreal application of pharmacologic agents for the induction of vitreous liquefaction and/or vitreoretinal separation, an approach termed pharmacologic vitreolysis. This article aims to review the available evidence regarding the use of pharmacologic agents in the treatment of VMA-related pathology. In addition, a discussion of vitreous molecular organization and principles of physiologic posterior vitreous detachment is provided to allow for a consideration of vitreolytic agent mode of action and molecular targets.
Keywords: macular edema, macular hole, microplasmin, pharmacologic vitreolysis, posterior vitreous detachment, vitreomacular traction syndrome
Introduction
Perhaps owing to its fundamentally “invisible” nature, the vitreous has long been an under recognized cause of macular pathology. More recently, the improved characterization of vitreomacular relationships facilitated by the evolution of optical coherence tomography (OCT) has led to an increasing recognition of the role played by the posterior vitreous in such disorders. Numerous investigators have found an increased prevalence of incomplete posterior vitreous detachment – a surrogate marker for persistent vitreomacular adhesion (VMA) – in association with a number of retinal disorders including idiopathic macular hole,1 vitreomacular traction syndrome (VMT),2 cystoid (CME)3 and diabetic macular edema (DME),4 neovascularization in diabetic retinopathy5 and retinal vein occlusion (RVO),6 exudative age-related macular degeneration (ARMD),7 and myopic traction maculopathy.8 The pathologic role of VMA in many of these disorders relates to the transmission of static and dynamic anteroposterior tractional forces to the macular surface. The specific pathologic phenotype produced depends on the size and strength of the adhesion, with more extensive vitreous separations and smaller areas of vitreous adhesion imparting greater tractional force.8,9 The role of vitreoretinal and/or vitreomacular adhesion (hereafter referred to collectively as VMA) in the development of neovascularization and nontractional macular edema is less well defined. It may be due in part to residual posterior cortical vitreous acting as a barrier to the flux of important intravitreal molecules10 (specifically oxygen, growth factors, and cytokines) or as a scaffold for the growth of fibrovascular proliferation arising from the retinal surface.11
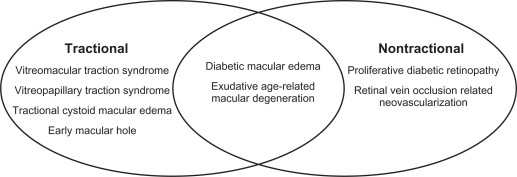
Of note, careful distinction should be made between pathologic (or “symptomatic”) VMA, a categorization that encompasses the pathologic entities described above, and nonpathologic (or “asymptomatic”) VMA in which persistent attachment of the posterior vitreous to the macula does not result in anatomic or functional impairment. This latter form of VMA is frequently noted incidentally on OCT imaging and represents a physiologic stage I posterior vitreous detachment (PVD).8,9 Taken in sum, these descriptions can be utilized to generate a mechanistic classification of disorders associated with pathologic VMA, detailed in Figure 1.
Figure 1.
Schematic representation of mechanistic classification of vitreomacular adhesion-related disease
With the introduction of pars plana vitrectomy by Machemer et al in 1971,12 a definitive solution for VMA-related disease became available to vitreoretinal surgeons. While implementation lagged in line with the delay in recognizing the etiologic role played by the posterior vitreous, the past two decades have seen a dramatic upswing in the application of vitrectomy. In many cases, elimination of vitreoretinal traction and complete separation of the posterior hyaloid from the retinal surface with removal of all vitreous gel lead to both anatomic and functional improvement. Though outcomes have improved with advancements in instrumentation and technique, the utility of vitrectomy remains limited by incomplete vitreoretinal separation and vitreous removal, associated complications, and high costs. Clinical and histologic studies have shown that residual cortical vitreous commonly adheres to the inner retinal surface following vitrectomy despite concentrated aspiration and peeling of the posterior hyaloid.13–15 Cortical vitreous remnants may organize into a fibrocellular epiretinal membrane with subsequent contraction causing macular pucker.16 Indeed, patients with pathologic VMA inherently have exaggerated adherence at the vitreoretinal interface, which in turn leads to a more difficult mechanical separation. Attempts to achieve a cleaner cleavage plane, through mechanical peeling of the internal limiting membrane (ILM), may increase the risk of surgical complications, including retinal hemorrhages, retinal breaks, nerve fiber layer (NFL) damage, and paracentral scotomas.17–19
In light of the inherent limitations associated with vitrectomy for the treatment of pathologic VMA, it is unsurprising that, over the past 15 years, investigators have increasingly examined alternative methods for induction of PVD. Though a few investigators have explored mechanical PVD induction with isolated intraocular gas injection20–22 and even LASIK (laser-assisted in-situ keratomileusis) suction rings,23,24 the bulk of efforts have focused on the use of pharmacologic agents to modify the molecular structure of the vitreous in order to eliminate its role in disease, a technique termed pharmacologic vitreolysis.25 In general, this involves the induction of vitreoretinal separation and/or liquefaction of vitreous gel either as monotherapy or in conjunction with vitrectomy. This article aims to review the available evidence to date regarding the application of pharmacologic agents in the endeavor of vitreolysis. A summary of vitreous molecular organization and principles of physiologic PVD will provide context for the consideration of vitreolytic agent mode of action and molecular targets. Following a careful analysis of specific indications for pharmacologic vitreolysis, the individual agents will be reviewed with summaries to include postulated mechanism of action, results of past pre-clinical and clinical trials (if available) as well as a discussion of any pending investigations. A final section on emerging trends and future directions will conclude the review.
Vitreous molecular organization and structure
Pharmacologic vitreolysis is largely an outgrowth of an evolving understanding of the molecular organization, structure, and physiology of the vitreous. This knowledge has allowed for the selection of appropriate agents to target key molecules and structures within the vitreous gel as well as at the vitreoretinal interface.
Consistent with its dual purpose of maintaining media clarity and mitigating possible concussive effects associated with ocular movement or trauma,26 the vitreous is a transparent viscoelastic extracellular gel matrix (ECM) composed of approximately 99% water.27 In the gel state, vitreous further maintains media clarity by acting as a barrier to limit cellular invasion27 and may also act to protect sensitive tissues such as the crystalline lens and trabecular meshwork from oxidative damage via its role in oxygen metabolism.28,29 These unique properties are a direct result of the molecular structure of vitreous gel, which consists of two complementary macromolecular structures: a fibrillar component, represented by collagen; and glycosaminoglycans (GAGs), chiefly hyaluronan with a lesser concentration of chondroitin sulfate. Vitreous collagen consists of heterotypic fibrils composed of a combination of collagen type II, type IX, and a hybrid type V/XI. The core of each fibril is composed of type V/XI collagen, which is surrounded by a sheath of fibrillar type II collagen as well as a regular arrangement of type IX collagen along the outermost surface of the fibril.27 The rope-like structures formed are largely resistant to proteolysis except by a few select proteases – bacterial collagenase, cathespin K, and certain matrix metalloproteinases.30 The heterotypic fibrils are arranged into interconnecting bundles that form an extended network, which provides shape, strength, and flexibility to the vitreous and allows it to resist tractional forces. As mentioned above, the GAG component is predominantly hyaluronan (>90%) along with chondroitin sulfate, which is found only in proteoglycan form (attached to a protein core) as either versican or collagen type IX.27 The negatively charged disaccharide moieties of hyaluronan attract a shell of water molecules that act to inflate the collagen matrix and provide swelling pressure to resist compressive forces. Chondroitin sulfate appears to be involved in the maintenance of interfibrillar spacing via interconnections between collagen fibrils mediated through links to opticin, a small leucine-rich repeat protein.31
As the site of adhesion between the posterior cortical vitreous and the ILM, the vitreoretinal interface plays a decisive role in the relationship between physiologic and pathologic VMA. The interface juxtaposes the ILM, a basement membrane secreted by underlying Müller cells, and the posterior hyaloid. Similar to basal membranes elsewhere in the body, the ILM is compromised chiefly of collagen type IV,32 which imparts a sheet-like conformation, in addition to several ECM proteins, notably laminin, fibronectin,33 and collagen type XVIII.34 Despite this expanding knowledge of vitreoretinal interface components, the mechanisms underlying VMA remain poorly understood. Histologic studies have demonstrated that vitreous collagen fibrils are oriented in an anteroposterior manner and, posterior to the vitreous base, run parallel to the ILM.35 However, at the vitreous base, vitreous collagen fibrils run perpendicular to and pass through the ILM where they insert to form an adhesion, considered unbreakable without proteolysis.36,37 This contrasts with the posterior vitreoretinal interface at which collagen fibrils do not insert into the ILM. Instead, VMA posterior to the vitreous base is thought to be fascial in nature and mediated by some form of “extracellular matrix glue” that connects cortical vitreous collagen fibrils with the ILM surface.38 ILM-associated molecules suggested as potential “glue” components include laminin and fibronectin, which have been demonstrated to have high affinity for collagen,39,40 as well as collagen type XVIII, which has been shown to be bound by the opticin molecules coating vitreous collagen fibrils.31
Principles of physiologic PVD
The process of physiologic, or age-related, PVD is well characterized from a mechanistic standpoint, but remains poorly understood on a molecular level. While the lack of an accurate molecular description has hampered the development of pharmacologic vitreolytics, improved understanding of the physical sequence of uncomplicated PVD has shed significant light on ways in which it may go awry and induce pathologic sequelae. Within the vitreous gel, two related processes, liquefaction (synchysis) and fibrillar collapse (syneresis), act as the primary motive force driving vitreoretinal separation.41,42 Liquefaction originates in the premacular cortical vitreous where it has been detected as early as 2 years of age in fixed autopsy specimens.43 Focal collections of liquefaction, or liquefied lacunae, subsequently form throughout the vitreous, increasing in number and size during adolescence and adulthood.41–44 This process culminates in the coalescence of extensive areas of synchysis, with approximately 70% of autopsy eyes obtained from donors aged 70 years and greater demonstrating at least 50% liquefaction of the vitreous body.41 As liquefaction progresses, the collagen fibrillar meshwork undergoes collapse. This leads to the aggregation of collagen into parallel bundles that form thick fibers, seen clinically as syneretic debris, which become progressively dense and tortuous with age.35,44 In line with the demonstrated positive correlation between the extent of synchysis/syneresis and PVD incidence,41,45,46 liquefaction with subsequent collapse appears to drive vitreoretinal separation following attenuation of the adhesion between the cortical vitreous and ILM of the posterior pole. Though previous investigators believed it to be an acute process,41,47 more recent studies indicate that PVD occurs over months to years, beginning as a shallow perifoveal vitreoretinal separation that expands gradually until complete PVD is achieved with acute separation of the vitreous from the optic disc margin.8,9
The weakening of the adhesion between the vitreous and the retina stands as a critical factor in the timing and progression of PVD. Multiple studies have demonstrated that the majority of patients less than age 60 have an intact vitreoretinal interface despite the presence of extensive liquefaction.41,45 After age 60, however, there is a much stronger correlation between PVD incidence and extent of liquefaction, indicating the presence of some factor that enhances the effect of liquefaction and collapse on PVD induction; this factor is likely a weakened adhesive force between the retina and vitreous.41,45 Variations in strength of this force likely determine whether a given PVD is accompanied by pathologic sequelae. In a subset of eyes, age-related PVD progresses normally except where especially firm vitreorentinal adhesions are encountered in the macula, at the optic disc margin, or at focal sites in the retinal periphery. In such eyes, dynamic (saccadic) vitreous traction is exerted upon the retina at the residual adhesion site(s), leading to various complications including macular hole, VMT, tractional DME, vitreopapillary traction syndrome (VPT), vitreous hemorrhage, and retinal tears.8,9 Sebag coined the term “anomalous PVD,” to describe the condition in which the extent of gel liquefaction and collapse exceed the attenuation of vitreorentinal adhesion.48 This condition results from disorders that cause premature vitreous liquefaction, including hereditary vitreoretinal syndromes, uveitis, vitreous hemorrhage, and high myopia, and increases the risk of PVD-associated morbidity.
