Abstract
Numerous epidemiological studies have consistently demonstrated that individuals who eat more fruits and vegetables (which are rich in carotenoids) and who have higher serum β-carotene levels have a lower risk of cancer, especially lung cancer. However, two human intervention trials conducted in Finland and in the United States have reported contrasting results with high doses of β-carotene supplementation increasing the risk of lung cancer among smokers. The failure of these trials to demonstrate actual efficacy has resulted in the initiation of animal studies to reproduce the findings of these two studies and to elucidate the mechanisms responsible for the harmful or protective effects of carotenoids in lung carcinogenesis. Although these studies have been limited by a lack of animal models that appropriately represent human lung cancer induced by cigarette smoke, ferrets and A/J mice are currently the most widely used models for these types of studies. There are several proposed mechanisms for the protective effects of carotenoids on cigarette smoke-induced lung carcinogenesis, and these include antioxidant/prooxidant effects, modulation of retinoic acid signaling pathway and metabolism, induction of cytochrome P450, and molecular signaling involved in cell proliferation and/or apoptosis. The technical challenges associated with animal models include strain-specific and diet-specific effects, differences in the absorption and distribution of carotenoids, and differences in the interactions of carotenoids with other antioxidants. Despite the problems associated with extrapolating from animal models to humans, the understanding and development of various animal models may provide useful information regarding the protective effects of carotenoids against lung carcinogenesis.
Introduction
Lung cancer is the most common cancer worldwide and has remained the leading cause of cancer death in the United States for the past 10 years [1]. A large body of observational, epidemiological studies have consistently demonstrated that consumption of carotenoids including β-carotene, lycopene, and β-cryptoxanthin and their higher serum levels is associated with a lower risk of cancer, most notably lung cancer [2–6]. As a result, a number of studies involving various animal models have been performed to investigate the effects of carotenoids on lung carcinogenesis and to establish biologic plausibility (Table 1). However, there is no adequate animal model available for the evaluation of the role of carotenoids in human lung carcinogenesis resulting from tobacco smoke. To date, previous studies have reported the different effects of carotenoids on lung carcinogenesis owing to various experimental conditions, including the dose of the carotenoid administered, the duration of the studies conducted, and the differences in the metabolism of carotenoids by different animals. Therefore, it is necessary to thoroughly examine the literatures published to date to develop and use appropriate animal models in future studies. The goals of this review were to summarize the animal models currently available and to identify the major hypotheses that have been identified for the effects of carotenoids on lung carcinogenesis.
Table 1.
Animal Model Studies in Carotenoids and Lung Carcinogenesis.
| Model | Carotenoids | Investigators | Reference |
| Ferret | β-Carotene | Kim et al. | Carcinogenesis [38] |
| Ferret | β-Carotene | Wang et al. | J Natl Cancer Inst [40] |
| Ferret | β-Carotene | van Helden et al. | Carcinogenesis [51] |
| Ferret | β-Carotene | Liu et al. | Carcinogenesis [47] |
| Ferret | β-Carotene | Fuster et al. | J Nutr Biochem [53] |
| Ferret | Lycopene | Liu et al. | Cancer Res [39] |
| Ferret | β-Cryptoxanthin | Liu et al. | Cancer Prev Res [54] |
| Mouse | α-Carotene | Tsuda et al. | IARC Sci Publ [59] |
| Mouse | β-Carotene or lycopene | Huang et al. | J Nutr [66] |
| Mouse | Lycopene | Guttenplan et al. | Cancer Lett [74] |
| Mouse | Lycopene | Kim et al. | Cancer Lett [137] |
| Mouse (A/J) | β-Carotene | Goralczyk | Nutr Cancer [55] |
| Mouse (A/J) | Apo-10′-lycopenoic acid | Lian et al. | Carcinogenesis [72] |
| Mouse (A/J) | Lycopene | Hecht et al. | Cancer Lett [73] |
| Mouse (A/J) | 9-cis-RA | Mernitz et al. | Cancer Lett [75] |
| Mouse (A/J) | β-Cryptoxanthin | Kohno et al. | Cancer Lett [77] |
| Rat | β-Carotene | Paolini et al. | Nature [87] |
| Hamster | β-Carotene | Furukawa et al. | Jpn J Cancer Res [90] |
| Hamster | β-Carotene | Al-Wadei and Schuller | Eur J Cancer [91] |
Lung Cancer and Cigarette Smoke
Lung cancer is believed to arise after a series of progressive pathologic changes that occur in preneoplastic or precancerous lesions present in the respiratory mucosa. The four major histologic types of lung cancer include squamous cell carcinoma, adenocarcinoma, small cell carcinoma, and large cell carcinoma [7]. However, there are also several preneoplastic lesions that have been identified, which include squamous metaplasia, dysplasia, and atypical adenomatous hyperplasia [8]. Although hyperplasia and squamous metaplasia are considered reactive and reversible changes, dysplasia and carcinoma in situ are the changes most frequently associated with the development of squamous cell carcinoma [7]. For adenocarcinoma, changes can include atypical adenomatous hyperplasia in peripheral airway cells [9].
Although other agents, including occupational carcinogens, can contribute to the incidence of lung cancer, cigarette smoking and exposure to second-hand smoke are the two main risk factors that have been associated with the development of lung cancer. A total of 90% of lung cancer cases are associated with exposure to tobacco smoke [10]. Animal studies have identified more than 62 carcinogens present in cigarette smoke, and 15 of these compounds have been confirmed to be carcinogenic in humans [11,12]. Particulate phase constituents of cigarette smoke include polycyclic aromatic hydrocarbons, which include well-known components such as benzo[a]pyrene (BaP) and tobacco-specific nitrosamines such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) [13]. NNK is a potent and selective lung carcinogen in various species, independent of the route of administration.
In addition to the carcinogenic compounds mentioned previously, cigarette smoke contains a variety of free radicals and reactive oxygen species, which have been shown to cause oxidative damage to various tissues of the human body [14]. Reactive oxygen species from cigarette smoke severely depletes both extracellular and intracellular antioxidants in humans. The resulting DNA damage and antioxidant depletion induced by cigarette smoke is considered to be a critical aspect of carcinogenesis that affects the lungs, esophagus, kidneys, pancreas, and cervix [15].
Because there are few known effective therapies available for the treatment of lung cancer, chemoprevention by dietary components, including carotenoids, could be an excellent way to reduce this devastating disease.
Carotenoids and Lung Carcinogenesis
Carotenoids are a class of lipophilic compounds with polyisoprenoid structures. There are six major types of carotenoids, and these include α-carotene, β-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin [16]. They are routinely detected in human plasma and tissue, and α-carotene, β-carotene, and β-cryptoxanthin are provitamin A carotenoids that can be cleaved to form vitamin A [17,18]. Evidence from a number of observational, epidemiological studies has consistently demonstrated an inverse association between the incidence of lung cancer and a high consumption of fruits and vegetables (which are rich in carotenoids). In particular, people with higher serum levels of β-carotene, lycopene, and cryptoxanthin have been associated with a lower risk of lung cancer [2–6]. In addition, carotenoids 1) function as antioxidants [19,20], 2) are precursors of retinoic acid (RA) [21,22], 3) enhance gap junction communication [23–25], 4) function as immune enhancers [26–28], 5) inhibit cell proliferation [29], 6) induce cell apoptosis [29,30], 7) suppress insulin-like growth factor 1 (IGF-1)-stimulated cell proliferation by inducing insulin-like growth factor-binding protein (IGFBP) [31], and 8) induce carcinogen-metabolizing enzymes [32]. However, in two intervention trials, the Alpha-Tocopherol, Beta-carotene Cancer Prevention Trial in Finland [33] and the β-Carotene and Retinol Efficacy Trial in the United States [34–36], an increased risk of lung cancer was associated with smokers and asbestos workers who consumed supplements containing β-carotene. In contrast, the Physicians' Health Study reported no adverse effects associated with β-carotene supplementation for lung cancer risk in either smokers or nonsmokers [37]. On the basis of the contrasting results obtained from these trials, attempts have been made to develop appropriate animal models to explain the reasons and mechanism(s) of the discrepancies. However, a limited number of animal models are available to address the effectiveness of dietary chemopreventive agents against smoke-induced lung cancer, and these models are associated with various advantages and disadvantages.
Animal Models of Carotenoids Research in Lung Cancer
Ferret Model Studies
Data from various animal models have demonstrated that carotenoids are associated with a protective effect during lung carcinogenesis. A ferret (Mustela putorious furo) model has been extensively used for studies of lung cancer and stomach cancer owing to the physiological similarities it shares with humans [38–41]. Specifically, ferrets and humans have similar carotenoids absorption [42,43], metabolism [40,44–46], tissue distribution and levels [39,40,47], biologic functions [39,40,47], and regulation of genes, including RA receptor β (RARβ) and IGFBP-3 [39,40]. In addition, the ferret has been used as a model for studies in inhalation toxicology owing to similarities in the lung architecture of ferret and human, a fact indicating that ferrets provide advantages over other animal models (e.g., mice, rats, or hamsters) in studies of lung carcinogenesis induced by cigarette smoke.
Daily exposure to cigarette smoke (equivalent to humans who smoke 1.5 packs per day) by ferrets for 2 to 6 months resulted in proliferative changes and squamous metaplasia in the lungs [39,47]. Further, the combination of NNK with smoke exposure for 6 months successfully induced various lung preneoplastic lesions and neoplasia in ferrets [8]. Importantly, the morphologic characteristics of these tumors, including squamous cell carcinoma and adenocarcinoma as well as preneoplastic lesions in ferret lungs, closely resemble those found in humans, indicating that this animal model is appropriate for the study of human lung epithelial malignancies. Because most nutrients block or delay the progression of the early phase of tumor rather than the late phase, the findings of preneoplastic lesions in this ferret model may be important in terms of cigarette smoke-related chemoprevention studies. A novel observation associated with the ferret model has been the development of a mixed cell type such as adenosquamous carcinoma with both glandular and squamous differentiation. This condition is similar to that of adenosquamous carcinomas that have been detected in humans [48,49].
