Abstract
In 2008, Pienta et al. (Transl Oncol. 2008;1:158–164) introduced the term ecological therapy for cancer treatment and, in particular, emphasized that destruction of the tumor microenvironment would be more effective than just killing the cells that inhabit it. Proposed here is an expansion on the idea of ecological therapy of cancer, incorporating 1) literature on species invasion, i.e., a right cancerous clone needs to be at the right place at the right time to actually invade its environment, and 2) the literature on niche construction, that is, the idea that once a tumor is formed, cancer cells they modify their microenvironment (niche construction) by changing pH through glycolysis, secreting growth factors and recruiting tumor-associated macrophages to promote cell growth, activating fibroblasts, evading predation from immune system, making the cancer that much more difficult to eradicate. Paleontological literature suggests that the largestmass extinctions occurred when environmental stress that would weaken the population was coupled with some pulse destructive event that caused extensive mortality. To have the same effect on cells in the tumor, rather than, or at least in addition to, killing the cells, one would also need to target the niche that they created for themselves.
Cancer as an Ecological System
During the past decade, it has become increasingly recognized that a tumor is not genetically homogeneous but is rather composed of many genetically diverse cancer cells [1,2]. If variability in the population is heritable and if it affects fitness, then the system is going to evolve, leading to competition for space and common resources and resulting in different clones being selected for or weeded out of the population due to natural selection. Genomic heterogeneity is one of the major reasons why we see acquired therapeutic resistance because cytotoxic therapy inevitably selects for resistant cells by applying a severe selective pressure on the entire heterogeneous cell population. Moreover, heterogeneity within even premalignant lesions has been shown to be indicative of a worse prognosis for the patient [3]. At the same time, prognosis for young cancer patients is typically more favorable, which can be attributed in part to the fact that younger tumors are less heterogeneous and hence are less likely to become resistant to therapy.
Another consequence of tumor heterogeneity is the possibility of so-called evolutionary suicide [4]—in their quest for higher growth rates, lower death rates, and increased competitiveness and with their ability to migrate out and colonize distant organs, cancer cells defy “cooperation” with somatic tissue, eventually killing the host and thus killing themselves. This evolutionary experiment is run within each cancer patient, sometimes leading to cancer cells committing evolutionary suicide at the expense of the host.
From an ecological perspective, one can look at this process as an attempt of new species (cancer cells), which have different metabolic and reproductive strategies compared with the “resident” population (somatic cells) to invade a new habitat (tissue). Successful invasion will result in the formation of a primary solid tumor. Such perspective might be able to provide a different viewpoint, allowing us to draw parallels with other ecological systems to find answers to such questions as “under what conditions can invasions occur?,” “how do invading species adapt to and modify their environment?,” and, most importantly, “what can be done to eradicate them?”
Mechanisms of Species Extinction
The mechanisms by which species in nature go extinct can generally be subdivided into two distinct categories—extrinsic factors, such as habitat modification, change in nutrient supply, and interactions with predators; and intrinsic factors, such as any change in the genotype, which eventually results in changes in the phenotype.
Intrinsic factors typically reflect how the species have been adapting to their environment over a long evolutionary time scale. From an evolutionary game theory point of view, individuals within the population have been moving toward an evolutionarily stable strategy (ESS), that is, a state when no individual within the population has an incentive to change his/her “strategy” in his/her interactions with the environment. As a result, theoretically, once the ESS is adopted in the population, natural selection alone becomes insufficient to allow invasion by a new mutant. (It is important to note that being at an ESS does not imply highest fitness in the sense of the largest difference between birth and death rates. It only implies resistance to invasion.)
However, invasions do happen. One of the frequent ways by which species can go extinct is when a more efficient or more proliferative competitor invades their habitat much like cancerous cells can invade and start outcompeting healthy cells in the tissues. Research in the area of invasion ecology has been focused particularly on this question.