To improve upon existing mechanical methods for the treatment of pathologic PVD, controlled induction of vitreous liquefaction and weakening of vitreorentinal adhesion will be necessary. Targeted pharmacologic agents, capable of altering the molecular organization of the vitreous gel and vitreoretinal interface, have the potential to provide such control.25 Unfortunately, the development of these agents has been slowed by the lack of accurate molecular descriptions of the processes in question. As mentioned above, the basis of vitreorentinal adhesion remains poorly described. The same is true of the physiologic weakening of vitreorentinal adhesion that accompanies aging. Investigators have proposed several possible age-related changes – including alterations in Müller cell function,49 thickening of the ILM,50 or the cumulative effects of incident light-generated free radicals51 – as a basis for this weakening. Similarly, models of age-related vitreous liquefaction, implicating incident light-generated free radicals52–54 or enzymatic degradation of collagen,55–57 have been suggested but remain largely unproven.
Indications for pharmacologic vitreolysis
From a pathophysiologic perspective, any manifestation of pathologic VMA should benefit from pharmacologic vitreoretinal separation, based on either release of anteroposterior traction (tractional VMA) or removal of premacular vitreous cortex (nontractional VMA). While this may be true in principle, the selection of appropriate indications for pharmacologic vitreolysis requires careful consideration of utilization models and therapeutic goals.
While early interest in pharmacologic vitreolysis focused on its application as an adjunct to vitrectomy and removal of fibrovascular proliferative membranes,58–60 investigators quickly realized its potential as a stand-alone therapy.61–63 Indeed, clinical applications of pharmacologic vitreolysis can be broadly grouped into two categories based on utilization model: pharmacology-assisted vitrectomy and pharmacologic PVD induction. In the former model, a pharmacologic agent administered preoperatively acts to either induce vitreous liquefaction, allowing for more rapid vitreous removal, or weaken the vitreorentinal adhesion, allowing for greater ease of mechanical PVD induction with a cleaner vitreoretinal separation.15,64–67 More rapid vitreous removal translates into shorter surgical times15,25,68 and possibly an increased ability to employ smaller gauge instrumentation,69,70 which are associated with reduced postoperative recovery times.71,72 Weakening of the vitreorentinal adhesion allows mechanical PVD creation without the use of high suction, which reduces the risk of iatrogenic tears73,74 and permits the use of smaller gauge instrumentation not capable of achieving such high levels of aspiration.69,75,76 Theoretically, it also facilitates cleaner vitreoretinal separation, avoiding cortical vitreous remnants and thereby reducing postoperative complications such as macular pucker,77 proliferative vitreoretinopathy (PVR),78,79 and persistence of diabetic retinopathy-related complications.80
When pharmacologic vitreolysis is used as stand-alone therapy, vitreolytic agents can be employed either as a definitive treatment in active VMA-related disease or as a prophylactic measure in conditions in which PVD is associated with an improved prognosis. Examples of the former include early macular holes, VMT, VPT, and tractional DME/CME,8,9,48 while the latter includes conditions such as DME,4 proliferative diabetic retinopathy (PDR),5,81,82 RVO,6,83–85 and ARMD.7,86,87 In all cases, the therapeutic effect results from induction of complete pharmacologic PVD. The basis of the prophylactic benefit of PVD in macular edema and neovascularization remains unclear, but likely relates to the barrier and scaffold functions played by posterior cortical vitreous discussed earlier. Of note, stand-alone vitreolytic therapy has also been utilized strictly for its liquefactive properties in conditions such as non-clearing vitreous hemorrhage;88,89 however, this indication is outside the scope of this review as it does not pertain to VMA-related disease.
As is the case with most ophthalmologic therapeutic interventions, pharmacologic vitreolysis is indicated in the conditions discussed above based on its potential to improve anatomical and functional outcomes. In addition to this primary therapeutic objective, ancillary goals should also be considered, including: decreased costs, based on shorter surgical times or decreased incidence of progressive disease requiring surgery; and greater access to therapy, based on the simple instrumentation involved and a possible transition to office-based procedures.25,68,90 A reduction in surgical cases, due to either a decreased incidence of advanced disease following prophylactic therapy or due to a transition to office-based treatment of VMA-related disease, would have the additional benefit of reducing patient exposure to vitrectomy-related complications – such as endophthalmitis, cataract, and iatrogenic breaks – as well as complications related to anesthesia.
Vitreolytic agents
Overview
The biochemical properties requisite in any potential vitreolytic agent include the ability to induce vitreous liquefaction (liquefactants), weakening of the vitreorentinal adhesion (interfactants), or both.25,90 These molecular alterations may be accomplished through either nonenzymatic, or more commonly, enzymatic means. Based on these characteristics, the available vitreolytic agents – including those previously tested as well as those that remain under development – can be classified as detailed in Table 1.25,90 The following section aims to review the mechanism of action of each agent and summarize the results of relevant pre-clinical and clinical trials.
Table 1.
Classification of pharmacologic vitreolytic agents
| Interfactants | Liquefactants | Combination | |
|---|---|---|---|
| Enzymatic | Dispase | Hyaluronidase Collagenase |
Plasmin Microplasmin tPA ± plasminogen Nattokinase Chondroitinase |
| Nonenzymatic | RGD peptides | Vitreosolvea |
Note:
Vitreosolve® (Vitreoretinal Technologies Inc, Irvine, CA).
Abbreviations: RGD, arginine-glycine-aspartate; tPA, tissue plasminogen activator.
Collagenase
Bacterial collagenase, purified from Clostridium histolyticum, is one of the few proteases known to cleave the type II collagen comprising the fibrillar network of the vitreous gel.30 In contrast to fibrillar collagen, the resulting proteolyzed fragments are soluble, allowing for spontaneous denaturation and further degradation by nonspecific proteases.91 Prolonged intravitreal incubation of escalating doses of bacterial collagenase in rabbits resulted in dose-dependent liquefaction, but was accompanied by ILM damage and disruption of retinal architecture at doses achieving clinically significant degrees of liquefaction.92 Using lower doses and shorter incubation periods, subsequent studies in a rabbit model of PVR demonstrated some focal fibrovascular membrane digestion prior to vitrectomy, although histologic and electrophysiologic toxicity was noted at incubation periods greater than 30 minutes.58,60 Later human pilot studies of collagenase-assisted vitrectomy resulted in hemorrhages on the surface of the retina as well as evidence of digestion of retinal vasculature on proliferative membranes,59,81 which confirmed earlier reports of severe retinal hemorrhages in rabbits.93
Chondroitinase
Although substrate specificity varies depending on the bacterial species of origin, chondroitinase catalyzes the depolymerization of various GAGs including chondroitin sulfate, hyaluronan, and dermatan sulfate.94 The role of chondroitin sulfate within the vitreous remains unclear. It is generally found in proteoglycan form as either versican, which is linked to hyaluronan, or as collagen IX, which coats the heterotypic vitreous collagen fibrils.27 Some investigators believe it may contribute to vitreous structure or spacing by linking the hyaluronan network to other integral ECM components,27 while others have reported it may play a role in vitreorentinal adhesion based on immunolocalization of chondroitin sulfate to the vitreous base and papillary margin.95 Early pre-clinical results examining the vitreolytic potential of chondroitinase were mixed. One group found intravitreal chondroitinase to have no effect on vitreous liquefaction or vitreorentinal adhesion in a rabbit model,93 while a second group found complete vitreous body disinsertion, including at the vitreous base, prior to vitrectomy in cynomologus monkeys as well as in a limited number of human cadaver eyes.95 This latter group also found no microscopic, immunohistochemical, or electroretinography (ERG) evidence of retinal toxicity up to 16 months following the procedure.95 Two studies comparing the liquefactive capacity of chondroitinase to hyaluronidase and plasmin in animal models demonstrated modest reduction in wet weight following 48-hour incubation of isolated vitreous gel as well as a mild enhancement of gel removal during standardized vitrectomy following 1 and 3 hours incubation.96,97 The significantly lower doses used in these studies did not appear to induce an accompanying gel collapse,96 but were associated with mild ILM or NFL damage in the vitrectomy-based study.97 In a later study evaluating PVD induction in an enzyme-assisted vitrectomy model in pigs, chondroitinase, at similarly low doses, failed to achieve a significant improvement in the rate of spontaneous PVD, the extent of PVD, or the degree of residual collagen detected on electron microscopy (EM) when compared with controls.98 Thus, initial encouraging results were unable to be duplicated in later trials using much lower doses, which did still demonstrate some limited toxicity.