Humans can absorb significant amounts of intact carotenoids (e.g., β-carotene and lycopene) and accumulate very high concentrations of carotenoids in the liver and peripheral tissues, whereas most animal species (e.g., mice, rats, guinea pigs, rabbits, and sheep) absorb virtually no carotenoids in their intact form, except at high doses [42]. The absorption of intact carotenoids and absorption efficiency between ferrets and humans are quite similar. Ferrets fed β-carotene at 4 or 20 mg/kg of body weight (BW) achieved high serum concentrations (15 and 41 µg/dl) and appreciable levels in the liver and adipose tissue, whereas rats fed the same levels of β-carotene achieved minimal serum levels with no detectable β-carotene in either the liver or the adipose tissue [50].
Wang et al. [40] reproduced the harmful effects of large doses of β-carotene shown in human intervention studies using ferrets. The study demonstrated that ferrets exposed to smoke and supplemented with a high dose of β-carotene (equivalent to 30 mg/d in humans as given in the β-Carotene and Retinol Efficacy Trial) for 6 months developed lung squamous metaplasia compared with controls. Further, smoke-exposed and high-dose β-carotene-supplemented animals showed lower RA levels in their lungs [40]. However, when ferrets received 0.8 mg of β-carotene/kg per day (equivalent to ∼10 mg/d in humans) by oral gavage, the malondialdehyde-derived DNA adduct, 3-(2-deoxy-β-d-erythro-pentafuranosyl)pyrimido[1,2-α]purin-10 (3H)-one deoxyguanosine (M1dG), induced by BaP was inhibited. In contrast, the levels of 8-oxo7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) were not affected [51]. This is probably due to the increased base excision repair capacity in this model. Unlike in the in vivo experiment, the physiological dose of β-carotene (5 µM) has been shown to enhance levels of 8-oxo-dG in A549 and BEAS-2B lung epithelial cells while inhibiting M1dG. This difference in the effects of β-carotene on M1dG adducts and 8-oxo-dG suggested a role for β-carotene as a prooxidant, or an antioxidant, depending on the type of radicals involved.
In a study of ferrets that received combined physiological doses of β-carotene (0.85 mg/kg BW per day), α-tocopherol (22 mg/kg BW per day), and ascorbic acid (3 mg/kg BW per day) for 6 months, the ferrets were found to be protected from NNK-induced lung carcinogenesis after long-term exposure to smoke [38]. In addition, plasma β-carotene concentrations were not reduced during this treatment regimen, indicating that β-carotene degradation may be prevented in the presence of α-tocopherol and ascorbic acid. Furthermore, lower levels of RA present in the lungs of the ferrets exposed to smoke and treated with NNK were completely restored to normal levels after administration of the combined supplementation [40,52]. This may be due to the enhanced central cleavage of β-carotene or to the inhibition of eccentric cleavage of β-carotene, resulting in an increase in RA production or in a decrease in RA catabolism, respectively. The presence of certain antioxidants was previously shown by Fuster et al. [53] to be important in determining the cancer-protective or cancer-promoting effects of β-carotene. In their study, ferrets were treated with two different concentrations of β-carotene (0.8 vs 3.2 mg/kg BW per day) as a water-soluble formulation containing dl-α-tocopherol and ascorbyl palmitate for 6 months. BaP, a primary lung carcinogen from tobacco smoke, was also administered orally in the presence or absence of β-carotene. When β-carotene was administered in combination with dl-α-tocopherol and ascorbyl palmitate, levels of jun protein and those of cyclin D1 messenger RNA (mRNA) were reestablished in the lungs of BaP-treated animals. Moreover, the prooxidant effects associated with a high dose of β-carotene were abolished.
Another carotenoid, lycopene, has shown anticancer effects against lung cancer. Lycopene supplementation at a low dose (1.1 mg/kg BW per day) and a high dose (4.3 mg/kg BW per day) for 9 weeks showed significantly higher plasma levels of IGFBP-3 and lower levels of IGF-I/IGFBP-3 in smoke-exposed ferrets than in those exposed to smoke alone [39]. Recently, the chemopreventive effects of β-cryptoxanthin against lung carcinogenesis have been demonstrated in a ferret model [54]. For example, both a low dose of β-cryptoxanthin (equivalent to 105 g/d in humans) and a high dose of β-cryptoxanthin (equivalent to 525 g/d in humans) were associated with anti-inflammatory and anticancer effects, including a reduction in levels of proinflammatory cytokines, tumor necrosis factor α, and transcription factors (nuclear factor κB and activator protein 1 [AP-1]) and 8-oxo-dG. The incidence of squamous metaplasia was also reduced in ferrets exposed to cigarette smoke. On the basis of these results, Liu et al. [54] proposed that the anticancer effects of β-cryptoxanthin may not be due to its provitamin A activity.
Despite the number of advantages of ferrets, there are several disadvantages associated with the ferret model. Vitamin A transport and metabolism in ferrets differ those in humans, and the cleavage rate of β-carotene is low in ferrets [55]. Additional disadvantages include the limited number of partial-length or full-length complementary DNA available from GenBank and the incomplete sequencing of the ferret genome [56]. Moreover, ferret-specific primers, probes, and antibodies are not commercially available for this model, which limits the investigation of molecular and immunologic mechanisms involved in carcinogenesis and further limits the usefulness of the ferret model.
Mouse Model Studies
Mouse model, particularly, the A/J mice model is the most commonly used animal model for lung carcinogenesis. Mice do not require much space and are inexpensive to perform experiments with compared to larger animals. Furthermore, genetic information for mice is widely available, along with mouse-specific primers and antibodies that are commercially available. As a result, there have been many advances in the development of genetically modified animal models, as well as xenograft tumor models. In combination, these models have enhanced our understanding of the molecular mechanisms related to cancer initiation and promotion. However, the use of rodent models for studies of tobacco smoke inhalation is still associated with many difficulties because rodents are obligatory nose breathers, and their complex nasal structures differ from those of humans [13]. In addition, it is known that most rodents do not accumulate carotenoids owing to the poor absorption, bioavailability, and conversion to vitamin A of carotenoids in this model [57,58]. Therefore, further development of murine models based on the purpose of the study to be conducted is still necessary.
Carcinogen-Induced Mouse Models. Two types of carotenoids were evaluated in a study of male and female B6C3F1 mice that were treated with N,N-diethylnitrosamine (DEN) and N-methyl-N-nitrosourea while receiving α-carotene (0.4 mg per mouse) and β-carotene (0.4 mg per mouse) three times a week by gavage for 20 weeks. After an analysis of the lung tissues, an association between administration of a-carotene and a reduction in both hyperplasia and adenomas was observed only in male mice [59].
In a second study, skin painting with N-nitroso-tri-chloroethylurea for 8 months was shown to induce squamous cell carcinoma in the lungs of various strains of mice [60]. However, the specificity of the lung tumors induced by these carcinogens and the application of this model to cigarette smoke-related lung cancer prevention require further investigation.
Xenograft Mouse Models. Xenograft mouse models have been used to investigate the relationship between carotenoids and RA, and various cancers such as prostate cancer, gliomas, and neuroblastoma [61,62]. A549 lung cancer cells have been widely used for lung cancer xenograft models to study the effect of chemotherapeutic drugs on lung carcinogenesis [63,64]. However, few studies have demonstrated the effects of nutrients on human lung carcinogenesis using xenograft animal models. Recently, Lu et al. [65] reported the chemopreventive effects to be associated with a γ-tocopherol-rich mixture of tocopherols in carcinogen-induced A/J mice and H1299 human lung cancer cell xenograft tumors. In another model, supplements of lycopene or β-carotene were administered two times per week for 12 weeks to athymic nude mice that had received tail vein injections of SK-Hep-1 cells [66]. This treatment regimen was associated with a decrease in tumor numbers and a decrease in the expression of matrix metalloproteinase 9 and vascular endothelial growth factor. Moreover, Schleicher et al. [67] demonstrated that retinoids (2-hydroxyethyl retinimade, N-[4-hydroxy-phenyl] all-trans-retinamide, and 13-cis-RA) have an antimetastatic potential in a malignant hamster melanoma cell line (HM1-F5) that was injected into athymic mice.
A/J Mice. The strain A mouse lung tumor model, particularly carcinogen-treated A/J mice, was the first murine model of lung cancer, and it has been extensively used to evaluate the chemopreventive efficacy of various carotenoids against lung carcinogenesis [68–70]. However, although A/J mice develop similar adenocarcinomas as those seen in humans [13], this mouse model frequently develops spontaneous Ki-ras mutations, and only lung adenoma and adenocarcinoma are induced by NNK in the presence or absence of cigarette smoke, respectively [68,69]. Unfortunately, premalignant lesions and squamous cell carcinomas do not usually develop in these mice in contrast with human malignancies.
In the study by Goralczyk et al. [71], which investigated the effect of β-carotene on NNK-induced lung carcinogenesis in A/J mice, animals were fed various doses of β-carotene as part of an enhanced diet containing 0.25% sodium cholate and 5% corn oil with the purpose of increasing β-carotene absorption. Plasma and lung concentrations of β-carotene reached similar levels to those detected in humans, and tumor multiplicity, the functional end point, was not significantly affected by β-carotene supplementation regardless of dose or time point of treatment in either NNK-treated or control animals. Moreover, β-carotene was not able to completely reverse the down-regulation of RARβ induced by NNK in these mice.
The metabolite of lycopene, apo-10′-lycopenoic acid, suppressed NNK-induced lung tumorigenesis in A/J mice [72]. The mice were given 10-, 40-, or 120-mg/kg diet of apo-10′-lycopenoic acid for 2 weeks before lung tumors were induced by the injection of NNK. After 16 weeks on the experimental diets, there were dose-dependent increases in plasma levels of apo-10′-lycopenoic acid and significant dose-dependent decreases in tumor multiplicity in the treated animals. However, tumor incidence was not affected by this treatment regimen. In contrast, when A/J mice received dietary lycopene-enriched tomato oleoresin for 8 weeks, whereas lung tumors were induced by tobacco smoke carcinogens, BaP and NNK, no effect on lung tumor multiplicity was observed [73]. Furthermore, when lycopene was supplemented, BaP-induced mutagenesis was found to be enhanced in both lung and colon tissues but slightly decreased in prostate [74]. In combination, these data suggest that lycopene or lycopene metabolites may have the potential to enhance lung carcinogenesis similar to β-carotene and its metabolites, and this effect may be organ-specific.