Habitat Invasion and Cancer
A number of mechanisms have been proposed to explain why some habitats are more or less susceptible to invasion, of which habitat modification is most often the common denominator [5–7]. Invasion can be facilitated when the “native” populations are more specialized toward their niche, whereas the invaders are “generalists”—perhaps less efficient in some aspects when compared with the natives but capable of taking on multiple roles and exploiting multiple resources [8,9]. Another, perhaps complementary, theory comes from David Tilman, whose research focus has primarily been on the questions of ecosystem stability and the effects on it of biodiversity. He suggests that ecosystems that are more diverse are less susceptible to invasion because greater biodiversity ensures more complete resource utilization [7,10,11]. Incomplete resource utilization allows for the formation of a new niche, which can be occupied by invaders. And, if the new niche has been available for an extended period, invaders not only will have time to find and occupy it but also will be able to “coevolve with it.” This phenomenon is known as niche construction [12], and it refers to a situation when the niche gets modified because of the metabolic activity of its occupants. The adaptations could also be different: an invader can modify the niche to be better suited for them than for any other species or it can exploit the niche in such a way as to make it uninhabitable by anyone, inducing increased migration (which could be an ecological explanation for the formation of metastases).
When it comes to cells within a tissue, one can argue that they are at an evolutionarily stable state and thus should not be prone to invasion by a cell that adapts a different metabolic or reproductive strategy. Another way of thinking about the “normal” state of cells in the tissue is that they are at an adaptive peak [13]. Therefore, in order for a cancerous clone to invade the population of healthy cells, something must take the healthy cells “off of the adaptive peak.”
It has been suggested [13–15] that aging is one such mechanism by which the somatic cells gradually slide off of the adaptive peak, allowing for the invasion of cancerous clones. It is possible that aging-associated decline in functionality of cells, tissues, and organs, caused by both intrinsic cell mechanisms, such as accumulated mutations, as well as damage caused by extrinsic factors, such as exposure to carcinogens, could be reducing fitness of the resident cell population over time. Some studies also suggest that mitochondrial function declines with age, possibly because of the accumulated damage from exposure to reactive oxygen species during the individual's life span [16–22]. Because most aerobic metabolism occurs in mitochondria, decline in mitochondrial function would cause loss of fitness advantage for somatic cells. If, for cancer initiation, one needs to not only have the right cancer clone (identification of what makes the right clone is the focus of molecular study of cancer genetics) but also have it in the right place at the right time, aging could provide the ever-increasing window of that “right time.”
Niches in a Human Body
It is, of course, not completely clear what defines a niche for a cell population in a human body. If one were to continue with the ecological analogy, one would have to include in the definition of nutrients (glucose, phosphorus, iron, lipids, and other materials necessary for cell growth and reproduction), space (including extracellular matrix, which is often destroyed by tumors), and predators (cells of the immune system), as well as other microorganisms, such as gut or skin bacteria. The niche would also be characterized by such factors as pH, blood flow, and rates at which cell metabolic products, dead cells, as well as external chemicals, such as certain carcinogens, are being washed out from the tissue. Other inhabitants of the niche, in this case the somatic cells, are, of course, also part of the environment. So, a significant modification in either of these components could hypothetically allow for the creation of a new niche that a budding primary tumor can occupy.
Interactions with the Predator: The Immune System
Many tumors are characterized by increased inflammation [23–26]. It is possible that, while the immune system is fighting an infection, immune cells secrete growth factors that premalignant cells also partake in, thus creating new growth factor-rich microenvironment [27,28]. If the inflammation, and thus inflow of growth factors, continues long enough, it can give the few cancerous clones the boost they need to start growing. A subsequent decrease in the inflammatory response may not be enough to stop the tumor from growing once the process has been initiated because some tumors either learn to secrete their own growth factors (the so-called hormone-secreting tumors like pituitary adenoma) or learn to manipulate other cells to secrete growth factors for them. A striking example of the latter is the existence of tumor-associated macrophages (TAMs) that accumulate preferentially in the poorly vascularized regions of tumors [26,29,30] and secrete cytokines that actually promote tumor growth [24,28,29,31]. Moreover, not only can these cytokines promote tumor growth but they have also been known to suppress activation of CD8+ T cells that are most efficient in tumor elimination [32–36].