Hyaluronidase
Hyaluronidase is an endoglycosidase capable of cleaving bulky hyaluronan molecules, in addition to other GAGs such as chondroitin sulfate, into much smaller fragments.99 The resulting decreased viscosity within the ECM allows for easier passage of material through the ECM. This property has already been exploited clinically to increase the absorption and dispersion of injected drugs such as anesthetics.100 As hyaluronan plays a critical role in maintaining the gel-like character of the vitreous, hyaluronidase possesses considerable potential as a liquefactive agent. Hyaluronidase-mediated vitreous liquefaction has been demonstrated both in vitro96 and in vivo,101,102 and more recently, in Phase III trials studying its potential in speeding the clearance of vitreous hemorrhage.88,89 Additionally, hyaluronidase was shown to significantly improve the extent of vitreous removal during standardized vitrectomy compared with untreated eyes after incubations of 1 or 3 hours.97 In view of the clear association between age-related PVD and vitreous liquefaction, hyaluronidase would seem a logical agent for pharmacologic induction of PVD. Initial studies examining low dose (1 U) intravitreal hyaluronidase in conjunction with perfluoropropane gas in a rabbit model exhibited only partial PVD following 3-day incubations; this contrasted with eyes receiving either hyaluronidase or gas alone, in which no PVD was detected.103 Using higher doses (5–20 U) of hyaluronidase alone in a similar rabbit model, two follow-up studies produced very different outcomes: one reported gradual development of PVD at 5–8 weeks in nearly all experimental eyes at the highest doses (10 and 20 U),62 while a later study reported no PVD in any experimental eye at 3 and 6 months using a similarly high dose (20 U).104 Although the source of the discrepancy is unclear, the authors of the latter study implicated inadequacy of the light microscopy and clinical examination for PVD determination employed in the former study compared with the EM techniques utilized in their own study.104 The poor performance of hyaluronidase (20 U) in PVD induction was confirmed by a separate group, again using EM to assess PVD status, though the incubation period in this study was considerably shorter at 1 week.105 In terms of safety, hyaluronidase was generally well tolerated up to doses of 20 U at incubation periods ranging from 3 to 28 days.62,102,103,105 Aside from transient vitreous haze noted at nearly all doses and incubation periods,62,102–105 there was no evidence of retinal structural toxicity, as assessed by EM,102,103,105 nor functional toxicity, as assessed by ERG.62,103,105 Reported toxicities at higher doses were mixed, with one group demonstrating disruption of retinal cellular anatomy at doses as low as 30 U (bovine hyaluronidase) for 1 week, while the large Phase III trial using doses of 55 and 75 IU (ovine hyaluronidase) reported only an increased rate of transient moderate-severe iritis.89 Despite impressive liquefactive capacity, hyaluronidase appears limited in terms of PVD induction as it has no demonstrated impact on the vitreoretinal interface. Investigators hypothesized that the induction of PVD by hyaluronidase follows liquefaction of central vitreous, with collapse and subsequent loss of support for cortical vitreous fibers, rendering it susceptible to separation by mechanical forces such as eye movements.62,104 In effect, hyaluronidase produces PVD through an increase in vitreoretinal traction and may worsen existing VMA-related pathologies.
Dispase
A neutral protease obtained from Bacillus polymyxa, dispase is known to cleave type IV collagen and fibronectin while laminin and other collagen subtypes are left largely unaltered.106 Given its activity against type IV collagen, a major component of the ILM, and fibronectin, postulated to be a factor in vitreorentinal adhesion, dispase was tabbed as a vitreolytic agent based on its potential to mediate vitreoretinal disinsertion. Interestingly, dispase was initially utilized intravitreally to generate an animal model of PVR without the addition of exogenous cells.107,108 This model was based on the ability of dispase to release endogenous cells – such as fibroblasts, macrophages, and glial cells – from their cellular attachments and recruit them into the vitreous. As PVR generation was achieved with prolonged incubations at very low doses (0.01–0.50 U),107,108 later studies investigating the utility of dispase in PVD induction used shorter incubation periods (15–120 minutes) at the same or higher doses.63,109–111 In effect, dispase was only evaluated as an agent for enzyme-assisted vitrectomy due to the fact that removal of the enzyme following a specified incubation would be required to avoid PVR development. In the two earliest such studies, dispase achieved significantly higher rates of spontaneous complete PVD in enucleated pig and human eyes as well as pig eyes in vivo at the majority of doses tested following 15 or 120 minutes of incubation.63,109 Neither study revealed significant toxicity, excepting transient vitreous haze.63,109 In contrast, a similar study of dispase-assisted vitrectomy in rabbits in vivo using greater doses and incubation times failed to show any effect on PVD induction, and enzyme use was associated with retinal and vitreous hemorrhages.110 Further evidence of dispase-associated toxicity was found in two follow-up studies in an in-vivo rabbit model: in addition to vitreous hemorrhage and cataract formation, histologic exam revealed disruption of ganglion cell and photoreceptor layers, while ERG testing revealed significant depression of a- and b-wave amplitudes.111,112 These studies did employ longer incubations (1 week), but clinical evidence of toxicity was noted as early as 30 minutes following injection.111,112 The reason for the conflicting results regarding the safety and efficacy of dispase in vitreolysis is unclear but may be related to the model used. Regardless, given its ability to generate PVR as well as its action on collagen IV, a major component of the ILM and lenticular capsule, the safety of dispase remains questionable at best.
Nattokinase
Originally discovered in the Japanese soybean cheese nattō, nattokinase is a serine protease produced by Bacillus subtilius.113 Although incompletely characterized, it is known to possess potent fibrinolytic effects likely due to its enhancement of plasmin activation via increased synthesis of tissue plasminogen activator (tPA)114 as well as inactivation of plasmin activator inhibitors.115 Additionally, nattokinase has direct proteolytic effects on collagen.116 Thus, the postulated PVD induction mechanism of nattokinase involves some combination of direct liquefaction and plasmin-mediated vitreoretinal dehiscence. In the rabbit model, intravitreal injection resulted in histologically confirmed PVD (eg, bare ILM) in all eyes at the two highest doses following 30-minute incubations; however, eyes exposed to the highest dose displayed subtle alterations in inner retinal architecture, parapapillary retinal hemorrhages, and ERG depression.116 To date, no follow-up investigations have been conducted.
Plasmin
Undoubtedly the most widely studied vitreolytic agent, plasmin is a serine protease with a critical role in fibrinolysis. In addition to fibrin, plasmin has been shown to directly degrade other ECM components including laminin and fibronectin,117–119 which have a postulated role in vitreorentinal adhesion.33 Plasmin may also indirectly generate increased levels of other nonspecific proteases such as matrix metalloproteinases120,121 and elastase,122 capable of cleaving further ECM structures. These downstream activities may enhance the primary action of plasmin – weakening of vitreoretinal insertion – or may allow for a limited degree of liquefaction. Initial studies of plasmin (1 U) with and without adjunctive vitrectomy61 or intraocular gas injection123 in a rabbit model indicated that such adjunctive procedures were necessary to achieve clean vitreoretinal separation. Similar results were obtained in human cadaver eyes using a plasmin-assisted vitrectomy model in which the addition of 1 U of plasmin was found to eliminate the cortical vitreous remnants noted on the ILM in untreated, vitrectomized eyes.15 Surprisingly, in follow-up studies by the same group in both enucleated pig124 and human eyes,119,125 short incubations (30–60 minutes) with similar doses of plasmin (1–3 U) also resulted in a bare ILM without the use of adjunctive techniques. Further in-vivo studies in rabbits, however, indicated that the previous results in cadaver eyes may have been related to specific properties of post-mortem eyes, as very low rates of complete PVD were found following prolonged exposure (1 week) to 1 U of plasmin.105,112 Better rates of complete PVD were achieved with higher doses (4 U).112 Several studies were able to demonstrate a correlation between both plasmin concentration and exposure time and the resultant extent of vitreoretinal separation.61,119,124 The safety profile of intravitreal plasmin in these pre-clinical trials was excellent. Excepting a consistent finding of transient vitreous haze,61,105,112,123 there were no reported toxicities despite thorough histologic examination with light and EM at doses up to 4 U and with exposure times ranging from 30 minutes to 1 week.61,105,112,119,124,125 No functional toxicity was detected by ERG in three studies,105,112,123 while a fourth found only transient depression of b-wave amplitudes.61 One group, using a plasmin-assisted vitrectomy model in enucleated pig eyes, reported rare ILM (0%–20%) and NFL (0%–10%) damage at doses of 3 and 30 U, with incubations of 1 and 3 hours.97
As plasmin is exceedingly unstable owing to rapid inactivation via autolysis and binding to α2-antiplasmin, clinical application typically requires activation of its proenzyme, plasminogen, with plasminogen activators immediately prior to use. Application in human trials is further complicated by the lack of an approved, commercially available plasminogen. In place of a commercial alternative, investigators have relied on the time-consuming and expensive process of autologous plasmin enzyme (APE) generation via harvesting a patient’s own plasma-derived plasminogen and purifying it via affinity chromatography.126 Numerous human pilot studies used this technique to examine outcomes of plasmin-assisted vitrectomy, with doses ranging from 0.03 U up to 2 U, in a variety of VMA-related disorders. Because of the exceptionally strong vitreorentinal adhesion found in pediatric patients,50 several groups examined the use of APE in the surgical treatment of pediatric traumatic macular holes,126,127 stage 5 retinopathy of prematurity,128,129 and complicated X-linked retinoschisis;130 all reported improved anatomic outcomes compared with previously published studies. Other investigators employed plasmin-assisted vitrectomy to treat full-thickness macular holes in patients without pre-existing PVD and found high rates of spontaneous PVD intraoperatively, which reduced overall surgical time.131–133 The application of APE prior to vitrectomy resulted in nonsignificant increases in spontaneous PVD and ease of PVD induction in eyes with tractional DME77,134,135 and complicated PDR.136 One comparative study reported significantly higher visual acuities at 1 year follow-up in patients with DME treated with APE compared with those not receiving such treatment.134 More recently, a small prospective study found that intravitreal APE without vitrectomy improved central macular thickness and visual acuity in eyes with macular edema complicating branch RVO.137 Of note, many groups reported varying degrees of vitreous liquefaction following APE administration,77,127,131,133,135,136 although several others either did not comment or noted the lack of liquefaction.134 In this collection of pilot studies, no investigator reported a clear enzyme-related complication.