In A/J mice that received 9-cis-RA for 4 months, NNK-induced lung tumor multiplicity was significantly lowered and RARβ expression was upregulated [75]. Furthermore, when 9-cis-RA was administered in combination with 1α,25-dihydroxyvitamin D3 (1,25D), this regimen was observed to effectively prevent the toxicity associated with 1,25D treatment, without compromising the chemopreventive efficacy of 1,25D during lung carcinogenesis [76]. In another study of male A/J mice, consumption of a citrus juice rich in β-cryptoxanthine (3.9 mg/100 g of juice) and hesperidin (100 mg/100 g of juice) for 21 weeks resulted in a 29% reduction in the incidence of NNK-induced lung tumors [77]. Moreover, the proliferating cell nuclear antigen-positive index of the lung tumors that developed was reduced in the citrus juice-treated group, suggesting that β-cryptoxanthine can mediate chemopreventive effects against lung carcinogenesis.
Transgenic Mouse Models. Using transgenic technology to express oncogenes or to repress tumor suppressor genes, murine transgenic models for lung carcinogenesis have been developed to study effective therapeutic interventions for lung cancer. In particular, because p53 mutations play an important role in cigarette smoke-induced lung carcinogenesis, NNK or BaP-induced lung tumor p53 mutant mouse model on A/J F1 background was developed [78]. In addition, targeted expression of genes in nonciliated Clara airway epithelial cells was produced using a Clara cell secretory protein (CCSP/CC10) promoter driving expression of SV40 T antigen (TAG) or other genes [79]. Although this TAG mice model is one of the most aggressive lung-directed models developed to date, these mice do not replicate the preneopalstic lesions and metastatic characteristics of lung cancer observed in humans.
A few transgenic animal models have been developed, which demonstrated the preventive effect of retinoids. For example, in the C3(1) SV40 large T/t-antigen (Tag) transgenic mouse, 9-cis-RA was found to inhibit mammary tumorigenicity [80]. In addition, a synthetic retinoid, N-(4-hydroxyphenyl)retinamide (4-HPR), was found to delay T-cell lymphoma development in transgenic mice overexpressing the pim-1 oncogene that were treated with N-ethyl-N-nitrosourea [81]. Recently, mouse knockout models of β-carotene 15,15′-monooxygenase 1 (Bcmo1-/-) mice [82] and carotene-9′10′-monooxygenase II (CMO-II-/-) mice [83] were developed. These models have been used to study carotenoid metabolism and the effects of β-carotene on hormone synthesis. Moreover, the continued use of these models in studies of various cancers could provide further evidence regarding the role of carotenoids in mediating carcinogenesis.
Rat Model Studies
Rat models have frequently been used to investigate the absorption, uptake, and tissue distribution of carotenoids. For example, F344 rats have been used to investigate the metabolism of lycopene, or lycopene metabolites, and their influence on prostate cancer [84–86]. In lung carcinogenesis studies, F344 rats are also commonly used to evaluate dietary interventions for cigarette smoke-induced lung lesions. On the basis of the substantial amounts of NNK contained in cigarette smoke, the total dose that is received after a lifetime of smoking was surprisingly close to the lowest total dose shown to induce tumors in these animals [68]. Furthermore, tumors can be induced by NNK in the lungs, nasal cavities, and livers of F344 rats after subcutaneous (s.c.) injections [68]. However, there is limited evidence regarding the chemopreventive effects of carotenoids on lung cancer in F344 rats. Therefore, the development of a reliable F344 rat model would be beneficial to understand dietary interventions, particularly in the study of carotenoids and lung carcinogenesis.
To understand the inconsistency of observational, epidemiological studies regarding the harmful effects of pharmacological doses of β-carotene, Paolini et al. [87] demonstrated a significant increase in the levels of several cytochrome (CYP) enzymes, including CYP 1A1, in the lungs of male Sprague-Dawley (SD) rats that had been supplemented with a very high dose of β-carotene (500 mg/kg BW). However, it is not clear whether this increase in enzyme levels was directly related to the high dose of β-carotene administered or was indirectly related to its metabolites that were generated. Additional variables that can be considered include diet composition. For example, the percentage of fat in the diet can affect serum concentrations of lipid-soluble carotenoids. Correspondingly, serum levels of lycopene have been shown to be almost two-fold higher in F344 rats that were fed lycopene-rich tomato carotenoid oleoresin at 500 ppm in a diet for 10 weeks [88] compared with the levels of serum lycopene in SD rats that were fed the same amount of lycopene for 18 weeks [89]. These differences in serum levels of lycopene could be due to the amount of fat in each diet (e.g., 4% corn oil in the grain-based diet vs 5% corn oil in the AIN-76A diet used, respectively), the strain-specific differences in terms of carotenoids absorption, and/or the presence of other carotenoids [89].
Other Animal Model Studies
Syrian golden hamsters have been used to study lung cancer induced by carcinogens such as BaP [10], N-dibutylnitrosamine [10], DEN [90], and NNK [68,91]. In humans, the most predominant histologic type of lung cancer is that of small airway-derived pulmonary adenocarcinomas (PACs), which are thought to arise from epithelial cells composed of Clara cells in the small airways in the lung periphery [92]. In hamsters, the trachea and stem bronchi are coated by a pseudostratified respiratory epithelium found in the large airway of humans, and these consist of basal cells and ciliated cells [10]. This is in contrast with the respiratory epithelia of the mice, which are composed of nonciliated Clara cells that are restricted to small airways, similar to bronchioles in humans. Furthermore, spontaneous and NNK-induced lung tumors in mice are derived from alveolar type II cells [93], whereas these lung tumors in hamsters are derived from small airway epithelia, similar to that of PACs in humans. Tumor expression in hamsters has also been shown to involve activating point mutations in K-ras [94] and overexpression of the epidermal growth factor receptor [95], similar to human tumors. Moreover, retinoid-induced inhibition of cell proliferation involving cAMP-dependent inhibition of extracellular signal-regulated kinase 1/2 phosphoryaltion in human large airway epithelial cells has been observed in hamster tumor model [96]. Therefore, because of the similarities in the features of PAC exhibited by humans and hamsters, NNK-induced PAC in Syrian golden hamsters has been used as a model of human small airway-derived PAC [97].
In one experiment, in which two groups of Syrian golden hamsters were treated with either BaP or unfiltered research cigarette smoke and one group of European hamsters was treated subcutaneously with N-dibutylnitrosamine, all developed atypical cilia in their tracheae and bronchi [10]. In another study, Syrian golden hamsters were treated with s.c. injections or oral swabs of NNK, and tumors were induced in the lungs, trachea, and nasal cavities of this model [68,98].
Hamster models have also been used to evaluate the protective effects of β-carotene on upper respiratory tumorigenesis (e.g., trachea and larynx) induced by exposure to smoke and DEN [90]. A total of 120 male Syrian hamsters received a single s.c. injection of 100 mg/kg BWDEN and were then exposed to smoke from nonfiltered cigarettes and supplemented with various doses of β-carotene (0%, 0.005%, 0.05%, or 0.25%) for 12 weeks. In this study, the incidence and multiplicity of hyperplasia were found to be dose dependent in relation to the amount of β-carotene administered.
Recently, Al Wadei and Schuller [91] demonstrated that β-carotene enhanced the development of NNK-induced PAC in Syrian golden hamsters. The development of PAC in the small airway epithelial cells of the hamsters was induced with NNK treatments (2.5 mg/100 g BW three times a week for 10 weeks). One week after the last NNK injection, β-carotene injections (5 µM β-carotene in 0.2 ml of sesame oil administered s.c. twice a week) were given for 1.5 years. This treatment group was then found to be associated with increased lung tumor multiplicity, tumor size, blood cell cAMP, and increased levels of cAMP response element binding and extracellular signal-regulated kinase 1/2 phosphorylation in lung tumor samples. In combination, these results suggest that lung tumorigenicity was enhanced by β-carotene through increased cAMP signaling.
As a species similar to ferret (Mustela putorius furo), lung carcinoma was developed in minks (Mustala vison) [99]. In this model, administration of nitrosonornicotine (11.9 mM/mink) or NNK (6.3 mM/mink) for 19 months induced the development of lung adenocarcinoma. Work is still ongoing to evaluate the effects of carotenoids on lung carcinogenesis in this model.
Levels of Carotenoids in Plasma and Lung Tissues Vary between Supplementation Studies Performed in Various Animal Models
Several studies have reported plasma and lung concentrations of carotenoids obtained from supplementation studies performed in various animal models, including humans (Table 2). However, it is difficult to interpret the effects of carotenoids from these studies because of the differences in the various supplementation methods and the duration of carotenoids administered. In addition, there are differences in the metabolism and absorption of carotenoids in animal models, along with differences in the units used for carotenoids concentrations detected in plasma and tissue. However, a consistent result is that plasma and lung tissue concentrations of carotenoids significantly increase in most animal models supplemented with carotenoids, particularly β-carotene and lycopene.
Table 2.
Carotenoid Levels in Plasma and Lung after Supplementation in Various Models.
| Model | Supplementation | Plasma* (nM) | Lung* (nmol/kg) | Reference |
| Human | β-Carotene | |||
| (20†) | 5.3‡ | [138] | ||
| Lycopene | ||||
| (30§) | 1300 | [100] | ||
| Ferret | β-Carotene | |||
| (2.4¶) | 109 ± 21 | 26.2 ± 1.7 | [40] | |
| Lycopene | ||||
| (1.1#) | 226 ± 35 | 342.2 ± 42.3 | [39] | |
| (4.3#) | 373 ± 60 | 1159.2 ± 145 | [39] | |
| β-Cryptoxanthin | ||||
| (7.5**) | 69 ± 9 | 31 ± 5 | [54] | |
| (37.5**) | 117 ± 20 | 63 ± 10 | [54] | |
| Rat | ||||
| F344 | Lycopene | |||
| (20††) | 139 ± 41 | [106] | ||
| Sprague-Dawley | β-Carotene | |||
| (500‡‡) | 0.43 ± 0.03§§ | [139] | ||
| Mouse | ||||
| A/J | β-Carotene | |||
| (120–3000¶¶) | 230 ± 60 to 3200 ± 660 | 340 ± 40 to 3400 ± 380 | [71] | |
| Nude mice | Lycopene | |||
| (20††) | 224 ± 51 | [106] | ||
| BALB/C | Lycopene | |||
| (20††) | 198 ± 52 | [106] |
Values are means ± SDs.