Cancer-Induced Niche Modification
Thus, tumor cells, after invading a newly formed niche, have ample ways to modify it as to make it suit their particular needs. A possible unifying mechanism could be as follows: a right cell (exhibiting one or more hallmarks of cancer) has been in the right place (having access to enough nutrients, such as carbon and phosphorus and other building materials) at the right time (during cell division or inflammation, getting access to growth factors, or simply in an older tissue, where the surrounding cells are not as fit). As the primary tumor outgrows its blood supply, an increasing number of cells switch to glycolytic metabolism. Glycolytic cells secrete lactic acid as a by-product of glucose metabolism, creating acidic microenvironment, which can become toxic to surrounding somatic cells [37–39], thus giving glycolytic cancer cells the competitive advantage even in the presence of oxygen.
Normally, glycolysis is upregulated only in a hypoxic microenvironment, where production of protein hypoxia-inducible factor 1 (HIF-1) is upregulated; under normoxic conditions, its oxygen-sensitive part HIF-1α is degraded through the ubiquitin-proteasome pathway [40,41]. However, in hypoxia, the presence of HIF-1α stimulates production of vascular endothelial growth factor (VEGF) and other angiogenesis-promoting factors to stimulate blood flow and bring in more oxygen to the supposedly hypoxic areas [40].
In the presence of a large enough number of glycolytic cells, an acidic microenvironment is created, in which HIF-1 production is upregulated, and, what is more important, HIF-1α, the oxygen-sensitive part of HIF-1, is not degraded. Lu et al. [42] provide evidence that lactate and pyruvate regulate hypoxia-inducible gene expression independently of hypoxia by stimulating the accumulation of HIF-1α at the site. It seems like the function of von Hippel-Lindau protein, a site of HIF-1α recognition by the proteosomes, is neutralized both in hypoxic conditions and in the areas of normoxic acidosis, thus allowing tumors to simulate hypoxia in normoxic conditions [43].
What does this lead to? Corzo et al. [44] showed that when HIF-1 is upregulated, activation of CD8+ T cells is suppressed, and expression of TAMs goes up. Also, HIF-1, because its primary purpose is to attract oxygen to hypoxic areas, stimulates production of VEGF, which has a number of different effects. For one, VEGF not only promotes angiogenesis but also downregulates activation of CD8+ T cells, allowing the tumor to grow unrestrained by the immune system [32].
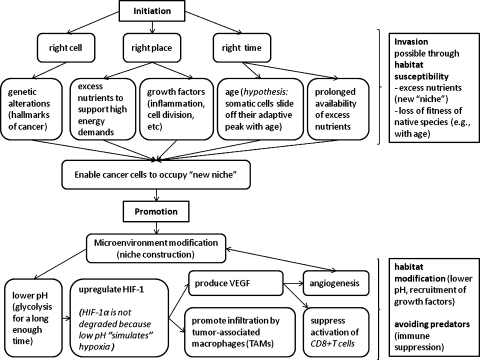
The process can be summarized as follows (see also Figure 1):
A mutated cell survives and starts proliferating in the tissue. Faced with decreasing oxygen availability, cells within the tumor start switching to glycolytic metabolism, which results in the creation of acidic microenvironment around the tumor.
HIF-1 is upregulated even in normoxic conditions, because von Hippel-Lindau protein, a binding site for HIF-1α-degrading proteosomes, becomes neutralized in areas of hypoxia and normoxic acidosis, thus allowing the tumor to simulate hypoxia in normoxic conditions. It has been shown that by-products of glycolysis, lactate and pyruvate, allow up-regulation of HIF-1 even in normoxia.