Plasminogen activators (tPA and urokinase [UK])
Potent fibrinolytic agents originally approved for a variety of nonophthalmologic vascular disorders including stroke, symptomatic coronary artery, and peripheral vascular occlusive disease, intraocular plasminogen activators have proven to be of modest utility in the treatment of several ophthalmologic conditions including post-surgical fibrin formation, submacular hemorrhage, and acute RVO.138,139 As serine proteases capable of activating plasminogen, both tPA and UK exert their effects indirectly through plasmin. Several groups turned to plasminogen activators as potential vitreolytic agents in lieu of plasmin based on: (1) the ease of access to a US Food and Drug Administration-approved commercially available formulation, (2) the safety of a recombinant molecule compared with blood derivatives in terms of microbial contamination, and (3) the established intraocular safety record based on its use for other indications.140 The challenge in working with plasminogen activators, however, lies in the need for clinically sufficient quantities of intraocular plasminogen, which outside the setting of pathologic blood–retinal barrier breakdown (ie, intraocular inflammation, hemorrhage, PDR),141 can typically only be achieved via iatrogenic blood–retinal barrier breakdown (ie, cryopexy)142 or exogenous administration.143,144 In a rabbit model, cryopexy followed by tPA and limited vitrectomy (static core vitrectomy only) induced PVD in all treated eyes, whereas no PVD was noted in eyes not receiving either cryopexy or tPA injection.142 Two later in-vivo rabbit studies examined the efficacy of UK plus exogenous plasminogen with differing results.143,144 Complete PVD was noted in all eyes at the two highest nontoxic doses of UK plus purified human plasminogen,143 but only occurred with the addition of intraocular sulfur hexafluoride following UK plus recombinant plasminogen in a later study.144 In human pilot studies, tPA-assisted vitrectomy in patients with complicated PDR was not associated with spontaneous PVD induction (assessed intraoperatively) nor with any significant improvement in postoperative functional outcomes.145 However, a subsequent report of tPA injection alone in patients with RVO-associated macular edema revealed post-treatment PVD in a majority of patients that correlated with improved functional and anatomic outcomes.146 Overall, plasminogen activators, much like plasmin, have a promising efficacy and safety record, but their therapeutic potential will continue to be limited by the need for adequate concentrations of intraocular plasminogen substrate. Assuring sufficient quantities of plasminogen, either through exogenous administration or blood–retinal barrier breakdown, inevitably leads to imprecise dosing.
Microplasmin
Another agent initially developed for systemic administration in the treatment of cerebral thromboembolic disease, microplasmin is a recombinant molecule, highly expressed in the Pischia pastoris yeast expression system, consisting solely of the catalytic domain of human plasmin.147 It has several advantages over plasmin: (1) it is commercially available, allowing investigators to avoid the time-intensive and expensive production of APE; (2) it is generated by recombinant techniques, assuring its sterility and avoiding the risk of microbial contamination; (3) it is much smaller than plasmin (22 versus 88 kDa), which theoretically would allow for greater penetration of epiretinal tissues; and (4) it is inherently more stable than plasmin and can be stored in citrate buffer prior to use.147–149 It is thus unsurprising – given the greater safety, ease of administration, and dosing accuracy – that investigators increasingly turned to microplasmin for the treatment of VMA-related pathologies in favor of either plasmin or plasminogen activators. The initial pre-clinical studies revealed achievement of complete PVD (eg, bare ILM) at doses ranging from 62.5–125.0 μg (equivalent to 1–2 U of plasmin) in enucleated human eyes with or without adjunctive intraocular gas.148 In-vivo studies similarly exhibited complete PVD following 7–21 day intravitreal microplasmin exposure with doses differing based on species; cat eyes achieved PVD at doses of 14.5–25.0 μg,148 while two separate studies in rabbits reported consistent PVD at doses of 125–250 μg.149,150 No histologic toxicity148–150 or functional toxicity149 was noted excepting persistent a- and b-wave depression on ERG testing in the 250 μg group only. A later study in enucleated pig eyes utilized a dose- (range 62.5–400 μg incubated for 1 or 2 hours) and exposure time-escalation (range 15–120 minutes after 125 μg administration) design.151 Their results demonstrated both time- and dose-dependent PVD development with a minimal effective dose of 125 μg and a minimal effective incubation period of 60 minutes with 125 μg dose. These values may have been somewhat inflated by the use of room temperature incubations. Although no disruption of cellular anatomy was noted in any experimental eye, serous-like retinal elevations were detected in 25% of eyes exposed to 400 μg for 120 minutes and scattered dendritic-like cells were found on the ILM in eyes receiving doses of 125 μg or higher for 120 minutes, with a greater number of cells in eyes administered higher doses.
Based on the promising anatomic and safety profile reported in these pre-clinical studies, a series of clinical trials was undertaken collectively entitled the Microplasmin Intravitreal Injections (MIVI) trials. MIVI-I was an uncontrolled Phase I/IIa clinical trial designed to assess safety and preliminary efficacy at various doses (25, 50, 75, and 125 μg for 24 hours) and exposures (2 hours, 24 hours, or 7 days following 25 μg administration) of microplasmin in enzyme-assisted vitrectomy for the treatment of VMA-related pathologies including tractional DME, VMT, and full-thickness macular holes.152 The incidence of spontaneous PVD as well as the ease of PVD induction intraoperatively were found to be both time- and dose-dependent, although no more than 50% of eyes in any one cohort developed spontaneous PVD. Of note, the three eyes in the highest dose cohort were noted to have vitreoschisis intraoperatively, and thus were incorrectly classified as spontaneous PVDs by ultrasound. Aside from a single retinal detachment noted shortly following microplasmin administration, there were no safety concerns ascribed to the study drug. MIVI-IIt (traction) was a follow-up Phase II prospective sham-controlled trial examining the efficacy of microplasmin alone for the treatment of VMT and early macular holes.153 In this trial, patients were divided into four cohorts and randomized 4:1 to microplasmin at doses of 75, 125, 175, and repeatable 125 μg doses or sham injection and followed with serial OCT and ultrasound exams. A significantly higher incidence of PVD was noted in the 125 μg cohort as well as the pooled (all doses) microplasmin cohort at 90 days, but not at the remaining time points (14, 28, and 180 days). At 28 days, nonsurgical release of VMA was achieved in 8%, 25%, 44%, and 27% of patients in the sham, 75, 125, and 175 μg cohorts, respectively, with no significant difference observed in the overall analysis or in pairwise comparisons. A larger multicenter Phase IIb prospective placebo-controlled trial, MIVI-III, was subsequently designed to evaluate three doses of microplasmin (25, 75, and 125 μg) compared with placebo administered 7 days prior to vitrectomy for the treatment of a variety of VMA-related disorders.69 No significant pairwise differences were found in terms of spontaneous PVD induction, but there was significantly greater progression of PVD from the date of microplasmin administration until surgery in the 125 μg cohort compared with placebo. Within the 125 μg cohort, there were also a significantly greater proportion of patients in the total cohort as well as within the subset of patients with macular holes who achieved resolution of their condition without the need for vitrectomy versus placebo. Two additional MIVI trials were conducted, but results have yet to be formally published. MIVI-II (TG-MV-002) is a Phase II sham-controlled non-vitrectomy study in patients with tractional DME that completed data collection in March 2010. MIVI-Trust (Traction Release without Surgical Treatment) (TM-MV-006, TM-MV-007) is a Phase III multicenter, randomized, placebo-controlled trial evaluating 125 μg of microplasmin alone for the treatment of focal VMA associated with subjective visual dysfunction, a concept the study designers have labeled “symptomatic VMA,” that completed data collection in July 2010. The preliminary results of this latter trial were reported at the 2011 ARVO (Association for Research in Vision and Ophthalmology) meeting and indicated a statistically significant improvement in the rate of pharmacological resolution of symptomatic VMA in the microplasmin group compared with placebo.154 Formal publication of results from both of these studies should be forthcoming in the near future.
RGD peptides
Widely expressed cell surface receptors, integrins, play a critical role in cellular-ECM adhesion and signaling.155 Binding to integrins is mediated through a specific binding motif – defined by the amino acid sequence arginine-glycine-aspartate (RGD) – present in a vast array of ECM components including laminin, fibronectin, and certain collagens.155 Synthetic RGD peptides are known to compete for integrin binding sites, which results in disruption of integrin-ECM interaction and subsequent loosening of attachments.156 As immunolocalization studies have identified integrins on the surface of the ILM,157 there has been considerable speculation regarding a possible role of synthetic RGD peptides in disrupting vitreorentinal adhesion, and by extension, a potential utility in vitreolysis. In a rabbit model, 24-hour incubation of intravitreal RGD peptides followed by limited vitrectomy – consisting of 30-second core vitrectomy with attempted PVD induction at low aspiration (≤30 mmHg) – resulted in a significantly greater extent of PVD in treated eyes compared with controls; however, only a single treated eye achieved complete PVD. Aside from focal retinal edema noted in half of the treated eyes, no toxicity was detected on clinical examination, EM analysis, or TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) apoptosis assay.158 Despite the modest success of this initial study, no further studies have been published.
Vitreosolve®
Vitreosolve® (Vitreoretinal Technologies Inc, Irvine, CA) is a proprietary nonenzymatic, urea-based molecule currently undergoing Phase II/III testing (PVD-301, PVD-302) in patients with non-proliferative diabetic retinopathy without pre-existing PVD. Given the proprietary nature of the agent, there is little information regarding its structure or mechanism. Preliminary results from an earlier, unpublished study demonstrated PVD induction in 45% of patients following a single 12 mg injection with the proportion rising to 75% following a second injection 30 days later.159 As Phase II/III trials are currently enrolling patients, final results may be pending for some time.