Milligrams per day for 5 to 8 years.
Micromoles per liter.
Milligrams per day for 28 days.
Milligrams per kilogram BW for 6 months.
Milligrams per kilogram BW for 9 weeks.
Micrograms per kilogram BW for 3 months.
Milligrams per kilogram BW every 2 days for 10 days.
Milligrams per kilogram BW for 5 days.
Micrograms per gram of tissue.
Milligram per kilogram feed for 4 weeks.
In humans, β-carotene (20 mg/d) was supplemented for 5 to 8 years in the Alpha-Tocopherol, Beta-carotene Cancer Prevention Trial, and this dose is 10-fold higher than the average intake of β-carotene in a typical American diet, which is approximately 2 mg/d. When plasma levels of β-carotene were assayed, an approximate 18-fold increase in levels of β-carotene was detected [16]. Similarly, when β-carotene (2.4 mg/kg BW, approximately 30 mg/d) was administered to ferrets for 6 months, the plasma concentration of β-carotene was increased approximately 20-fold [40]. In another study, when dietary lycopene (30 mg/d) was supplemented for 3 weeks in humans, the plasma concentration of lycopene was 1300 nM [100]. However, human epidemiological studies have identified a reference range for plasma lycopene to be 290 to 350 nM, and the level of plasma lycopene detected in the ferret model administered lycopene supplement (equivalent to 15 mg/d in humans) was 226 nM [39,101,102]. Taken together, these studies indicated similarities of absorption and accumulation of these carotenoids between ferrets and humans.
However, cigarette smoke exposure inhibited the supplementation-induced elevation of plasma and lung tissue level of β-carotene or lycopene [39,40]. These results are consistent with many epidemiological studies, which reported that smokers had lower plasma/serum concentrations of β-carotene than nonsmokers do [103,104]. Furthermore, it has been hypothesized that these results represent a smoke-induced oxidization of β-carotene that generates a number of transient oxidative metabolites, as well as smoke-induced isomerization and degradation of lycopene.
Whereas rodent models have been widely used in carotenoids research, the suitability of these models has been questioned based on the poor absorption of carotenoids exhibited by these models relative to that of humans [85,105]. For example, Huang et al. [106] performed a study where lycopene supplements (20 mg/kg BW every 2 days) were administered to various rodent models (F344 rats, nude mice, and BALB/c mice) for 10 days, and then plasma levels of lycopene were compared. Of these three strains, nude mice (224 ± 51 nM) exhibited the highest levels of lycopene followed by BALB/c mice (198 ± 52 nM) and F344 rats (139 ± 41 nM). It is possible that the differences in lycopene plasma concentrations were due to the differences in the plasma lipoprotein profiles of these rodent species. However, the results from this comparison did indicate that nude mice are more appropriate than BALB/c mice or F344 rats for the investigation of in vivo effects of lycopene in humans.
Possible Mechanisms for the Effects of Carotenoids on Smoke-Induced Lung Carcinogenesis
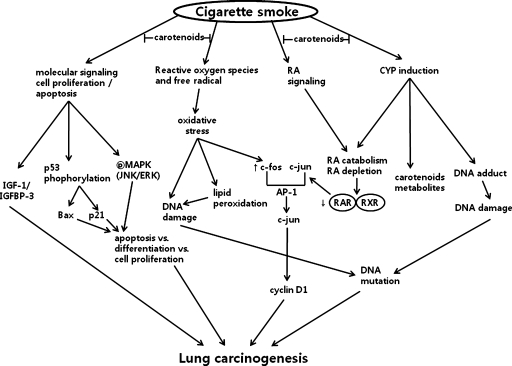
There are several hypotheses that have been developed to explain the protection against lung carcinogenesis provided by carotenoids, and these are outlined in Figure 1. These hypotheses involved 1) antioxidant and prooxidant effects, 2) the effects of carotenoids on levels of RA and its signaling, 3) induction of CYP enzymes, 4) and signaling pathways involved in apoptosis, proliferation, and differentiation.
Figure 1.
Possible mechanism(s) of the protective effect of carotenoids on smoke induced lung carcinogenesis. For details, see text. CYP indicates cytochrome P450; ERK, extracellular signal-regulated kinase; IGF-1, insulin-like growth factor 1; IGFBP-3, IGF binding protein 3; JNK, c-jun N-terminal kinase; MAPK, mitogen-activated protein kinase; RA, retinoic acid; RAR, retinoic acid receptor; RXR, retinoid X receptor.
Antioxidant and Prooxidant Effects of Carotenoids
Antioxidant effects mediated by carotenoids have been well characterized and have been shown to prevent oxidative damage [107]. Cigarette smoke contains free radicals as well as carcinogens. Therefore, smokers develop higher levels of free radicals, higher levels of lipid peroxidation products, and lower levels of antioxidants in their plasma and lungs compared with nonsmokers [108–110]. Palozza et al. [111] have demonstrated the antioxidant effect of β-carotene by inhibiting cigarette smoke condensate (tar)-induced lipid peroxidation. Antioxidant properties of many carotenoids have been believed to play important roles in anticarcinogenic actions. Of the carotenoids identified to date, lycopene has been associated with the most powerful antioxidant activity [112]. Numerous in vivo studies using tomatoes, or tomato products, have demonstrated that the antioxidant properties of lycopene can mediate a decrease in DNA damage, low-density lipoprotein oxidation, and lipid peroxidation [113–116]. It has also been proposed that carotenoids have prooxidant properties based on their interactions with free radicals present in cigarette smoke, or carotenoids oxygenated products produced by high consumption of carotenoids, particularly in the oxidative environment of a smoker's lung [117,118]. Correspondingly, the formation of β-apo-carotenals from all-trans-β-carotenes was higher when lung extracts from ferrets exposed to cigarette smoke were compared with lung extracts of ferrets that were not exposed to cigarette smoke. Further, these oxidative metabolites might lower the levels of RA in lungs of these animals.
Some observations support the hypothesis that a complex network of intracellular antioxidants has a critical role in preventing the oxidative damage induced by cigarette smoke. Previously, in vitro studies have detected strong interactions between β-carotene, α-tocopherol, and ascorbic acid that result in the protection from oxidative damage. Furthermore, these antioxidants have been shown to regenerate each other from their radical cation and enhance their antioxidant efficiency mutually [119]. In a ferret model, Kim et al. [52] also found that the combination of the three antioxidants mediates a potential chemopreventive effect against smoke-induced lymphocyte DNA damage. In combination, these observations support the hypothesis that combinations of antioxidants may mediate complex signaling interactions that result in the prevention of DNA damage induced by cigarette smoke.
Effects of Carotenoids on Levels of RA and RA Signaling
It has been shown that low levels of RA may interfere with retinoid signal transduction, resulting in an enhancement of cell proliferation and an increased potential for malignant transformation [120]. In ferrets, exposure to cigarette smoke and/or high doses of β-carotene (30 mg/d) was found to induce CYP enzymes in the lungs, to enhance RA catabolism, and to decrease levels of RARβ [40,121]. However, the decreased level of RA induced by cigarette smoke exposure and NNK is restored by β-carotene supplementation in the presence of other antioxidants including α-tocopherol and ascorbic acid [38]. In addition, oxidative stress induced by high doses of β-carotene (e.g., 2.4 mg/kg per day, for 6 months) and exposure to cigarette smoke was associated with increase in levels of AP-1, a complex composed of c-jun and c-fos [40]. Antiproliferative functions of RA are mediated by RAR/RXR-dependent repression of AP-1 activity. Moreover, AP-1 sites have been identified in various genes that are involved in cellular proliferation, transformation, and death [122]. Correspondingly, it has been hypothesized that lower levels of RA and RARβ down-regulation induced by cigarette smoke may reduce the inhibitory effects of retinoids on AP-1. This could then enhance lung cell proliferation and induce the development of preneoplastic lesions and therefore the potential for lung carcinogenesis. However, a recent study has demonstrated that levels of AP-1 were unaffected by various doses of β-carotene in the presence of DL-α-tocopherol and ascorbyl palmitate in ferrets exposed to BaP [53]. These contradictory results may be due to differences in the β-carotene formulations used, and therefore, additional studies need to be performed. In addition, numerous studies have also reported that RA prevents abnormal cell proliferation by upregulating mitogen-activated protein kinase-phosphatase-1 (MKP-1), resulting in dephosphorylation of mitogen-activated protein kinase [123–125]. Accordingly, levels of MKP-1 were found to correlate with the concentrations of RA detected in the lungs of ferrets [126].
Effects of Carotenoids on the Induction of CYP Enzymes
It has been shown that the CYP family of enzymes is activated by carcinogens present in cigarettes [11,127]. In particular, the induction of CYP1A1 and CYP1A2 by cigarette smoke enhances RA catabolism and increases levels of carotenoid metabolites, including β-apo-8′-carotenal. For example, in a comparison of ferrets exposed to cigarette smoke versus untreated controls, levels of β-apo-8′-carotenal were found to be three-fold higher in the lung extracts collected from ferrets exposed to cigarette smoke [40,128]. In the study by Gradelet et al. [128], a nonspecific CYP inhibitor, liaroxole, was used to demonstrate that P450 enzymes induced by eccentric cleavage breakdown products of β-carotene are involved in the degradation of RA. On the basis of these results, it is hypothesized that the induction of CYPs has the capacity to induce low levels of RA in the lungs of ferrets exposed to cigarette smoke and/or high doses of β-carotene, thereby providing a possible explanation for the enhanced lung carcinogenesis observed in studies involving high doses of β-carotene supplementation and smoke exposure. However, although it has been observed that significant increases in the levels of several CYP enzymes, including CYP1A1, have been detected in the lungs of animals supplemented with high doses of β-carotene [121], there is no sufficient evidence to indicate that carotenoids prevent increases in CYP enzymes levels in animals with lung cancer.