As the production of HIF-1 increases, activation of CD8+ T cells decreases (immune system evasion), and recruitment of TAMs increases, thus providing more growth factors for tumor cells.
As HIF-1 concentration increases, so does the production of VEGF because the main purpose of HIF-1 is to attract more blood vessels to restore oxygen supply, thus promoting angiogenesis. VEGF has also been shown to downregulate CD8+ T-cell activation through suppression of maturation of antigen-presenting cells, such as dendritic cells, thus also suppressing the antitumor immune response.
Figure 1.
Schematic representation of the possible mechanism of tumor initiation and progression from an ecological point of view. Tumor initiation corresponds to the mechanism of species invasion and is hypothesized to be possible when the environment is permissive, in particular, when there are excess nutrients (new niche) and when competitors (somatic cells) are less fit compared with the invaders. Tumor promotion corresponds to niche colonization and modification by the invading species through pH alteration, recruitment of growth factors, and others, as well as avoidance of predators (immune suppression).
Reverse Conservation Biology and Mass Extinctions: Lessons from Paleontology
A naturally arising question is then: “If a niche has been created, and if the tumor cells had had the chance to occupy it and settle in it, how can one get rid of them?” Just reversing the initial conditions that had led to the formation of the niche might not be sufficient because, as it was pointed out previously, the tumor cells themselves had the chance to modify their microenvironment. Just targeting the population of tumor cells would also simply free up the space and nutrients to be used by the resistant clones, which could have previously been held back because of space and nutrient limitations, imposed on them by the less aggressive but more abundant cell clones.
A possible answer to this question comes from paleontology and, in particular, from the studies performed to analyze the conditions that precede mass species extinctions that have occurred for the past several million years. Arens and West [45] have suggested, based on their analysis of geologic record of impact factors and continental flood basalts, that mass extinctions occurred more frequently and were more destructive, when pulse disturbances (such as marine anoxic incursions) that cause extensive mortality, were accompanied by press disturbances (such as climate or sea level change) that weakened and destabilized populations over many generations preceding the pulse disturbance.
In cancer treatment, chemotherapy and radiation therapy act like pulse disturbances for a population, causing extensive cell mortality and, as a result, not only selecting for the resistant clones but also freeing up the “niche” that can now be easily (or at least much easier than before) colonized by them. Perhaps, weakening the population through continuous microenvironmental stress before applying the pulse would be more likely to cause mass extinction of cancer cells. That is, rather than just kill the tumor cells, one also needs to eliminate their niche or at least make it less habitable for those cells that might survive after therapy.
One way to do this could be to reverse the adaptations that the tumor cells made for themselves. For instance, Robey et al. [46] demonstrated in mouse models of metastatic breast cancer that neutralizing acidic tumor microenvironment with sodium bicarbonate reduced formation of spontaneous metastases, an approach similar to what J. Pepper termed targeting the public goods [47]. Counteracting the cells' attempts at modifying their microenvironment poses less of a selective pressure on the cell population and is thus much less likely to propagate evolution of resistant clones.
Blocking growth factors that facilitate tumor growth would be another approach, whether tumors secrete them themselves or “steal” them from tricked macrophages [28]. For instance, VEGF has been identified to be a key mediator of angiogenesis in cancer: when tumors start outgrowing their blood supply, they upregulate VEGF production, which, in turn, promotes the formation of new blood vessels [48]. Blocking VEGF receptors in tumors, accompanied by blocking of c-met pathway, has been shown to halt tumor growth in mouse models [49]. Not only could this be due to vasculature normalization, which has been suggested to actually keep tumors from spreading because their environment is acceptable enough for them to not need to migrate out, but also because it is through growth factors like VEGF that tumors suppress the activation of cytotoxic lymphocytes by blocking the maturation of myeloid-derived suppressor cells [32,34]. Thus as a side effect, there could be an additional activation of the tumor-specific immune response coming from neutralizing tumor-induced changes in the microenvironment.