Conclusion
Medical therapy of VMA-related disorders is an exciting and promising area of investigation but currently remains an incompletely realized technology. To facilitate the identification of new agents and maximize the therapeutic potential of current vitreolytics, additional efforts are needed to expand present knowledge of vitreous molecular structure and physiology. Future work on existing agents will undoubtedly draw on the studies reviewed above, though care must be taken in evaluating this body of literature. Two characteristics of previous study design warrant further discussion: PVD definition and clinical model. The modality used to assess for the presence of PVD varies significantly between studies – ranging from clinical examination to ultrasonography to EM – thus complicating inter-study comparison. Moreover, the definition of complete PVD differed significantly and was often related to the assessment modality employed; complete PVD by microscopy typically signified the absence of collagen fibrils on the small specimen of ILM examined, while OCT and ultrasound looked more macroscopically – though perhaps less sensitively – at the vitreoretinal interface. The considerable variability in choice of clinical models also impacts study outcomes. Physiologic PVD is not known to occur in either rabbit61 or pig eyes124 – indicating a distinctly different vitreous physiology – and differences in ILM thickness might exaggerate perceived toxicities in certain models.36 Additionally, use of cadaver models may be confounded by differences in temperature, pH, solubility parameters, and electrolyte concentrations, which vary depending on the duration of the post-mortem period.160
Study design issues notwithstanding, many of the agents examined to date have shown the capacity to produce significant degrees of vitreous liquefaction or weakening of vitreorentinal adhesion. Unfortunately, these changes are rarely induced concurrently or in the appropriate proportions to induce a safe and reliable PVD. Agents acting either solely through liquefaction (liquefactants such as hyaluronidase and collagenase) or vitreoretinal interface disruption (interfactants such as dispase or RGD peptides) are non-physiologic and have the potential to worsen existing tractional pathology. Plasmin-based agents have shown some ability to concurrently liquefy vitreous and weaken vitreorentinal adhesion; however, the presence of liquefaction is inconsistent and its extent is quite variable.127,131,161 Moreover, some investigators suggest that plasmin/microplasmin-associated liquefaction is actually due to drug-related inflammation and that this change occurs several hours after plasmin exerts its effect on the vitreoretinal interface.161 Such temporal separation of the two components of PVD induction may ultimately reduce efficacy. This combination of factors likely accounts for the surprisingly low rates of spontaneous PVD noted in human trials of plasmin and microplasmin, which were routinely less than 50%.69,77,134,152,153 As no single agent currently appears sufficient for routine PVD production, the future of pharmacologic vitreolysis will likely involve the simultaneous administration of different agents with distinct roles in PVD production.51,90 Two in vivo pre-clinical studies, one in rabbits105 and the second in diabetic rats,162 have utilized this approach with excellent results. In these studies, 80%–100% of eyes treated with a combination of hyaluronidase and plasmin/microplasmin achieved spontaneous PVD without adjunctive procedure compared with much lower rates in eyes treated with the individual agents alone (0%–12.5%). Future studies could explore the use of different doses and combinations of existing agents and could permit the application of agents previously found to be associated with significant toxicity as combination therapy may allow the use of lower doses. Other promising concepts deserving of further exploration include the use of nonenzymatic agents, which offer the potential for vitreolysis without collateral damage to adjacent ECM structures, and the identification of particular agents for specific clinical indications.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Akiba J, Quiroz MA, Trempe CL. Role of posterior vitreous detachment in idiopathic macular holes. Ophthalmology. 1990;97(12):1610–1613. doi: 10.1016/s0161-6420(90)32368-0. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe NS. Vitreous traction at the posterior pole of the fundus due to alterations in the vitreous posterior. Trans Am Acad Ophthalmol Otolaryngol. 1967;71(4):642–652. [PubMed] [Google Scholar]
- 3.Roldan M, Serrano JM. Macular edema and vitreous detachment. Ann Ophthalmol. 1989;21(4):141–148. [PubMed] [Google Scholar]
- 4.Nasrallah FP, Jalkh AE, Van Coppenolle F, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95(10):1335–1339. doi: 10.1016/s0161-6420(88)33004-6. [DOI] [PubMed] [Google Scholar]
- 5.Akiba J, Arzabe CW, Trempe CL. Posterior vitreous detachment and neovascularization in diabetic retinopathy. Ophthalmology. 1990;97(7):889–891. doi: 10.1016/s0161-6420(90)32486-7. [DOI] [PubMed] [Google Scholar]
- 6.Akiba J, Kado M, Kakehashi A, Trempe CL. Role of the vitreous in posterior segment neovascularization in central retinal vein occlusion. Ophthalmic Surg. 1991;22(9):498–502. [PubMed] [Google Scholar]
- 7.Robison CD, Krebs I, Binder S, et al. Vitreomacular adhesion in active and end-stage age-related macular degeneration. Am J Ophthalmol. 2009;148(1):79–82e72. doi: 10.1016/j.ajo.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537–567. [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371–382.e371. doi: 10.1016/j.ajo.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Ranchod TM, Goldenberg DT, Trese MT. Pharmacologic vitreodynamics. Int Ophthalmol Clin. 2009;49(2):135–140. doi: 10.1097/IIO.0b013e31819fd66b. [DOI] [PubMed] [Google Scholar]
- 11.Chu TG, Lopez PF, Cano MR, et al. Posterior vitreoschisis. An echographic finding in proliferative diabetic retinopathy. Ophthalmology. 1996;103(2):315–322. doi: 10.1016/s0161-6420(96)30698-2. [DOI] [PubMed] [Google Scholar]
- 12.Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(4):813–820. [PubMed] [Google Scholar]
- 13.Sonoda KH, Sakamoto T, Enaida H, et al. Residual vitreous cortex after surgical posterior vitreous separation visualized by intravitreous triamcinolone acetonide. Ophthalmology. 2004;111(2):226–230. doi: 10.1016/j.ophtha.2003.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Russell SR, Hageman GS. Optic disc, foveal, and extrafoveal damage due to surgical separation of the vitreous. Arch Ophthalmol. 2001;119(11):1653–1658. doi: 10.1001/archopht.119.11.1653. [DOI] [PubMed] [Google Scholar]
- 15.Gandorfer A, Ulbig M, Kampik A. Plasmin-assisted vitrectomy eliminates cortical vitreous remnants. Eye (Lond) 2002;16(1):95–97. doi: 10.1038/sj.eye.6700064. [DOI] [PubMed] [Google Scholar]
- 16.Sebag J, Gupta P, Rosen RR, Garcia P, Sadun AA. Macular holes and macular pucker: the role of vitreoschisis as imaged by optical coherence tomography/scanning laser ophthalmoscopy. Trans Am Ophthalmol Soc. 2007;105:121–129. discussion 129–131. [PMC free article] [PubMed] [Google Scholar]
- 17.Han DP, Abrams GW, Aaberg TM. Surgical excision of the attached posterior hyaloid. Arch Ophthalmol. 1988;106(7):998–1000. doi: 10.1001/archopht.1988.01060140144042. [DOI] [PubMed] [Google Scholar]
- 18.Vander JF, Kleiner R. A method for induction of posterior vitreous detachment during vitrectomy. Retina. 1992;12(2):172–173. doi: 10.1097/00006982-199212020-00013. [DOI] [PubMed] [Google Scholar]
- 19.Haritoglou C, Ehrt O, Gass CA, Kristin N, Kampik A. Paracentral scotomata: a new finding after vitrectomy for idiopathic macular hole. Br J Ophthalmol. 2001;85(2):231–233. doi: 10.1136/bjo.85.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thresher RJ, Ehrenberg M, Machemer R. Gas-mediated vitreous compression: an experimental alternative to mechanized vitrectomy. Graefes Arch Clin Exp Ophthalmol. 1984;221(5):192–198. doi: 10.1007/BF02134139. [DOI] [PubMed] [Google Scholar]
- 21.Miller B, Miller H, Ryan SJ. Experimental vitreous syneresis. Arch Ophthalmol. 1985;103(9):1385–1388. doi: 10.1001/archopht.1985.01050090137049. [DOI] [PubMed] [Google Scholar]
- 22.Lincoff H, Kreissig I, Jakobiec F, Iwamoto T, Vitolo J, Shapiro R. Gas vitrectomy in a primate model. Graefes Arch Clin Exp Ophthalmol. 1986;224(3):215–217. doi: 10.1007/BF02143056. [DOI] [PubMed] [Google Scholar]
- 23.Luna JD, Artal MN, Reviglio VE, Pelizzari M, Diaz H, Juarez CP. Vitreoretinal alterations following laser in situ keratomileusis: clinical and experimental studies. Graefes Arch Clin Exp Ophthalmol. 2001;239(6):416–423. doi: 10.1007/s004170100295. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesh P, Vajpayee RB, Garg S. Induction of posterior vitreous separation using LASIK suction ring may have a potential role in the management of diabetic macular edema. Med Hypotheses. 2006;66(6):1137–1139. doi: 10.1016/j.mehy.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Sebag J. Pharmacologic vitreolysis. Retina. 1998;18(1):1–3. doi: 10.1097/00006982-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Foulds WS. Is your vitreous really necessary? The role of the vitreous in the eye with particular reference to retinal attachment, detachment and the mode of action of vitreous substitutes. Eye (Lond) 1987;1(Pt 6):641–664. doi: 10.1038/eye.1987.107. [DOI] [PubMed] [Google Scholar]