The carcinogen metabolites of BaP, one of the most important smoke-derived carcinogens, have the capacity to bind DNA and form DNA adducts. Salgo et al. [129] has shown that fractions containing β-carotene metabolites, but not β-carotene itself, were able to facilitate the binding of carcinogen metabolites present in cigarette smoke to DNA. Moreover, an induction of BALB/c 3T3 cell transformation by BaP has been shown to be significantly enhanced in the presence of β-carotene [130]. However, it is not clear whether the increase in cell transforming activity associated with β-carotene is due to β-carotene itself or due to its metabolites. Therefore, these studies indicated that β-carotene may directly decrease the binding of BaP metabolites to DNA, whereas β-carotene metabolites from high doses of β-carotene may facilitate the binding of BaP metabolites to DNA and enhance lung carcarcinogenesis in smokers.
Effects of Carotenoids on Signaling Pathways Involving in Apoptosis, Proliferation, and Differentiation
Insulin-like growth factors are mitogens that play an important role in regulating cell proliferation, differentiation, and apoptosis [131]. IGFBP-3 regulates the bioactivity of IGF-1 by sequestering IGF-1 away from its receptor, thereby inhibiting the mitogenic activity of IGF-1 [132]. IGF-1 and IGFBP-3 have been implicated in the pathogenesis of lung cancer, as well as other malignancies, and lung cancer risk is associated with higher plasma levels of IGF-1 and/or lower levels of IGFBP-3 [131,133]. In a ferret model, administration of lycopene supplements was associated with an increase in plasma levels of IGFBP-3, an inhibition of cigarette smoke-induced squamous metaplasia, a decrease in proliferating cell nuclear antigen, and a decrease in the phosphorylation of BAD [39]. In another model, exposure of ferrets to cigarette smoke and NNK affected mRNA levels of IGFBP-3 in different tissues. In contrast, a combination of antioxidant supplements (e.g., β-carotene, α-tocopherol, and ascorbic acid) had no effect on plasma levels of IGF-1/IGFBP-3 and mRNA expression of IGF-1/IGFBP-3 in the lung and liver [52]. Additional studies are needed to further address the mechanisms that mediate tissue-specific expression of IGF and IGFBP-3.
It has been demonstrated that high doses of β-carotene enhanced the phosphorylation of JNK and p53, as well as total p53 expression, induced by cigarette smoke [126]. Moreover, physiological doses of β-carotene in the presence of α-tocopherol and ascorbic acid have been shown to block up-regulation of JNK phosphorylation and p53 phosphorylation at Ser-15 [38]. In contrast, lycopene has been shown to attenuate elevated levels of p53 induced by cigarette smoke [39]. In a ferret model, exposure to cigarette smoke has been associated with a significant decrease in levels of p21, a promoter of differentiation response to cellular stress [41]. Similarly, supplementation with lycopene has been shown to prevent attenuation of p21, thereby inhibiting cell growth [39]. Additional study has also demonstrated that a combination of antioxidants (e.g., β-carotene, α-tocopherol, and ascorbic acid) play an important role in apoptosis by regulating Bax levels during lung carcinogenesis [38].
Conclusions
Dietary modifications that delay lung cancer growth have the potential to reduce morbidity and mortality and decrease medical cost and treatments. For this reason, appropriate animal studies are required to investigate the effect of dietary modification on carcinogenesis. Accordingly, a number of animal models have been developed and used for lung carcinogenesis studies. However, there remain several important methodological issues to be solved to study the chemopreventive effects of carotenoids in lung carcinogenesis, including strain-specific and diet-specific effects, and differences in the absorption and distribution of carotenoids supplemented (e.g., pure lycopene vs pure carotenoids vs carotenoid mixtures vs a combination with other antioxidants), which may affect the uptake, metabolism, and storage of carotenoids. Additional factors to consider include doses, timings of supplementation, and routes of administration in relation to the induction of carcinogenesis studied [134–136]. Furthermore, the contribution of interactions between carotenoids and other antioxidants including vitamin C, α-tocopherol, and flavonoids should be considered. In most animal studies, carotenoid levels in plasma and tissue are often not described, or the doses of carotenoids administered are very high. Therefore, it is difficult to interpret the effects of carotenoids using existing animal models.
Exposure to cigarette smoke is a key factor in studies of high-dose carotenoid supplementation. Therefore, the dose effects of carotenoids on lung carcinogenesis in response to smoke exposure in animal models should be thoroughly examined before large-scale intervention trials are established, particularly when doses of carotenoids that greatly exceed normal dietary levels are being considered. It is anticipated that the development of additional carcinogen-induced or transgenic animal models will facilitate an elucidation of the mechanisms responsible for the effects of carotenoids on lung carcinogenesis, leading to improvements in prevention and treatment.
Acknowledgments
The authors thank Yunsook Lim (Kyung Hee University, Seoul, Korea) for constructive comments.
Footnotes
This review was not supported by grants.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Mayne ST. β-Carotene, carotenoids, and disease prevention in humans. FASEB J. 1996;10:690–701. [PubMed] [Google Scholar]
- 3.Comstock GW, Alberg AJ, Huang HY, Wu K, Burke AE, Hoffman SC, Norkus EP, Gross M, Cutler RG, Morris JS, et al. The risk of developing lung cancer associated with antioxidants in the blood: ascorbic acids, carotenoids, α-tocopherol, selenium, and total peroxyl radical absorbing capacity. Am J Epidemiol. 2008;168:831–840. doi: 10.1093/aje/kwn328. [DOI] [PubMed] [Google Scholar]
- 4.Yuan JM, Ross RK, Chu XD, Gao YT, Yu MC. Prediagnostic levels of serum β-cryptoxanthin and retinol predict smoking-related lung cancer risk in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2001;10:767–773. [PubMed] [Google Scholar]
- 5.Yuan JM, Stram DO, Arakawa K, Lee HP, Yu MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2003;12:890–898. [PubMed] [Google Scholar]
- 6.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65–81. doi: 10.1016/s0272-5231(03)00061-3. viii. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y, Liu XS, Liu C, Smith DE, Russell RM, Wang XD. Induction of pulmonary neoplasia in the smoke-exposed ferret by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK): a model for human lung cancer. Cancer Lett. 2006;234:209–219. doi: 10.1016/j.canlet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 9.Colby TV, Wistuba II, Gazdar A. Precursors to pulmonary neoplasia. Adv Anat Pathol. 1998;5:205–215. doi: 10.1097/00125480-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Reznik-Schuller H. Ciliary alterations in hamster respiratory tract epithelium after exposure to carcinogens and cigarette smoke. Cancer Lett. 1975;1:7–13. doi: 10.1016/s0304-3835(75)94558-9. [DOI] [PubMed] [Google Scholar]
- 11.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 13.Hecht SS, Kassie F, Hatsukami DK. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9:476–488. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakayama T, Kodama M, Nagata C. Generation of hydrogen peroxide and superoxide anion radical from cigarette smoke. Gann. 1984;75:95–98. [PubMed] [Google Scholar]
- 15.Rojas E, Valverde M, Sordo M, Ostrosky-Wegman P. DNA damage in exfoliated buccal cells of smokers assessed by the single cell gel electrophoresis assay. Mutat Res. 1996;370:115–120. doi: 10.1016/0165-1218(96)00062-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang X-D. Carotenoid oxidative/degradative products and their biological activities. In: Krinsky NI, editor. Carotenoids in Health and Disease. New York, NY: Marcel Dekker; 2004. pp. 313–335. [Google Scholar]
- 17.Goodman DS, Huang HS. Biosynthesis of vitamin A with rat intestinal enzymes. Science. 1965;149:879–880. doi: 10.1126/science.149.3686.879. [DOI] [PubMed] [Google Scholar]
- 18.Olson JA, Hayaishi O. The enzymatic cleavage of β-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc Natl Acad Sci USA. 1965;54:1364–1370. doi: 10.1073/pnas.54.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krinsky NI. Actions of carotenoids in biological systems. Annu Rev Nutr. 1993;13:561–587. doi: 10.1146/annurev.nu.13.070193.003021. [DOI] [PubMed] [Google Scholar]
- 20.Burton GW, Ingold KU. β-Carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 21.Napoli JL, Race KR. Biogenesis of retinoic acid from β-carotene. Differences between the metabolism of β-carotene and retinal. J Biol Chem. 1988;263:17372–17377. [PubMed] [Google Scholar]
- 22.Wang XD, Krinsky NI. The bioconversion of β-carotene into retinoids. Subcell Biochem. 1998;30:159–180. doi: 10.1007/978-1-4899-1789-8_7. [DOI] [PubMed] [Google Scholar]