It is also important to remember that different processes take place on different time scales, and so they may be influencing each other in less obvious ways than anticipated [50,51]. Biochemical and metabolic reactions take place on the scale of seconds and minutes, whereas cell growth and expansion occur on the scale of days. Hence, modification of the environment that causes changes on one scale might have delayed effects on the processes that take place on a different time scale.
Also, some nutrients can be functionally replaced (different carbon sources), while others cannot—for instance, nothing but phosphorus can be used for building of DNA, RNA, and ribosomes. Jin et al. [52] conducted an experiment where an increased amount of phosphorus led to increased tumor growth in mouse models, supporting the hypothesis that phosphorus could be a limiting reagent for cell proliferation [53]. Changing the amount of phosphorus present (through phosphorus enriched diet, for instance) would change the composition of the cell microenvironment, creating a new niche for phosphorus-greedy tumor cells to invade. Glucose transporters are also highly upregulated in cancer cells to accommodate the high demand for glucose [54], so a sustained diet that is high in carbohydrates would also allow cancer cells to not worry about the drawbacks of glycolysis. Caloric restriction has also been implied to improve mitochondrial function [55,56], so limiting carbohydrate intake could hypothetically give somatic tissue back some competitive advantage (benign boost).
Although changing what constitutes the “right cell” and the “right time” may not be possible, the composition of the “right place,” the microenvironment, could potentially be manipulated. Lessons from ecology suggest that it could be of vital importance both for disease prevention and for more successful treatment.
Acknowledgments
The author thanks Carlo Maley for valuable comments and suggestions about the article.
Footnotes
This project has been partially supported by grants from the National Science Foundation (DMPS-0838705), the National Security Agency (H98230-09-1-0104), the Alfred P. Sloan Foundation, and the Office of the Provost of Arizona State University.
References
- 1.Stratton M, Campbell P, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Cancer Genome Consortium, author. Hudson TJ, Anderson W, Aretz A, Barker AD, Bell C, Bernabé RR, Bhan MK, Calvo F, Eorola I, et al. International network of cancer genome projects. Nature. 2010;464:993–998. doi: 10.1038/nature08987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 4.Rankin DJ, Lopez-Sepulcre A. Can adaptation lead to extinction? Oikos. 2005;111:616–619. [Google Scholar]
- 5.Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol Evol. 2007;22:489–496. doi: 10.1016/j.tree.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, Hastings A, Holt RD, Mayfield MM, et al. Ecological and evolutionary insights from species invasions. Trends Ecol Evol. 2007;22:465–471. doi: 10.1016/j.tree.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Tilman D, Wedin D, Knops J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature. 1996;379:718–720. [Google Scholar]
- 8.Parker JD, Burkepile DE, Hay ME. Opposing effects of native and exotic herbivores on plant invasions. Science. 2006;311:1459–1461. doi: 10.1126/science.1121407. [DOI] [PubMed] [Google Scholar]
- 9.Callaway RM, Thelen GC, Rodriguez A, Holben WE. Soil biota and exotic plant invasion. Nature. 2004;427:731–733. doi: 10.1038/nature02322. [DOI] [PubMed] [Google Scholar]
- 10.Tilman D. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA. 2004;101:10854–10861. doi: 10.1073/pnas.0403458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilman D, Lehman CL, Thomson KT. Plant diversity and ecosystem productivity: theoretical considerations. Proc Natl Acad Sci USA. 1997;94:1857–1861. doi: 10.1073/pnas.94.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odling-Smee FJ, Laland KN, Feldman MW. Niche Construction: The Neglected Process in Evolution. Princeton, NJ: Princeton University Press; 2003. [Google Scholar]