- 27.Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19(3):323–344. doi: 10.1016/s1350-9462(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 28.Shui YB, Holekamp NM, Kramer BC, et al. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009;127(4):475–482. doi: 10.1001/archophthalmol.2008.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holekamp NM. The vitreous gel: more than meets the eye. Am J Ophthalmol. 2010;149(1):32–36. doi: 10.1016/j.ajo.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 30.Bishop PN. Vitreous as a substrate for vitreolysis. Dev Ophthalmol. 2009;44:7–19. doi: 10.1159/000223939. [DOI] [PubMed] [Google Scholar]
- 31.Hindson VJ, Gallagher JT, Halfter W, Bishop PN. Opticin binds to heparan and chondroitin sulfate proteoglycans. Invest Ophthalmol Vis Sci. 2005;46(12):4417–4423. doi: 10.1167/iovs.05-0883. [DOI] [PubMed] [Google Scholar]
- 32.Heegaard S, Jensen OA, Prause JU. Structure and composition of the inner limiting membrane of the retina. SEM on frozen resin-cracked and enzyme-digested retinas of Macaca mulatta. Graefes Arch Clin Exp Ophthalmol. 1986;224(4):355–360. doi: 10.1007/BF02150029. [DOI] [PubMed] [Google Scholar]
- 33.Kohno T, Sorgente N, Ishibashi T, Goodnight R, Ryan SJ. Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci. 1987;28(3):506–514. [PubMed] [Google Scholar]
- 34.Ponsioen TL, van Luyn MJ, van der Worp RJ, van Meurs JC, Hooymans JM, Los LI. Collagen distribution in the human vitreoretinal interface. Invest Ophthalmol Vis Sci. 2008;49(9):4089–4095. doi: 10.1167/iovs.07-1456. [DOI] [PubMed] [Google Scholar]
- 35.Sebag J, Balazs EA. Morphology and ultrastructure of human vitreous fibers. Invest Ophthalmol Vis Sci. 1989;30(8):1867–1871. [PubMed] [Google Scholar]
- 36.Foos RY. Vitreoretinal juncture; topographical variations. Invest Ophthalmol. 1972;11(10):801–808. [PubMed] [Google Scholar]
- 37.Wang J, McLeod D, Henson DB, Bishop PN. Age-dependent changes in the basal retinovitreous adhesion. Invest Ophthalmol Vis Sci. 2003;44(5):1793–1800. doi: 10.1167/iovs.02-0802. [DOI] [PubMed] [Google Scholar]
- 38.Sebag J. Molecular biology of pharmacologic vitreolysis. Trans Am Ophthalmol Soc. 2005;103:473–494. [PMC free article] [PubMed] [Google Scholar]
- 39.Terranova VP, Rohrbach DH, Martin GR. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- 40.Engvall E, Ruoslahti E, Miller EJ. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foos RY, Wheeler NC. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982;89(12):1502–1512. doi: 10.1016/s0161-6420(82)34610-2. [DOI] [PubMed] [Google Scholar]
- 42.Sebag J. The vitreous: structure, function, and pathobiology. New York: Springer-Verlag; 1989. [Google Scholar]
- 43.Kishi S, Shimizu K. Posterior precortical vitreous pocket. Arch Ophthalmol. 1990;108(7):979–982. doi: 10.1001/archopht.1990.01070090081044. [DOI] [PubMed] [Google Scholar]
- 44.Sebag J. Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol. 1987;225(2):89–93. doi: 10.1007/BF02160337. [DOI] [PubMed] [Google Scholar]
- 45.O’Malley P. The pattern of vitreous syneresis: a study of 800 autopsy eyes. In: Irvine AR, O’Malley C, editors. Advances in vitreous surgery. Springfield, IL: Thomas; 1976. pp. 17–33. [Google Scholar]
- 46.Larsson L, Osterlin S. Posterior vitreous detachment. A combined clinical and physiochemical study. Graefes Arch Clin Exp Ophthalmol. 1985;223(2):92–95. doi: 10.1007/BF02150952. [DOI] [PubMed] [Google Scholar]
- 47.Linder B. Acute posterior vitreous detachment and its retinal complications. Acta Ophthalmol. 1966;87(Suppl):1–108. [Google Scholar]
- 48.Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefes Arch Clin Exp Ophthalmol. 2004;242(8):690–698. doi: 10.1007/s00417-004-0980-1. [DOI] [PubMed] [Google Scholar]
- 49.Kloti R. Experimental occlusion of retinal and ciliary vessels in owl monkeys. I. Technique and clinical observations of selective embolism of the central retinal artery system. Exp Eye Res. 1967;6(4):393–399. doi: 10.1016/s0014-4835(67)80014-9. [DOI] [PubMed] [Google Scholar]
- 50.Sebag J. Age-related differences in the human vitreoretinal interface. Arch Ophthalmol. 1991;109(7):966–971. doi: 10.1001/archopht.1991.01080070078039. [DOI] [PubMed] [Google Scholar]
- 51.Sebag J. Is pharmacologic vitreolysis brewing? Retina. 2002;22(1):1–3. doi: 10.1097/00006982-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Ueno N, Sebag J, Hirokawa H, Chakrabarti B. Effects of visible-light irradiation on vitreous structure in the presence of a photosensitizer. Exp Eye Res. 1987;44(6):863–870. doi: 10.1016/s0014-4835(87)80048-9. [DOI] [PubMed] [Google Scholar]
- 53.Akiba J, Ueno N, Chakrabarti B. Mechanisms of photo-induced vitreous liquefaction. Curr Eye Res. 1994;13(7):505–512. doi: 10.3109/02713689408999882. [DOI] [PubMed] [Google Scholar]
- 54.Kakehashi A, Ueno N, Chakrabarti B. Molecular mechanisms of photochemically induced posterior vitreous detachment. Ophthalmic Res. 1994;26(1):51–59. doi: 10.1159/000267374. [DOI] [PubMed] [Google Scholar]
- 55.Brown DJ, Bishop P, Hamdi H, Kenney MC. Cleavage of structural components of mammalian vitreous by endogenous matrix metalloproteinase-2. Curr Eye Res. 1996;15(4):439–445. doi: 10.3109/02713689608995835. [DOI] [PubMed] [Google Scholar]
- 56.Vaughan-Thomas A, Gilbert SJ, Duance VC. Elevated levels of proteolytic enzymes in the aging human vitreous. Invest Ophthalmol Vis Sci. 2000;41(11):3299–3304. [PubMed] [Google Scholar]
- 57.Los LI, van der Worp RJ, van Luyn MJ, Hooymans JM. Age-related liquefaction of the human vitreous body: LM and TEM evaluation of the role of proteoglycans and collagen. Invest Ophthalmol Vis Sci. 2003;44(7):2828–2833. doi: 10.1167/iovs.02-0588. [DOI] [PubMed] [Google Scholar]
- 58.Moorhead LC, Chu HH, Garcia CA. Enzyme-assisted vitrectomy with bacterial collagenase. Time course and toxicity studies. Arch Ophthalmol. 1983;101(2):265–274. doi: 10.1001/archopht.1983.01040010267018. [DOI] [PubMed] [Google Scholar]
- 59.Moorhead LC, Radtke N. Enzyme-assisted vitrectomy with bacterial collagenase. Pilot human studies. Retina. 1985;5(2):98–100. doi: 10.1097/00006982-198500520-00007. [DOI] [PubMed] [Google Scholar]
- 60.Moorhead LC, Redburn DA, Kirkpatrick DS, Kretzer F. Bacterial collagenase. Proposed adjunct to vitrectomy with membranectomy. Arch Ophthalmol. 1980;98(10):1829–1839. doi: 10.1001/archopht.1980.01020040681018. [DOI] [PubMed] [Google Scholar]
- 61.Verstraeten TC, Chapman C, Hartzer M, Winkler BS, Trese MT, Williams GA. Pharmacologic induction of posterior vitreous detachment in the rabbit. Arch Ophthalmol. 1993;111(6):849–854. doi: 10.1001/archopht.1993.01090060139038. [DOI] [PubMed] [Google Scholar]
- 62.Harooni M, McMillan T, Refojo M. Efficacy and safety of enzymatic posterior vitreous detachment by intravitreal injection of hyaluronidase. Retina. 1998;18(1):16–22. doi: 10.1097/00006982-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Tezel TH, Del Priore LV, Kaplan HJ. Posterior vitreous detachment with dispase. Retina. 1998;18(1):7–15. doi: 10.1097/00006982-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Trese MT. Enzymatic-assisted vitrectomy. Eye (Lond) 2002;16(4):365–368. doi: 10.1038/sj.eye.6700193. [DOI] [PubMed] [Google Scholar]
- 65.Trese MT. Enzymatic vitreous surgery. Semin Ophthalmol. 2000;15(2):116–121. doi: 10.3109/08820530009040002. [DOI] [PubMed] [Google Scholar]
- 66.Gandorfer A. Pharmacologic vitreolysis. Dev Ophthalmol. 2007;39:149–156. doi: 10.1159/000098505. [DOI] [PubMed] [Google Scholar]
- 67.Gandorfer A. Enzymatic vitreous disruption. Eye (Lond) 2008;22(10):1273–1277. doi: 10.1038/eye.2008.29. [DOI] [PubMed] [Google Scholar]
- 68.Bhisitkul RB. Anticipation for enzymatic vitreolysis. Br J Ophthalmol. 2001;85(1):1–2. doi: 10.1136/bjo.85.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benz MS, Packo KH, Gonzalez V, et al. A placebo-controlled trial of microplasmin intravitreous injection to facilitate posterior vitreous detachment before vitrectomy. Ophthalmology. 2010;117(4):791–797. doi: 10.1016/j.ophtha.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Hermel M, Prenner J, Alabdulrazzak M, Dailey W, Hartzer M. Effect of intravitreal plasmin on vitreous removal through a 25-gauge cutting system in the rabbit in vivo. Graefes Arch Clin Exp Ophthalmol. 2009;247(3):331–334. doi: 10.1007/s00417-008-1000-7. [DOI] [PubMed] [Google Scholar]
- 71.Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25(2):208–211. doi: 10.1097/00006982-200502000-00015. [DOI] [PubMed] [Google Scholar]
- 72.Fujii GY, De Juan E, Jr, Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109(10):1807–1812. doi: 10.1016/s0161-6420(02)01179-x. discussion 1813. [DOI] [PubMed] [Google Scholar]
- 73.Ramkissoon YD, Aslam SA, Shah SP, Wong SC, Sullivan PM. Risk of iatrogenic peripheral retinal breaks in 20-G pars plana vitrectomy. Ophthalmology. 2010;117(9):1825–1830. doi: 10.1016/j.ophtha.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 74.Tan HS, Mura M, de Smet MD. Iatrogenic retinal breaks in 25-gauge macular surgery. Am J Ophthalmol. 2009;148(3):427–430. doi: 10.1016/j.ajo.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Hubschman JP, Gupta A, Bourla DH, Culjat M, Yu F, Schwartz SD. 20-, 23-, and 25-gauge vitreous cutters: performance and characteristics evaluation. Retina. 2008;28(2):249–257. doi: 10.1097/IAE.0b013e31815ec2b3. [DOI] [PubMed] [Google Scholar]
- 76.Teixeira A, Chong LP, Matsuoka N, et al. Vitreoretinal traction created by conventional cutters during vitrectomy. Ophthalmology. 2010;117(7):1387–1392.e1382. doi: 10.1016/j.ophtha.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Asami T, Terasaki H, Kachi S, et al. Ultrastructure of internal limiting membrane removed during plasmin-assisted vitrectomy from eyes with diabetic macular edema. Ophthalmology. 2004;111(2):231–237. doi: 10.1016/j.ophtha.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Bonnet M. The development of severe proliferative vitreoretinopathy after retinal detachment surgery. Grade B: a determining risk factor. Graefes Arch Clin Exp Ophthalmol. 1988;226(3):201–205. doi: 10.1007/BF02181181. [DOI] [PubMed] [Google Scholar]