- 23.Sies H, Stahl W. Carotenoids and intercellular communication via gap junctions. Int J Vitam Nutr Res. 1997;67:364–367. [PubMed] [Google Scholar]
- 24.Zhang LX, Cooney RV, Bertram JS. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis. 1991;12:2109–2114. doi: 10.1093/carcin/12.11.2109. [DOI] [PubMed] [Google Scholar]
- 25.Stahl W, Eisenbrand G. Comparative study on the influence of two 2-chloroethylnitrosoureas with different carbamoylating potential towards glutathione and glutathione-related enzymes in different organs of the rat. Free Radic Res Commun. 1991;14:271–278. doi: 10.3109/10715769109088956. [DOI] [PubMed] [Google Scholar]
- 26.Santos MS, Meydani SN, Leka L, Wu D, Fotouhi N, Meydani M, Hennekens CH, Gaziano JM. Natural killer cell activity in elderly men is enhanced by β-carotene supplementation. Am J Clin Nutr. 1996;64:772–777. doi: 10.1093/ajcn/64.5.772. [DOI] [PubMed] [Google Scholar]
- 27.Watson RR, Prabhala RH, Plezia PM, Alberts DS. Effect of β-carotene on lymphocyte subpopulations in elderly humans: evidence for a dose-response relationship. Am J Clin Nutr. 1991;53:90–94. doi: 10.1093/ajcn/53.1.90. [DOI] [PubMed] [Google Scholar]
- 28.Hughes DA. Effects of carotenoids on human immune function. Proc Nutr Soc. 1999;58:713–718. doi: 10.1017/s0029665199000932. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, Zick A, Sharoni Y, Levy J. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4:177–186. [PubMed] [Google Scholar]
- 30.Stahl W, von Laar J, Martin D, Emmerich T, Sies H. Stimulation of gap junctional communication: comparison of acyclo-retinoic acid and lycopene. Arch Biochem Biophys. 2000;373:271–274. doi: 10.1006/abbi.1999.1510. [DOI] [PubMed] [Google Scholar]
- 31.Karas M, Amir H, Fishman D, Danilenko M, Segal S, Nahum A, Koifmann A, Giat Y, Levy J, Sharoni Y. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr Cancer. 2000;36:101–111. doi: 10.1207/S15327914NC3601_14. [DOI] [PubMed] [Google Scholar]
- 32.Edes TE, Thornton W, Jr, Shah J. β-Carotene and aryl hydrocarbon hydroxylase in the rat: an effect of β-carotene independent of vitamin A activity. J Nutr. 1989;119:796–799. doi: 10.1093/jn/119.5.796. [DOI] [PubMed] [Google Scholar]
- 33.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, et al. α-Tocopherol and β-carotene supplements and lung cancer incidence in the α-tocopherol, β-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 34.Omenn GS, Goodman G, Thornquist M, Grizzle J, Rosenstock L, Barnhart S, Balmes J, Cherniack MG, Cullen MR, Glass A, et al. The β-Carotene and Retinol Efficacy Trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos-exposed workers. Cancer Res. 1994;54:2038s–2043s. [PubMed] [Google Scholar]
- 35.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Effects of a combination of β carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 36.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, et al. Risk factors for lung cancer and for intervention effects in CARET, the β-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 37.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, et al. Lack of effect of long-term supplementation with β carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y, Chongviriyaphan N, Liu C, Russell RM, Wang XD. Combined antioxidant (β-carotene, α-tocopherol and ascorbic acid) supplementation increases the levels of lung retinoic acid and inhibits the activation of mitogen-activated protein kinase in the ferret lung cancer model. Carcinogenesis. 2006;27:1410–1419. doi: 10.1093/carcin/bgi340. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003;63:3138–3144. [PubMed] [Google Scholar]
- 40.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given β-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–66. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Russell RM, Wang XD. Lycopene supplementation prevents smoke-induced changes in p53, p53 phosphorylation, cell proliferation, and apoptosis in the gastric mucosa of ferrets. J Nutr. 2006;136:106–111. doi: 10.1093/jn/136.1.106. [DOI] [PubMed] [Google Scholar]
- 42.Wang XD, Krinsky NI, Marini RP, Tang G, Yu J, Hurley R, Fox G, Russell RM. Intestinal uptake and lymphatic absorption of β-carotene in ferrets: a model for human β-carotene metabolism. Am J Physiol. 1992;263:G480–G486. doi: 10.1152/ajpgi.1992.263.4.G480. [DOI] [PubMed] [Google Scholar]
- 43.Gugger ET, Bierer TL, Henze TM, White WS, Erdman JW., Jr β-Carotene uptake and tissue distribution in ferrets (Mustela putorius furo) J Nutr. 1992;122:115–119. doi: 10.1093/jn/122.1.115. [DOI] [PubMed] [Google Scholar]
- 44.Wang XD, Tang GW, Fox JG, Krinsky NI, Russell RM. Enzymatic conversion of β-carotene into β-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues. Arch Biochem Biophys. 1991;285:8–16. doi: 10.1016/0003-9861(91)90322-a. [DOI] [PubMed] [Google Scholar]
- 45.Wang XD, Russell RM, Marini RP, Tang G, Dolnikowski GG, Fox JG, Krinsky NI. Intestinal perfusion of β-carotene in the ferret raises retinoic acid level in portal blood. Biochim Biophys Acta. 1993;1167:159–164. doi: 10.1016/0005-2760(93)90157-5. [DOI] [PubMed] [Google Scholar]
- 46.Wang XD, Russell RM, Liu C, Stickel F, Smith DE, Krinsky NI. β-Oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from β-apocarotenoic acids. J Biol Chem. 1996;271:26490–26498. [PubMed] [Google Scholar]
- 47.Liu C, Wang XD, Bronson RT, Smith DE, Krinsky NI, Russell RM. Effects of physiological versus pharmacological β-carotene supplementation on cell proliferation and histopathological changes in the lungs of cigarette smoke-exposed ferrets. Carcinogenesis. 2000;21:2245–2253. doi: 10.1093/carcin/21.12.2245. [DOI] [PubMed] [Google Scholar]
- 48.McDowell EM, McLaughlin JS, Merenyl DK, Kieffer RF, Harris CC, Trump BF. The respiratory epithelium. V. Histogenesis of lung carcinomas in the human. J Natl Cancer Inst. 1978;61:587–606. [PubMed] [Google Scholar]
- 49.Brambilla E, Moro D, Veale D, Brichon PY, Stoebner P, Paramelle B, Brambilla C. Basal cell (basaloid) carcinoma of the lung: a new morphologic and phenotypic entity with separate prognostic significance. Hum Pathol. 1992;23:993–1003. doi: 10.1016/0046-8177(92)90260-a. [DOI] [PubMed] [Google Scholar]
- 50.Wang XD, Krinsky NI, Tang GW, Russell RM. Retinoic acid can be produced from excentric cleavage of β-carotene in human intestinal mucosa. Arch Biochem Biophys. 1992;293:298–304. doi: 10.1016/0003-9861(92)90399-h. [DOI] [PubMed] [Google Scholar]
- 51.van Helden YG, Keijer J, Heil SG, Pico C, Palou A, Oliver P, Munnia A, Briede JJ, Peluso M, Franssen-van Hal NL, et al. β-Carotene affects oxidative stress-related DNA damage in lung epithelial cells and in ferret lung. Carcinogenesis. 2009;30:2070–2076. doi: 10.1093/carcin/bgp186. [DOI] [PubMed] [Google Scholar]
- 52.Kim Y, Lian F, Yeum KJ, Chongviriyaphan N, Choi SW, Russell RM, Wang XD. The effects of combined antioxidant ( β-carotene, α-tocopherol and ascorbic acid) supplementation on antioxidant capacity, DNA single-strand breaks and levels of insulin-like growth factor-1/IGF-binding protein 3 in the ferret model of lung cancer. Int J Cancer. 2007;120:1847–1854. doi: 10.1002/ijc.22320. [DOI] [PubMed] [Google Scholar]
- 53.Fuster A, Pico C, Sanchez J, Oliver P, Zingaretti MC, Murano I, Morroni M, Hoeller U, Goralczyk R, Cinti S, et al. Effects of 6-month daily supplementation with oral β-carotene in combination or not with benzo[a]pyrene on cell-cycle markers in the lung of ferrets. J Nutr Biochem. 2008;19:295–304. doi: 10.1016/j.jnutbio.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Liu C, Bronson RT, Russell RM, Wang XD. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage and squamous metaplasia in ferrets. Cancer Prev Res (Phila) 2011 doi: 10.1158/1940-6207.CAPR-10-0384. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goralczyk R. β-Carotene and lung cancer in smokers: review of hypotheses and status of research. Nutr Cancer. 2009;61:767–774. doi: 10.1080/01635580903285155. [DOI] [PubMed] [Google Scholar]
- 56.Bruder CE, Yao S, Larson F, Camp JV, Tapp R, McBrayer A, Powers N, Granda WV, Jonsson CB. Transcriptome sequencing and development of an expression microarray platform for the domestic ferret. BMC Genomics. 2010;11:251. doi: 10.1186/1471-2164-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferreira AL, Yeum KJ, Liu C, Smith D, Krinsky NI, Wang XD, Russell RM. Tissue distribution of lycopene in ferrets and rats after lycopene supplementation. J Nutr. 2000;130:1256–1260. doi: 10.1093/jn/130.5.1256. [DOI] [PubMed] [Google Scholar]
- 58.Johansson MB, Karlsson BW. Lipoproteins in serum of rat, mouse, gerbil, rabbit, pig and man studied by electrophoretical and immunological methods. Comp Biochem Physiol B. 1976;54:495–500. doi: 10.1016/0305-0491(76)90128-0. [DOI] [PubMed] [Google Scholar]
- 59.Tsuda H, Iwahori Y, Asamoto M, Baba-Toriyama H, Hori T, Kim DJ, Uehara N, Iigo M, Takasuka N, Murakoshi M, et al. Demonstration of organotropic effects of chemopreventive agents in multiorgan carcinogenesis models. IARC Sci Publ. 1996:143–150. [PubMed] [Google Scholar]