- 13.Marusyk A, DeGregori J. Declining cellular fitness with age promotes cancer initiation by selecting for adaptive oncogenic mutations. Biochim Biophys Acta. 2008;1785:1–11. doi: 10.1016/j.bbcan.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeGregori J. Evolved tumor suppression: why are we so good at not getting cancer? Cancer Res. 2011;71:3739. doi: 10.1158/0008-5472.CAN-11-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry CJ, Marusyk A, Zaberezhnyy V, Adane B, Degregori J. Declining lymphoid progenitor fitness promotes aging-associated leukemogenesis. Proc Natl Acad Sci USA. 2010;107:21713–21718. doi: 10.1073/pnas.1005486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer. 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Druzhyna NM, Wilson GL, LeDoux SP. Mitochondrial DNA repair in aging and disease. Mech Ageing Dev. 2008;129:383–390. doi: 10.1016/j.mad.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 19.Linford NJ, Schriner SE, Rabinovitch PS. Oxidative damage and aging: spotlight on mitochondria. Cancer Res. 2006;66:2497–2499. doi: 10.1158/0008-5472.CAN-05-3163. [DOI] [PubMed] [Google Scholar]
- 20.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 22.Shigenaga MK. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 24.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 25.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimshaw MJ, Balkwill FR. Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation—a potential mechanism. Eur J Immunol. 2001;31:480–489. doi: 10.1002/1521-4141(200102)31:2<480::aid-immu480>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21:3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79:991–995. doi: 10.1038/sj.bjc.6690158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 34.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–331. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 37.Fang JS, Gillies RD, Gatenby RA. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin Cancer Biol. 2008;18:330–337. doi: 10.1016/j.semcancer.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 39.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 40.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin-proteasome system. Biophysics. 2008;9:679–689. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 43.Mekhail K, Gunaratnam L, Bonicalzi ME, Lee S. HIF activation by pH-dependent nucleolar sequestration of VHL. Nat Cell Biol. 2004;6:642–647. doi: 10.1038/ncb1144. [DOI] [PubMed] [Google Scholar]
- 44.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn J-I, Cheng P, Cho H-I, Celis E, Quiceno DG, Cheng P, Padhya T, et al. HIF-1 regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arens NC, West ID. Press-pulse: a general theory of mass extinction? Paleobiology. 2008;34:456–471. [Google Scholar]
- 46.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pepper JW. Defeating pathogen drug resistance: guidance from evolutionary theory. Evolution. 2008;62:3185–3191. doi: 10.1111/j.1558-5646.2008.00525.x. [DOI] [PubMed] [Google Scholar]
- 48.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 49.You W-K, McDonald DM. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep. 2008;41:833–839. doi: 10.5483/bmbrep.2008.41.12.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levin SA. Multiple scales and the maintenance of biodiversity. Ecosystems. 2000;3:498–506. [Google Scholar]
- 51.Menge DNL, Pacala SW, Hedin LO. Emergence and maintenance of nutrient limitation over multiple timescales in terrestrial ecosystems. Am Nat. 2009;173:164–175. doi: 10.1086/595749. [DOI] [PubMed] [Google Scholar]
- 52.Jin H, Xu C-X, Lim H-T, Park S-J, Shin J-Y, Chung Y-S, Park SC, Chang SH, Youn HJ, Lee KH, et al. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am J Respir Crit Care Med. 2009;179:59–68. doi: 10.1164/rccm.200802-306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elser JJ, Kyle MM, Smith MS, Nagy JD. Biological stoichiometry in human cancer. PLoS One. 2007;2:e2028. doi: 10.1371/journal.pone.0001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Schwer B, Eckersdorff M, Li Y, Silva JC, Fermin D, Kurtev MV, Giallourakis C, Comb MJ, Alt FW, Lombard DB. Calorie restriction alters mitochondrial protein acetylation. Aging Cell. 2009;8:604–606. doi: 10.1111/j.1474-9726.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]