- 79.Capeans C, Lorenzo J, Santos L, et al. Comparative study of incomplete posterior vitreous detachment as a risk factor for proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 1998;236(7):481–485. doi: 10.1007/s004170050109. [DOI] [PubMed] [Google Scholar]
- 80.Gandorfer A, Rohleder M, Grosselfinger S, Haritoglou C, Ulbig M, Kampik A. Epiretinal pathology of diffuse diabetic macular edema associated with vitreomacular traction. Am J Ophthalmol. 2005;139(4):638–652. doi: 10.1016/j.ajo.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi M, Trempe CL, Maguire K, McMeel JW. Vitreoretinal relationship in diabetic retinopathy. A biomicroscopic evaluation. Arch Ophthalmol. 1981;99(2):241–245. doi: 10.1001/archopht.1981.03930010243003. [DOI] [PubMed] [Google Scholar]
- 82.Jalkh A, Takahashi M, Topilow HW, Trempe CL, McMeel JW. Prognostic value of vitreous findings in diabetic retinopathy. Arch Ophthalmol. 1982;100(3):432–434. doi: 10.1001/archopht.1982.01030030434009. [DOI] [PubMed] [Google Scholar]
- 83.Kado M, Trempe CL. Role of the vitreous in branch retinal vein occlusion. Am J Ophthalmol. 1988;105(1):20–24. doi: 10.1016/0002-9394(88)90115-8. [DOI] [PubMed] [Google Scholar]
- 84.Hikichi T, Konno S, Trempe CL. Role of the vitreous in central retinal vein occlusion. Retina. 1995;15(1):29–33. doi: 10.1097/00006982-199515010-00006. [DOI] [PubMed] [Google Scholar]
- 85.Avunduk AM, Cetinkaya K, Kapicioglu Z, Kaya C. The effect of posterior vitreous detachment on the prognosis of branch retinal vein occlusion. Acta Ophthalmol Scand. 1997;75(4):441–442. doi: 10.1111/j.1600-0420.1997.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 86.Krebs I, Brannath W, Glittenberg C, Zeiler F, Sebag J, Binder S. Posterior vitreomacular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007;144(5):741–746. doi: 10.1016/j.ajo.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 87.Lee SJ, Lee CS, Koh HJ. Posterior vitreomacular adhesion and risk of exudative age-related macular degeneration: paired eye study. Am J Ophthalmol. 2009;147(4):621–626.e621. doi: 10.1016/j.ajo.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Kuppermann BD, Thomas EL, de Smet MD, Grillone LR. Pooled efficacy results from two multinational randomized controlled clinical trials of a single intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140(4):573–584. doi: 10.1016/j.ajo.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 89.Kuppermann BD, Thomas EL, de Smet MD, Grillone LR. Safety results of two phase III trials of an intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140(4):585–597. doi: 10.1016/j.ajo.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 90.Sebag J. Pharmacologic vitreolysis – premise and promise of the first decade. Retina. 2009;29(7):871–874. [Google Scholar]
- 91.Mandl I. Collagenase. Science. 1970;169(951):1234–1238. doi: 10.1126/science.169.3951.1234. [DOI] [PubMed] [Google Scholar]
- 92.O’Neill R, Shea M. The effects of bacterial collagenase in rabbit vitreous. Can J Ophthalmol. 1973;8(2):366–370. [PubMed] [Google Scholar]
- 93.Quiroz H, Buzney SM, Furukawa H. Enzymatically induced posterior vitreous detachment [abstract] Invest Ophthalmol Vis Sci (Suppl) 1984;25:307. [Google Scholar]
- 94.Prabhakar V, Raman R, Capila I, Bosques CJ, Pojasek K, Sasisekharan R. Biochemical characterization of the chondroitinase ABC I active site. Biochem J. 2005;390(Pt 2):395–405. doi: 10.1042/BJ20050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hageman GS, Russell SR. Chondroitinase-mediated disinsertion of the primate vitreous body [abstract] Invest Ophthalmol Vis Sci (Suppl) 1994;35:1260. [Google Scholar]
- 96.Bishop PN, McLeod D, Reardon A. Effects of hyaluronan lyase, hyaluronidase, and chondroitin ABC lyase on mammalian vitreous gel. Invest Ophthalmol Vis Sci. 1999;40(10):2173–2178. [PubMed] [Google Scholar]
- 97.Staubach F, Nober V, Janknecht P. Enzyme-assisted vitrectomy in enucleated pig eyes: a comparison of hyaluronidase, chondroitinase, and plasmin. Curr Eye Res. 2004;29(4–5):261–268. doi: 10.1080/02713680490516747. [DOI] [PubMed] [Google Scholar]
- 98.Hermel M, Schrage NF. Efficacy of plasmin enzymes and chondroitinase ABC in creating posterior vitreous separation in the pig: a masked, placebo-controlled in vivo study. Graefes Arch Clin Exp Ophthalmol. 2007;245(3):399–406. doi: 10.1007/s00417-006-0388-1. [DOI] [PubMed] [Google Scholar]
- 99.Ludowieg J, Vennesland B, Dorfman A. The mechanism of action of hyaluronidase. J Biol Chem. 1961;236:333–339. [PubMed] [Google Scholar]
- 100.Menzel EJ, Farr C. Hyaluronidase and its substrate hyaluronan: biochemistry, biological activities and therapeutic uses. Cancer Lett. 1998;131(1):3–11. doi: 10.1016/s0304-3835(98)00195-5. [DOI] [PubMed] [Google Scholar]
- 101.Foulds WS, Allan D, Moseley H, Kyle PM. Effect of intravitreal hyaluronidase on the clearance of tritiated water from the vitreous to the choroid. Br J Ophthalmol. 1985;69(7):529–532. doi: 10.1136/bjo.69.7.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gottlieb JL, Antoszyk AN, Hatchell DL, Saloupis P. The safety of intravitreal hyaluronidase. A clinical and histologic study. Invest Ophthalmol Vis Sci. 1990;31(11):2345–2352. [PubMed] [Google Scholar]
- 103.Kang SW, Hyung SM, Choi MY, Lee J. Induction of vitreolysis and vitreous detachment with hyaluronidase and perfluoropropane gas. Korean J Ophthalmol. 1995;9(2):69–78. doi: 10.3341/kjo.1995.9.2.69. [DOI] [PubMed] [Google Scholar]
- 104.Hikichi T, Kado M, Yoshida A. Intravitreal injection of hyaluronidase cannot induce posterior vitreous detachment in the rabbit. Retina. 2000;20(2):195–198. [PubMed] [Google Scholar]
- 105.Wang Z-L, Zhang X, Xu X, Sun X-D, Wang F. PVD following plasmin but not hyaluronidase: implications for combination pharmacologic vitreolysis therapy. Retina. 2005;25(1):38–43. doi: 10.1097/00006982-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 106.Stenn KS, Link R, Moellmann G, Madri J, Kuklinska E. Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J Invest Dermatol. 1989;93(2):287–290. doi: 10.1111/1523-1747.ep12277593. [DOI] [PubMed] [Google Scholar]
- 107.Frenzel EM, Neely KA, Walsh AW, Cameron JD, Gregerson DS. A new model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1998;39(11):2157–2164. [PubMed] [Google Scholar]
- 108.Kralinger MT, Kieselbach GF, Voigt M, et al. Experimental model for proliferative vitreoretinopathy by intravitreal dispase: limited by zonulolysis and cataract. Ophthalmologica. 2006;220(4):211–216. doi: 10.1159/000093073. [DOI] [PubMed] [Google Scholar]
- 109.Oliveira LB, Tatebayashi M, Mahmoud TH, Blackmon SM, Wong F, McCuen BW., 2nd Dispase facilitates posterior vitreous detachment during vitrectomy in young pigs. Retina. 2001;21(4):324–331. doi: 10.1097/00006982-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Jorge R, Oyamaguchi EK, Cardillo JA, Gobbi A, Laicine EM, Haddad A. Intravitreal injection of dispase causes retinal hemorrhages in rabbit and human eyes. Curr Eye Res. 2003;26(2):107–112. doi: 10.1076/ceyr.26.2.107.14516. [DOI] [PubMed] [Google Scholar]
- 111.Zhu D, Chen H, Xu X. Effects of intravitreal dispase on vitreoretinal interface in rabbits. Curr Eye Res. 2006;31(11):935–946. doi: 10.1080/02713680600932142. [DOI] [PubMed] [Google Scholar]
- 112.Wang F, Wang Z, Sun X, Xu X, Zhang X. Safety and efficacy of dispase and plasmin in pharmacologic vitreolysis. Invest Ophthalmol Vis Sci. 2004;45(9):3286–3290. doi: 10.1167/iovs.04-0026. [DOI] [PubMed] [Google Scholar]
- 113.Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43(10):1110–1111. doi: 10.1007/BF01956052. [DOI] [PubMed] [Google Scholar]
- 114.Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 1990;84(3):139–143. doi: 10.1159/000205051. [DOI] [PubMed] [Google Scholar]
- 115.Urano T, Ihara H, Umemura K, et al. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis Cleaves and inactivates plasminogen activator inhibitor type 1. J Biol Chem. 2001;276(27):24690–24696. doi: 10.1074/jbc.M101751200. [DOI] [PubMed] [Google Scholar]
- 116.Takano A, Hirata A, Ogasawara K, et al. Posterior vitreous detachment induced by nattokinase (subtilisin NAT): a novel enzyme for pharmacologic vitreolysis. Invest Ophthalmol Vis Sci. 2006;47(5):2075–2079. doi: 10.1167/iovs.05-0130. [DOI] [PubMed] [Google Scholar]
- 117.Liotta LA, Goldfarb RH, Brundage R, Siegal GP, Terranova V, Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- 118.Uemura A, Nakamura M, Kachi S, et al. Effect of plasmin on laminin and fibronectin during plasmin-assisted vitrectomy. Arch Ophthalmol. 2005;123(2):209–213. doi: 10.1001/archopht.123.2.209. [DOI] [PubMed] [Google Scholar]
- 119.Li X, Shi X, Fan J. Posterior vitreous detachment with plasmin in the isolated human eye. Graefes Arch Clin Exp Ophthalmol. 2002;240(1):56–62. doi: 10.1007/s004170100351. [DOI] [PubMed] [Google Scholar]
- 120.Takano A, Hirata A, Inomata Y, et al. Intravitreal plasmin injection activates endogenous matrix metalloproteinase-2 in rabbit and human vitreous. Am J Ophthalmol. 2005;140(4):654–660. doi: 10.1016/j.ajo.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 121.Baramova EN, Bajou K, Remacle A, et al. Involvement of PA/plasmin system in the processing of pro-MMP-9 and in the second step of pro-MMP-2 activation. FEBS Lett. 1997;405(2):157–162. doi: 10.1016/s0014-5793(97)00175-0. [DOI] [PubMed] [Google Scholar]