- 60.Wang Y, Zhang Z, Yan Y, Lemon WJ, LaRegina M, Morrison C, Lubet R, You M. A chemically induced model for squamous cell carcinoma of the lung in mice: histopathology and strain susceptibility. Cancer Res. 2004;64:1647–1654. doi: 10.1158/0008-5472.can-03-3273. [DOI] [PubMed] [Google Scholar]
- 61.Limpens J, Schroder FH, de Ridder CM, Bolder CA, Wildhagen MF, Obermuller-Jevic UC, Kramer K, van Weerden WM. Combined lycopene and vitamin E treatment suppresses the growth of PC-346C human prostate cancer cells in nude mice. J Nutr. 2006;136:1287–1293. doi: 10.1093/jn/136.5.1287. [DOI] [PubMed] [Google Scholar]
- 62.Karmakar S, Banik NL, Ray SK. Combination of all-trans retinoic acid and paclitaxel-induced differentiation and apoptosis in human glioblastoma U87MG xenografts in nude mice. Cancer. 2008;112:596–607. doi: 10.1002/cncr.23223. [DOI] [PubMed] [Google Scholar]
- 63.Jang SH, Ryu PD, Lee SY. Dendrotoxin-κ suppresses tumor growth induced by human lung adenocarcinoma A549 cells in nude mice. J Vet Sci. 2011;12:35–40. doi: 10.4142/jvs.2011.12.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng J, Dai B, Fang B, Bekele BN, Bornmann WG, Sun D, Peng Z, Herbst RS, Papadimitrakopoulou V, Minna JD, et al. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PLoS One. 2010;5:e14124. doi: 10.1371/journal.pone.0014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu G, Xiao H, Li GX, Picinich SC, Chen YK, Liu A, Lee MJ, Loy S, Yang CS. A γ-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis. 2010;31:687–694. doi: 10.1093/carcin/bgp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang CS, Liao JW, Hu ML. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J Nutr. 2008;138:538–543. doi: 10.1093/jn/138.3.538. [DOI] [PubMed] [Google Scholar]
- 67.Schleicher RL, Moon RC, Patel MK, Beattie CW. Influence of retinoids on growth and metastasis of hamster melanoma in athymic mice. Cancer Res. 1988;48:1465–1469. [PubMed] [Google Scholar]
- 68.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 69.Witschi H. Chemoprevention of lung cancer. Methods Mol Med. 2003;75:739–754. doi: 10.1385/1-59259-324-0:739. [DOI] [PubMed] [Google Scholar]
- 70.Freemantle SJ, Dmitrovsky E. Cyclin E transgenic mice: discovery tools for lung cancer biology, therapy, and prevention. Cancer Prev Res (Phila) 2010;3:1513–1518. doi: 10.1158/1940-6207.CAPR-10-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goralczyk R, Wertz K, Lenz B, Riss G, Buchwald Hunziker P, Geatrix B, Aebischer P, Bachmann H. β-Carotene interaction with NNK in the AJ-mouse model: effects on cell proliferation, tumor formation and retinoic acid responsive genes. Biochim Biophys Acta. 2005;1740:179–188. doi: 10.1016/j.bbadis.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–1574. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 73.Hecht SS, Kenney PM, Wang M, Trushin N, Agarwal S, Rao AV, Upadhyaya P. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–130. doi: 10.1016/s0304-3835(98)00326-7. [DOI] [PubMed] [Google Scholar]
- 74.Guttenplan JB, Chen M, Kosinska W, Thompson S, Zhao Z, Cohen LA. Effects of a lycopene-rich diet on spontaneous and benzo[a]pyrene-induced mutagenesis in prostate, colon and lungs of the lacZ mouse. Cancer Lett. 2001;164:1–6. doi: 10.1016/s0304-3835(00)00705-9. [DOI] [PubMed] [Google Scholar]
- 75.Mernitz H, Smith DE, Zhu AX, Wang XD. 9-cis-Retinoic acid inhibition of lung carcinogenesis in the A/J mouse model is accompanied by increased expression of RAR-β but no change in cyclooxygenase-2. Cancer Lett. 2006;244:101–108. doi: 10.1016/j.canlet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Mernitz H, Smith DE, Wood RJ, Russell RM, Wang XD. Inhibition of lung carcinogenesis by 1α,25-dihydroxyvitamin D3 and 9-cis retinoic acid in the A/J mouse model: evidence of retinoid mitigation of vitamin D toxicity. Int J Cancer. 2007;120:1402–1409. doi: 10.1002/ijc.22462. [DOI] [PubMed] [Google Scholar]
- 77.Kohno H, Taima M, Sumida T, Azuma Y, Ogawa H, Tanaka T. Inhibitory effect of mandarin juice rich in β-cryptoxanthin and hesperidin on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced pulmonary tumorigenesis in mice. Cancer Lett. 2001;174:141–150. doi: 10.1016/s0304-3835(01)00713-3. [DOI] [PubMed] [Google Scholar]
- 78.Lubet RA, Zhang Z, Wiseman RW, You M. Use of p53 transgenic mice in the development of cancer models for multiple purposes. Exp Lung Res. 2000;26:581–593. doi: 10.1080/01902140150216684. [DOI] [PubMed] [Google Scholar]
- 79.de Seranno S, Meuwissen R. Progress and applications of mouse models for human lung cancer. Eur Respir J. 2010;35:426–443. doi: 10.1183/09031936.00124709. [DOI] [PubMed] [Google Scholar]
- 80.Wu K, Kim HT, Rodriquez JL, Munoz-Medellin D, Mohsin SK, Hilsenbeck SG, Lamph WW, Gottardis MM, Shirley MA, Kuhn JG, et al. 9-cis-Retinoic acid suppresses mammary tumorigenesis in C3(1)-simian virus 40 T antigen-transgenic mice. Clin Cancer Res. 2000;6:3696–3704. [PubMed] [Google Scholar]
- 81.McCormick DL, Johnson WD, Rao KV, Bowman-Gram T, Steele VE, Lubet RA, Kelloff GJ. Comparative activity of N-(4-hydroxyphenyl)-all-trans-retinamide and α-difluoromethylornithine as inhibitors of lymphoma induction in PIM transgenic mice. Carcinogenesis. 1996;17:2513–2517. doi: 10.1093/carcin/17.11.2513. [DOI] [PubMed] [Google Scholar]
- 82.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, et al. CMO1 deficiency abolishes vitamin A production from β-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–33561. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 83.Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW., Jr Loss of carotene-9′,10′-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr. 2010;140:2134–2138. doi: 10.3945/jn.110.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell JK, Stroud CK, Nakamura MT, Lila MA, Erdman JW., Jr Serum testosterone is reduced following short-term phytofluene, lycopene, or tomato powder consumption in F344 rats. J Nutr. 2006;136:2813–2819. doi: 10.1093/jn/136.11.2813. [DOI] [PubMed] [Google Scholar]
- 85.Boileau TW, Clinton SK, Erdman JW., Jr Tissue lycopene concentrations and isomer patterns are affected by androgen status and dietary lycopene concentration in male F344 rats. J Nutr. 2000;130:1613–1618. doi: 10.1093/jn/130.6.1613. [DOI] [PubMed] [Google Scholar]
- 86.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. J Nutr. 2006;136:1552–1557. doi: 10.1093/jn/136.6.1552. [DOI] [PubMed] [Google Scholar]
- 87.Paolini M, Cantelli-Forti G, Perocco P, Pedulli GF, Abdel-Rahman SZ, Legator MS. Co-carcinogenic effect of β-carotene. Nature. 1999;398:760–761. doi: 10.1038/19655. [DOI] [PubMed] [Google Scholar]
- 88.Zhao Z, Khachik F, Richie JP, Jr, Cohen LA. Lycopene uptake and tissue disposition in male and female rats. Proc Soc Exp Biol Med. 1998;218:109–114. doi: 10.3181/00379727-218-44283a. [DOI] [PubMed] [Google Scholar]
- 89.Cohen LA. A review of animal model studies of tomato carotenoids, lycopene, and cancer chemoprevention. Exp Biol Med (Maywood) 2002;227:864–868. doi: 10.1177/153537020222701005. [DOI] [PubMed] [Google Scholar]
- 90.Furukawa F, Nishikawa A, Kasahara K, Lee IS, Wakabayashi K, Takahashi M, Hirose M. Inhibition by β-carotene of upper respiratory tumorigenesis in hamsters receiving diethylnitrosamine followed by cigarette smoke exposure. Jpn J Cancer Res. 1999;90:154–161. doi: 10.1111/j.1349-7006.1999.tb00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Wadei HA, Schuller HM. β-Carotene promotes the development of NNK-induced small airway-derived lung adenocarcinoma. Eur J Cancer. 2009;45:1257–1264. doi: 10.1016/j.ejca.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen GD, Perou CM, Whyte RI, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adissu HA, Schuller HM. Antagonistic growth regulation of cell lines derived from human lung adenocarcinomas of Clara cell and aveolar type II cell lineage: implications for chemoprevention. Int J Oncol. 2004;24:1467–1472. [PubMed] [Google Scholar]
- 94.Oreffo VI, Lin HW, Padmanabhan R, Witschi H. K-ras and p53 point mutations in 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced hamster lung tumors. Carcinogenesis. 1993;14:451–455. doi: 10.1093/carcin/14.3.451. [DOI] [PubMed] [Google Scholar]
- 95.Schuller HM, Cekanova M. NNK-induced hamster lung adenocarcinomas over-express β2-adrenergic and EGFR signaling pathways. Lung Cancer. 2005;49:35–45. doi: 10.1016/j.lungcan.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 96.Al-Wadei HA, Schuller HM. Cyclic adenosine monophosphate-dependent cell type-specific modulation of mitogenic signaling by retinoids in normal and neoplastic lung cells. Cancer Detect Prev. 2006;30:403–411. doi: 10.1016/j.cdp.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schuller HM, Witschi HP, Nylen E, Joshi PA, Correa E, Becker KL. Pathobiology of lung tumors induced in hamsters by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the modulating effect of hyperoxia. Cancer Res. 1990;50:1960–1965. [PubMed] [Google Scholar]
- 98.Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- 99.Koppang N, Rivenson A, Dahle HK, Hoffmann D. A study of tobacco carcinogenesis, LIII: carcinogenicity of N′-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in mink (Mustela vison) Cancer Lett. 1997;111:167–171. doi: 10.1016/s0304-3835(96)04507-7. [DOI] [PubMed] [Google Scholar]