- 122.Wachtfogel YT, Abrams W, Kucich U, Weinbaum G, Schapira M, Colman RW. Fibronectin degradation products containing the cytoadhesive tetrapeptide stimulate human neutrophil degranulation. J Clin Invest. 1988;81(5):1310–1316. doi: 10.1172/JCI113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hikichi T, Yanagiya N, Kado M, Akiba J, Yoshida A. Posterior vitreous detachment induced by injection of plasmin and sulfur hexafluoride in the rabbit vitreous. Retina. 1999;19(1):55–58. doi: 10.1097/00006982-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 124.Gandorfer A, Putz E, Welge-Lussen U, Gruterich M, Ulbig M, Kampik A. Ultrastructure of the vitreoretinal interface following plasmin assisted vitrectomy. Br J Ophthalmol. 2001;85(1):6–10. doi: 10.1136/bjo.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gandorfer A, Priglinger S, Schebitz K, et al. Vitreoretinal morphology of plasmin-treated human eyes. Am J Ophthalmol. 2002;133(1):156–159. doi: 10.1016/s0002-9394(01)01252-1. [DOI] [PubMed] [Google Scholar]
- 126.Margherio AR, Margherio RR, Hartzer M, Trese MT, Williams GA, Ferrone PJ. Plasmin enzyme-assisted vitrectomy in traumatic pediatric macular holes. Ophthalmology. 1998;105(9):1617–1620. doi: 10.1016/S0161-6420(98)99027-3. [DOI] [PubMed] [Google Scholar]
- 127.Wu WC, Drenser KA, Trese MT, Williams GA, Capone A. Pediatric traumatic macular hole: results of autologous plasmin enzyme-assisted vitrectomy. Am J Ophthalmol. 2007;144(5):668–672. doi: 10.1016/j.ajo.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 128.Tsukahara Y, Honda S, Imai H, et al. Autologous plasmin-assisted vitrectomy for stage 5 retinopathy of prematurity: a preliminary trial. Am J Ophthalmol. 2007;144(1):139–141. doi: 10.1016/j.ajo.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 129.Wu W-C, Drenser KA, Lai M, Capone A, Trese MT. Plasmin enzyme-assisted vitrectomy for primary and reoperated eyes with stage 5 retinopathy of prematurity. Retina. 2008;28(Suppl 3):S75–S80. doi: 10.1097/IAE.0b013e318158ea0e. [DOI] [PubMed] [Google Scholar]
- 130.Wu W-C, Drenser KA, Capone A, Williams GA, Trese MT. Plasmin enzyme-assisted vitreoretinal surgery in congenital X-linked retinoschisis: surgical techniques based on a new classification system. Retina. 2007;27(8):1079–1085. doi: 10.1097/IAE.0b013e31806196d0. [DOI] [PubMed] [Google Scholar]
- 131.Trese MT, Williams GA, Hartzer MK. A new approach to stage 3 macular holes. Ophthalmology. 2000;107(8):1607–1611. doi: 10.1016/s0161-6420(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 132.Rizzo S, Pellegrini G, Benocci F, Belting C, Baicchi U, Vispi M. Autologous plasmin for pharmacologic vitreolysis prepared 1 hour before surgery. Retina. 2006;26(7):792–796. doi: 10.1097/01.iae.0000244266.83395.16. [DOI] [PubMed] [Google Scholar]
- 133.Sakuma T, Tanaka M, Inoue M, Mizota A, Souri M, Ichinose A. Efficacy of autologous plasmin for idiopathic macular hole surgery. Eur J Ophthalmol. 2005;15(6):787–794. doi: 10.1177/112067210501500621. [DOI] [PubMed] [Google Scholar]
- 134.Azzolini C, D’Angelo A, Maestranzi G, et al. Intrasurgical plasmin enzyme in diabetic macular edema. Am J Ophthalmol. 2004;138(4):560–566. doi: 10.1016/j.ajo.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 135.Sakuma T, Tanaka M, Inoue J, Mizota A, Souri M, Ichinose A. Use of autologous plasmin during vitrectomy for diabetic maculopathy. Eur J Ophthalmol. 2006;16(1):138–140. doi: 10.1177/112067210601600122. [DOI] [PubMed] [Google Scholar]
- 136.Hirata A, Takano A, Inomata Y, Yonemura N, Sagara N, Tanihara H. Plasmin-assisted vitrectomy for management of proliferative membrane in proliferative diabetic retinopathy: a pilot study. Retina. 2007;27(8):1074–1078. doi: 10.1097/IAE.0b013e3180592beb. [DOI] [PubMed] [Google Scholar]
- 137.Udaondo P, Diaz-Llopis M, Garcia-Delpech S, Salom D, Romero FJ. Intravitreal plasmin without vitrectomy for macular edema secondary to branch retinal vein occlusion. Arch Ophthalmol. 2011;129(3):283–287. doi: 10.1001/archophthalmol.2011.8. [DOI] [PubMed] [Google Scholar]
- 138.Kamei M, Estafanous M, Lewis H. Tissue plasminogen activator in the treatment of vitreoretinal diseases. Semin Ophthalmol. 2000;15(1):44–50. doi: 10.3109/08820530009037850. [DOI] [PubMed] [Google Scholar]
- 139.Glacet-Bernard A, Kuhn D, Vine AK, Oubraham H, Coscas G, Soubrane G. Treatment of recent onset central retinal vein occlusion with intravitreal tissue plasminogen activator: a pilot study. Br J Ophthalmol. 2000;84(6):609–613. doi: 10.1136/bjo.84.6.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Le Mer Y, Korobelnik JF, Morel C, Ullern M, Berrod JP. TPA-assisted vitrectomy for proliferative diabetic retinopathy: results of a double-masked, multicenter trial. Retina. 1999;19(5):378–382. doi: 10.1097/00006982-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 141.Esser P, Heimann K, Bartz-Schmidt KU, Walter P, Krott R, Weller M. Plasminogen in proliferative vitreoretinal disorders. Br J Ophthalmol. 1997;81(7):590–594. doi: 10.1136/bjo.81.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hesse L, Nebeling B, Schroeder B, Heller G, Kroll P. Induction of posterior vitreous detachment in rabbits by intravitreal injection of tissue plasminogen activator following cryopexy. Exp Eye Res. 2000;70(1):31–39. doi: 10.1006/exer.1999.0772. [DOI] [PubMed] [Google Scholar]
- 143.Unal M, Peyman GA. The efficacy of plasminogen-urokinase combination in inducing posterior vitreous detachment. Retina. 2000;20(1):69–75. doi: 10.1097/00006982-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 144.Men G, Peyman GA, Genaidy M, et al. The role of recombinant lysine-plasminogen and recombinant urokinase and sulfur hexafluoride combination in inducing posterior vitreous detachment. Retina. 2004;24(2):199–209. doi: 10.1097/00006982-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 145.Hesse L, Kroll P. TPA-assisted vitrectomy for proliferative diabetic retinopathy. Retina. 2000;20(3):317–318. doi: 10.1097/00006982-200003000-00021. [DOI] [PubMed] [Google Scholar]
- 146.Murakami T, Takagi H, Ohashi H, et al. Role of posterior vitreous detachment induced by intravitreal tissue plasminogen activator in macular edema with central retinal vein occlusion. Retina. 2007;27(8):1031–1037. doi: 10.1097/IAE.0b013e318074bc39. [DOI] [PubMed] [Google Scholar]
- 147.Nagai N, Demarsin E, Van Hoef B, et al. Recombinant human microplasmin: production and potential therapeutic properties. J Thromb Haemost. 2003;1(2):307–313. doi: 10.1046/j.1538-7836.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 148.Gandorfer A, Rohleder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004;45(2):641–647. doi: 10.1167/iovs.03-0930. [DOI] [PubMed] [Google Scholar]
- 149.Sakuma T, Tanaka M, Mizota A, Inoue J, Pakola S. Safety of in vivo pharmacologic vitreolysis with recombinant microplasmin in rabbit eyes. Invest Ophthalmol Vis Sci. 2005;46(9):3295–3299. doi: 10.1167/iovs.04-1517. [DOI] [PubMed] [Google Scholar]
- 150.Chen W, Huang X, Ma XW, Mo W, Wang WJ, Song HY. Enzymatic vitreolysis with recombinant microplasminogen and tissue plasminogen activator. Eye (Lond) 2008;22(2):300–307. doi: 10.1038/sj.eye.6702931. [DOI] [PubMed] [Google Scholar]
- 151.de Smet MD, Valmaggia C, Zarranz-Ventura J, Willekens B. Microplasmin: ex vivo characterization of its activity in porcine vitreous. Invest Ophthalmol Vis Sci. 2009;50(2):814–819. doi: 10.1167/iovs.08-2185. [DOI] [PubMed] [Google Scholar]
- 152.de Smet MD, Gandorfer A, Stalmans P, et al. Microplasmin intravitreal administration in patients with vitreomacular traction scheduled for vitrectomy: the MIVI I trial. Ophthalmology. 2009;116(7):1349–1355. 1355.e1341–e1342. doi: 10.1016/j.ophtha.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 153.Stalmans P, Delaey C, de Smet MD, van Dijkman E, Pakola S. Intravitreal injection of microplasmin for treatment of vitreomacular adhesion: results of a prospective, randomized, sham-controlled phase II trial (the MIVI-IIT trial) Retina. 2010;30(7):1122–1127. doi: 10.1097/IAE.0b013e3181e0970a. [DOI] [PubMed] [Google Scholar]
- 154.Dugel PU, Group M-TS. A single injection of ocriplasmin for the treatment of symptomatic vitreomacular adhesion (sVMA): results of the Phase III MIVI-TRUST Program [abstract] Invest Ophthalmol Vis Sci (Suppl) 2011;52:6628. [Google Scholar]
- 155.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 156.Gehlsen KR, Argraves WS, Pierschbacher MD, Ruoslahti E. Inhibition of in vitro tumor cell invasion by Arg-Gly-Asp-containing synthetic peptides. J Cell Biol. 1988;106(3):925–930. doi: 10.1083/jcb.106.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brem RB, Robbins SG, Wilson DJ, et al. Immunolocalization of integrins in the human retina. Invest Ophthalmol Vis Sci. 1994;35(9):3466–3474. [PubMed] [Google Scholar]
- 158.Oliveira LB, Meyer CH, Kumar J, et al. RGD peptide-assisted vitrectomy to facilitate induction of a posterior vitreous detachment: a new principle in pharmacological vitreolysis. Curr Eye Res. 2002;25(6):333–340. doi: 10.1076/ceyr.25.6.333.14234. [DOI] [PubMed] [Google Scholar]
- 159.Karageozian HL. Determine the safety and efficacy of Vitreosolve® administered intravitreally to induce a complete posterior vitreous detachment (PVD) in non proliferative diabetic retinopathy human subjects. Invest Ophthalmol Vis Sci (Suppl) 2005;46:5453. [Google Scholar]
- 160.McLaughlin PS, McLaughlin BG. Chemical analysis of bovine and porcine vitreous humors: correlation of normal values with serum chemical values and changes with time and temperature. Am J Vet Res. 1987;48(3):467–473. [PubMed] [Google Scholar]
- 161.Gandorfer A, Kampik A. Pharmacologic vitreolysis combining the two enzymes plasmin and hyaluronidase. Retina. 2005;25(5):674. doi: 10.1097/00006982-200507000-00025. author reply 674–675. [DOI] [PubMed] [Google Scholar]
- 162.Zhi-Liang W, Wo-Dong S, Min L, Xiao-Ping B, Jin J. Pharmacologic vitreolysis with plasmin and hyaluronidase in diabetic rats. Retina. 2009;29(2):269–274. doi: 10.1097/IAE.0b013e3181923ff0. [DOI] [PubMed] [Google Scholar]