- 100.van Breemen RB, Xu X, Viana MA, Chen L, Stacewicz-Sapuntzakis M, Duncan C, Bowen PE, Sharifi R. Liquid chromatography-mass spectrometry of cis- and all-trans-lycopene in human serum and prostate tissue after dietary supplementation with tomato sauce. J Agric Food Chem. 2002;50:2214–2219. doi: 10.1021/jf0110351. [DOI] [PubMed] [Google Scholar]
- 101.Vogt TM, Mayne ST, Graubard BI, Swanson CA, Sowell AL, Schoenberg JB, Swanson GM, Greenberg RS, Hoover RN, Hayes RB, et al. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US blacks and whites. Am J Epidemiol. 2002;155:1023–1032. doi: 10.1093/aje/155.11.1023. [DOI] [PubMed] [Google Scholar]
- 102.Lu QY, Hung JC, Heber D, Go VL, Reuter VE, Cordon-Cardo C, Scher HI, Marshall JR, Zhang ZF. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:749–756. [PubMed] [Google Scholar]
- 103.Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr. 1996;126:129–137. doi: 10.1093/jn/126.1.129. [DOI] [PubMed] [Google Scholar]
- 104.Marangon K, Herbeth B, Lecomte E, Paul-Dauphin A, Grolier P, Chancerelle Y, Artur Y, Siest G. Diet, antioxidant status, and smoking habits in French men. Am J Clin Nutr. 1998;67:231–239. doi: 10.1093/ajcn/67.2.231. [DOI] [PubMed] [Google Scholar]
- 105.Lee CM, Boileau AC, Boileau TW, Williams AW, Swanson KS, Heintz KA, Erdman JW., Jr Review of animal models in carotenoid research. J Nutr. 1999;129:2271–2277. doi: 10.1093/jn/129.12.2271. [DOI] [PubMed] [Google Scholar]
- 106.Huang CS, Chuang CH, Hu ML. Effects of lycopene supplementation on plasma and tissue lycopene levels in various rodent strains. Int J Vitamin Nutr Res. 2006;76:377–384. doi: 10.1024/0300-9831.76.6.377. [DOI] [PubMed] [Google Scholar]
- 107.Palozza P, Krinsky NI. Antioxidant effects of carotenoids in vivo and in vitro: an overview. Methods Enzymol. 1992;213:403–420. doi: 10.1016/0076-6879(92)13142-k. [DOI] [PubMed] [Google Scholar]
- 108.Handelman GJ, Packer L, Cross CE. Destruction of tocopherols, carotenoids, and retinol in human plasma by cigarette smoke. Am J Clin Nutr. 1996;63:559–565. doi: 10.1093/ajcn/63.4.559. [DOI] [PubMed] [Google Scholar]
- 109.Begum AN, Terao J. Protective effect of quercetin against cigarette tar extract-induced impairment of erythrocyte deformability. J Nutr Biochem. 2002;13:265–272. doi: 10.1016/s0955-2863(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 110.Wurzel H, Yeh CC, Gairola C, Chow CK. Oxidative damage and antioxidant status in the lungs and bronchoalveolar lavage fluid of rats exposed chronically to cigarette smoke. J Biochem Toxicol. 1995;10:11–17. [PubMed] [Google Scholar]
- 111.Palozza P, Serini S, Trombino S, Lauriola L, Ranelletti FO, Calviello G. Dual role of β-carotene in combination with cigarette smoke aqueous extract on the formation of mutagenic lipid peroxidation products in lung membranes: dependence on Po2. Carcinogenesis. 2006;27:2383–2391. doi: 10.1093/carcin/bgl074. [DOI] [PubMed] [Google Scholar]
- 112.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 113.Wertz K, Siler U, Goralczyk R. Lycopene: modes of action to promote prostate health. Arch Biochem Biophys. 2004;430:127–134. doi: 10.1016/j.abb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 114.Rao AV, Ray MR, Rao LG. Lycopene. Adv Food Nutr Res. 2006;51:99–164. doi: 10.1016/S1043-4526(06)51002-2. [DOI] [PubMed] [Google Scholar]
- 115.Stahl W, Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- 116.Agarwal S, Rao AV. Carotenoids and chronic diseases. Drug Metabol Drug Interact. 2000;17:189–210. doi: 10.1515/dmdi.2000.17.1-4.189. [DOI] [PubMed] [Google Scholar]
- 117.Mayne ST, Handelman GJ, Beecher G. β-Carotene and lung cancer promotion in heavy smokers—a plausible relationship? J Natl Cancer Inst. 1996;88:1513–1515. doi: 10.1093/jnci/88.21.1516-a. [DOI] [PubMed] [Google Scholar]
- 118.Arora A, Willhite CA, Liebler DC. Interactions of β-carotene and cigarette smoke in human bronchial epithelial cells. Carcinogenesis. 2001;22:1173–1178. doi: 10.1093/carcin/22.8.1173. [DOI] [PubMed] [Google Scholar]
- 119.Weitberg AB, Corvese D. Effect of vitamin E and β-carotene on DNA strand breakage induced by tobacco-specific nitrosamines and stimulated human phagocytes. J Exp Clin Cancer Res. 1997;16:11–14. [PubMed] [Google Scholar]
- 120.Bogos K, Renyi-Vamos F, Kovacs G, Tovari J, Dome B. Role of retinoic receptors in lung carcinogenesis. J Exp Clin Cancer Res. 2008;27:18. doi: 10.1186/1756-9966-27-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu C, Russell RM, Wang XD. Exposing ferrets to cigarette smoke and a pharmacological dose of β-carotene supplementation enhance in vitro retinoic acid catabolism in lungs via induction of cytochrome P450 enzymes. J Nutr. 2003;133:173–179. doi: 10.1093/jn/133.1.173. [DOI] [PubMed] [Google Scholar]
- 122.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 123.Hirsch DD, Stork PJ. Mitogen-activated protein kinase phosphatases inactivate stress-activated protein kinase pathways in vivo. J Biol Chem. 1997;272:4568–4575. doi: 10.1074/jbc.272.7.4568. [DOI] [PubMed] [Google Scholar]
- 124.Lee HY, Sueoka N, Hong WK, Mangelsdorf DJ, Claret FX, Kurie JM. All-trans-retinoic acid inhibits Jun N-terminal kinase by increasing dual-specificity phosphatase activity. Mol Cell Biol. 1999;19:1973–1980. doi: 10.1128/mcb.19.3.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chung J, Chavez PR, Russell RM, Wang XD. Retinoic acid inhibits hepatic Jun N-terminal kinase-dependent signaling pathway in ethanol-fed rats. Oncogene. 2002;21:1539–1547. doi: 10.1038/sj.onc.1205023. [DOI] [PubMed] [Google Scholar]
- 126.Liu C, Russell RM, Wang XD. Low dose β-carotene supplementation of ferrets attenuates smoke-induced lung phosphorylation of JNK, p38, MAPK, and p53 proteins. J Nutr. 2004;134:2705–2710. doi: 10.1093/jn/134.10.2705. [DOI] [PubMed] [Google Scholar]
- 127.Palozza P, Simone R, Mele MC. Interplay of carotenoids with cigarette smoking: implications in lung cancer. Curr Med Chem. 2008;15:844–854. doi: 10.2174/092986708783955400. [DOI] [PubMed] [Google Scholar]
- 128.Gradelet S, Leclerc J, Siess MH, Astorg PO. β-Apo-8′-carotenal, but not β-carotene, is a strong inducer of liver cytochromes P4501A1 and 1A2 in rat. Xenobiotica. 1996;26:909–919. doi: 10.3109/00498259609052493. [DOI] [PubMed] [Google Scholar]
- 129.Salgo MG, Cueto R, Winston GW, Pryor WA. β Carotene and its oxidation products have different effects on microsome mediated binding of benzo[a]pyrene to DNA. Free Radic Biol Med. 1999;26:162–173. doi: 10.1016/s0891-5849(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 130.Perocco P, Paolini M, Mazzullo M, Biagi GL, Cantelli-Forti G. β-Carotene as enhancer of cell transforming activity of powerful carcinogens and cigarette-smoke condensate on BALB/c 3T3 cells in vitro. Mutat Res. 1999;440:83–90. doi: 10.1016/s1383-5718(99)00009-1. [DOI] [PubMed] [Google Scholar]
- 131.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 132.Liu B, Lee HY, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, Cohen P. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-α regulate transcriptional signaling and apoptosis. J Biol Chem. 2000;275:33607–33613. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- 133.Giovannucci E. Insulin-like growth factor-I and binding protein-3 and risk of cancer. Horm Res. 1999;51(suppl 3):34–41. doi: 10.1159/000053160. [DOI] [PubMed] [Google Scholar]
- 134.Saffiotti U, Montesano R, Sellakumar AR, Borg SA. Experimental cancer of the lung. Inhibition by vitamin A of the induction of tracheobronchial squamous metaplasia and squamous cell tumors. Cancer. 1967;20:857–864. doi: 10.1002/1097-0142(1967)20:5<857::aid-cncr2820200545>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 135.Nettesheim P, Williams ML. The influence of vitamin A on the susceptibility of the rat lung to 3-methylcholanthrene. Int J Cancer. 1976;17:351–357. doi: 10.1002/ijc.2910170311. [DOI] [PubMed] [Google Scholar]
- 136.Dahl AR, Grossi IM, Houchens DP, Scovell LJ, Placke ME, Imondi AR, Stoner GD, De Luca LM, Wang D, Mulshine JL. Inhaled isotretinoin (13-cis retinoic acid) is an effective lung cancer chemopreventive agent in A/J mice at low doses: a pilot study. Clin Cancer Res. 2000;6:3015–3024. [PubMed] [Google Scholar]
- 137.Kim DJ, Takasuka N, Kim JM, Sekine K, Ota T, Asamoto M, Murakoshi M, Nishino H, Nir Z, Tsuda H. Chemoprevention by lycopene of mouse lung neoplasia after combined initiation treatment with DEN, MNU and DMH. Cancer Lett. 1997;120:15–22. doi: 10.1016/s0304-3835(97)00281-4. [DOI] [PubMed] [Google Scholar]
- 138.IARC Working Group on the Evaluation of Cancer Preventive Agents, author. IARC Handbook on Cancer Prevention. Lyon, France: IARC; 1998. Carotenoids. [Google Scholar]
- 139.Paolini M, Antelli A, Pozzetti L, Spetlova D, Perocco P, Valgimigli L, Pedulli GF, Cantelli-Forti G. Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in β-carotene supplemented rats. Carcinogenesis. 2001;22:1483–1495. doi: 10.1093/carcin/22.9.1483. [DOI] [PubMed] [Google Scholar]